Abstract
Phencyclidine (PCP) and other N-methyl-D-aspartate (NMDA) receptor antagonists have been shown to be neurotoxic to developing brains and to result in schizophrenia-like behaviors later in development. Prevention of both effects by antischizophrenic drugs suggests the validity of PCP neurodevelopmental toxicity as a heuristic model of schizophrenia. Lithium is used for the treatment of bipolar and schizoaffective disorders and has recently been shown to have neuroprotective properties. The present study used organotypic corticostriatal slices taken from postnatal day 2 rat pups to investigate the protective effect of lithium and the role of the phosphatidylinositol-3 kinase (PI-3K)/Akt and mitogen-activated protein kinase kinase/extracellular regulated kinase (MEK/ERK) pathways in PCP-induced cell death. Lithium pretreatment dose-dependently reduced PCP-induced caspase-3 activation and DNA fragmentation in layer II-IV of the cortex. PCP elicited time-dependent inhibition of the MEK/ERK and PI-3K/Akt pathways, as indicated by dephosphorylation of ERK1/2 and Akt. The pro-apoptotic factor glycogen synthase kinase-3β (GSK-3β) was also dephosphorylated at serine 9 and thus activated. Lithium prevented PCP-induced inhibition of the two pathways and activation of GSK-3β. Furthermore, blocking either PI-3K/Akt or MEK/ERK pathway abolished the protective effect of lithium, while inhibiting GSK-3β activity mimicked the protective effect of lithium. However, no crosstalk between the two pathways was found. Finally, specific GSK-3β inhibition did not prevent PCP-induced dephosphorylation of Akt and ERK. These data strongly suggest that the protective effect of lithium against PCP-induced neuroapoptosis is mediated through independent stimulation of the PI-3K/Akt and ERK pathways and suppression of GSK-3β activity.
Introduction
Phencyclidine and other antagonists of the N-methyl-D-aspartate (NMDA) receptor-ion channel complex such as MK-801 and ketamine have been shown to cause schizophrenia-like symptoms in adult humans (Javitt and Zukin, 1991) and have been used in animals to model this disease (du Bois and Huang, 2007). More recently, it has been shown that rats treated with PCP or ketamine during early postnatal development demonstrated schizophrenia-like behavioral changes during early adolescence or as young adults (Wang et al., 2001; Harris et al., 2003; Fredriksson et al., 2004). The ability of olanzapine to prevent PCP-induced neuroapoptosis and behavioral deficits suggests the usefulness of this model in studying the pathophysiology of schizophrenia (Wang et al., 2001). Determining the precise mechanisms of PCP-induced neurodevelopmental apoptosis may help form appropriate strategies for selecting druggable targets for the treatment of schizophrenia.
During the third trimester in humans and the first three postnatal weeks in rodents, neuronal survival critically depends upon trophic support from neurotrophins, such as brain-derived neurotrophin factor (BDNF) and nerve growth factor (NGF), as well as neurotransmitters, such as glutamate (Pettmann and Henderson, 1998). It has been postulated that deprivation of glutamatergic trophic support disrupts normal neuronal circuitry formation and may underlie the expression of some mental diseases in later life, including schizophrenia (du Bois and Huang, 2007). The NMDAR is thought to be the principle mediator of glutamate trophic activity in the central nervous system (Balazs et al., 1988). The mechanisms underlying NMDAergic trophic support are not completely understood, though involvement of pro-survival signaling transductions coupled to NMDAR has been proposed. For example, the extracellular signal-regulated kinase (ERK) signaling has been implicated in NMDA-mediated protection against glutamate toxicity in cerebellar granule neurons (CGN) (Zhu et al., 2005). Furthermore, in developing brain, NMDAR blockade by MK-801 decreased ERK activity, though ERK activation by transgenic synRas only partially prevented MK-801-induced neuronal death in neonatal rats (Hansen et al., 2004), implying the involvement of other pathways.
The phosphotydlinositide-3 kinase (PI-3K)/Akt pathway is another well established pro-survival pathway and has been demonstrated to mediate the pro-survival action of NMDA in CGN (Zhang et al. 1998). PI-3K/Akt promotes cell survival by phosphorylating and inhibiting pro-apoptotic proteins such as caspase-9 (Cardone et al., 1998) and the serine/threonine kinase, glycogen synthase kinase-3β (GSK-3β) (Cross et al., 1995). Recently, our laboratory demonstrated that PCP, a NMDAR blocker, inhibits the PI-3K/Akt pathway and activates GSK-3β both in cortical cell culture and in intact neonatal rats (Lei et al., 2008). Importantly, activation of the PI-3K/Akt pathway by enhancing synaptic NMDAR strength prevented PCP-induced neuroapoptosis (Lei et al., 2008). Furthermore, consistent with reports from other laboratories (Takadera and Ohyashiki, 2004; Takadera et al., 2004), we found that the anti-apoptotic property of the PI-3K/Akt pathway is largely mediated by inhibiting activity of GSK-3β, as GSK-3 siRNA and lithium, prevented PCP-induced neuronal death (Lei et al., 2008).
Lithium is currently used in the treatment of bipolar disorder, as well as schizoaffective disorder, though its therapeutic mechanism is uncertain (Burgess et al., 2001). Lithium has been shown to inhibit GSK-3β activity through direct or indirect mechanisms (Klein and Melton, 1996; Kirshenboim et al., 2004). Recently, increasing evidence suggests that lithium also protects against apoptosis induced by a variety of insults in cultured neurons, including growth factor withdrawal (Jin et al., 2005), β-amyloid administration (Alvarez et al., 1999), and glutamate treatment (Nonaka et al., 1998). Consequently, several targets other than GSK-3β have been proposed to account for lithium protection. For example, lithium has been shown to act on ERK, PI-3K, Akt, and phospholipase C (Kang et al., 2003; Pardo et al., 2003; Sasaki et al., 2006). Therefore, it is possible that lithium may prevent PCP-induced cell death in primary neuronal culture through mechanisms in addition to GSK-3β inhibition.
This study was designed to investigate the potential protective effect of lithium on PCP-induced cell death as well as the underlying mechanisms in organotypic brain slice culture, an in vitro model that resembles living tissue in situ more closely than primary neuronal culture (Vickers and Fisher, 2004). We hypothesize that lithium protects against PCP-induced cell death by acting on the PI-3K/Akt and ERK pathways in organotypic culture.
Methods
Animals
Timed pregnant female Sprague–Dawley rats were obtained on day 14 or 18 of pregnancy from Charles River. They were housed individually with a regular 12-hour light: 12-hour dark cycle (lights on at 07:00 h. off at 19:00 h) with food and water available ad libitum. On postnatal day (PN) 2.5, the pups were killed by decapitation and their brains were removed and processed for slice culture as described below.
The protocol under which this study was performed was approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee.
Reagents
PCP was acquired from the National Institute on Drug Abuse (Rockville, MD, USA) and dissolved in distilled water. Slice culture media including Hank's balanced salt solution, heat inactivated horse serum, OPTI-MEM medium, neurobasal medium and B-27 supplement were purchased from Invitrogen Corporation (Carlsbad, CA, USA). 10% D-(+)-Glucose solution, 200 mM L-glutatmine, and penicillin/streptomycin solution were purchased from Sigma-Aldrich (St. Louis, MO, USA). 7-amino-4-trifluorocumarin (AFC), the caspase-3 substrate acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluorocumarin (Ac-DEVD-AFC) and the caspase-3 inhibitor z-DEVD-FMK were purchased from MP Biomedicals (Livermore, CA, USA). Primary antibodies against phopho- GSK-3β (Ser9), phosphor-AKT (Ser473), phospho-p44/42 ERK (Thr202/Tyr204), total AKT, total ERK, total GSK-3 were obtained from Cell Signaling Technology, Inc. (Danvers, MA 01923). Mouse monoclonal anti-actin antibody, HRP-conjugated anti-mouse and anti-rabbit secondary antibodies were obtained from Chemicon. Deoxynucleotidyl transferase (TdT) and biotin-16-dUTP were purchased from Roche Diagnostics (Indianapolis, IN, USA). ABC Elite Kit and Vector SG peroxidase substrate were from Vector Laboratories (Burlingame, CA, USA). LY294002 (PI-3K inhibitor), AR-A014418 (GSK-3β inhibitor), PD98059 (MEK inhibitor) were purchased from CalBiochem/EMD Biosciences. Akt inhibitor, triciribine (TCN, Yang et al., 2004), was a generous gift from the laboratory of Dr. Xiaodong Cheng in UTMB. Lithium chloride was purchased from Sigma (St. Louis, MO, USA).
Drug treatments
All experiments with brain slice cultures were done on day in vitro (DIV) 10. For experiments using PCP only, PCP (3 μM) was added to the medium at indicated time points before sampling. For experiments using lithium chloride (LiCl) or AR-A014418 (AR-A), drugs were added either 30 min (LiCl) or one hour (AR-A) before PCP (3 μM) and left in the medium with PCP until sampling. For experiments using PI-3K, Akt, or ERK inhibitors, slices were preincubated with individual kinase inhibitors for an hour before LiCl treatment (10 mM) and then challenged with 3 μM PCP 30 minutes later without drug removal.
Organotypic slice culture
This study used cultures of organotypic brain slices, an in vitro model that conserves the biologically relevant structural and functional features of in vivo tissues (Vickers and Fisher, 2004), while also allowing manipulation of drugs that can not easily gain access to the brain in vivo. Corticostriatal slice cultures were prepared as previously prescribed (Wang and Johnson, 2007). In brief, two day-old rat pups were sacrificed by decapitation. The brains were removed quickly and cut into 400-μm-thick coronal sections under sterile conditions. Three adjacent frontal corticostriatal slices with morphology comparable to levels between A5.3 and A6.8 mm in P10 rats (Sherwood and Timiras, 1970) were placed and cultured in inserts with a porous and translucent membranes (Culture Plate Insert, MILLIPORE Co, Bedford, MA) at the interface between medium and a CO2-enriched atmosphere. The initial culture medium was a mixture of 25% inactivated horse serum, 25% Hank's balanced salt solution, and 50% OPTI-MEM culture medium, supplemented with 25 mM D-glucose and 1% penicillin/streptomycin. On DIV 3, the medium was switched to serum-free Neurobasal medium supplemented with 25 mM D-glucose, 1 mM glutamine, 2% B-27, and 1% penicillin/streptomycin. The medium was changed twice a week thereafter. Slices were ready for experimental conduction on DIV 10.
Caspase-3 Activity Assay
Slice samples used for caspase-3 activity assay were always collected 12 hours after PCP treatment. Caspase-3 activity of slice was measured as previously described (Wang and Johnson, 2007). Briefly, slices were sonicated in ice-cold lysis buffer containing 25 mM HEPES (pH 7.4), 5 mM MgCl2, 1.5 mM EDTA, 1.0 mM EGTA, 1 mM dithiothreitol, 0.1% Triton X-100 and 1% protease inhibitor cocktail. After sitting on ice for 15 min, sonicates were centrifuged at 13,000 g for 5 min at 4°C. The supernatants were then collected for measurement of caspase-3 activity. Protein level of the samples was measured by using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). To measure the enzyme activity, each sample was prepared in two parallel sets. One set consisted of an equal volume of supernatant and assay buffer [100 mM HEPES (pH 7.4), 2 mM dithiothreitol, 0.1% CHAPS, and 1% sucrose]. The other set was a mix of equal volume of the same supernatant and assay buffer containing the selective caspase-3 inhibitor, z-DEVD-FMK (0.5 μM). After incubation at room temperature for 15 min, the caspase-3 substrate, Ac-DEVD-AFC (25 μM), was added and the samples were then incubated at 37°C for 60 min. Fluorescence resulting from cleavage of the substrate was monitored using the microplate fluorometer (Fluoroskan Ascent, Labsystems, Helsinki, Finland) at excitation and emission wavelengths of 405 and 510 nm, respectively. 7-Amino-4-trifluoromethyl-cumarin (AFC) was used as a fluorescent standard. Caspase-3 activity was calculated as the difference of enzyme activities in samples incubated without and with caspase-3 inhibitor and then normalized to protein level. Final caspase-3 activity shown in figures was presented as percentage of control group.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)
Slice samples used for TUNEL were always collected 12 hours after PCP treatment.Slices were first rinsed with 0.01 M PBS (pH 7.2) and then fixed with ice-cold 2% paraformaldehyde in 0.1 M PB (pH 7.2) at room temperature for 1 hour. After washing with 0.01M PBS (pH 7.2), slices were dehydrated and rehydrated in a sequential ethanol (70%, 90%, 100%), incubated with pepsin (0.04% in 10 mM HCl) for 15 min and endogenous peroxidase was then quenched with 3.0% hydrogen peroxide in methanol for 10 min. After washing with PBS and pre-incubation with TdT (terminal deoxynucleotidyl transferase) reaction buffer (30 mM Tris-HCl, pH 7.2, 140 mM Na cacodylate and 1 mM CoCl2) for 15 min, slices were incubated with biotin-16-dUTP (10 nmoles/ml) and TdT (200 U/ml) in the TdT buffer in a humidified chamber for 2 hours at 37°C. Slices were then washed in PBS, incubated with ABC reagents for 60 min and stained with a filtered mixture of Vector SG peroxidase substrate. For quantification, stained TUNEL-positive cells were counted in photomicrograph of one microscopic field of each slice using a computer-based image analysis program, SimplePCI (Compix Inc. Imaging Systems, Cranberry Township, PA).
Western blot analysis
Slices were collected and sonicated in 0.3 ml of lysis buffer (50 mM HEPES, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 20 mM NaF, 0.5% NP40) containing the protease inhibitors cocktail and phenylmethlylsulfonyl fluoride (PMSF, 10 μl/ml), phosphatase inhibitor cocktail I and II (Sigma). After sitting on ice for 15 min, sonicates were centrifuged at 10,000 g for 10 min. The supernatants were collected for immediate use or stored at −80 °C. After measuring protein concentration, equal amounts of protein (30 μg) were loaded and separated on 10% SDS-polyacrylamide gels with a Tris–glycine running buffer system and then transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking in 5% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST), the membranes were incubated overnight at 4°C with specific antibodies [anti-phospho-Ser-9-GSK-3β (1:2,000), anti-phospho-Ser473-Akt (1:2,000), anti-phospho-Thr202/Tyr204 ERK1/2 (1: 2,000)] and then incubated with HRP-conjugated anti-mouse or anti-rabbit secondary antibody for 1 hour at room temperature (RT). After extensive washes in TBST, blots were visualized by enhanced chemiluminescence (ECLplus) according to the manufacturer's instruction. To ensure equivalent protein loading, membranes were stripped and re-probed with anti-actin antibody, which was subsequently used to normalize the western analyses.
Total protein levels of ERK, Akt, and GSK-3β in the same samples were determined in another membrane. Membranes were incubated with anti-ERK, anti-Akt, or anti-GSK-3β antibodies at a dilution of 1:1,000 for 2 hours at RT and then with secondary antibodies for 1 hour. Blots were developed as indicated above. Equivalent protein loading was determined by stripping and re-probing the membrane with anti-actin antibody.
The bands were scanned and densitometrically analyzed using an automatic image analysis system (Alpha Innotech Corporation, San Leandro, CA). All target proteins were quantified by normalizing to β-actin re-probed on the same membrane and then calculated as percentage of control group.
Statistical analysis
All experimental data are presented as mean ± S.E. One-way ANOVA or two-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons, was used to determine differences among more than two groups. Differences were considered significant when p<0.05.
Results
It has been previously demonstrated that PCP causes neuronal apoptosis in developing rat brain (Wang et al., 2001; Wang and Johnson, 2007) as well as in primary culture of dissociated neurons (Lei et al., 2008) and in organotypic corticostriatal slices (Wang and Johnson, 2007). PCP and dizolcipine (MK-801) are relatively potent blockers of open NMDA channels. In primary culture of dissociated cortical neurons, PCP-induced neurotoxicity is prevented by 10 μM NMDA (Lei et al., 2008). This strongly suggests that PCP-induced apoptosis is due to blockade of NMDA receptors. To further support this contention, we compared the potency of MK-801 and PCP as NMDA channel blockers to cause caspase-3 activation in cultured organotypic corticostriatal slices. As ionization is known to affect NMDA binding potency, it is important to note that the pKa values for MK-801 and PCP are 8.37 and 8.5, respectively (Dravid et al., 2007). The EC50 values for caspase-3 activation were determined to be 23±3 nM for MK-801 and 426±160 nM for PCP. The corresponding Ki values from published binding experiments are 3.8 nM and 52 nM (Javitt and Zukin, 1989). The calculated EC50/Ki values for MK-801 and PCP are 6.5 and 8.2, respectively. The similarity in these values for PCP and MK-801 provides additional support for the contention that the PCP-induced increase in caspase-3 activity is mediated by blockade of the NMDA receptor.
Lithium chloride protects corticostriatal slices from PCP-induced neurotoxicity
Our previous study has demonstrated that staining of caspase-3 immunoreactivity was well correlated with caspase-3 enzymatic activity as well as with TUNEL staining (Wang and Johnson, 2007). Therefore, in the current study we used both caspase-3 activation and DNA fragmentation as detected by TUNEL as two indices of PCP-induced apoptosis. In this study, corticostriatal slices were first incubated with a series concentration of lithium (0, 0.3, 1, 3, 10 mM). 30 minutes later, PCP (3 μM) was added to the medium without washing lithium out. 12 hours after PCP treatment, slices were collected for either caspase-3 enzymatic assay or TUNEL staining. As previously reported, PCP caused robust caspase-3 activation and DNA fragmentation (as indicated by positive TUNEL staining) that was primarily restricted to layers II to IV in the cortex (Wang and Johnson, 2007). Lithium pretreatment prevented PCP-induced caspase-3 activation (F 6, 35 =23.365, p<0.001) and DNA fragmentation (F 5, 30 =8.671, p=0.001) in a concentration-dependent manner (Fig. 1). Lithium showed no protective effect at either 0.3 or 1 mM (p>0.05 vs. PCP). 3 mM lithium partially inhibited PCP-induced caspase-3 activation (p>0.05 vs. control, p>0.05 vs. PCP) and DNA fragmentation (p<0.05 vs. PCP; p<0.05 vs. control), while 10 mM lithium completely blocked PCP-induced cell death (p<0.001 vs. PCP). 10 mM lithium did not cause any toxicity to the slice (p>0.05 vs. control).
Fig.1.
Effect of lithium on PCP-induced cell death. Corticostriatal slices were pretreated with various concentrations of lithium chloride (LiCl) on DIV 10 and then challenged with PCP (3 μM) 30 minutes later without washing LiCl out. Cell death was assessed 12 hours after PCP treatment by measuring caspase-3 activity or TUNEL staining in the cortex. Untreated slices were used as control (CTRL). A. Lithium blocked PCP-induced caspase-3 activation dose-dependently. B. Representative pictures showing TUNEL-positive staining in the cortex. C. Quantitative analysis of TUNEL-positive staining. * p< 0.05 vs. CTRL; # p<0.05 vs. PCP. One-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons.
PCP inhibits the PI-3K/Akt/GSK-3β and MEK/ERK pathways
Upon activation, NMDAR allows calcium influx, leading to activation of the PI-3K/Akt/GSK-3β and MEK/ERK signaling pathways, which have been shown to mediate the pro-survival effect of NMDAR (Zhang et al., 1998; Lei et al., 2008). Therefore, we proposed that PCP may cause cell death by inhibiting the two NMDAR-coupled pro-survival pathways. To test this idea, PCP (3 μM) was added to medium on DIV 10 and incubated with slices for 0 hour, 30 minutes, 2 hours, 4 hours, 8 hours or 24 hours before sampling. Untreated slices were used as controls and are presented as “0 h after PCP” in the figure. Slices were then homogenized and total protein was extracted for western blot analysis of phosphorylation level as well as total level of Akt, GSK-3β, and ERK1/2, as described in material and methods. As shown in figure 2, PCP caused time-dependent decrease in phosphorylation of ERK (F6, 55=13.742, p<0.001), Akt (F6, 69=14.298, p<0.001), and GSK-3β (F6, 57=5.898, p<0.001) without changing total protein levels (data not shown). However, the kinetics of dephosphorylation of the three kinases induced by PCP was different. PCP showed a fast and strong inhibitory effect on the ERK1/2 activity. 30 minutes after PCP treatment, p-ERK1/2 level already decreased to 49.8 % of the control level (p<0.05) and was stable at this level thereafter until 24 hour at the end of sampling (38.9 % of control, p<0.05). Compared to the action on p-ERK1/2, PCP-induced dephosphorylation of Akt and GSK-3 β was slower. No significant decrease of p-Akt was observed at the time points of 30 min and 1 h (p>0.05). 2 hours after PCP treatment, p-Akt decreased to 79.1% of control level (p<0.05) and continue to decrease with time. At 24 hours, p-Akt already decreased to 56.1 % of control (p<0.05). Significant decrease of p-GSK-3β was observed at 1 h after PCP treatment (74.2 % of control, p<0.05) and lasted until 24 hours (48 % of control, p<0.05). These data suggest that PCP may cause cell death through inhibiting the PI-3K/Akt/GSK-3β and MEK/ERK1/2 pro-survival pathways.
Fig. 2.
Temporal effect of PCP on phosphorylation of ERK1/2, Akt and GSK-3β in cultured corticostriatal slices. Corticostriatal slices were incubated with PCP (3 μM) for 30minutes, 1, 2 , 4 , 8, or 24 hours on DIV 10 and total protein was extracted for western blotting analysis of phosphorylation of Thr202/Tyr204-ERK1/2, Ser473-AKT and Ser9-GSK-3β. Untreated slices were used as control and presented as “0 h after PCP” in the figure. Phosphorylation levels of ERK, Akt, and GSK-3β were normalized to β-actin re-probed on the same membrane and then calculated as percentage of control (0h after PCP). * p-ERK, p< 0.05 vs. control (0 h); # p-Akt, p< 0.05 vs. control (0 h); ^ p- GSK-3β, p< 0.05 vs. control (0 h). One-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons.
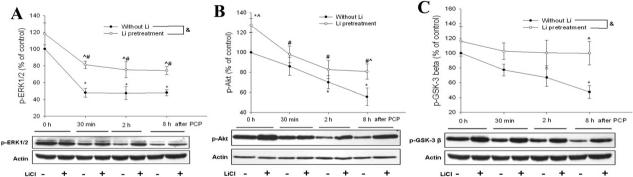
Lithium prevents PCP-induced inhibition of the PI-3K/Akt/GSK-3βand MEK/ERK pathways
To determine whether PI-3K/Akt/GSK-3β and MEK/ERK1/2 pathways were also involved in lithium's prevention of PCP-induced cell death, we first examined the effects of lithium on the kinetics of dephosphorylation of ERK, Akt, and GSK-3β induced by PCP. Slices were incubated with PCP (3 μM) for 0 h, 30 min, 2 h, or 8 h in the presence or absence of LiCl (10 mM) that was added 30 minutes before PCP. Phosphorylation of ERK1/2, Akt and GSK-3β was measured by western blot analysis. Two-way analysis of variance (ANOVA), with lithium treatment and time as two independent factors, revealed that pre-incubating slices with lithium for 30 minutes effectively prevented PCP-evoked dephosphorylation of ERK, Akt, GSK-3β (p<0.001, “without Li” vs “Li pretreatment”) (Fig. 3). More specifically, lithium pretreatment did not increase basal p-ERK1/2 (p>0.05), but significantly attenuated the PCP-induced decrease in p-ERK1/2 at the three time points examined (30 min, 2 h, 8 h) (p<0.05). Pre-incubating slices with lithium alone for 30 minutes increased basal p-Akt and p-GSK-3β by 27.2% (p<0.05) and 16.3% (p>0.05), respectively. This stimulating effect of lithium was suppressed 30 minutes after addition of PCP to the medium. Furthermore, the level of p-Akt and p-GSK-3β decreased significantly in the “without Li” group at the 2 h and 8 h time points (p<0.05), while they were still near the basal level in the “Li pretreatment” group (p>0.05). At 8 h, “Li pretreatment” significantly increased p-Akt and p-GSK-3β relative to the “without Li” group (p<0.05).
Fig. 3.
Effects of lithium on temporal kinetics of PCP-induced dephosphorylation of ERK1/2 (A), Akt (B) and GSK-3β (C) in cultured corticostriatal slices. On DIV 10, corticostriatal slices, preincubated with or without lithium chloride (LiCl, 10 mM) for 30 minutes, were incubated with PCP (3 μM) for 0, 30 minutes, 2 hours, or 8 hours. Untreated slices were as control and presented as “0 h after PCP” in the “without Li” group. The group treated with LiCl (10 mM) alone for 30 minutes was presented as “0h after PCP” in the “Li pretreatment” group. Phosphorylation levels of ERK (A), Akt (B), and GSK-3β (C) were normalized to β-actin re-probed on the same membrane and presented as percentage of control. &: p<0.05, “without Li” vs. “Li pretreatment”, two-way ANOVA, with time and LiCl treatment as two independent factors. * p<0.05 vs. control; # p<0.05 vs. “0h after PCP” of the “Li pretreatment” group; ^ p<0.05, “without Li” vs. “Li pretreatment” at the same time point; Student-Newman-Keuls test for multiple comparisons following two-way ANOVA.
The role of PI-3K/Akt/GSK-3β pathway in lithium-mediated protection
As has been shown above, we observed that PCP inhibits, while lithium pretreatment stimulates the PI-3K/Akt pathway. In order to investigate whether stimulation of this pathway contributes to the neuroprotective effects of lithium, we inhibited the PI-3K/Akt pathway pharmacologically with PI-3K inhibitor, LY294002 (30 μM), or with Akt inhibitor, TCN (10 μM). These inhibitors were incubated individually with the slice alone, or followed by lithium (10 mM) an hour later. Some slices were challenged with PCP (3 μM) 30 minutes after lithium treatment. Cell death was assessed 12 hours after PCP treatment by measuring caspase-3 activity and TUNEL-positive cells in the cortex. As shown in figure 4 and 5, pre-exposure to LY294002 (LY) or TCN completely abolished the protective effects of lithium (p<0.05 vs. PCP+Li; p>0.05 vs. PCP). We also noticed that LY294002 or TCN alone induced significant caspase-3 activation (P<0.005 and p<0.001, respectively vs. control) and caused widely distributed apoptosis in the cortex. It is conceivable that the residual neurotoxicity observed in the inhibitor+PCP+Li group is due to the intrinsic toxicity of the inhibitors; however, we also observed that the neurotoxicity induced by these inhibitors was largely reversed by lithium (p<0.001, Li+TCN vs. TCN; p<0.05, Li+LY vs. LY), thereby making this possibility unlikely. It is also notable that both PI-3K and Akt inhibitors potentiated neurotoxicity of PCP (p<0.05, PCP+inhibitor vs. PCP). Similar results were also observed with another PI-3K inhibitor, wortmannin (data not shown).
Fig. 4.

Inhibition of PI-3K blunts the protective effects of lithium on PCP-induced cell death. Corticostriatal slices were preincubated with LY294002 (LY, 30 μM) for 1 hour before lithium chloride (LiCl, 10 mM) treatment. PCP (3 μM) was added 30 minutes after LiCl. Cell death was assessed 12 hours after PCP treatment by measuring caspase-3 activity or TUNEL staining in the cortex. Untreated slices were used as control (CTRL). A. Effect of LY on lithium prevention of caspase-3 activation induced by PCP. B. Representative TUNEL-positive staining in the cortex. * p< 0.05 vs. control; # p<0.05 vs. PCP alone; ^ p<0.05 vs. PCP+Li.; & p<0.05 vs. LY alone. One-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons. .
Fig. 5.

Akt inhibition diminishes the protective effects of lithium on PCP-induced cell death. Corticostriatal slices were preincubated with Akt inhibitor, triciribine (TCN, 10 μM)) for 1 hour before lithium chloride (LiCl, 10 mM) treatment. PCP (3 μM) was added 30 minutes after LiCl. Cell death was assessed 12 hours after PCP treatment by measuring caspase-3 activity or TUNEL staining in the cortex. Untreated slices were used as control (CTRL). A. Effect of TCN on lithium prevention of caspase-3 activation induced by PCP. B. Representative TUNEL-positive staining in cortex. * p< 0.05 vs. control; # p<0.05 vs. PCP; ^ p<0.05 vs. PCP+Li.; & p<0.05 vs. TCN alone. One-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons.
Inhibition of PI-3K/Akt leads to dephosphorylation and activation of the pro-apoptotic factor GSK-3β (Pap and Cooper, 1998). Accordingly, we observed that PCP caused a significant decrease in p-GSK-3β (Fig.2) that was prevented by lithium pretreatment (Fig. 3). To further investigate the role of GSK-3β, we used a selective GSK-3β inhibitor, AR-A014418 (Bhat et al., 2003). Slices were preincubated with the indicated concentrations of AR-A014418 for an hour before exposure to PCP (3 μM). It was found that AR-A014418 inhibited PCP-induced caspase-3 activation (F5, 24=30.449, p<0.001) and DNA fragmentation as measured by TUNEL in the cortex in a dose-dependent manner (Fig. 6). Another specific GSK-3β inhibitor, SB216763, was also found to protect slices from PCP-induced cell death (data not shown). Taken together, these data indicate that activation of the PI-3K/Akt pathway and the resulting inhibition of GSK-3β plays a critical role in lithium protection of PCP neurotoxicity.
Fig. 6.

Effect of the GSK-3β inhibitor, AR-A014418, on PCP-induced cell death. Corticostriatal slices were pretreated with indicated concentrations of AR-A014418 on DIV 10 for 1 hour and then challenged with PCP (3 μM). Cell death was assessed 12 hours after PCP treatment by measuring caspase-3 activity or TUNEL staining in the cortex. Untreated slices were used as control (CTRL). A. AR-A014418 (AR-A) blocked PCP-induced caspase-3 activation. B. Representative TUNEL-positive staining in the cortex. * p< 0.05 vs. control; # p<0.05 vs. PCP. One-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons.
The role of MEK/ERK pathway in lithium-mediated protection
The MEK/ERK pathway is another possible signaling transducer involved in the trophic action of NMDAR activity in developing brain (Zhu et al., 2005). We have demonstrated that PCP inhibits ERK1/2 activity and lithium prevents the inhibition (Figs. 2 and 3). To investigate the contribution of MEK/ERK1/2 pathway activation to the protective effect of lithium, we used a MEK inhibitor, PD98059, to block ERK activation pharmacologically. Slices were treated with 30 μM PD98059 alone, or followed by lithium (10 mM) an hour later and then challenged with 3 μM PCP. Cell death was assessed 12 hours after PCP treatment. Unlike LY294002 or TCN, PD98059 alone did not cause significant caspase-3 activation (p>0.05, vs. control) or DNA fragmentation in the cortex (Fig. 7). However, the neuroprotection afforded by lithium against the PCP insult was significantly reduced by PD98059 exposure (p<0.001, vs. PCP+Li) (Fig. 7). This finding was confirmed with another MEK inhibitor, U0126 (data not shown). These data indicate that the MEK/ERK signaling pathway is also involved in lithium's protection against PCP neurotoxicity.
Fig. 7.

MEK inhibition attenuates the protective effects of lithium against PCP-induced cell death. Corticostriatal slices were preincubated with PD98059 (PD, 30 μM) for 1 hour before lithium chloride (LiCl, 10 mM) treatment. PCP (3 μM) was added 30 minutes after LiCl. Cell death was assessed 12 hours after PCP treatment by measuring caspase-3 activity or TUNEL staining in the cortex. Untreated slices were used as control (CTRL). A. Effect of PD98059 on lithium inhibition of caspase-3 activation induced by PCP. B. Representative TUNEL-positive staining in the cortex. * p< 0.05 vs. control; # p<0.05 vs. PCP; ^ p<0.05 vs. PCP+Li. One-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons.
Lack of crosstalk between the PI-3K/Akt/GSK-3β and MEK/ERK pathways
The preceding data has shown that both the PI-3K/Akt/GSK-3β and MEK/ERK pathways were affected by PCP application and were involved simultaneously in the protective effects of lithium against PCP-induced neurotoxicity. Considering that PCP-induced dephosphorylation of ERK1/2 occurred much earlier than that of either Akt or GSK-3β (Fig. 2), we questioned whether ERK could be involved in either the inhibition of the PI-3K/Akt pathway by PCP or its activation by lithium pretreatment. To answer this question, we determined the phosphorylation levels of ERK, Akt, and GSK-3β in slices pre-incubated with either PI-3K or MEK inhibitors in the presence or absence of lithium and/or PCP. As expected, the PI-3K inhibitor, LY294002, specifically decreased the basal level of p-Akt and p-GSK-3β (p<0.001, vs. control), but had no effect on p-ERK1/2, while the MEK/ERK inhibitor, PD98059, selectively inhibited basal activity of ERK1/2. Furthermore, though lithium prevented PCP-induced dephosphorylation of ERK1/2, Akt and GSK-3β, LY294002 pre-exposure abolished the preventive effect of lithium on p-Akt and p-GSK-3β (p<0.001 vs. PCP+Li), but not that on p-ERK (p>0.05 vs. PCP+Li). Analogously, PD98059 blocked the preventive effect of lithium on p-ERK1/2 (p<0.001, vs. PCP+Li), but not that on p-Akt or p-GSK-3β (Fig. 8). These data strongly suggest that although both the MEK/ERK and PI-3K/Akt pathways are involved in lithium protection, there is no crosstalk between their activation, at least at the level of Akt and ERK.
Fig. 8.
Relationship between MEK/ERK and PI-3K/Akt/GSK-3β pathways. Corticostriatal slices were pretreated with PI-3K inhibitor, LY294002 (LY), or ERK inhibitor, PD98059 (PD) for 1 hour before they were incubated lithium chloride (LiCl, 10 mM) and then challenged with PCP (3 μM) 30 minutes later. Slices were collected 8 hours after PCP treatment for western blot analysis of phosphorylation of Thr202/Tyr204-ERK1/2, Ser473-Akt and Ser9-GSK-3β. Untreated slices were used as control (CTRL). Phosphorylation levels of ERK, Akt, and GSK-3β were normalized to β-actin re-probed on the same membrane and calculated as percentage of the control. PLLY: PCP+Li+LY; PPDL: PCP+PD+Li. * p< 0.05 vs. control; # p<0.05 vs. PCP; ^ p<0.05 vs. PCP+Li. One-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons.
The role of GSK-3β in lithium protection
Lithium is a well known GSK-3β inhibitor (Klein and Melton, 1996). In the above study, we observed that the PI-3K inhibitor, LY294002, blocked lithium's prevention of PCP-induced dephosphorylation of GSK-3β, suggesting that lithium may inhibit GSK-3β activity indirectly in this model. To further investigate the role of GSK-3β in lithium's protection against the PCP insult, we measured the activity of Akt and ERK in slices pretreated with the specific GSK-3β inhibitor, AR-A014418. Figure 9 shows that AR-A014418 alone had no effect on the basal phosphorylation level of Akt (p>0.01, vs. control) or ERK (p>0.1, vs. control). Moreover, although AR-A014418 blocked PCP-induced cell death (Fig. 6), it did not alter the inhibitory effect of PCP on p-Akt (p>0.05, PCP vs. PCP+AR-A) or p-ERK (p>0.1, PCP vs. PCP+AR-A) (Fig. 9). These data indicate that GSK-3β is the critical downstream factor that mediates protective effect of lithium.
Fig. 9.

Effect of the GSK-3β inhibitor, AR-A014418, on PCP-induced dephosphorylation of Akt and ERK. Slices were pre-incubated with AR-A014418 (AR-A, 10 μM) on DIV 10 for 1 hour and then challenged with PCP (3 μM). Slices were collected 8 hours after PCP treatment and used for western blot analysis of phosphorylation of Thr202/Tyr204-ERK1/2 and Ser473-Akt. Untreated slices were used as control (CTRL). Phosphorylation levels of ERK and Akt were normalized to β-actin re-probed on the same membrane and calculated as percentage of control (CTRL). * p< 0.05 vs. control. One-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons.
Discussion
This study found that pre-incubation with lithium protected corticostriatal slices from PCP neurotoxicity by a mechanism that prevented caspase-3 activation. Our data strongly suggest that the protective effect of lithium is mediated through stimulation of the PI-3K/Akt and MEK/ERK pathways and inhibition of GSK-3β indirectly.
The PI-3K/Akt/ GSK-3β pathway has been most convincingly implicated in neuronal survival afforded by neurotrophic factors and by NMDAR in cerebellar granule neurons (Zhang et al., 1998; Xifro et al., 2005). NMDAR may activate PI-3K via Ras activation through calcium influx from the channel (Hetman and Kharebava, 2006). It has been demonstrated that the NR2B subunit, when phosphorylated at the tyrosine residue near the C-terminal, recruits the binding domain of the regulatory p85 subunit of PI-3Kinase via the SH2 domain, thus leading to activation of PI-3K (Hisatsune et al., 1999). This lab has previously showed that PCP treatment reduced the activity of Akt, a downstream effector of PI-3K, both in dissociated cortical culture and in intact rat pups (Lei et al., 2008). Furthermore, Lei and co-workers demonstrated that inhibitors of either Akt or PI-3K attenuated the protection against PCP neurotoxicity afforded by activation of L-type calcium channels or potentiation of synaptic activity in dissociated cortical culture, indicating that inhibition of PI-3K/Akt signaling may account for PCP-induced cell death (Lei et al., 2008).
Here, we show that PCP caused similar inhibition of Akt activity in organotypic culture (Fig.2). In the presence of lithium, inhibition of Akt by PCP was significantly reduced (Fig.2). In addition, PI-3K or Akt inhibitors abolished the protective properties of lithium (Fig. 4 and 5), indicating that lithium may protect against the PCP insult through activating the PI-3K/Akt pathway. However, we also observed that blocking the PI-3K/Akt pathway with either LY294002 or TCN caused significant toxicity in the brain slices. It is conceivable that the neurotoxicity observed in the PCP+Li+inhibitor experimental group may be the result of the intrinsic or residual toxicity of the inhibitor itself. To exclude this possibility, we included a Li+inhibitor group in the analysis. We found that lithium attenuated the toxicity of the inhibitors greatly. These data suggest that blockade of the PI-3K/Akt pathway abolishes the protective effect of lithium against PCP. Together, these results strongly support our hypothesis that the PI-3K/Akt pathway is an essential anti-apoptotic pathway involved in lithium's protection against PCP-induced cell death.
The Ras/MEK/ERK pathway is another possible player in PCP neurotoxicity and lithium protection in this model. This pathway is activated by calcium influx through NMDA receptors (Krapivinsky et al., 2003) and has been studied extensively in NMDAR-mediated long-term potentiation (LTP) (Thomas and Huganir, 2004). Decreased ERK activity has been observed in neonatal rats injected with MK-801 (Hansen et al., 2004). In addition, Hansen et al (2004) demonstrated that transgenic synRas activation inhibited MK-801-induced developmental neurodegeneration by 40%, indicating a significant role of ERK inhibition in NMDAR blockade-induced neurotoxicity. In accordance with these findings, we also observed a rapid and long-lasting inhibition of ERK1/2 by PCP in cultured corticostriatal slices (Fig. 2), probably by uncoupling NMDAR and ERK signaling via blockade of calcium influx. Lithium pretreatment prevented PCP-inhibition of ERK1/2, as well as PCP-induced apoptosis (Fig. 1&3). Furthermore, blocking ERK1/2 activity with the MEK inhibitor, PD98059, abolished lithium protection (Fig. 7). These data further suggest that the MEK/ERK1/2 pathway plays a role in NMDAR-blockade-induced neurotoxicity in the developing brain and that activation of the MEK/ERK1/2 pathway is involved in lithium protection in this model.
The PI-3K/Akt and MEK/ERK pathways play different roles in neuroprotection observed under different conditions. For example, activation of the PI-3K/Akt pathway was shown to be necessary for BDNF protection against serum withdrawal in cortical neurons, but not for BDNF-mediated protection against DNA damage (Hetman et al., 1999). In contrast, activation of the MEK/ERK pathway, but not the PI-3K/Akt pathway, was required for protection both by low concentrations of NMDA against glutamate excitotoxicity in cerebellar granule neurons (Zhu et al., 2005) and for BDNF protection against camptothecin-induced apoptosis in cortical culture (Hetman et al., 1999). Under certain situations, activation of both pathways is required. For example, protection of BDNF against glutamate-induced apoptosis in hippocampal neurons is mediated by both pathways, though crosstalk between the two pathways has been reported (Almeida et al., 2005). In this study, we found that blocking either the PI-3K/Akt or the MEK/ERK pathway attenuated lithium protection, suggesting involvement of both pathways. However, no crosstalk between these pathways was found, as the MEK inhibitor, PD98059, only specifically suppressed the stimulating effect of lithium on PCP-induced inhibition of ERK, while it had no effect on p-Akt or p-GSK-3β. Further, the PI-3K inhibitor, LY294002, selectively blocked the preventive effect of lithium on PCP-induced dephosphorylation of Akt and GSK-3β, but not ERK (Fig. 8). These data strongly suggest that though the two pathways equally contributed to lithium protection, their stimulation by lithium is independent. However, it is possible that they may share other common targets downstream.
In this model, our data suggest that GSK-3β is likely such a downstream common target. Indeed, Takadera and co-workers have shown that GSK-3β inhibitors prevented ethanol-, ketamine-, and ifenprodil-induced apoptosis in cortical cell cultures by inactivation of caspase-3 (Takadera and Ohyashiki 2004; Takadera et al. 2004). Recently, our laboratory reported that inhibition of GSK-3β by siRNA or other GSK-3β inhibitors, including lithium, blocked PCP-induced cell death in dissociated cortical cell cultures (Lei et al., 2008). Consistent with these reports, the present study using organotypic corticostriatal slice cultures found that PCP caused time-dependent activation of GSK-3β by decreasing phosphorylation at serine 9. Lithium and another specific GSK-3β inhibitor, AR-A014418, also prevented PCP-induced cell death. Noticeably, unlike lithium, AR-A014418 did not alter PCP-inhibition of Akt and ERK activity, implying that neither Akt nor ERK activation is required for the protection afforded through GSK-3β inhibition. This strongly suggests that GSK-3β is the critical downstream factor that mediates PCP-induced cell death as well as lithium-mediated protection. In addition, the PI-3K inhibitor, LY294002, attenuated the inhibitory effect of lithium on GSK-3β (Fig. 8), indicating that unlike AR-A014418, lithium inhibited GSK-3β activity indirectly in this system. It is quite possible that stimulation of the PI-3K/Akt and MEK/ERK pathways by lithium may finally converge on GSK-3β inhibition to counteract PCP-induced neurotoxicity. However, this hypothesis is confounded by the observation that the MEK inhibitor, PD98059 showed no effect on phosphorylation of GSK-3β at serine 9. It has been reported that ERK activation protects cortical neurons from GSK-3β activation-induced apoptosis through an unknown mechanism that is independent of serine 9 phosphorylation (Hetman et al., 2002; Habas et al., 2006). Thus, it is possible that in this model, PI-3K/Akt and ERK may be activated by lithium through different mechanisms to suppress the pro-apoptotic activity of GSK-3β.
In summary, these data confirmed our previous report of the role of the PI-3K/Akt/GSK-3β survival pathway in PCP-induced apoptosis in dissociated culture (Lei et al., 2008) and extend these findings by demonstrating that PCP also inhibits the MEK/ERK1/2 survival pathway in corticostriatal slices. Most importantly, the present study demonstrates that the protective effect of lithium against PCP-induced neuroapoptosis in the cortical slice is mediated through co-stimulation of the PI-3K/Akt and MEK/ERK pathways as well as through the indirect suppression of GSK-3β activity. Finally, the ability of lithium to provide neuroprotection against PCP-induced neurotoxicity lends support to this paradigm as a cellular model of the symptoms of schizophrenia, mania and other disorders that are well known to be significantly dampened by lithium therapy.
Acknowledgements
The authors would also like to thank Dr. Xiaodong Cheng and Mr. Zhenyu Ji for valuable discussions of PI-3K/Akt signaling.
This work was supported by NIH grants DA-02073 (to KMJ).
Abbreviations
- PI-3K
phosphotydylinositide-3 kinase
- GSK-3β
Glycogen Synthase Kinase 3 β
- MEK
mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- ERK
extracellular signal-regulated kinase
- PCP
phencyclidine
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling
References
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS Lett. 1999;453:260–264. doi: 10.1016/s0014-5793(99)00685-7. [DOI] [PubMed] [Google Scholar]
- Balazs R, Jorgensen OS, Hack N. N-methyl-d-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience. 1988;27:437–451. doi: 10.1016/0306-4522(88)90279-5. [DOI] [PubMed] [Google Scholar]
- Bhat R, Xue Y, Berg S, Hellberg S, Ormo M, Nilsson Y, Radesater AC, Jerning E, Markgren PO, Borgegard T, Nylof M, Gimenez-Cassina A, Hernandez F, Lucas JJ, Diaz-Nido J, Avila J. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem. 2003;278:45937–45945. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- Burgess S, Geddes J, Hawton K, Townsend E, Jamison K, Goodwin G. Lithium for maintenance treatment of mood disorders. Cochrane Database Syst Rev. 2001:CD003013. doi: 10.1002/14651858.CD003013. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, French A, Balster R, Murray TF, Traynelis SF. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol. 2007;581:107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Bois TM, Huang XF. Early brain development disruption from NMDA receptor hypofunction: relevance to schizophrenia. Brain Res Rev. 2007;53:260–270. doi: 10.1016/j.brainresrev.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behavioural Brain Research. 2004;153:367–376. doi: 10.1016/j.bbr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Habas A, Kharebava G, Szatmari E, Hetman M. NMDA neuroprotection against a phosphatidylinositol-3 kinase inhibitor, LY294002 by NR2B-mediated suppression of glycogen synthase kinase-3beta-induced apoptosis. J Neurochem. 2006;96:335–348. doi: 10.1111/j.1471-4159.2005.03543.x. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Briem T, Dzietko M, Sifringer M, Voss A, Rzeski W, Zdzisinska B, Thor F, Heumann R, Stepulak A, Bittigau P, Ikonomidou C. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol Dis. 2004;16:440–453. doi: 10.1016/j.nbd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur J Neurosci. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- Hetman M, Hsuan SL, Habas A, Higgins MJ, Xia Z. ERK1/2 antagonizes glycogen synthase kinase-3beta-induced apoptosis in cortical neurons. J Biol Chem. 2002;277:49577–49584. doi: 10.1074/jbc.M111227200. [DOI] [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- Hetman M, Kharebava G. Survival signaling pathways activated by NMDA receptors. Curr Top Med Chem. 2006;6:787–799. doi: 10.2174/156802606777057553. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Umemori H, Mishina M, Yamamoto T. Phosphorylation-dependent interaction of the N-methyl-D-aspartate receptor epsilon 2 subunit with phosphatidylinositol 3-kinase. Genes Cells. 1999;4:657–666. doi: 10.1046/j.1365-2443.1999.00287.x. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Biexponential kinetics of [3H]MK-801 binding: evidence for access to closed and open N-methyl-D-aspartate receptor channels. Mol Pharmacol. 1989;35:387–393. [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jin N, Kovacs AD, Sui Z, Dewhurst S, Maggirwar SB. Opposite effects of lithium and valproic acid on trophic factor deprivation-induced glycogen synthase kinase-3 activation, c-Jun expression and neuronal cell death. Neuropharmacology. 2005;48:576–583. doi: 10.1016/j.neuropharm.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Noh JS, Bae YS, Gwag BJ. Calcium-dependent prevention of neuronal apoptosis by lithium ion: essential role of phosphoinositide 3-kinase and phospholipase Cgamma. Mol Pharmacol. 2003;64:228–234. doi: 10.1124/mol.64.2.228. [DOI] [PubMed] [Google Scholar]
- Kirshenboim N, Plotkin B, Shlomo SB, Kaidanovich-Beilin O, Eldar-Finkelman H. Lithium-mediated phosphorylation of glycogen synthase kinase-3beta involves PI3 kinase-dependent activation of protein kinase C-alpha. J Mol Neurosci. 2004;24:237–245. doi: 10.1385/JMN:24:2:237. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Lei G, Xia Y, Johnson KM. The role of Akt-GSK-3beta signaling and synaptic strength in phencyclidine-induced neurodegeneration. Neuropsychopharmacology. 2008;33:1343–1353. doi: 10.1038/sj.npp.1301511. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Pardo R, Andreolotti AG, Ramos B, Picatoste F, Claro E. Opposed effects of lithium on the MEK-ERK pathway in neural cells: inhibition in astrocytes and stimulation in neurons by GSK3 independent mechanisms. J Neurochem. 2003;87:417–426. doi: 10.1046/j.1471-4159.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- Pettmann B, Henderson CE. Neuronal cell death. Neuron. 1998;20:633–647. doi: 10.1016/s0896-6273(00)81004-1. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Han F, Shioda N, Moriguchi S, Kasahara J, Ishiguro K, Fukunaga K. Lithium-induced activation of Akt and CaM kinase II contributes to its neuroprotective action in a rat microsphere embolism model. Brain Res. 2006;1108:98–106. doi: 10.1016/j.brainres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Takadera T, Ohyashiki T. Glycogen synthase kinase-3 inhibitors prevent caspase-dependent apoptosis induced by ethanol in cultured rat cortical neurons. Eur J Pharmacol. 2004;499:239–245. doi: 10.1016/j.ejphar.2004.07.115. [DOI] [PubMed] [Google Scholar]
- Takadera T, Sakamoto Y, Ohyashiki T. NMDA receptor 2B-selective antagonist ifenprodil-induced apoptosis was prevented by glycogen synthase kinase-3 inhibitors in cultured rat cortical neurons. Brain Res. 2004;1020:196–203. doi: 10.1016/j.brainres.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Vickers AEM, Fisher RL. Organ slices for the evaluation of human drug toxicity. Chemico-Biological Interactions. 2004;150:87–96. doi: 10.1016/j.cbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: implications for schizophrenia. Neuroscience. 2001;107:535–550. doi: 10.1016/s0306-4522(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. The role of caspase-3 activation in phencyclidine-induced neuronal death in postnatal rats. Neuropsychopharmacology. 2007;32:1178–1194. doi: 10.1038/sj.npp.1301202. [DOI] [PubMed] [Google Scholar]
- Xifro X, Malagelada C, Minano A, Rodriguez-Alvarez J. Brief exposure to NMDA produces long-term protection of cerebellar granule cells from apoptosis. Eur J Neurosci. 2005;21:827–840. doi: 10.1111/j.1460-9568.2005.03935.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, Sebti SM, Cheng JQ. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- Zhang FX, Rubin R, Rooney TA. N-Methyl-D-aspartate inhibits apoptosis through activation of phosphatidylinositol 3-kinase in cerebellar granule neurons. A role for insulin receptor substrate-1 in the neurotrophic action of n-methyl-D-aspartate and its inhibition by ethanol. J Biol Chem. 1998;273:26596–26602. doi: 10.1074/jbc.273.41.26596. [DOI] [PubMed] [Google Scholar]
- Zhu D, Wu X, Strauss KI, Lipsky RH, Qureshi Z, Terhakopian A, Novelli A, Banaudha K, Marini AM. N-methyl-D-aspartate and TrkB receptors protect neurons against glutamate excitotoxicity through an extracellular signal-regulated kinase pathway. J Neurosci Res. 2005;80:104–113. doi: 10.1002/jnr.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]