Abstract
The purpose of this study was to investigate the effect of cancer- and normal basement membrane-derived extracellular matrix to modulate the phenotype of bladder cancer cell lines. Five lines, varying in malignancy from papilloma to highly undifferentiated and invasive and immortalized human urothelial cells, were grown on two extracellular matrix preparations, Matrigel and SISgel. Matrigel represents matrix remodeled by malignancy while SISgel, obtained from small intestine submucosa (SIS), represents the normal matrix supporting differentiated cell growth. On Matrigel, regardless of the content of growth factors, the invasive lines displayed an invasive phenotype, while the low grade lines grew as papillary structures. In contrast, when the same cells were grown on SISgel, they grew as a layer of cells one to 5 cells thick, failed to invade, and expressed cell-surface E-cadherin. Unlike breast cancer cells, neutralization of β1, β4 and α6 integrins altered cell-cell and cell-matrix adhesiveness but did not alter the phenotype. When invasive cells were grown on mixtures of SISgel and Matrigel, the phenotype changed gradually, not abruptly, indicating that factors within the gel reversibly alter the phenotypic expression of invasion. In summary, the phenotype of bladder cancer cells growing in tissue-like 3-dimensional culture is highly plastic, and malignant properties such as invasion and papillary growth can be suppressed by the matrix.
Keywords: Phenotype, bladder cancer, invasion, E-cadherin, extracellular matrix
Each tissue, such as the urothelium that lines the urinary bladder, also represents a complex ecosystem of interacting cell types that acts as a system. Homeostatic signals tightly regulate proliferation, differentiation and apoptosis as well as tissue architecture. In the normal urinary bladder, the epithelial system includes basal and intermediate transitional epithelial cells that maintain a direct cytoplasmic connection to the lamina propria (1), the terminally differentiated umbrella cells that maintain the impermeability of the bladder, as well as neurons and occasional glands. The epithelium overlays a stroma containing smooth muscle cells, blood vessels and connective tissue cells, all of which are in chemical communication with the epithelial cells through cytokines and the extracellular matrix (2). The bladder urothelium maintains a very low rate of proliferation (1), but responds to injury with a rapid burst of growth that is all the more remarkable given the long lifetime of months and the quiescence of the usual umbrella cell (2). How the urothelial structure is maintained is understood only in general terms.
The cell-extracellular matrix interaction is a major modulator of cellular phenotype. The survival of normal cells is dependent upon establishing connections through their surface integrins, and failure to do so results in apoptosis (3). Bissell and coworkers (4) suggested that integrin signaling through the extracellular matrix was the major force in determining whether breast cancer or normal breast cells exhibited an organized, normal phenotype or a disorganized cancer phenotype. Integrins also organize multilayered urothelium and modulate cell-substrate adhesion (5). The α6β4 integrin is often overexpressed in bladder cancers (6). Other molecules in the matrix also play a crucial role in modulating the phenotype of epithelial cells. Among these are proteoglycans such as decorin and biglycan that regulate differentiation (7), and perlecan, which regulates matrix assembly (8). Others, such as the proteoglycan bamacan (9) and laminin, are associated with malignancy and invasion (10). Together, these findings suggest that epigenetic factors both maintain homeostasis and can interrupt the homeostasis that generally inhibits carcinogenesis and progression (11).
In this communication we show that bladder cancer cells grow in radically different phenotypes on different matrix preparations. Matrigel, the extracellular matrix product of the EHS sarcoma, has long been known to support growth of tumor cells in a phenotype similar to that expressed in vivo (12). Another gel product, SISgel, and the original porcine small intestine submucosa (SIS) from which it is produced, is used as a matrix for reconstruction of bladder (13) and vascular tissue (14) as well as for a substrate that promotes the growth of normal cells (15). Growth on Matrigel serves as a model for growth after remodeling the matrix by malignant cells (16), while growth on SIS or SISgel models the effects of normal matrix homeostatic mechanisms on malignant cells.
Materials and Methods
3-Dimensional cell culture
SV-HUC-1, TCCSUP, RT4 and J82 cells were obtained from the American Type Culture Collection, Bethesda, MD, USA. According to the ATCC, the SV-HUC-1 cell line was immortalized with SV-40 but is neither tumorigenic nor produces virus. The TCCSUP line was from a grade IV tumor, the J82 line was from an anaplastic tumor while the RT4 cells were derived from a papilloma. All cancer cells arc reported to be tumorigenic in one or more models. The 253 JP and 253 JB-V cells were provided by Dr. Colin Dinney (17). The former is derived from a low-grade tumor, while the latter is a metastatic variant cloned in Dr. Dinney's laboratory after 5 passages of 253 JP cells in the bladder walls of nude mice. After receipt of cells, stocks differing only in one passage from those received were frozen, and the cells used in these studies were no more than 12 passages from the original stock. Three dimensional gel cultures with Matrigel (obtained as both standard and “growth factor-depleted” formulations from Becton-Dickinson, Bedford, MA, USA) were performed by layering 0.8 ml of ice-cold Matrigel onto polyethylene terephthalate membranes of 6-well cell culture inserts (Falcon, Becton-Dickinson Labware, Franklin Lakes, NJ, USA). Gels were solidified at 37°C. For culture on SISgel, 8 ml of SISgel (Cook Biotech, W. Lafayette, IN, USA) were mixed with 1.0 ml 10 × PBS and then the pH was adjusted to 7.4 with 25 μl aliquots of ice-cold 1 M NaOH. Ice-cold SISgel (0.8 ml) was layered onto each membrane of the 6-well cell culture inserts and allowed to solidify overnight at 37°C. For experiments with laminin, 125 μg and 250 μg of human recombinant laminin (Upstate Biologicals, Lake Placid, NY, USA) was added to the SISgel. Confluent cells were trypsinized with 1 ml 0.25% trypsin; 1.0 mM EDTA (Life Technologies, Rockville, MD, USA). Trypsinized cells were resuspended in 2 ml of respective media and 500,000 cells aliquoted onto solidified SISgel or Matrigel discs. Two ml of medium (Minimal Essential Media containing 1× nonessential amino acids, L-glutamine and pyruvate, Life Technologies), containing 1% Fetal Calf Serum (Life Technologies), were layered beneath the transwell supports in 6-well plates such that an air bubble did not form. The cells were allowed to adhere to the gels for 72 hours before the media were replenished. Cultures were grown for 14 days with media changes twice per week. Cultures were harvested for microscopic sectioning by excising the gel with a scalpel from the bottom. The gel was slid into a 60 mm Petri dish, cell side up, and all liquid was removed. The gel was encased in 3% agar to prevent loss of cells, fixed with 10% buffered formalin overnight and embedded in paraffin. A 5 mm section was cut and stained with hematoxylin and eosin.
Integrin neutralization
The purified antibodies were obtained in an azide-free solution at 1 mg/ml concentration from BD PharMingen (San Diego, CA, USA). For studies to neutralize β1 integrin, the function-inhibiting antibody purified from the hamster anti-rat IgM CD29Ha2/5 clone was used. For the anti-α6 antibody studies, the GoH3 rat monoclonal clone was used. These are the same antibodies that were used previously by Bissell and coworkers (4), who also proved the specificity of the antibody for the respective integrins. The antibody (35 μg) was delivered to the top of the gel in small aliquots of 2-5 μl that were allowed to sink into the gel for 6 hours prior to addition of cells. The amount of antibody needed was determined experimentally by titration by adding 5, 10, 25, 35 and 50 μg per well. At least 35 μg was necessary to see an effect on the phenotype. These concentrations are similar to those reported as being effective in modulating breast cancer cell phenotype4. An isotype control antibody (mouse IgM purified anti-human Cyclin H from BD Pharmingen that would not be expected to be expressed on the cell surface) was used at the same concentration to ensure that any effect was not due to the nonspecific presence of an antibody in the gel. An additional control consisted of purified b1 integrins (α1β1 and an α5β1 (Chemicon, Temecula, CA, USA) added at equimolar quantities to the antibody and then added to the gel as described above. A separate function-inhibiting IgG anti-β1 antibody, Clone P5D2 (18) (Chemicon), also was used as described above.
Immunohistochemical analysis
E-cadherin protein expression was assayed using a mouse monoclonal IgG1-k (clone 4A2 C7) (Zymed Laboratories, San Francisco, CA, USA). The 5 μm sections were dewaxed and the epitope liberated with heat-induced citrate retrieval, as specified by the manufacturer. The tissue was labeled with the primary antibody diluted 1:100 and incubated for 30 minutes. A secondary system of goat anti-mouse labeled with horseradish peroxidase and AEC chromogen (Zymed) was used. Positive controls consisted of normal kidney and normal bladder. An isotype-control unrelated antibody was used as a negative control. Negative controls were consistently negative. Cells were photographed using a Nikon Diaphot microscope equipped with a Hammamatsu Color Chilled 3CCD camera. Images of stained sections were captured using a Nikon Microphot FXA microscope equipped with the same camera.
Results
Phenotype as a function of extracellular matrix
As shown in Figure 1, the SV-HUC-1 cells grew as an ordered and organized multilayer that completely covered the SISgel. On Matrigel, these cells also grew as an organized layer, but failed to cover the gel. As shown in transverse sections (Figure 2) and by phase contrast from above (Figure 3), in every case, the SISgel modulated the malignant phenotype. On Matrigel, the TCCSUP and J82 cells displayed an invasive phenotype characterized by nodules or nests of tumor cells that invaded deeply into the gel while linked on the surface by cellular processes or strands that connected the nodules (Figures 2A and 3A). A process, denoted by the arrow in Figure 2A, appears to consist of a single thickness of cells growing atop the gel. A radically different phenotype was displayed on the SISgel, where the phase contrast photograph of the aggressive TCCSUP line (Figure 3B) shows the cells grew as a uniform cell layer. Examination of the stained section (Figure 2B) confirms the TCCSUP cells are minimally invasive in SISgel and grow as a one or two cell layer on the gel surface. The J82 line behaved very similarly to the TCCSUP line (Figure 2C and D), though the J82 line tended to organize into layers two or more cells thick when allowed to grow for a longer time. A separate phase contrast photograph is not shown. On Matrigel, the 253 JB-V (Figure 2E) and 253 JP (Figure 2G) lines grew as papillary clumps, with the 253 JB-V cells showing invasion (Figure 2E), but on SISgel these cells grew as thin cell layers (Figure 2F, H). Figure 3C and D shows the 253 JP line growing at less than confluence on Matrigel and SISgel to illustrate the papillary nature of their growth. On Matrigel the cells grow both vertically as papillary clusters and spread horizontally, eventually covering the gel. On SISgel (Figure 3D) they grow only horizontally as a thin cell layer that eventually covers the gel. The RT4 cells grew as large papillary structures on the Matrigel but on SISgel as a multilayer that appeared very similar to a normal cell layer (Figure 2 E, F and Figure 3 I, J).
Figure 1.
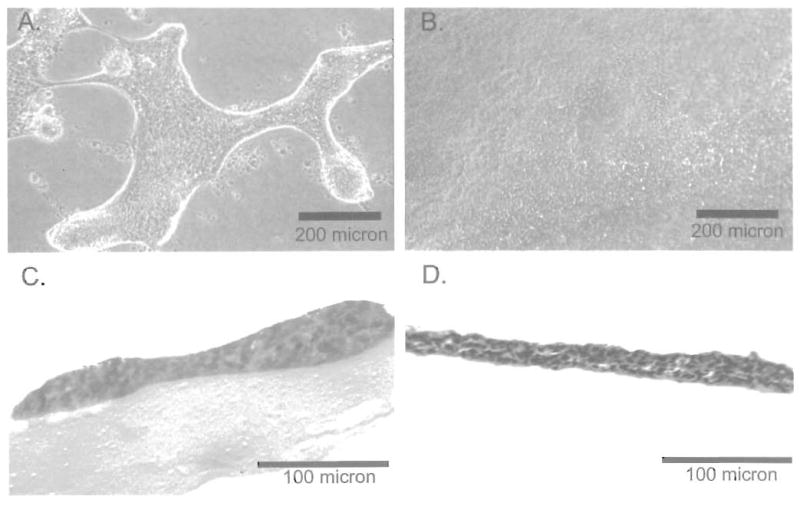
Immortalized normal urothelial cells on Matrigel (A, C) and SISgel (B, D). The top row shows the cells by phase contrast viewed from the top, while the lower row shows hematoxylin and eosin-labeled transverse sections.
Figure 2.
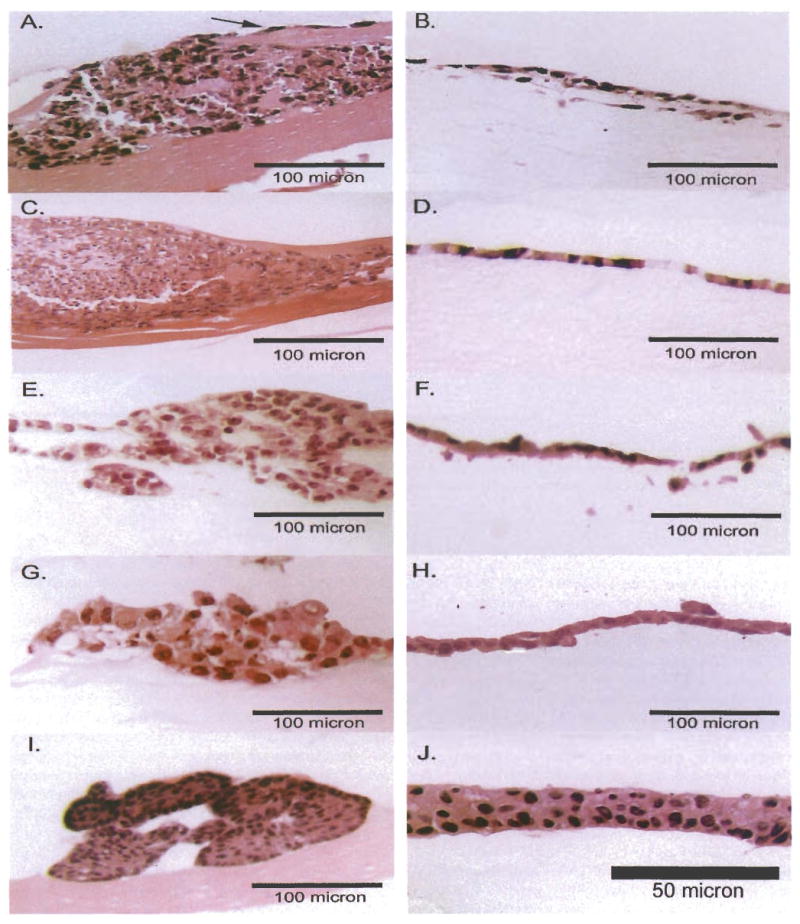
Hematoxylin and eosin-labeled transverse sections of 3-dimensional cultures. Cells grown on Matrigel are shown on the left and on SISgel on the right. A, B: TCCSUP, a highly undifferentiated line. The arrow indicates one of the connecting processes. C, D: J82 line, also a highly undifferentiated line. E, F: 253JB-V line, a metastatic phenotype derived from the 253JP line. G, H, 253 JP line, a low-grade tumor line: I, J: RT4, a papilloma line.
Figure 3.
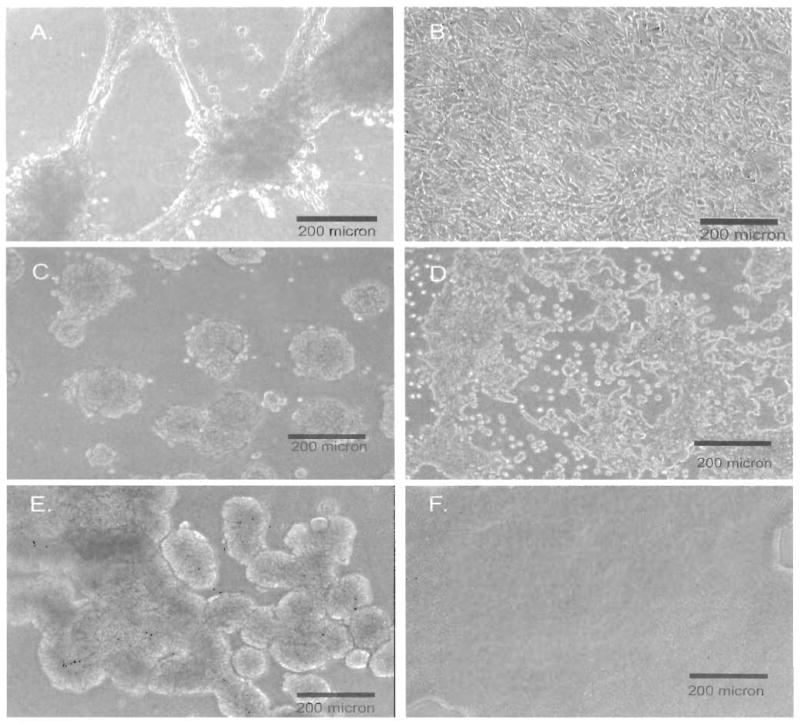
Phase contrast images of cells grown in 3-dimensional gel culture. Cells growing on Matrigel are shown on the left and on SISgel on the right. (A) Growth of TCCSUP on Matrigel at two weeks demonstrating an aggressive phenotype with invasive nodules connected by processes. (B) Growth of TCCSUP on SISgel showing a nonagressive, layer-like phenotype. (C) Growth of 253 JP cells growing at less than confluence in a cluster or papillary phenotype on Matrigel. (D) Growth of 253 JP cells on SISgel at less than confluence demonstrating the thinness of the layer. (E) Growth of RT4 cells on Matrigel demonstrating a papilloma phenotype. (F) Growth of RT4 cells on SISgel demonstrating the evenness of the layer formed.
Several variables were tested to determine if they affected the modulation of phenotype by the gels. The growth peptides found in Matrigel (19) do not seem to be a factor because the phenotype of cells growing on the growth factor-depleted formulation was identical in all cases to that seen in ordinary Matrigel (not shown). When TCCSUP cells that were growing as a normalized layer on SISgel were transferred to Matrigel they resumed growing in the phenotype characteristic of Matrigel, and when cells growing on Matrigel were transferred to SISgel, they exhibited the morphology typical of growth on SISgel. Thus, the different phenotypes seen on Matrigel and SISgel do not represent selection for a specific genotype from the heterogeneous culture of cells on plastic but are induced by components of the gels themselves. This is further emphasized by the results of mixing the two gels, as shown in Figure 4. As the proportion of SISgel is increased, the phenotype gradually becomes less invasive. The addition of 125 or 250 μg laminin per 0.8 ml SISgel had no effect on the phenotype (not shown).
Figure 4.
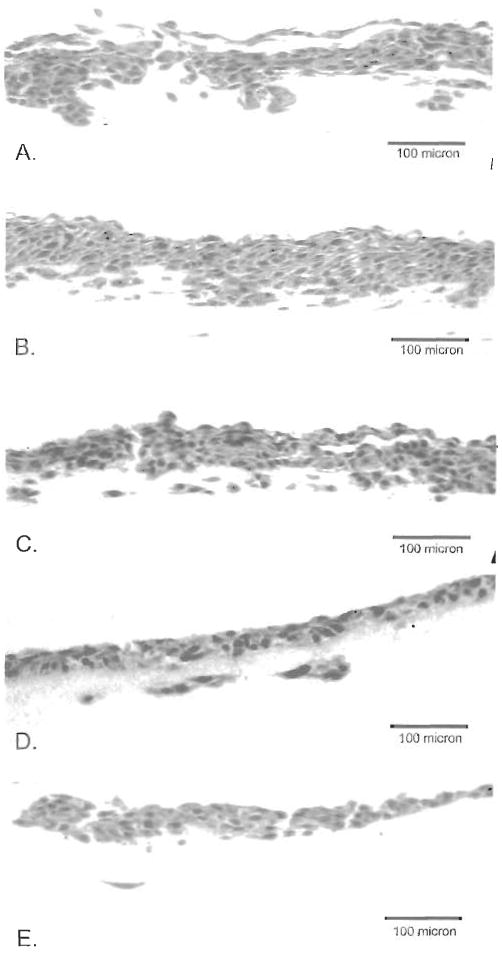
Growth of TCCSUP cells on mixtures of Matrigel and SISgel. (A) 100% Matrigel, (B) 75% Matrigel, 25% SISgel, (C) 50% Matrigel, 50% SISgel, (D) 75% SISgel, 25% Matrigel, (E) 100% SISgel. Note the progressive inhibition of invasion as the fraction of SISgel increases.
Neutralization of integrins
Because the relative strengths of integrin-mediated cell-cell and cell-matrix interactions were such key factors in establishing the phenotype of breast cancer cells in Matrigel (4), the role of α6, β4 and β1 integrins were investigated using function-inhibiting antibodies to modulate the relative strengths of these interactions. The results are summarized in Figure 5 and Table I. In general, these antibodies altered the adhesiveness of the cells to each other or to the matrix, or to both, but the large-scale change in phenotype reported with gland-forming breast cancer cells were not seen with the layer-forming urothelial cells. Reduced adhesiveness was identified from the presence of small, spherical cells seen by phase contrast but a paucity of cells seen in transverse sections. The one exception was that incorporation of the multivalent IgM antibody to β1 integrin into SISgel elicited an invasive phenotype from TCCSUP cells. Interestingly, the bivalent IgG antibody did not (Figure 5). However this invasive phenotype is quite different from the invasive phenotype seen in Matrigel (Figure 2A, 3A). The surface layer of cells failed to adhere, but the invading cells displayed a completely unorganized invasive phenotype with reduced cell-cell adhesiveness. Treatment of the equally invasive J82 cells with the IgM antibody failed to produce invasion, even if the amount of antibody was increased 8-fold (Table I). Control experiments with an unrelated IgM anti-cyclin H antibody showing no effect demonstrated the effect was specific. Premixing of either α1β1 or α5β1 integrins in equimolar amounts to the IgM antibody induced a phenotype that failed to adhere to the SISgel as did incorporation of the integrins themselves into the SISgel, but no invasion was elicited. Both the anti-β4 and anti-α6 integrin antibodies produced identical nonadherent phenotypes.
Figure 5.
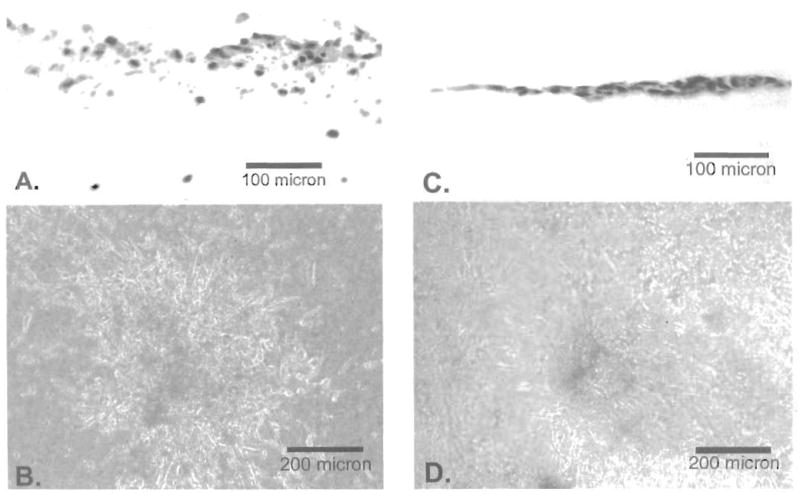
Effect of neutralization of β1 integrins on phenotype of TCCSUP bladder cancer cells on SISgel. (A, B) 35 mg of polyvalent CD29Ha2/5 IgM anti-β1 integrin antibody per gel. (C,D) 35 μg of divalent P5D2 IgG anti-β1 antibody per gel.
Table I. Phenotypes observed in the presence of exogenous integrins and integrin-blocking antibodies.
| Substance added to gel | Gel | Cell line | Amt. | Phenotype observed |
|---|---|---|---|---|
| CD29Ha2/5 IgM anti-β1 Integrin | S | TCCSUP | 35 μg | Unorganized invasive |
| P5D2 IgG anti-β1 Integrin | S | TCCSUP | 35 μg | Nonadherent |
| CD29Ha2/5 IgM anti-β1 Integrin | M | TCCSUP | 35 μg | Organized invasive (no effect) |
| IgM Anti-cyclin H control | S | TCCSUP
J82 |
35 μg | Single layered (no effect) |
| Integrin α1β1 | S | TCCSUP | 11.3 μg | Nonadherent |
| Integrin α5β1 | S | TCCSUP | 8.5 μg | Nonadherent |
| CD29Ha2/5 IgM anti-β1 Integrin
(function-inhibiting) + Integrin α1β1 or α5β1 |
S | TCCSUP | 35 μg | Nonadherent |
| Rat GoH3 IgG anti-α6 Integrin | M | TCCSUP
J82 RT4 |
35 μg | Nonadherent. |
| IgG anti-β4 Integrin | M | TCCSUP | 35 μg | Nonadherent |
| Rat GoH3 IgG anti-α6 Integrin | S | TCCSUP | 35 μg | Nonadherent |
| IgG anti-β4 Integrin | M | RT4 | 35 μg | Nonadherent |
| Rat GoH3 IgG anti-α6 Integrin | S | RT4 | 35 μg | Nonadherent |
| CD29Ha2/5 IgM anti-β1 Integrin | S | J82 | 35 μg
70 μg 280 μg |
Single layered (no effect) |
We were also interested in determining if the phenotypic switching was associated with modulation of known biomarkers of aggressiveness, such as loss of E-cadherin expression by aggressive bladder cancers (20). The phenotypic plasticity was accompanied by changes in the expression of the cell adhesion molecule, E-cadherin, as shown in Figure 6. The cells growing on the surface of the Matrigel (Figure 6A) express E-cadherin, but expression does not appear to be limited to the cell periphery, as is the case for differentiated cells. Thus, the main failure seems to be in organizing E-cadherin rather than in a failure to synthesize the protein. In invasive nodules, the cells at the surface continue to express E-cadherin, but the invasive cells fail completely to synthesize the protein (Figure 6B). Figure 6C shows that cells growing on SISgel in the presence of the IgM function inhibiting anti-βl integrin antibody. Interestingly these cells seem to express E-cadherin, even while expressing the invasive phenotype.
Figure 6.
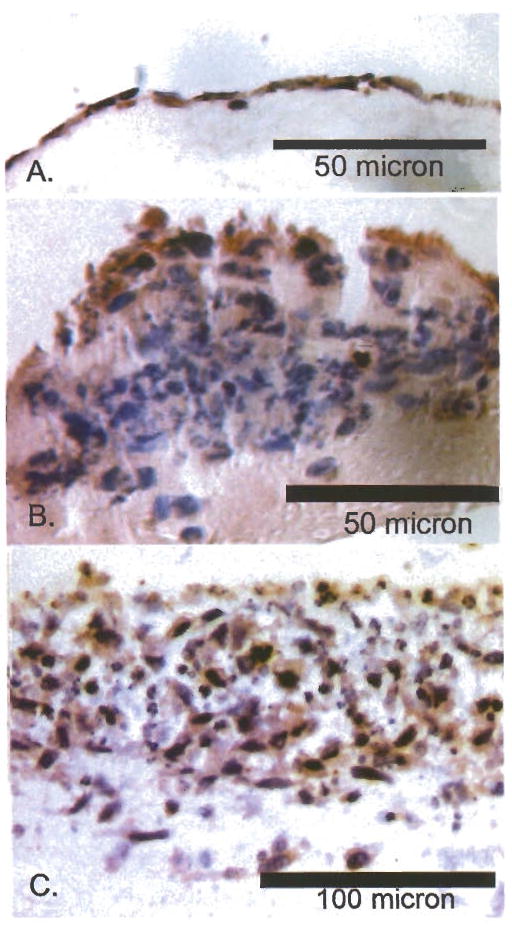
Immunohistochemical labeling of E-cadherin in TCCSUP cells with modulation of phenotype. Immunoreactivity is shown by the brownish-red stain against hematoxylin and eosin counterstain (blue). E-cadherin was expressed diffusely in all cases where it was expressed and was not organized at cell surfaces. (A) Growth on SISgel showing very diffuse expression by cells on the gel surface. (B) Growth on Matrigel showing expression by surface cells and lack of expression by invasive cells. (C) Growth on SISgel in the presence of antibody neutralizing β1 integrin illustrating heterogeneity of expression of E-cadherin.
Discussion
The ability of cells to self-organize into tissue structures is highly dependent upon their ability to interact with matrix and other cells (2). In this study we show that the phenotype of bladder cancer cell lines growing in 3-dimensional “organotypic” cultures is remarkably plastic and depends upon epigenetic factors involving cell surface-extracellular matrix interactions. Simply by changing the supporting matrix, the phenotype can be altered from one characteristic of the original tumor to a minimally aggressive phenotype expressing a layered structure resembling that of normal urothelium. The phenotype is not independent of the degree of malignancy of the cells, and more malignant cells seem to have lost some of their ability to self-organize into multilayered structures. Malignant progression therefore represents a progressive loss of normal differentiation and growth controls arising from cell-matrix interactions.
Figure 7 illustrates a model that shows that many of the apparently complex phenotypic changes could result from a few simple changes in the cells and differences in properties of the gels. In ordered vertical growth the cells spread laterally because a relatively strong cell-matrix (c-m) interaction overwhelms the cell-cell (c-c) affinity. Layers form sequentially in organized vertical growth after the first layer becomes confluent. Of the cancer cells, only the RT4 cells retain the ability to form multiple layers on SISgel. On Matrigel, the affinity for matrix is weaker than the cell-cell affinity, thus favoring vertical growth. This hypothesis is supported by the observation that the cells adhere to SISgel within an hour, but up to 10 hours is required for adherence to Matrigel. Normal cells, including the SV-HUC-1 cells, further replication is terminated by differentiation. Papillary growth occurs because the cell-cell affinity is relatively stronger than the cell-matrix affinity and because the cells have lost the ability to terminate replication. Moreover, such growth is chaotic because of the lack of discrete layer formation. The invasive phenotype involves chaotic invasion en masse, rather than as individual cells. This phenotype resembles that described previously for aggressive lung cancer cells grown on Matrigel (21). Cells on the gel surface expressed the cell-adhesion molecule E-cadherin, while those that invaded did not, suggesting that loss of E-cadherin represents a manifestation of the invasive phenotype. The invasive phenotype involves further loss of the ability to grow outward. This level of organization must be inherent in the self-assembly of the system and does not represent selection for particular cell types on one gel as opposed to the other.
Figure 7.
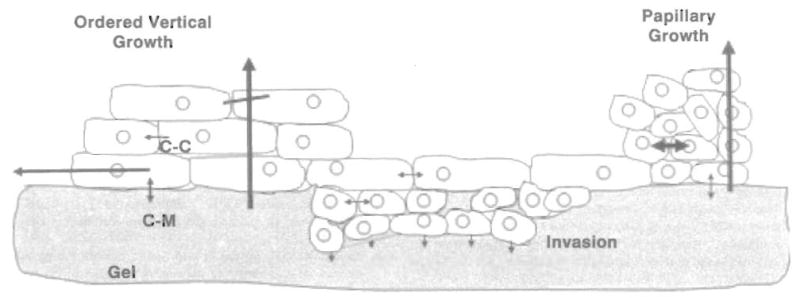
Schematic illustrating growth control and cell-matrix and cell-cell interactions in producing normal, papillary and invasive growth. In ordered vertical growth the strong cell-matrix (c-m) interaction causes the cells to spread laterally, forming a single layer on SISgel that then develops additional layers. Replication is terminated after some number of layers form, as indicated by the diagonal line through the vertical arrow. Cells self-organize into papillary structures when the cell-cell (c-c) affinity is stronger than the cell-matrix affinity, but the organization is chaotic due to lack of appropriate organizing morphogens. The invasive phenotype is heterogeneous, with the cells on the surface displaying a stronger cell-cell affinity than the invading cells. Invasion involves matrix degradation along the invasion front, as indicated by the series of arrows, and a distinct preference for growing downward into the gel in a chaotic manner.
Disruption of either α6 or β4 integrin interactions inhibited the ability of the cells to adhere to either gel, as would be predicted from reports that the α6β4 integrin was the principal integrin involved in substratum adhesion (5). However, the phenotypic changes seen in Figures 2 and 3 could not be reproduced by neutralization of α6 or β4 integrins. Likewise, neutralization of β1 integrins in SISgel or Matrigel failed to switch the phenotype, even though self-organization in normal urothelium reportedly involves α2β1 and α3β1 integrins (5), which are differentially regulated and mainly involved in homotypic cell-cell interactions and the maintenance of a stratified morphology. Only the IgM anti-β1 integrin antibody induced invasion in SISgel. However, the phenotype clearly is not the same as the phenotype seen in Matrigel. The most likely mechanism is that the invasion is induced in this one cell type by pathways linked to β1 signaling produced by clustering the integrins and not adherence to matrix (22). Clearly, the β1 integrins play a major role in cell-cell adherence, and both anti-β1 integrin antibodies altered homotypic cell adherence and matrix adherence without producing the phenotypic switching phenomenon. These findings are in direct contrast to the behavior of breast cells, for which the neutralizing IgM anti-β1 integrin antibody normalized the growth pattern in Matrigel of a malignant breast cell line, whereas the growth pattern of the parental, untransformed line could be deranged by neutralizing α6 integrin (4). These differences, observed with the same reagents, suggest that the rules for a glandular epithelium such as breast-derived cells are different from those governing the phenotype of a layered epithelium such as the urothelium and that these findings with bladder are unique and not recapitulations of the same phenomenon seen with breast cells.
The lack of a mechanistic role for the integrins in producing the phenotypic modulation suggests elements in the gel are responsible. Some connective tissue elements that modulate phenotype have been identified; overexpression of the proteoglycan bamacan facilitates the malignant phenotype (9), while expression of the proteoglycan decorin induced differentiation and quiescence (23). Both Matrigel and SISgel are composed predominately of collagen, but Matrigel contains laminin (24) and the proteoglycans bamacan and perlecan (9,25) that promote the malignant phenotype. However, addition of laminin in the approximate concentrations seen in Matrigel failed to alter the phenotype. SISgel is composed predominately of collagen I, with some collagen IV and contains an admixture of growth factors (26) and cell-surface molecules that facilitate growth of normal cells (15). The selection for the malignant phenotype by Matrigel does not appear to be due to growth factor-induced de-differentiation, because Matrigel also promotes differentiation of numerous cell types (27) and the growth factor-depleted product produced no detectable difference in phenotype. Our hypothesis is that SISgel suppresses the malignant phenotype.
Clearly, the genetic errors in one cell type are the same, regardless of the phenotype expressed on matrix gels. What is different is how the cells interact and interconnect, a crucial factor in self-organization (28). Taken together, these results suggest that the view of cancer representing the inevitable result of genetic errors is an oversimplification and that epigenetic factors can suppress or induce many of the features of the malignant phenotype. These findings may have clinical significance and may provide an explanation for the dormancy of metastatic cells and the presence of p53-mutant cells in normal-appearing epithelium (29). Clearly, additional work will be necessary to identify the mechanisms by which matrix can suppress malignant behavior. We suggest that our model modulating the phenotype between an aggressive and normalized phenotype in the absence of genetic differences should provide an important new means for identifying mechanisms by which tumor cells acquire aggressive phenotypes. In turn, this knowledge may lead to identification of markers for aggressiveness that can be used for pathologic prognostication or to identify potential targets for chemotherapy or chemoprevention that could suppress progression or metastasis.
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance of Jean Coffman and Norma McElwee and the gift of the 253 JP and 253 JB-V cells from Dr. Colin Dinney. This work was supported, in part, by a grant from the National Cancer Institute, CA 75322, to REH.
Abbreviations
- SIS
small intestine submucosa
- FCS
fetal calf serum
References
- 1.Jost SP, Gosling JA, Dixon JS. The morphology of normal human bladder urothelium. J Anat. 1989;167:103. [PMC free article] [PubMed] [Google Scholar]
- 2.Baskin LS, Hayward SW, Sutherland RA, DiSandro MS, Thomson AA. Cellular signaling in the bladder. Front Biosci. 1997;2:d592. doi: 10.2741/a215. [DOI] [PubMed] [Google Scholar]
- 3.Bates RC, Linez LF, Burns GF. Involvement of integrins in cell survival. Cancer Metastasis Rev. 1995;14:191. doi: 10.1007/BF00690291. [DOI] [PubMed] [Google Scholar]
- 4.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Southgate J, Kennedy W, Hutton KA, Trejdosiewicz LK. Expression and in vitro regulation of integrins by normal human urothelial cells. Cell Adhes Commun. 1995;3:231. doi: 10.3109/15419069509081289. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Bachman NJ, Nair TS, Goldsmith S, Liebert M, Grossman HB, Lomax MI, Carey TE. Beta 4 integrin transfection of UM-UC-2 (human bladder carcinoma) cells: stable expression of a spontaneous cytoplasmic truncation mutant with rapid loss of clones expressing intact beta 4. Cancer Res. 1997;57:38. [PubMed] [Google Scholar]
- 7.Iozzo RV. The family of the small leucine-rich proteoglycans: Key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 8.Iozzo RV, Pillarisetti J, Sharma B, Murdoch AD, Danielson KG, Uitto J, Mauviel A. Structural and functional characterization of the human perlecan gene promoter. Transcriptional activation by transforming growth factor-beta via a nuclear factor 1-binding element. J Biol Chem. 1997;272:5219. doi: 10.1074/jbc.272.8.5219. [DOI] [PubMed] [Google Scholar]
- 9.Ghiselli G, Iozzo RV. Overexpression of bamacan/SMC3 causes transformation. J Biol Chem. 2000;275:20235. doi: 10.1074/jbc.C000213200. [DOI] [PubMed] [Google Scholar]
- 10.Stadler E, Dziadek M. Extracellular matrix penetration by epithelial cells is influenced by quantitative changes in basement membrane components and growth factors. Exp Cell Res. 1996;229:360. doi: 10.1006/excr.1996.0381. [DOI] [PubMed] [Google Scholar]
- 11.Yamasaki H, Omori Y, Zaidan-Dagli ML, Mironov N, Mesnil M, Krutovskikh V. Genetic and epigenetic changes of intercellular communication genes during multistage carcinogenesis. Cancer Detect Prev. 1999;23:273. doi: 10.1046/j.1525-1500.1999.99037.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamura K, Kibbey MC, Jun SH, Kleinman HK. Effect of Matrigel and laminin peptide YIGSR on tumor growth and metastasis. Semin Cancer Biol. 1993;4:259. [PubMed] [Google Scholar]
- 13.Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995;46:396. doi: 10.1016/S0090-4295(99)80227-1. [DOI] [PubMed] [Google Scholar]
- 14.Sandusky GE, Lantz GC, Badylak SF. Healing comparison of small intestine submucosa and ePTFE grafts in the canine carotid artery. J Surg Res. 1995;58:415. doi: 10.1006/jsre.1995.1064. [DOI] [PubMed] [Google Scholar]
- 15.Badylak SF, Record R, Lindberg K, Hodde J, Park K. Small intestinal submucosa: a substrate for in vitro cell growth. J Biomater Sci Polym Ed. 1998;9:863. doi: 10.1163/156856298x00208. [DOI] [PubMed] [Google Scholar]
- 16.Iozzo RV. Tumor stroma as a regulator of neoplastic behavior. Agonistic and antagonistic elements embedded in the same connective tissue. Lab Invest. 1995;73:157. [PubMed] [Google Scholar]
- 17.Dinney CP, Fishbeck R, Singh RK, Eve B, Pathak S, Brown N, Xie B, Fan D, Bucana CD, Fidler IJ. Isolation and characterization of metastatic variants from human transitional cell carcinoma passaged by orthotopic implantation in athymic nude mice. J Urol. 1995;154:1532. [PubMed] [Google Scholar]
- 18.Seltzer JL, Lee AY, Akers KT, Sudbeck B, Southon EA, Wayner EA, Eisen AZ. Activation of 72-kDa type IV collagenase/gelatinase by normal fibroblasts in collagen lattices is mediated by integrin receptors but is not related to lattice contraction. Exp Cell Res. 1994;213:365. doi: 10.1006/excr.1994.1211. [DOI] [PubMed] [Google Scholar]
- 19.Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- 20.Mialhe A, Levacher G, Champelovier P, Martel V, Serres M, Knudsen K, Seigneurin D. Expression of E-, P-, n-cadherins and catenins in human bladder carcinoma cell lines. J Urol. 2000;164:826. doi: 10.1097/00005392-200009010-00057. [DOI] [PubMed] [Google Scholar]
- 21.Strohmaier AR, Spring H, Spiess E. Three-dimensional analysis of the substrate-dependent invasive behavior of a human lung tumor cell line with a confocal laser scanning microscope. Histochem Cell Biol. 1996;105:179. doi: 10.1007/BF01462290. [DOI] [PubMed] [Google Scholar]
- 22.Buckley CD, Rainger GE, Bradfield PF, Nash GB, Simmons DL. Cell adhesion: more than just glue. Mol Membr Biol. 1998;15:167. doi: 10.3109/09687689709044318. [DOI] [PubMed] [Google Scholar]
- 23.Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of erbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem. 2000;275:35153. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- 24.Sweeney TM, Kibbey MC, Zain M, Fridman R, Kleinman HK. Basement membrane and the SIKVAV laminin-derived peptide promote tumor growth and metastases. Cancer Metastasis Rev. 1991;10:245. doi: 10.1007/BF00050795. [DOI] [PubMed] [Google Scholar]
- 25.Couchman JR, Kapoor R, Sthanam M, Wu RR. Perlecan and basement membrane chondroitin sulfate proteoglycan (Bamacan) are two basement membrane chondroitin dermatan sulfate proteoglycans in the Engelbreth-Holm-Swarm tumor matrix. J Biol Chem. 1996;271:9595. doi: 10.1074/jbc.271.16.9595. [DOI] [PubMed] [Google Scholar]
- 26.Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478. [PubMed] [Google Scholar]
- 27.Royce LS, Kibbey MC, Mertz P, Kleinman HK, Baum BJ. Human neoplastic submandibular intercalated duct cells express an acinar phenotype when cultured on a basement membrane matrix. Differentiation. 1993;52:247. doi: 10.1111/j.1432-0436.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 28.Waliszewski P, Molski M, Konarski J. Self-similarity, collectivity, and evolution of fractal dynamics during retinoid-induced differentiation of cancer cell population. Fractals. 1999;7:139. [Google Scholar]
- 29.Koch WM, Boyle JO, Mao L, Hakim J, Hruban RH, Sidransky D. p53 gene mutations as markers of tumor spread in synchronous oral cancers. Arch Otholaryngol Head Neck Surg. 1994;120:943. doi: 10.1001/archotol.1994.01880330029006. [DOI] [PubMed] [Google Scholar]


