Abstract
Sex differences in behavior can be attributed to differences in steroid hormones. Sex chromosome complement can also influence behavior, independent of gonadal differentiation. The mice used for this work combined a spontaneous mutation of the Sry gene with a transgene for Sry that is incorporated into an autosome thus disassociating gonad differentiation from sex chromosome complement. The resulting genotypes are XX and XY− females (ovary-bearing) along with XXSry and XY−Sry males (testes-bearing). Here we report results of basic behavioral phenotyping conducted with these mice. Motor coordination, use of olfactory cues to find a food item, general activity, foot shock threshold, and behavior in an elevated plus maze were not affected by gonadal sex or sex chromosome complement. In a one-way active avoidance learning task females were faster to escape an electric shock than males. In addition, sex chromosome complement differences were noted during social interactions with submissive intruders. Female XY− mice were faster to follow an intruder than XX female mice. All XY− mice spent more time sniffing and grooming the intruder than the XX mice, with XY− females spending the most amount of time in this activity. Finally, XX females were faster to display an asocial behavior, digging, and engaged in more digging than XXSry male mice. All of these behaviors were tested in gonadectomized adults, thus, differences in circulating levels of gonadal steroids cannot account for these effects. Taken together, these data show that sex chromosome complement affects social interaction style in mice.
Keywords: Affective disorders, Autism, Depression, Anxiety, Sexual differentiation, X inactivation, Cognition, Pain
Introduction
Sex differences in behavior are common among sexually reproducing species. The neural bases of these behaviors are, in part, sculpted by the actions of gonadal steroids and genes downstream of the steroid receptors during development (MacLusky and Naftolin, 1981; Morris et al., 2004). This concept has been a major force behind decades of important research, yet, the discovery of “exceptions” to this rule has stimulated alternative hypotheses including the examination of sex chromosome complement as a source for neural and behavioral differences between males and females (Carruth et al., 2002; De Vries et al., 2002).
To manipulate sex chromosome complement independently of gonadal steroid exposure we use a mouse model that incorporates a spontaneous mutation of the Sry gene on the Y sex chromosome (referred to as Y−) and a Sry transgene that randomly inserts on an autosome, thus, testes determination is disassociated from sex chromosome complement (De Vries et al., 2002). As a result, all individuals possessing the Sry transgene develop testes and have a male external phenotype, regardless of their sex chromosome complement while individuals lacking the transgene have external female secondary sex characteristic and ovaries. Several other mouse models with sex chromosome mutations exist (Bouma et al., 2005; Isles et al., 2004; Reisert et al., 2002) and to distinguish the model we use from others we refer to the mice used in this study as the four core genotypes (FCG).
Using the FCG we, and others, have identified several sexually dimorphic behaviors that can be attributed to sex chromosome complement including; parental-like responses to pups, attack latencies toward intruders, habit formation in a food reinforcement paradigm, and pain sensitivity in the tail flick and formalin tests (Gatewood et al., 2006; Gioiosa et al., 2008; Quinn et al., 2007). Some of these behaviors require activation by gonadal steroid hormones (Gatewood et al., 2006) and in other tests sex chromosome effects have been demonstrated in gonadectomized animals thus eliminating the influence of circulating steroids (Gioiosa et al., 2008; Quinn et al., 2007). Of course, even when gonads are removed, differences in adult behavior may still be attributed to differences in gonadal hormone concentrations during development. Perinatal T levels have not been directly measured in the FCG. However, two sexually dimorphic measures that reflect perinatal T; numbers of tyrosine hydroxylase containing cells in the anterior paraventricular nucleus (AVPV) and anogenital distances (De Vries et al., 2002; Rissman, unpublished data) are not affected by sex chromosome complement.
Basic behavioral phenotyping of the FCG mice has been limited. In the present study we tested adult, gonadectomized, mice from the FCG on a battery of basic behavioral measures. Some of the behaviors have been reported to be sexually dimorphic, others have not (Bridges and Starkey, 2004; Craft et al., 2004; Imhof et al., 1993; Johnston and File, 1991). We also tested more complex behaviors; anxiety as assessed in an elevated plus maze, one-way active avoidance escape learning, and social interactions with passive intruders. In most behavior tests no gonadal sex or sex chromosome complement effects were noted. In the minority of tests either gonadal sex or sex chromosome complement did affect behavioral outcomes. In particular, interactions with intruders were influenced by sex chromosome complement revealing a difference in social interaction style in mice with XX versus XY chromosome complements.
Materials and methods
Animals and surgery
The mouse colony was bred and maintained in the University Of Virginia School Of Medicine, Jordan Hall Animal Facility. Development and production of the four core genotype (FCG) mouse model has been described previously (De Vries et al., 2002; Gatewood et al., 2006). Briefly, the cross uses males carrying a 129/SvEv-Gpi1c Y chromosome (Simpson et al., 1997) with an 11 kb deletion removing the testis-determining gene Sry (Gubbay et al., 1992). The Sry deletion (designated Y−) is complemented by the insertion of a fully penetrant Sry transgene [derived from the transgenic line C57BL/6Ei-YARK/JTgN (Sry-129)2Ei] located on an autosome (Mahadevaiah et al., 1993, 1998). The Y− chromosome and the Sry transgene segregate independently, and when XX females and XY−Sry males are paired the offspring include; XX females, XY− females, XY−Sry males, and XXSry males. We define males as testes-bearing individuals and females possess ovaries. Mice were genotyped between 10 and 21 days of age by PCR for the YMT2/B-related Ssty gene subfamily present on the Y long arm, which detects the Y− chromosome (Turner et al., 2000). In our colony, the Y− chromosome and Sry transgene have been fully crossed (over 10 generations) into the C57BL/6J strain.
All mice used in these studies were group housed by sex from weaning (20–21 days of age) until they were gonadectomized, at 2–4 months of age. After surgery all subjects were individually housed. Mice were maintained on a 12:12 light/dark cycle (lights off at 1900 EDT) and received food (#7912; Harlan Teklad, Madison, WI) and water ad libitum. Surgery was conducted under ketamine/xylazine anesthesia (0.1 ml/20 g body weight). After gonads were removed each mouse was given a s.c. injection of 0.9% sodium chloride (for rehydration), a topical analgesic (0.25% Bupivacaine) and kept warm until they awakened. Submissive intruders were C57BL/6J male mice that had been bulbectomized (Rowe and Edwards, 1971) at least 4 weeks prior to use in the social interaction tests. Behavior tests began no sooner than 10 days post surgery. The observers were blind to the sex chromosome complement and gonadal sex of the subjects. All tests, except social interaction, were performed during the last 3–4 h of the lighted portion of the day. Prior to each test mice were transported to the testing room at least 15 min before testing.
Three cohorts of mice were used for the current study. The first cohort (n=40) was tested for olfaction, motor coordination, and one-way active avoidance escape learning, in that order. The second cohort (n=32) was tested for open field activity and foot shock sensitivity. The final cohort (n=54) was tested in the elevated plus maze for anxiety and then used for social interaction testing. All testing and surgical procedures were approved by the University of Virginia Animal Care and Use Committee.
Cohort 1
Olfactory test
Basic olfactory functioning was measured by observing the time taken to discover a food item, Cocoa Puffs™ (General Mills), buried in clean bedding. To habituate mice (n=10, XX; n=11, XY−; n=9, XXSry; n=10, XY−Sry) to this novel food prior to testing, one Cocoa Puff™ was placed in their home cage, on the top of the bedding for four consecutive days. Mice readily investigated and then ate the food item. On the fourth day their standard chow was removed overnight. Twenty-four hours later, mice were briefly removed from their home cages, a single Cocoa Puff™ was buried in their bedding, and then the resident was returned to the cage. The latency (s) from the placement of the resident in the cage to the discovery of the Cocoa Puff™ was recorded.
Motor coordination
To test mice for balance and motor coordination an accelerating rotarod was used. Training occurred over three consecutive days. Each mouse (n=10, XX; n=11, XY−; n=9, XXSry; n=10, XY−Sry) was placed on the rotarod (Med Associates, Inc.; ENV 576M) for four consecutive trials, each lasting for up to 60 s. The latency to fall off the rotarod on each trial was noted with the speed set at a constant 10 rpm. On the fourth day each mouse was placed on the rotarod and the apparatus was set to constantly accelerate from 4 to 40 rpm during the session. The time from the start to fall off the rotarod (s) was recorded for each mouse. After each use the rotarod was cleaned with ethyl alcohol.
One-way active avoidance learning
To assess one-way active avoidance learning, mice were tested in a shuttle escape box. On the test day the mouse (n=10, XX; n=11, XY−; n=9, XXSry; n=8, XY−Sry) was placed on one side of the shuttle box with the door between the two sides shut. At 24 s intervals the grid floor was electrified to 20 mA, the door immediately opened and the mouse was then able to cross to the other side. Across 30 trials, of 24 s each the latency to cross to the other side once the shock was delivered was recorded. After each subject, the shuttle box was thoroughly cleaned with ethyl alcohol. The shuttle box (Med Associates, Inc.; ENV 010MC) measured 44 cm×17.3 cm and was made of Plexiglas and the metal grid floor was controlled by an 8 channel I/R controller (Med Associates, Inc.; ENV 253C) and divided into two equal sections by an automatic guillotine door (Med Associates, Inc.; ENV 010B).
Cohort 2
Open field activity
Open field activity was evaluated in a clear rectangular Plexiglas box (18.8 cm×39.4 cm), with a clear Plexiglas top. Subjects (n=8, XX; n=8, XY−; n=7, XXSry; n=9, XY−Sry) were placed in one corner of the box and allowed to explore the area for 10 min. On the floor was a grid square pattern divided into 14 equal quadrants (10 peripheral and 4 central squares; 18.6 cm×39 cm). The boxes were cleaned with ethyl alcohol between each use. Trials were videotaped from above. The total number of grids crossed, the amount of time (s) spent in squares adjacent to the wall, number of rears, and the numbers of feces present at the end of the 10 min trial were scored.
Foot shock thresholds
To determine shock thresholds, mice were tested in the same shuttle box apparatus described for active avoidance learning. On the testing day subjects (n=8, XX; n=8, XY−; n=7, XXSry; n=9, XY−Sry) were placed on the right side of the shuttle box, with the door between the two sides closed. Beginning at 0.05 mA, the shock was on for 15 s followed by 45 s off. The current was increased in 0.01 mA increments until the mouse flinched and/or vocalized, at which time the current was immediately terminated. After each mouse, the shuttle box was thoroughly rinsed with water and ethyl alcohol.
Cohort 3
Elevated Plus Maze (EPM)
At the start of the test, each mouse (n=13, XX; n=15, XY−; n=16, XXSry; n=10, XY−Sry) was placed in the center of the clean EPM, facing an open arm, and allowed to explore the maze for 5 min. Between each animal the EPM was thoroughly cleaned with ethyl alcohol. All sessions were video recorded from above and behaviors scored included number of crosses into the open and closed arms and the time (s) spent in the open and closed arms. The floors and walls of the elevated plus maze (ENV-560A; Med Associates, Inc., St. Albans, VT) were black polypropylene. Each runway measured 6.1 cm wide and 34.9 cm long and was raised 71.25 cm from the floor. The walls on the closed arms were 20.3 cm high.
Social interaction
A resident–intruder paradigm (Gatewood et al., 2006) was used to assess social interactions. Three to 5 days before testing, social experience was given with an ovary-intact C57BL/6J female that was placed in the home cage of each mouse (n=10, XX; n=11, XY−; n=10, XXSry; n=11, XY−Sry) for 2 min. On three consecutive days, during the first 4 h after lights off, under red-light illumination, a bulbectomized gonad-intact C57BL/6J male mouse (intruder) was placed in the home cage with the subject mouse (resident). The same intruder was used in each dyadic pairing across all 3 days. All behavior was videotaped using VideoWave (Roxio) and the behavior of the resident was scored during a 6 min test. The following behaviors were noted: 1) the latency to contact the intruder; 2) the latency, duration, and number of times the resident sniffed/groomed the body of the intruder; 3) the amount of time the resident was inactive and in physical contact with the intruder; 4) the latency, duration, and number of times the resident spent digging in the cage bedding; and 5) the latency, duration, and number of times the resident followed the intruder. If an attack occurred, the intruder was immediately removed and the test terminated. This happened twice, once in a test with an XXSry resident and again with an XY−Sry resident.
Statistics
The data for olfaction, motor coordination, open field activity, foot shock threshold, anxiety and mean social interactions averaged over all 3 days were analyzed using a two-way analysis of variance (ANOVA). A repeated measures ANOVA was used to analyze the one-way active avoidance (collected over 30 trials), data. For all analyses the two factors were sex chromosome complement (XX versus XY) and gonadal sex (male versus female). Post hoc pairwise comparison tests were performed using the Fisher's LSD Multiple Comparison Test. An alpha level of 0.05 was set for all tests.
In the social interaction tests two individuals attacked the intruder on one of their three testing days and thus those tests were ended early. We adjusted all the scores for the length of the test in each case and this did not affect any of the statistical outcomes.
Results
No sex chromosome complement or gonadal sex effects on food detection, motor coordination, activity in the open field, foot shock threshold, or anxiety
There were no significant effects of sex chromosome complement or gonadal sex on the latency to find the hidden Cocoa Puff™ (chromosomal, F(1,39)=0.09 and gonadal, F(1,39)=0.33; Table 1). We noted a trend for an interaction (F(1,39)=3.89, P=0.056), with XX females taking the longest to find the food item (mean=18 s) and XXSry males finding the food fastest (mean=7 s). The amount of time spent on an accelerating rotarod prior to falling was not significantly affected by sex chromosome complement or gonadal sex (chromosomal, F(1,39)=0.79, gonadal, F(1,39)=0.03), nor was there an interaction between the factors (F(1,39)=0.25; Table 1). In the open field there were no behavioral differences between the groups in terms of the total number of grids crossed, time spent adjacent to the wall, numbers of rears or feces deposited (chromosomal factor, F(1,31)=0.00, 0.06, 0.63, 0.22; gonadal factor, F(1,31)=1.96, 0.02, 0.06, 0.16; interaction, F(1,31)=0.05, 0.42, 0.09, 1.82; respectively, Table 2). There were no significant effects on foot shock threshold (chromosomal, F(1,31)=1.34; gonadal, F(1,31)=1.59; interaction, F(1,31)=0.10; Table 2).
Table 1.
Mean±SEM scores for the olfactory test, rotarod, and elevated plus maze
| Behavioral test | XX | XY | XXSry | XY−Sry |
|---|---|---|---|---|
| Latency to find the hidden food (s) | 17.9±7.3 (10) | 8.3±0.7 (11) | 7.1±0.7 (9) | 14.2±4.0 (10) |
| Latency from start to fall from the accelerating rotarod (s) | 402.6±211.7 (10) | 487.6±150.5 (11) | 333.9±162.5 (9) | 636.3±307.1 (10) |
| Elevated plus maze | ||||
| Total time spent in the open arms (s) | 61.5±15.8 (13) | 59.2±8.5 (15) | 60.1±6.2 (16) | 61.2±7.1 (10) |
| Total number of crosses into the open arms | 7.2±1.4 (13) | 8.1±0.69 (15) | 8.4±0.83 (16) | 10.6±1.0 (10) |
| Total number of crosses into the open and closed arms | 19.2±3.1 (13) | 21.3±1.1 (15) | 22.2±1.3 (16) | 25.1±1.2 (10) |
In all tests mice were gonadectomized and tested 10–14 days later. Numbers in () represent Ns.
Table 2.
Mean±SEM scores for the open field test and foot shock threshold
| Behavioral test | XX (8) | XY− (8) | XXSry(7) | XY−Sry (9) |
|---|---|---|---|---|
| Open field test | ||||
| Number of grids crossed | 331.75±27 | 337.5±24.1 | 300.14±25.7 | 294.6±28 |
| Total time spent in the outer squares (s) | 440.75±10.3 | 452.9±8.69 | 447.7±21.4 | 442.3±12.5 |
| Total number of rears | 75±10.7 | 80.25±5.0 | 74.3±14.1 | 86±11.3 |
| Total number of feces | 1.25±0.4 | 0.5±0.27 | 0.86±0.4 | 1.22±0.49 |
| Foot shock threshold | ||||
| Pain threshold (mA) | 0.13±0.0 | 0.12±0.09 | 0.14±0.01 | 0.13±0.0 |
In all tests mice were gonadectomized and tested 10–14 days later. Numbers in () represent Ns.
No effects of sex chromosome complement or gonadal sex on anxiety were noted in the elevated plus maze (EPM). The amount of time spent in the open arms was not different between the groups for any parameters (chromosomal, F(1,53)=0; gonadal, F(1,53)=0; interaction, F(1,53)=0.03). The number of crosses made into the open arms was not significantly affected by any of the factors (chromosomal, F(1,53)=2.27; gonadal, F(1,53)=3.27; interaction, F(1,53)=0.47). Likewise the total number of crosses made into the open and the closed arms was not influenced by sex chromosome complement (F(1,53)=1.76), gonadal sex (F(1,53)=3.17), nor was there an interaction (F(1,53)=0.05; Table 1).
One-way active avoidance escape behavior is influenced by gonadal sex
The latency to escape from a mild shock over 30 trials (of 24 s) each, was significantly influenced by gonadal sex (F(1,227)=5.01, P<0.032). Females were significantly faster to escape than males (P<0.05; Fig. 1). However there was no effect of sex chromosome complement (F(1,227)=1.33) nor was an interaction detected (F(1,227)=0.03, Fig. 1).
Fig. 1.
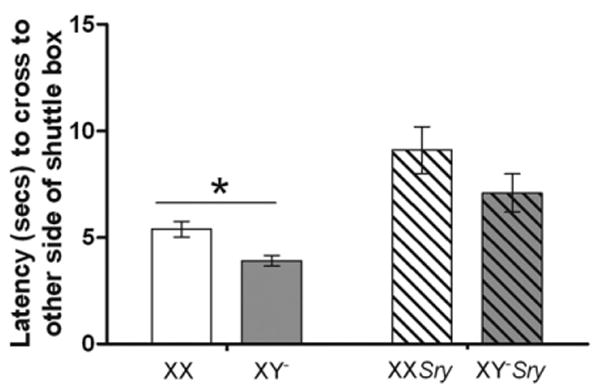
Mean (±SEM) scores for escape behavior (s) in an active avoidance task (n=10 XX; n=11 XY−; n=9 XXSry; n=8 XY−Sry). In all tests mice were gonadectomized and tested 10–14 days later. Solid colored histograms are data from gonadal females, striped histograms are data from gonadal males. XX genotypes have a white background and gray backgrounds are XY−. * Gonadal females have significantly shorter latencies to escape than gonadal males (P<0.05).
Social interactions are influenced by sex chromosome complement
In general, we found that XY− individuals of both sexes engaged in more sniffing and grooming of the intruder than did the XX mice. Interestingly XY− females were faster to follow the intruder than XX females. Digging, an asocial behavior, was displayed more often and sooner in XX females as compared to XXSry males.
Total time spent sniffing and grooming the intruder was affected by sex chromosome complement (F(1,40)=4.03; P=0.05) as was the number of sniffing and grooming bouts (F(1,40)=6.87; P<0.012; Fig. 2). The XY− individuals engaged in more sniffing and grooming for a longer total duration than did the XX mice (P<0.05). We did not detect an effect of gonadal sex (for bout numbers, F(1,40)=0.02 and for duration, F(1,40)=0) or an interaction (for bout numbers, F(1,40)=1.15, and for duration F(1,40)=2.86). The latencies to begin sniffing and grooming the intruders were equivalent for all the groups (chromosomal, F(1,40)=1.55; gonadal, F(1,40)=0.22; interaction, F(1,40)=1.68; Table 3).
Fig. 2.

Mean (±SEM) scores for sniffing and grooming the body of the intruder. A) Total time spent sniffing and grooming (s). B) Number of bouts of sniffing and grooming. All mice were gonadectomized and tested for social interaction in a resident–intruder paradigm 10–14 days later (n=10 XX; n=11 XY−; n=10 XXSry; n=11 XY−Sry). Solid colored histograms are data from gonadal females, striped histograms are data from gonadal males. XX genotypes have a white background and gray backgrounds are XY−. **Mice with the XX sex chromosome complement spent significantly less time sniffing and displayed significantly fewer bouts of these behaviors than mice with a XY− sex chromosome complement (P<0.05).
Table 3.
Mean (±SEM) scores for the non-significant social interaction behaviors
| Behavioral test | XX (10) | XY−(11) | XXSry(10) | XY−Sry(11) |
|---|---|---|---|---|
| Sniffing/grooming | ||||
| Latency (s) | 26.4±8.4 | 6.7±1.3 | 20.0±7.7 | 19.3±10.3 |
| Following | ||||
| Total time (s) | 1.1±0.33 | 4.1±1.4 | 2.3±0.68 | 4.0±0.84 |
| Digging | ||||
| Number of occurrences | 16.8±2.4 | 10.7±2.6 | 3.9±1.5 | 12.5±3.1 |
All mice were gonadectomized and tested 10–14 days later. Numbers in () represent Ns.
Another behavior, latency to follow the intruder, showed an interaction between the two main factors (F(1,40)=6.57, P<0.015). The interaction was driven by differences between the two gonadal female groups. Females with an XX genotype were slower to follow intruders than XY− females (P<0.05; Fig. 3). No significant effects of sex chromosome complement (F(1,40)=1.07) or gonadal sex (F(1,40)=0) alone were noted. Total time spent following the intruder was not significantly different with respect to sex chromosome complement (F(1,40)=3.17), gonadal sex (F(1,40)=0.25), nor was an interaction detected (F(1,40)=0.12; Table 3).
Fig. 3.
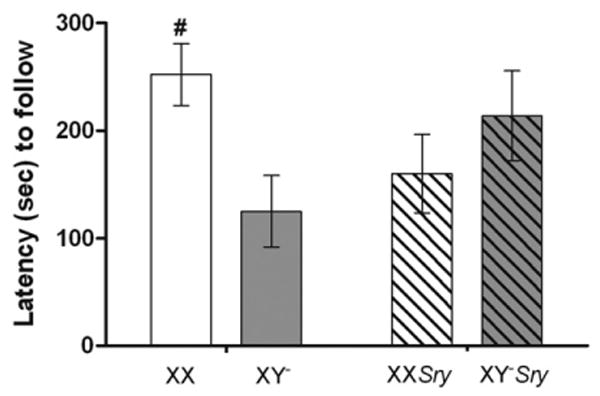
Mean (±SEM) latencies to follow the intruder. All mice (n=10 XX; n=11 XY−; n=10 XXSry; n=11 XY−Sry) were gonadectomized and tested for social interaction in a resident–intruder paradigm 10–14 days later. Solid colored histograms are data from gonadal females, striped histograms are data from gonadal males. XX genotypes have a white background and gray backgrounds are XY−. #XX females are significantly slower to follow than XY− female mice. (P<0.05).
The final behavior we scored in the social interaction tests was digging. This behavior is asocial, typically the resident withdrew from the intruder to another part of the cage and pawed and dug the bedding there. Interactions between sex chromosomes and gonadal sex were significant for both latencies to dig (F(1,40)=5.26, P<0.03) and the amount of time spent digging (F(1,40)=4.27, P<0.05; Fig. 4). For both aspects of this behavior the interaction was caused by the differences between XX females and XXSry males (P<0.05). Females (XX) were faster to start to dig and spent more time digging than did the XXSry males. Sex chromosome complement (latencies, F(1,40)=0; time spent, F(1,40)=0.17) was not significant as an independent factor nor was gonadal sex (latencies, F(1,40)=1.27; time spent, F(1,40)=1.36). Number of digging bouts did not differ between the groups (chromosomal, F(1,40)=0.14; gonadal, F(1,40)=1.82; interaction, F(1,40)=3.79; Table 3).
Fig. 4.
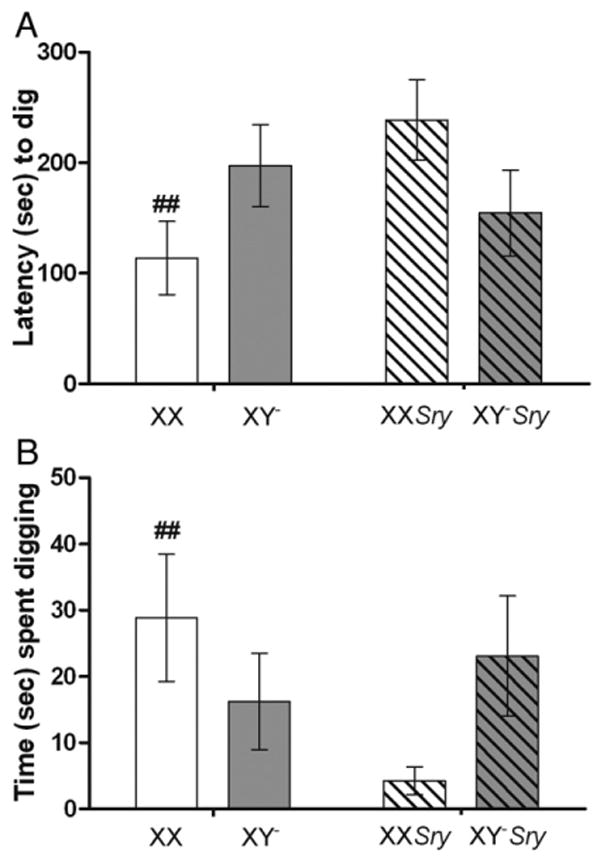
Mean (±SEM) scores for digging behavior. A) Latency (s) to begin digging. B) Total time (s) spent digging. All mice (n=10 XX; n=11 XY−; n=10 XXSry; n=11 XY−Sry) were gonadectomized and tested for social interaction in a resident–intruder paradigm 10–14 days later. Solid colored histograms are data from gonadal females, striped histograms are data from gonadal males. XX genotypes have a white background and gray backgrounds are XY−. ##XX females are significantly faster to dig and spend more time digging than XXSry male mice (P<0.05).
Discussion
The social interaction tests produced the most interesting data set. Here, using gonadectomized mice, we found an effect of sex chromosome complement on sniffing and grooming the body of the intruder. Mice with the XX genotype, irrespective of gonadal sex, spent less time and engaged in fewer bouts of sniffing and grooming the intruders than mice with the XY− genotype. Although the interaction failed to achieve significance, the trend suggests that the effects were caused by differences between the two female groups since the values for the two male groups are intermediate, between the two extreme female groups. In the two other behaviors recorded; following the intruder and digging, XX females took significantly longer to follow the intruder than XY− females. Digging, an asocial behavior, was demonstrated more rapidly and for a longer period of time by XX females than all other mice, however, the only significant difference was with XXSry mice. These differences between the XX females and the other mice, are consistent with our previous aggression and parental behavior findings (Gatewood et al., 2006) in which XX females stood out from the other three genotypes. In those tests, XX females treated with testosterone were slower to attack an intruder, and with intact gonads XX females were faster to retrieve, and retrieved more pups than mice in the three other groups (XY−, XXSry, and XY−Sry). The social interaction tests were conducted in a similar manner to the resident–intruder tests the only difference was that in the present tests the residents were gonadectomized. For this reason almost no attack behavior was displayed by mice of any genotype, but XX females were less likely to engage socially with the intruder which given their hormonal status may be similar to avoiding an aggressive interaction.
The fact that group differences in sniffing, grooming and following were driven primarily by differences in the two female genotypes suggests that sex differences in hormone concentrations prior to gonadectomy may influence these behaviors. Because females lack the Sry transgene, it is possible that the exposure of the gonadal males to testosterone at some point in development masks any sex chromosome differences that otherwise may have been observed. One way to test this hypothesis is to castrate males shortly after birth to see if this unmasks an affect of sex chromosomes on behavior. In contrast, digging differed by sex, but only in mice with XX sex chromosome complement. Therefore, the lack of difference between the two XY−groups may be the result of gene(s) on the Y chromosome or overexpression of X chromosome genes in XX individuals.
Sex chromosome complement effects can be caused by three main mechanisms: numbers of X chromosomes, presence/absence of the Y chromosome, or parent of origin effects for the X chromosome and its genes (imprinting). In humans and mice, XX and XY individuals have differences in the expression levels of the X chromosome genes that fail to escape X-inactivation (Carrel and Willard, 2005; Nguyen and Disteche, 2006). Several X genes are over-expressed in female as compared with male brains (Xu et al., 2002, 2005, 2006) and work in the FCG indicates that the difference is independent of gonadal sex. Sex differences in neural tube defects (females>males) have been noted in mice mutant for the p53 transcription factor, a line with high rates of spontaneous embryonic lethality caused by neural tube closure failure (Cranston et al., 1997). When the p53 mice were crossed with the FCG, XX individuals of both sexes were more likely to die in utero than XY− individuals (Chen et al., 2008). To ask if the presence of two X chromosomes was involved in neural tube-related lethality a second study used the XY* mouse to generate offspring (these are very close to an XO genotype; Burgoyne et al., 1998; Eicher et al., 1991). The XX individuals were the most likely to have neural tube deficits, suggesting that the X genes that escape inactivation may increase the risk of this developmental disorder.
The case for specific roles for Y chromosome genes has not yet been made in the FCG, and perhaps the existence of homologous X genes makes this less likely (Xu et al., 2002). However some behavioral differences between male mice might be attributed to differences in the genetic source of the Y chromosome (Selmanoff et al., 1977; Shrenker and Maxson, 1982; Sluyter et al., 1999). In addition, we have found that an extra Y− chromosome can have affects on male copulatory behavior (Park et al., in press).
In the human condition of Turner's Syndrome (45,XO) a variety of cognitive deficits have been reported (Skuse, 2000), the severity of which has been linked to a parent of origin effect in the XO girls (Skuse, 1999). In a mouse model of Turner's Syndrome (39,XO) XO mice are more fearful than their XX female littermates (Davies and Wilkinson, 2006; Isles et al., 2004), and a candidate X-linked imprinted gene, with a potential association to this behavior, has been identified (Davies et al., 2005; Raefski and O'Neill, 2005). The mechanism(s) that underlie the sex chromosome effects we report here, social interaction style, is of interest to our laboratory and currently under investigation.
The main goal of this study was to determine if any basic behavioral characteristics differed by genotype in mice of the FCG. We selected behaviors that were relevant to the types of behaviors influenced by sex chromosome complement (Gatewood et al., 2006). For example differences in latencies to retrieve pups or to behave aggressively toward intruders might be affected by olfactory abilities, activity levels, motor coordination, and/or anxiety. Here we found that none of these behaviors were affected by gonadal or chromosomal sex. Olfaction is particularly important for social interactions and our finding of no differences in latency to find a food item, most mice finding the buried food in less than 20 s, confirms pervious data which require more complex olfactory function. Olfactory preference for male- versus female-soiled bedding, social recognition (Gatewood et al., 2006) and time spent sniffing an anaesthetized male or female mouse (De Vries et al., 2002) have been investigated using the FCG. In the preference studies we noted the previously described gonadal sex effects; males spend more time sniffing females and their odors than do females. Moreover mice in all the genotypes were able to differentiate one ovariectomized female from another. Taken together we have no reason to attribute behavioral differences between the genotypes to olfactory deficits, but tests of more complex olfactory abilities are underway.
Interestingly, we did not find genotype differences in threshold sensitivity to foot shock, unlike another study using the FCG in which XX mice were more sensitive to pain than XY− mice in the formalin and hotplate tests (Gioiosa et al., 2008). The differences in our results are likely because of the different pain paradigms employed. We did, however, detect a sex difference in the latency to escape a shock in a one-way active avoidance task; here females were faster than males. These findings are similar to previous ones in which gonad-intact female C57BL/6J mice learned to avoid a shock faster than males (Levi et al., 2008). Thus, while all mice in the FCG have the same thresholds for responses to foot shock the pain may be more motivating in gonadal females than males.
Overall, the findings of this study lend further support to the role of sex chromosomes in the expression of sexually dimorphic behaviors in the mouse. This may be important in light of the number of affective disorders in humans that are expressed in a sexually dimorphic manner (Holden, 2005; Jamain et al., 2003). Some of these neuro-behavioral diseases have been linked to sex chromosomes (i.e. Fragile X, autism, 45,XO) others have not (i.e. ADHD). Perhaps investigations of sex differences, as related to sex chromosome genes, will open up new research opportunities and new genetic targets for these illnesses.
Acknowledgments
The authors would like to thank Aileen Wills for care of the animals and Savera Shetty for genotyping. We thank Dr. Marcia McDuffie for verification of our crosses into C57BL/6J. This work was supported by T32 DK007646, R01 NS55218, and T32 GM008328.
References
- Bouma GJ, Albrecht KH, Washburn LL, Recknagel AK, Churchill GA, Eicher EM. Gonadal sex reversal in mutant Dax1 XY mice: a failure to upregulate Sox9 in pre-Sertoli cells. Development. 2005;132(13):3045–3054. doi: 10.1242/dev.01890. [DOI] [PubMed] [Google Scholar]
- Bridges NJ, Starkey NJ. Sex differences in Mongolian gerbils in four tests of anxiety. Physiol Behav. 2004;83(1):119–127. doi: 10.1016/j.physbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet. 1998;80(1–4):37–40. doi: 10.1159/000014954. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434(7031):400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5(10):933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- Chen X, Watkins R, Delot E, Reliene R, Schiestl RH, Burgoyne PS, Arnold AP. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev Neurobiol. 2008;68(2):265–273. doi: 10.1002/dneu.20581. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8(5):397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Cranston A, Bocker T, Reitmair A, Palazzo J, Wilson T, Mak T, Fishel R. Female embryonic lethality in mice nullizygous for both Msh2 and p53. Nat Genet. 1997;17(1):114–118. doi: 10.1038/ng0997-114. [DOI] [PubMed] [Google Scholar]
- Davies W, Wilkinson LS. It is not all hormones: alternative explanations for sexual differentiation of the brain. Brain Res. 2006;1126(1):36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- Davies W, Isles A, Smith R, Karunadasa D, Burrmann D, Humby T, Ojarikre O, Biggin C, Skuse D, Burgoyne P, Wilkinson L. Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects on cognitive function in mice. Nat Genet. 2005;37(6):625–629. doi: 10.1038/ng1577. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22(20):9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, King TR, Eppig JT, Washburn LL. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet. 1991;57(4):221–230. doi: 10.1159/000133152. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26(8):2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008;53:124–130. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay J, Vivian N, Economou A, Jackson D, Goodfellow P, Lovell-Badge R. Inverted repeat structure of the Sry locus in mice. Proc Natl Acad Sci U S A. 1992;89(17):7953–7957. doi: 10.1073/pnas.89.17.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden C. Sex and the suffering brain. Science. 2005;308(5728):1574. doi: 10.1126/science.308.5728.1574. [DOI] [PubMed] [Google Scholar]
- Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav Brain Res. 1993;56(2):177–180. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Isles AR, Davies W, Burrmann D, Burgoyne PS, Wilkinson LS. Effects on fear reactivity in XO mice are due to haploinsufficiency of a non-PAR X gene: implications for emotional function in Turner's syndrome. Hum Mol Genet. 2004;13(17):1849–1855. doi: 10.1093/hmg/ddh203. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34(1):27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49(2):245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Levi Y, Kofman O, Schwebel M, Shaldubina A. Discrimination and avoidance learning in adult mice following developmental exposure to diisopropylfluorophosphate. Pharmacol Biochem Behav. 2008;88(4):438–445. doi: 10.1016/j.pbb.2007.09.017. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211(4488):1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Lovell-Badge R, Burgoyne PS. Tdy-negative XY, XXY and XYY female mice: breeding data and synaptonemal complex analysis. J Reprod Fertil. 1993;97(1):151–160. doi: 10.1530/jrf.0.0970151. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Odorisio T, Elliott DJ, Rattigan A, Szot M, Laval SH, Washburn LL, McCarrey JR, Cattanach BM, Lovell-Badge R, Burgoyne PS. Mouse homologues of the human AZF candidate gene RBM are expressed in spermatogonia and spermatids, and map to a Y chromosome deletion interval associated with a high incidence of sperm abnormalities. Hum Mol Genet. 1998;7(4):715–727. doi: 10.1093/hmg/7.4.715. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7(10):1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nat Genet. 2006;38(1):47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]
- Park JH, Burns-Cusato M, Dominguez-Salazar E, Riggan A, Shetty S, Arnold AP, Rissman EF. Effects of sex chromosome aneuploidy on male sexual behavior. Genes Brain and Behavior. doi: 10.1111/j.1601-183X.2008.00397.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10(11):1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Raefski AS, O'Neill MJ. Identification of a cluster of X-linked imprinted genes in mice. Nat Genet. 2005;37(6):620–624. doi: 10.1038/ng1567. [DOI] [PubMed] [Google Scholar]
- Reisert I, Karolczak M, Beyer C, Just W, Maxson SC, Ehret G. Sry does not fully sex-reverse female into male behavior towards pups. Behav Genet. 2002;32(2):103–111. doi: 10.1023/a:1015297622509. [DOI] [PubMed] [Google Scholar]
- Rowe FA, Edwards DA. Olfactory bulb removal: influences on the aggressive behaviors of male mice. Physiol Behav. 1971;7(6):889–892. doi: 10.1016/0031-9384(71)90059-x. [DOI] [PubMed] [Google Scholar]
- Selmanoff MK, Goldman BD, Maxson SC, Ginsburg BE. Correlated effects of the Y-chromosome of mice on developmental changes in testosterone levels and intermale aggression. Life Sci. 1977;20(2):359–365. doi: 10.1016/0024-3205(77)90332-0. [DOI] [PubMed] [Google Scholar]
- Shrenker P, Maxson SC. The Y chromosomes of DBA/1Bg and DBA/2Bg compared for effects on intermale aggression. Behav Genet. 1982;12(4):429–434. doi: 10.1007/BF01065634. [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16(1):19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Skuse DH. Genomic imprinting of the X chromosome: a novel mechanism for the evolution of sexual dimorphism. J Lab Clin Med. 1999;133(1):23–32. doi: 10.1053/lc.1999.v133.a94575. [DOI] [PubMed] [Google Scholar]
- Skuse DH. Imprinting, the X-chromosome, and the male brain: explaining sex differences in the liability to autism. Pediatr Res. 2000;47(1):9–16. doi: 10.1203/00006450-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Marican CC, Roubertoux PL, Crusio WE. Radial maze learning in two inbred mouse strains and their reciprocal congenics for the non-pseudoautosomal region of the Y chromosome. Brain Res. 1999;835(1):68–73. doi: 10.1016/s0006-8993(99)01385-2. [DOI] [PubMed] [Google Scholar]
- Turner JM, Mahadevaiah SK, Benavente R, Offenberg HH, Heyting C, Burgoyne PS. Analysis of male meiotic “sex body” proteins during XY female meiosis provides new insights into their functions. Chromosoma. 2000;109(6):426–432. doi: 10.1007/s004120000097. [DOI] [PubMed] [Google Scholar]
- Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet. 2002;11(12):1409–1419. doi: 10.1093/hmg/11.12.1409. [DOI] [PubMed] [Google Scholar]
- Xu J, Taya S, Kaibuchi K, Arnold AP. Sexually dimorphic expression of Usp9x is related to sex chromosome complement in adult mouse brain. Eur J Neurosci. 2005;21(11):3017–3022. doi: 10.1111/j.1460-9568.2005.04134.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Watkins R, Arnold AP. Sexually dimorphic expression of the X-linked gene Eif2s3x mRNA but not protein in mouse brain. Gene Expr Patterns. 2006;6(2):146–155. doi: 10.1016/j.modgep.2005.06.011. [DOI] [PubMed] [Google Scholar]


