Abstract
The serotonin system is strongly implicated in the pathophysiology and therapeutic alleviation of stress-related disorders such as anxiety and depression. Serotonergic modulation of the acute response to stress and the adaptation to chronic stress is mediated by a myriad of molecules controlling serotonin neuron development (Pet-1), synthesis (tryptophan hydroxylase 1 and 2 isozymes), packaging (vesicular monoamine transporter 2), actions at presynaptic and postsynaptic receptors (5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, 5-HT3A, 5-HT4, 5-HT5A, 5-HT6, 5-HT7), reuptake (serotonin transporter), and degradation (monoamine oxidase A). A growing body of evidence from preclinical rodents models, and especially genetically modified mice and inbred mouse strains, has provided significant insight into how genetic variation in these molecules can affect the development and function of a key neural circuit between the dorsal raphe nucleus, medial prefrontal cortex and amygdala. By extension, such variation is hypothesized to have a major influence on individual differences in the stress response and risk for stress-related disease in humans. The current article provides an update on this rapidly evolving field of research.
Keywords: Stress, Gene, Strain, Mouse, Inbred, Serotonin, Serotonin transporter, Tryptophan hydroxylase, VMAT2, MAOA, 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, 5-HT3, Prefrontal cortex, Hippocampus, Amygdala, Anxiety, Depression, Emergence test, Forced swim test, Restraint
1. Introduction
The work of Graeff et al. has contributed greatly to our understanding of the role of serotonin (5-hydroxytryptamine) in orchestrating the behavioral response to psychological stress (Graeff et al., 1996). Their work adds to a large corpus of data compiled over the past couple of decades which has uncovered the major enzymes, transporters and receptors that subserve the serotonergic system's modulation of both the acute response to stress and the adaptation to chronic stress. In concert, a more nascent literature has begun to identify sources of genetic variation within these molecules. These parallel lines of research have led to new ways of thinking about how genetically driven differences in serotonin function might predispose certain individuals to stress-related neuropsychiatric conditions such as depression and anxiety disorders and how we can better identify and therapeutically treat these people.
The present article provides an update on this rapidly growing field. The focus is studies that have utilized mice with modifications in serotonin genes to assess stress-related phenotypes. These data are placed in the context of salient pharmacological, neurochemical and electrophysiological findings in both rats and mice and, where applicable, findings of genetic influences from studies in humans. The review is also limited in focus to a neural circuit connecting the serotonergic dorsal raphe nucleus to the medial prefrontal cortex (mPFC) and amygdala, given growing evidence implicating this circuit in rodent stress-related responses and human stress-related disease (Drevets, 2001; Ressler and Mayberg, 2007). For a discussion of the mesencephalic tectum system as a site of serotonin's stress-related actions (see Graeff, 2004) and accompanying articles in this Special Issue. There have also been a number of excellent recent reviews dealing with the theme of serotonin genetics and stress, and the reader is referred to these for a complimentary perspective (Ansorge et al., 2007; Gross and Hen, 2004; Joca et al., 2007; Lucki, 1998; Maier et al., 2006; Neumeister et al., 2004a; Southwick et al., 2005). Lastly, for a comprehensive review of the pharmacology of serotonin's stress-related actions see Griebel, 1995; Menard and Treit, 1999; Millan, 2003.
2. Serotonin release
Serotonin is a major modulatory neurotransmitter, the actions of which are determined by various synthesizing and catabolizing enzymes, pre- and post-synaptic receptors, and molecules controlling intracellular trafficking and extracellular transport (Fig. 1). Serotonin producing neurons originate in the dorsal and median raphe nuclei located in the midbrain (Dahlstrom and Fuxe, 1964). Despite there being few serotonin neurons relative to total neuron number in brain (Jacobs and Azmitia, 1992), the serotonin system, and particularly the dorsal raphe nucleus (DRN), extensively innervates most of the brain including the key corticolimbic structures involved in the regulation of stress such as the mPFC, septum, extended amygdala, and hippocampus (Ma et al., 1991; Steinbusch, 1981). There is further topographical organization within the DRN itself. Although the possible significance of this organization will not be discussed here, Lowry and colleagues among others have shown that certain DRN subregions appear to be preferentially involved in stress and anxiety (Abrams et al., 2004, 2005; Lowry, 2002; Maier and Watkins, 2005; Silva et al., 2002).
Fig. 1.
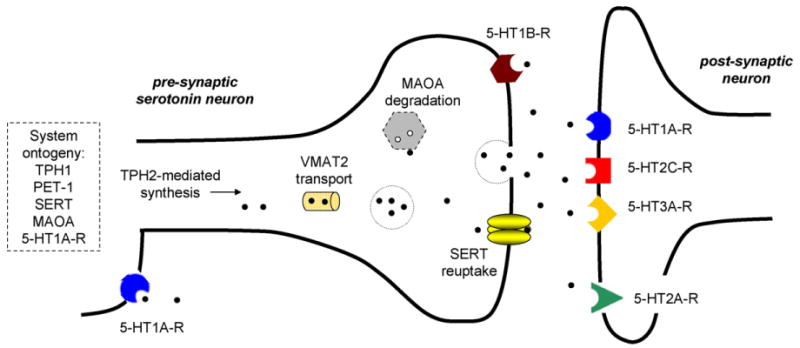
Major molecular components of the serotonin system. The serotonin system is shaped during ontogeny by various molecules including the peripheral synthesizing enzyme tryptophan hydroxylase 1 (TPH1), the serotonin neuron precursor PET-1, the serotonin transporter (SERT) and serotonin degrading enzyme monoamine oxidase A (MAOA), and the 5-HT1A-R receptor subtype. Within the adult pre-synaptic neuron, serotonin is synthesized by the rate-limiting enzyme tryptophan hydroxylase 2 (TPH2) and packaged and transported via vesicular monoamine transporter 2 (VMAT2) to the synapse for release. Following release, extracellular concentrations of serotonin of regulated by SERT-mediated reuptake and by pre-synaptic autoreceptors at both terminals (5-HT1B-R) and soma (5-HT1A-R). Internalized serotonin is deaminated by MAOA. Extracellular serotonin acts on multiple post-synaptic receptor subtypes located synaptically, as in 5-HT1A-R, 5-HT2C-R, and 5-HT3A-R, as well as extra-synaptically, as may be the case for 5-HT2A-R. The 5-HT4-R, 5-HT5A-R, 5-HT6-R, and 5-HT7-R receptor subtypes are also important functional components of the system these are not depicted here for the sake of clarity.
Under most waking states rodent serotonin neurons show slow, regular tonic activity (Jacobs and Azmitia, 1992). Following exposure to stressors such as inescapable shock serotonin neuron activity is increased, as evidenced by increased immediate-early gene expression in the DRN (Amat et al., 2005; Grahn et al., 1999; Takase et al., 2004). A large literature also demonstrates that extracellular fluid concentrations (ECF) of serotonin are increased in both the vicinity of the DRN and the corticolimbic targets of the serotonin system, including the mPFC, amygdala and hippocampus. The stressors shown to cause this increase in ECF, which is almost certainly due to increased serotonin release, range from exposure to a predator, tail pinch, elevated platform exposure, inescapable shock, social defeat, to restraint/immobilization (Adell et al., 1997; Amat et al., 2001; Bland et al., 2003; Funada and Hara, 2001; Grahn et al., 1999; Hajos-Korcsok et al., 2003; Jordan et al., 1994; Kang et al., 2005; Kawahara et al., 1993; Keck et al., 2005; Keeney et al., 2006; Kehr et al., 2001; Kirby et al., 1995, 1997; Kirby and Lucki, 1998; Maswood et al., 1998; Miyata et al., 2007; Mokler et al., 2007; Petty et al., 1994; Price et al., 2002; Renard et al., 2003; Rex et al., 2005; Rueter and Jacobs, 1996; Storey et al., 2006; Takahashi et al., 2000; Thorre et al., 1997; Yoshioka et al., 1995; Yoshitake et al., 2004). It is important to note however that not all studies have found increases in forebrain ECF serotonin in response to stress and there are actually some reports of stress-induced decreases (e.g., Kirby et al., 1997; Rueter and Jacobs, 1996). The reason for these apparent discrepancies is not clear but could be a function of the type of stressor used and other methodological factors, as well as the brain region examined. Another possibility is that the variability reflects genuine biological differences in the capacity for regulating stress-induced serotonin release that different studies are inadvertently tapping – including genetic diversity between different strains of rat or mouse employed. Whatever the reasons, this issue intimates from the outset the complexity of the serotonergic modulation of the central nervous system's response to stress.
3. Serotonin synthesis
The magnitude of stress-induced serotonin release will be dependent in part on the amount of serotonin available in the presynaptic terminal. In rodent, the synthesis of serotonin from L-tryptophan (McGeer and McGeer, 1973) in the brain is controlled by the rate-limiting enzyme tryptophan hydroxylase type 2 (Tph2) (Walther and Bader, 2003). Inhibition of Tph2 activity by chemical agents such as para-chlorophenylalanine methyl ester hydrochloride (PCPA) markedly decreases levels of serotonin (Fratta et al., 1973). PCPA-induced depletion of serotonin blocks the antidepressant-like effects of serotonin reuptake inhibitors (SRIs) in rodent assays for these effects, such as the forced swim test (Page et al., 1999) (for a discussion of these tests (see Cryan and Holmes, 2005)). This is consistent with the finding that upregulation of Tph2 mRNA levels in the DRN following chronic SRI treatment correlates with the magnitude of the antidepressant-like response in the forced swim test (Shishkina et al., 2007). Perhaps surprisingly however, serotonin depletion does not seem to affect the behavioral response during exposure to stressful situations – for example, as measured by the level of active swimming relative to passive immobility in the forced swim test. The absence of effects on baseline stress responsivity could be due to compensatory changes within the serotonin system following PCPA insult.
Compensatory adaptations do not however appear capable of mitigating the consequences of genetically driven loss of Tph2 function. In the mouse, a single-nucleotide polymorphism (SNP) has been identified in the mouse Tph2 gene that leads to a substitution of arginine to proline at position 447 and a significant decrease in brain serotonin levels (Zhang et al., 2004) (for genomic location of this and other mouse genes discussed in this article, see Fig. 2). The BALB/cJ and DBA/2J inbred mouse strains that carry the 1473G allele exhibit significantly lower levels of serotonin in the frontal cortex and striatum relative to the C57BL/6J and 129X1/SvJ strains (with 1473C) (Zhang et al., 2004) (see also Hackler et al., 2006). Interestingly, the BALB/cJ and DBA/2J strains also tend to exhibit higher levels of anxiety-like behavior and stress-reactivity than C57BL/6J, and BALB/cJ also show greater sensitivity to the antidepressant-like effects of SRI's (Anisman et al., 2001; Belzung and Griebel, 2001; Dulawa et al., 2004). The implication here is that the low functioning mouse Tph2 variant could account for the abnormal anxiety- and stress-related phenotype of these and possibly other mouse strains (Zhang et al., 2004). Further work will be needed to substantiate and extend this hypothesis. For example, because Tph2-mediated serotonin synthesis is known to be regulated by glucocorticoids in C57BL/6J (Clark et al., 2007; Clark et al., 2005) it would be of interest to examine whether this mechanism is impaired in strains with the low-expressing SNP such as DBA/2J and BALB/cJ (Table 1).
Fig. 2.
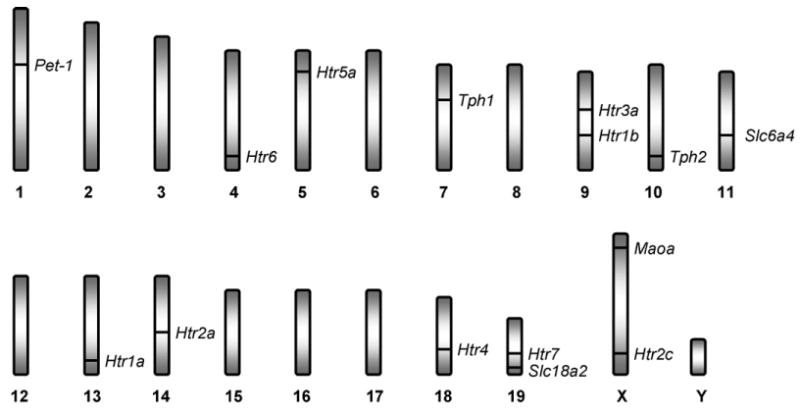
Approximate genomic location of serotonin system genes implicated in stress-related phenotypes in the murine genome. Htr1a = 5-HT1A receptor (13 58.0 centimorgans (cM)), Htr1b = 5-HT1B receptor (9 46.0 cM), Htr2a = 5-HT2A receptor (14 41.5 cM), Htr2c = 5-HT2C receptor (X 66.15 cM), Htr3a = 5-HT3A receptor (19 A5.3), Htr4 = 5-HT4 receptor (18 D3), Htr5a = 5-HT5A receptor (5 15.0 cM), Htr6 = 5-HT6 receptor (4 64.9 cM), Htr7 = 5-HT7 receptor (19 33.0 cM), Maoa = monoamine oxidase A (X 5.2 cM), Slc6a4 = serotonin transporter (11 42.0 cM), Slc18a2 = vesicular monoamine transporter 2 (19 D3), Tph1 = tryptophan hydroxylase 1 (7 23.5 cM), Tph2 = tryptophan hydroxylase 2 (10 D2).
Table 1.
Summary of stress-related phenotypes associated with experimentally induced mouse gene mutations and naturally occurring variation in mouse and man
| Gene | Mouse gene mutation | Mouse gene variant | Human gene variant |
|---|---|---|---|
| Htr1a | KO: increased anxiety-like behavior, autonomic response to stress, fear bias to ambiguity, decreased ‘depression-like’ behavior (postnatal KO mimics anxiety-related phenotype) | – | −1019C/G SNP associated with depression and panic |
| Htr1b | KO: locomotor hyperactivity, impulsivity p11 KO: increased ‘depression-like’ behavior p11 overexpression: decreased ‘depression-like’ behavior | – | G816C SNP associated with depression |
| Htr2a | KO: decreased anxiety-like behavior (rescued by expressing 5-HT2A-R in cortex) | – | 102 T/C SNP, Tyr452 SNP preliminary association with anxiety and depression |
| Htr2c | KO: decreased anxiety-like behavior, HPA-axis response to serotonin, increased antidepressant-like response | Lesser RNA splicing in strains with increased anxiety-like behavior and stress reactivity | |
| Htr3a | KO: decreased anxiety-like behavior, HPA-axis stress response, increased conditioned fear, depression-like behavior (females) | – | C178T SNP associated with trait anxiety, reduced prefrontal-amygdala response to threatening faces |
| Htr4 | KO: decreased anxiety-like behavior, attenuated gut motility and feeding response to stress | – | – |
| Htr5a | KO: no change in anxiety-like behavior | – | – |
| Htr6 | KO: increased anxiety-like behavior | – | – |
| Htr7 | KO: decreased anxiety-like behavior, ‘depression-like’ behavior | – | – |
| Maoa | KO: increased anxiety-like behavior, conditioned fear, aggression | – | Rare loss-of-function variant associated with impulsive aggression in Dutch family Loss-expressing VNTR associated with antisocial personality in maltreated children, abnormal prefrontal-amygdala function and structure |
| Pet-1 | KO: increased anxiety-like behavior, aggression | – | – |
| Slc6a4 | KO: increased anxiety-like behavior, HPA-axis stress response, ‘depression-like’ behavior, impaired fear extinction, reduced aggression, abnormal prefrontal-amygdala neuronal morphology | – | Low-expressing ‘s allele’ associated with increases harm avoidance, depression after trauma, lesser antidepressant efficacy, abnormal prefrontal-amygdala function and structure |
| Overexpression: decreased anxiety-like behavior | Rare I425V associated with obsessive compulsive disorder | ||
| Slc18a2 | KO: increased ‘depression-like’ behavior | Slc18a2 in QTL associated with antidepressant efficacy | – |
| Tph1 | KO dams bear offspring with gross brain abnormality | – | – |
| Tph2 | – | Low functioning C1473G SNP present in strains with increased anxiety-like behavior and stress reactivity | Uncommon G1463A SNP associated with increased depression |
| G(-844)T SNP associated with increased amygdala reactivity to threatening faces 218A/C SNP associated with increased anxiety and depression |
Htr1a = 5-HT1A receptor; Htr2a = 5-HT2A receptor; Htr2c = 5-HT2C receptor; Htr3a = 5-HT3A receptor; Htr4 = 5-HT4 receptor; Htr5a = 5-HT5A receptor; Htr6 = 5-HT6 receptor; Htr7 = 5-HT7 receptor; KO = knockout; Maoa = monoamine oxidase A; QTL = quantitative trait locus; Slc6a4 = serotonin transporter; Slc18a2 = vesicular monoamine transporter 2 (Vmat2); Tph1 = tryptophan hydroxylase 1; Tph2 = tryptophan hydroxylase 2.
Whether genetic control of human TPH2 might affect human stress-related disease has generated a lot of interest. A loss-of-function SNP (G1463A) in the human TPH2 gene has been associated with increased incidence of depression; although it is not yet clear whether this association extends beyond a certain subpopulation of patients (Delorme et al., 2006; Zhang et al., 2006; Zill et al., 2004). In addition, using functional magnetic resonance imaging (fMRI) Hariri and colleagues have recently shown that a more common SNP (G(−844)T) in the promoter region of the TPH2 gene predicts exaggerated amygdala responses to threatening faces (Brown et al., 2005). Finally, there is preliminary evidence that a 218A/C TPH2 polymorphism is also linked to increased rates of anxiety disorders and depressive illness (Christiansen et al., 2007). Taken together, these data provide encouraging support for the notion that genetic variation in the control of serotonin synthesis, via Tph2, can affect stress-related behaviors and the neural systems supporting these behaviors, both in mouse and in man.
Although present to some extent in the rodent DRN (Gundlah et al., 2005), the tryptophan hydroxylase isoform Tph1 largely governs peripheral serotonin synthesis. However, even if Tph1 did not act directly in the brain (which is still not certain), the porous blood–brain barrier in developing rodents could render the developing brain highly sensitive to the effects of variation in Tph1 gene function during ontogeny. Intriguingly in this context, mouse dams in which the Tph1 gene is knocked out produce offspring that exhibit gross abnormalities in brain development, regardless of the offspring's own Tph1 genotype (Cote et al., 2007). This demonstrates that loss of the maternal supply of peripheral serotonin can have profound consequences for the brain. The question of whether gene variation in the human TPH1 or mouse Tph1 gene impacts the development of corticolimbic circuits mediating stress responses has not yet been answered, but represents an important avenue for future work. It also raises the more general issue of how serotonin gene variation can influence stress responses by acting during ontogeny; a critical issue that will be revisited throughout this article.
Deneris and colleagues have shown that the pheochromocytoma 12 ETS domain transcription factor-1, Pet-1, is essential for the generation of serotonin neurons, as evidenced by the ability of gene knockout of mouse Pet-1 to dramatically disrupt the differentiation of serotonin neurons during embryogenesis (Hendricks et al., 2003). Adult Pet-1 knockout mice exhibit a 70% loss of serotonin neurons, an 89% decrease in serotonin tissue content in cortex and hippocampus, and significant decrease in Tph2, serotonin transporter and Vmat2 mRNA expression in serotonin neurons that are extant (Hendricks et al., 2003). Preliminary phenotypic analysis of these mice demonstrated an increase in anxiety-like behavior in some of the standard assays such as the novel open field and elevated zero-maze (for description and discussion of these tests, see Cryan and Holmes, 2005), as well as an increase in conspecific aggression (Hendricks et al., 2003). Pet-1 mice represent a powerful model for studying the stress-related consequences of genetically driven lifelong loss of brain serotonin (see also Lmx1b knockout mice (Zhao et al., 2006b)) and further data on these mice is eagerly anticipated.
4. Serotonin vesicular packaging
Following its synthesis, serotonin must be properly packaged into secretory vesicles and transported to the presynaptic terminal prior to release. The vesicular monoamine transporter type 2 (VMAT2) has an important role in this process (Hoffman et al., 1998). An early indication that VMAT2 might contribute to stress-related pathology stemmed from the serendipitous discovery that pharmacological inhibition of VMAT by reserpine treatment produced striking depressive-like symptoms in humans (Freis, 1954). Indeed this was one of the key findings that subsequently led to the monoamine-deficiency hypothesis of depression.
‘Reserpinization’ of rodents causes a suppression of motor activity that has been likened to a depressive-like state, which probably reflects the combined effects of central depletion of dopamine and norepinephrine as well as serotonin (Bourin, 1990). In addition, repeatedly exposing rats to immobilization or forced swimming has been found to increase or decrease VMAT2 expression (Rusnak et al., 2001; Zucker et al., 2005). Beyond these data there is still surprisingly little investigation of VMAT2's possible role in modulating the stress response until a recent report in a mouse model of loss of Vmat2 (Slc18a2) gene function (Fukui et al., 2007).
Complete knockout of Slc18a2 is lethal, but mice lacking one copy of the gene are viable and develop with significantly reduced brain serotonin (as well as dopamine and norepinephrine) (Fon et al., 1997). Fukui et al. found that Vmat2-deficient mice exhibit normal anxiety-like behaviors on the standard test assays, but show an exaggerated corticosterone response to forced swim stress and a number of behavioral abnormalities indicative of an increased ‘depression-related’ phenotype—i.e., poor escape learning in the passive avoidance test and reduced sucrose preference (an assay for anhedonia) (Fukui et al., 2007). These mice also showed an increased ‘depression-like’ phenotype in the forced swim test and tail suspension tests, in a manner that was reversible by antidepressant treatment (Fukui et al., 2007). This latter observation is noteworthy in the context of recent work by Lucki and colleagues demonstrating that variation in the magnitude of the antidepressant-like response between two inbred mouse strains (BALB/c, A/J) was associated with a quantitative trait locus in the chromosomal region where Slc18a2 resides (Crowley et al., 2006) (see Fig. 2). Taken together, these rodent data suggest that variation in human SLC18A2 could influence risk for depression as well as antidepressant efficacy. However, although numerous SNPs have been identified in SLC18A2 there is not yet any hard evidence linking any specific variants with these phenotypes.
5. Serotonin reuptake
Serotonin released as a result of stress will be removed from the extracellular space by the high-affinity serotonin transporter (SERT, 5-HTT). SERT has an important role in determining the magnitude and duration of serotonin's activity on its presynaptic and postsynaptic receptors, because while other monoamine transporters can take up serotonin under certain conditions, SERT is the principal active means of removing substrate from the synaptic space (Blakely et al., 1991) and has a particularly prominent extrasynaptic role in regulating serotonin volume transmission (Torres and Amara, 2007). SERT is best known as the initial molecular target for the SRI class of antidepressants and anxiolytics. Precisely how SERT mediates the effects of these drugs is less certain. The time lag between drug action and clinical efficacy alone demonstrates that SERT blockade is not in and of itself sufficient to alleviate stress-related symptoms and likely represents the initial stage upstream of a complex process eventually leading to therapeutic outcome (Blier and de Montigny, 1994; Duman et al., 1997; Manji et al., 2001).
There is evidence of reduced SERT binding in the PFC and amygdala of depressives (Oquendo et al., 2007; Parsey et al., 2006). However, despite one study reporting no gross alteration in SERT-mediated reuptake in the rat forebrain under acute stress (Martin et al., 2000), and evidence of decreased DRN SERT mRNA in chronically socially stressed rats (McKittrick et al., 2000) (cf. Arborelius et al., 2004; Vazquez et al., 2000) it is not fully clear how SERT function is altered by stress. Notwithstanding, alterations in SERT-mediated reuptake would be predicted to be an important mechanism by which the serotonin system can self-regulate the amount of synaptic serotonin evoked by stress. For example, SERT upregulation could explain why stress-induced increases in extracellular serotonin are sometimes lost with repeated stress (Kirby and Lucki, 1998). Conversely, a failure of SERT-mediated serotonin clearance would significantly limit regulation of ECF serotonin and thereby compromise the dynamic capacity of the system to respond and adapt to stress. This is borne out at the genetic level by a growing body of data from mouse and human studies.
Gene knockout of the mouse SERT gene (Slc6a4) produces loss of serotonin clearance and a consequent increase in ECF levels of serotonin in forebrain (Mathews et al., 2004; Montanez et al., 2003). A loss of 5-HT1A-R binding and autoreceptor function in these knockout mice further exacerbates ECF levels for the reasons discussed below (Bouali et al., 2003; Fabre et al., 2000; Gobbi et al., 2001; Li et al., 1999, 2000, 2004). These neurochemical changes are associated with an increase in anxiety-like behavior in a battery of the standard test assays (Ansorge et al., 2004; Carroll et al., 2007; Fox et al., 2007; Holmes et al., 2002a, 2003b, 2003c; Kalueff et al., 2007; Lira et al., 2003; Zhao et al., 2006a). Interestingly, transgenic overexpression of the SERT gene produces the opposite phenotype—i.e., reduced anxiety-like behavior (Jennings et al., 2006). In response to exposure to a variety of stressors such as saline injection and predator odor or low maternal care, SERT knockout or SERT-deficient mice display an exaggerated glucocorticoid catecholamine response and in some cases an enhancement of the anxiety-like phenotype (Adamec et al., 2006; Carola et al., 2007; Li et al., 1999, 2004; Tjurmina et al., 2004). In terms of behavioral responses to stress, these mice exhibit an increased ‘depression-related’ phenotype on the forced swim test, tail suspension test and passive avoidance task (Ansorge et al., 2004; Holmes et al., 2002b; Lira et al., 2003). Interestingly, SERT knockout mice have been found to show elevated levels of rapid eye movement (or paradoxical) sleep; an abnormality that is also found in human depression (Alexandre et al., 2006; Wisor et al., 2003). Finally and not surprisingly, SERT knockout mice are insensitive to the antidepressant-like effects of drugs that principally target SERT, such as fluoxetine, but not other monoamine transporters, such as desipramine (Holmes et al., 2002b).
A noteworthy extension of these data is that the increased depression-related phenotype seen in SERT knockout mice appears to be exacerbated by repeated stress exposure (Wellman et al., 2007; Zhao et al., 2006a). In fact, in some instances the phenotype is restricted to these repeated stress conditions and this appears to depend upon the genetic background strain onto which the SERT knockout is crossed, as in the case of backcrossing into the relatively stress-resilient C57BL/6 strain (Holmes et al., 2003b; Zhao et al., 2006a). One interpretation of these findings is that consequences of loss of Slc6a4 in the mouse is determined both by the chronicity of the stressor and by epistatic interactions with other genetic factors that can confer risk or resilience against the effects of Slc6a4 deletion (Holmes and Hariri, 2003). In support of this hypothesis is recent data demonstrating that stress-related interactions with SERT and brain-derived nerve growth factor (for further discussion, see Carola et al., 2007; Ren-Patterson et al., 2005, 2006).
The mouse Slc6a4 model finds strong parallels in the human literature. There has been enormous interest in a common variable number tandem repeat (VNTR) polymorphism in the promoter region of human SERT gene (SLC6A4). Of all the variants thus far identified in serotonin genes, this polymorphism has undoubtedly been the most widely studied. The short (s) allelic version of the polymorphism is associated with reduced SERT brain expression and lesser serotonin reuptake in vitro (Lesch et al., 1996; Little et al., 1999). This variant is considered a loss-of-function variant although functionality is contingent upon additional sources of variation in the SLC6A4 (Zalsman et al., 2006). Without reiterating a literature that has been exhaustively covered elsewhere (for reviews and meta-analyses (see Anguelova et al., 2003b; Lotrich and Pollock, 2004; Rees et al., 1997; Serretti et al., 2007b), individuals carrying the s allele have been found to be at moderately increased risk for anxiety disorders and depression, and to respond unfavorably to antidepressant drugs. Moreover, reminiscent of the stress-related phenotype observed in the SERT knockout mouse, the risk for major depression in individuals carrying the s allele is particularly pronounced, and perhaps even limited to, cases in which there is a history of exposure to traumatic life events (for discussion, see Caspi and Moffitt, 2006; Hariri and Holmes, 2006; Uher and McGuffin, 2007). Finally, as in SERT knockout mice, human s allele carriers also have significantly reduced 5-HT1A-R binding in brain (David et al., 2005) (cf. Lee et al., 2005).
Recent studies, both in rodent and human, provide some insight into the corticolimbic locus of the stress-related phenotype associated with loss of SERT gene function. SERT knockout mice display increased spine density in the basolateral nucleus of the amygdala and elongation of neuronal dendrites in the mPFC (Wellman et al., 2007). In humans, Hariri and colleagues first demonstrated that exposure to threatening faces, a stimulus that reliably engages the amygdala in humans, produces a relatively greater amygdala response in s allele carriers than long (l) allele carriers, as measured by fMRI (Hariri et al., 2002; Munafo et al., 2007). Subsequent studies found that this amygdala hyperactivity is related to a functional ‘uncoupling’ between the circuit connecting the amygdala with the anterior cingulate region of the PFC (Heinz et al., 2005; Pezawas et al., 2005). These data support a model in which loss of SERT gene function leads to a possible failure of cortical systems to exert sufficient inhibitory control over the amygdala during stressful situations, thereby catalyzing the development of chronic pathological states such as depression (Hariri and Holmes, 2006; Pezawas et al., 2005).
It is worth pointing out at his juncture that the adverse phenotypic consequences of loss of SERT function at the genetic level in human and mouse differ from the therapeutically beneficial (anxiolytic, antidepressant) effects of chronic treatment with SRIs (note bene—acute SRI treatment can often produce increased anxiety in rodent and human (Griebel, 1995; Holmes and Rodgers, 2003; Millan, 2003; Salchner and Singewald, 2002; Silva and Brandao, 2000)). The reason for this ostensible paradox between genetic and pharmacological inactivation of SERT is not yet understood, but one potentially crucial factor is that loss of SERT gene function may exert its long-term phenotypic effects by acting during brain development (Ansorge et al., 2007; Holmes et al., 2003a). A number of observations support this hypothesis. First, treating non-mutant rats and mice with SERT-blocking SRIs such as fluoxetine during the first weeks of life (corresponding to late pregnancy in humans) mimics the increased anxiety- and depression-like phenotype of Slc6a4 knockout (Andersen et al., 2002; Ansorge et al., 2004, 2008; Feng et al., 2001b; Hilakivi and Hilakivi, 1987; Maciag et al., 2006a, 2006b). Second, Alexandre and colleagues have shown that either depleting serotonin levels with PCPA or inhibiting 5-HT1A-Rs with WAY 100635 during the first few weeks of life, both of which presumably block some of the actions of excessive neonatal serotonin, is sufficient to rescue some of the depression-related abnormalities in adult SERT knockout mice (Alexandre et al., 2006).
That SERT inactivation during development can have such profound effects is perhaps not surprising considering that serotonin is a major developmental signal (Sodhi and Sanders-Bush, 2004; Whitaker-Azmitia et al., 1996). The rodent neonatal period also corresponds to a time of high transient expression of SERT (but not other monoamine transporters) on non-serotonin neurons (Lebrand et al., 1998). SERT knockout mice exhibit cytoarchitectural malformations and functional deficiencies in various sensory cortical regions that can be rescued by depleting the excess of neonatal serotonin (via PCPA treatment) in these mice (Esaki et al., 2005; Gaspar et al., 2003). This poses the question of whether the PFC-amygdala abnormalities resulting from genetic variation in mouse and human SERT also have their origins in ontogeny. The answer could have important implications for the field.
The VNTR promoter polymorphism is not the only potentially interesting variant in the human SLC6A4 gene. An intron 2 VNTR polymorphism in SLC6A4 is also associated, albeit inconclusively, with depression and suicide (Anguelova et al., 2003a, 2003b). Moreover, this variant as well as a rare gain-of-function mutation (I425V), has recently been implicated in various other psychiatric conditions with a major stress component, including autism and obsessive compulsive disorder (Ozaki et al., 2003; Sutcliffe et al., 2005). It is likely that additional sources of variation in SLC6A4 will be uncovered and these may also prove to influence serotonin regulation of the stress response (Wendland et al., 2008).
6. Serotonin degradation
Serotonin cleared from the extracellular space by SERT and returned to the cell undergoes oxidative deamination into inert metabolites by the mitochondrial enzyme monoamine oxidase A (MAOA). The rate at which MAOA degrades serotonin is therefore another determinant of serotonin availability (Shih et al., 1999). MAOA inhibitors were some of the first drugs to exert demonstrable antidepressant activity in humans (Klein and Fink, 1962). Treatment with these drugs increases serotonin tissue levels in the rodent brain and produces an antidepressant-like response on the forced swim and tail suspension test (Fukui et al., 2007; Holmes et al., 2002b). On the other hand, these drugs increase anxiety-like behavior and cause a resistance to habituation to the effects of chronic mild stress (Griebel, 1995; Holmes and Rodgers, 2003; Menard and Treit, 1999; Millan, 2003; Ward et al., 1998).
The effects of constitutive gene knockout of the mouse Maoa gene are in some ways similar to MAOA inhibitors. Maoa knockout mice have increased ECF serotonin in the PFC and hippocampus and display exaggerated unconditioned and conditioned fear behaviors, as well as increased conspecific aggression (Cases et al., 1995; Dubrovina et al., 2006; Evrard et al., 1999; Kim et al., 1997; Popova et al., 2000). However, these mice also display an attenuated glucocorticoid response to acute and chronic stressors (Popova et al., 2006) that may relate to the significant reduction in dorsal raphe neuron activity observed in these mice (Evrard et al., 1999).
A very rare point mutation in the human MAOA gene causing loss of MAOA function causes impulsive aggression in men within a Dutch family (Brunner et al., 1993). This variant is unlikely to influence stress or emotional traits in the general population, but it does provide a dramatic example of the consequences of loss of MAOA. A more common VNTR in the MAOA promoter region has been linked to various social and emotional abnormalities. Individuals carrying a lesser expressing form of this variant that were also maltreated as children have been found to be at greater risk of developing an antisocial, violent personality, in some but not all studies (e.g., Caspi et al., 2002; Huizinga et al., 2006; Kim-Cohen et al., 2006). The same variant is coupled with a range of structural and functional abnormalities in the corticolimbic system. Specifically, the low-expressing form of the variant is associated with an augmented amygdala (and hippocampal) response and an attenuated PFC response to threatening faces (Buckholtz et al., 2007; Meyer-Lindenberg et al., 2006). In addition, there is a decrease in anterior cingulate, amygdala, insula, and hypothalamus grey matter volume, and increased orbitofrontal cortex volume in these individuals (Buckholtz et al., 2007; Meyer-Lindenberg et al., 2006).
Both at the neural and behavioral level, the phenotypic consequences of genetically driven loss of MAOA in the mouse and human differ in certain ways to the effects of SERT gene loss. Although both lead to an increased stress-reactivity, there is a tendency for this to manifest as increased aggression in the case of loss of Maoa/MAOA but increased harm avoidance in the case of Slc6a4/SLC6A4 deficiency. The reason for this distinction is likely related to the fact that disruption of Maoa/MAOA leads to a significant increase in norepinephrine and dopamine as well as serotonin (as demonstrated in the mouse (Cases et al., 1995))—producing a more complex milieu of neurochemical changes during development and adulthood than would be wrought by an increase in serotonin alone.
7. Role of 5-HT1A receptors
Serotonin released into the extracellular space acts at multiple pre-synaptic and post-synaptic located receptors that are organized in a hugely complex pattern of regional and subcellular distribution. For excellent overviews of serotonin receptor pharmacology and signaling mechanisms (see Barnes and Sharp, 1999; Hoyer et al., 2002; Kroeze and Roth, 1998).
The 5-HT1A-R subserves two critical roles within the serotonin system in regards to stress—as an autoreceptor regulating DRN neuron activity and as a postsynaptic receptor mediating serotonin's action on corticolimbic regions including the mPFC and amygdala. Activation of 5-HT1A-R autoreceptors located on the soma and dendrites of serotonin cells (Sotelo et al., 1990; Verge et al., 1986) inhibits DRN neuronal firing and consequent forebrain release (Clifford et al., 1998; Sprouse and Aghajanian, 1986). This provides an essential mechanism by which the serotonin system can self-regulate its activity under conditions of stimulation, such as stress exposure. This mechanism appears to be compromised by chronic stress. For example, Lanfumey and colleagues have shown that corticosterone or chronic mild stress desensitizes 5-HT1A-R autoreceptor control of DRN firing (via a glucocorticoid receptor-dependent mechanism) (Froger et al., 2004; Laaris et al., 1995, 1997; Lanfumey et al., 1999) (see also Gartside et al., 2003). Along similar lines, the loss of 5-HT1A-R autoreceptor function caused by gene knockout of the mouse Htr1a exacerbates the increase in ECF concentrations in the DRN resulting from mild stress exposure (Amargos-Bosch et al., 2004; Bortolozzi et al., 2004; He et al., 2001).
Genetically driven or stress-induced loss of 5-HT1A-R auto-receptor function would prevent some of the plastic changes that occur in the DRN with chronic stress. Maier and colleagues have shown that rats repeatedly exposed to inescapable shock exhibit a passive behavioral response even when escape from shock becomes available in a phenomenon termed ‘learned helplessness’ (Seligman, 1972). Inescapable, but not escapable, stress not only activates DRN neurons (Grahn et al., 1999; Maswood et al., 1998), but ‘sensitizes’ these neurons to subsequent stress (Amat et al., 1998). The development or expression of this sensitization is prevented by pharmacological stimulation of 5-HT1A-Rs in the DRN—presumably by inhibiting stress-induced serotonin neuron activation (Maier et al., 1995).
As noted, 5-HT1A-R function plays a prominent role in mediating serotonin's postsynaptic actions, and these receptors are densely expressed in the mPFC (Clement et al., 1996; Kia et al., 1996b; Landry and Di Paolo, 2003; Pompeiano et al., 1992; Wright et al., 1995). Application of selective 5-HT1A-R agonists such as 8-OH-DPAT directly into the mPFC inhibits the spontaneous and glutamate receptor-mediated firing of the major projection neurons therein (Araneda and Andrade, 1991; Ashby et al., 1994; Borsini et al., 1995; Cai et al., 2002). Likewise, inhibitory actions of serotonin on these neurons is reversed by co-application of selective 5-HT1A-R antagonists such as WAY 100635 (Cai et al., 2002; Casanovas et al., 1999; Hajos et al., 2003; Tanaka and North, 1993).
5-HT1A-Rs can not only control serotonergic responses to stress via their actions as autoreceptors, but can indirectly influence DRN activity via their postsynaptic actions. There is an important projection from the mPFC back to the DRN (Peyron et al., 1998) (as well as recently identified pathway from the subthalamic nucleus to the DRN (Temel et al., 2007)). The glutamatergic mPFC projection synapses onto GABA interneurons that in turn negatively regulate DRN activity (Hajos et al., 1998). Using the aforementioned learned helplessness paradigm, Maier and colleagues have provided compelling evidence that the mPFC-DRN pathway determines whether stress is ‘perceived’ as uncontrollable and, as a result, the extent to which stress activates the serotonergic system. This is best illustrated by the finding that inactivation of the PFC promotes DRN activation, forebrain serotonin release and learned helplessness behavior in response to stress (for further discussion, see Maier et al., 2006; Maier and Watkins, 2005). By recruiting the mPFC-DRN pathway, activation of 5-HT1A-Rs in the mPFC causes a decrease in DRN firing and suppresses forebrain serotonin release (Celada et al., 2001; Gartside et al., 2000; Hajos et al., 1998, 1999, 2001, 2003; Martin-Ruiz et al., 2001; Martin-Ruiz and Ugedo, 2001; Puig et al., 2005). It is not yet certain how the activation of (inhibitory) 5-HT1A-Rs in the mPFC can stimulate the projection to the DRN, but may stem from their action at GABA interneurons in mPFC (Aznar et al., 2003; Santana et al., 2004). Whatever the precise mechanism of action, intra-mPFC actions of 5-HT1A-Rs (and as described later, other serotonin receptors) is a critical means of system-level regulation of the serotonergic response to stress. In this regard, a loss in mPFC 5-HT1A-R binding following stress or glucocorticoid excess (Crayton et al., 1996; Preece et al., 2004) (cf. Arborelius et al., 2004) could significantly impact this form of regulation.
Beyond the mPFC and DRN, 5-HT1A-Rs are present in the amygdala (Clement et al., 1996; Pazos and Palacios, 1985). The level of 5-HT1A-R binding in basolateral nucleus of the amygdala (BLA) is increased by maternal separation stress in rats (Vicentic et al., 2006) and Octodon degus (Ziabreva et al., 2003). Under non-stressed conditions, application of 5-HT1A-R agonists (e.g., 8-OH-DPAT) inhibits the excitability of neurons in the BLA, while the inhibitory actions of 5-HT on these neurons can be prevented by 5-HT1A-R antagonist co-application (Cheng et al., 1998; Stein et al., 2000; Wang et al., 1999). However 5-HT1A-Rs can also suppress GABA interneuron activity in the BLA (Koyama et al., 1999, 2002), which might account for the minimal 5-HT1A-R agonist activity in the BLA reported in some studies (Rainnie, 1999). As such, the net effect of stress-induced release in this area of the brain critical for anxiety and fear processing will be determined by the relative balance of 5-HT1A-R actions on excitatory and inhibitory neurons. This ambiguity appears to manifest at the behavioral level. Direct administration of 5-HT1A-R agonists (ipsapirone, buspirone, 8-OH-DPAT) into the rat BLA amygdala has been shown to reduce anxiety-like behavior in some tests (e.g., shock-induced vocalization), increase anxiety in others (social interaction) and have no or variable activity in others (e.g., elevated plus-maze, shock-punished conflict) (Gonzalez et al., 1996; Griebel, 1995; Hodges et al., 1987; Menard and Treit, 1999; Millan, 2003; Schreiber and De Vry, 1993; Zangrossi Junior and Graeff, 1994).
In addition to having a major role in controlling fear and anxiety, the amygdala also regulates the hypothalamic–pituitary–adrenal (HPA) axis response to stress via a pathway from the main output nucleus of the amygdala, the central nucleus, to the paraventricular nucleus of the hypothalamus (PVN) (Swanson and Petrovich, 1998). Serotonin can influence the HPA axis response to stress not only via the amygdala, but also via the mPFC, the hippocampus and direct innervation from the raphe (for review, see Herman et al., 2005). Intra-amygdala (and in some cases systemic) administration of 5-HT1A-R agonists can attenuate both the behavioral and neuroendocrine responses to various stressors, including restraint, forced swim and conditioned fear stimuli (Krysiak et al., 2000; Li et al., 2006; Rittenhouse et al., 1992). Conversely, although mRNA for the receptor is not abundant in the paraventricular nucleus of the hypothalamus (Heisler et al., 2007b), 5-HT1A-R agonists such as 8-OH-DPAT injected directly into this structure stimulates the HPA-axis (Calogero et al., 1990, 1993; Gilbert et al., 1988; Pan and Gilbert, 1992). A pro-HPA-axis action of 5-HT1A-Rs would be consistent with the observation that systemic administration of the 5-HT1A-R agonist flesinoxan increased c-Fos expression in corticotropin-releasing hormone (CRH)-labeled cells in the PVN of the hypothalamus (as well as the central nucleus of the amygdala) (Compaan et al., 1996, 1997).
The complex and sometimes opposing actions of serotonin at 5-HT1A-R on different cell types and multiple nodes within the DRN-mPFC-amygdala circuit might explain why 5-HT1A-R agonists never reached their full potential as clinical anxiolytics and antidepressants (Blier and Ward, 2003; Holmes, 2008). This complexity also suggests that genetically driven loss of 5-HT1A-R function should have pleiotropic consequences for stress-related phenotypes. This is indeed so in the case of gene knockout of mouse Htr1a. Adrien and colleagues have shown that 5-HT1A-R knockout mice display a profile of increased paradoxical sleep and insensitivity to stress-induced changes in sleep patterns (Boutrel et al., 2002) that is similar to that seen in other mouse models of depression (Popa et al., 2006). On the other hand, 5-HT1A-R knockout mice display a profile in the tail suspension test that is more consistent with an antidepressant-like response, and a normal profile on the ‘differential-reinforcement-of-low-rate 36-seconds’ test for depression-related behavior (Heisler et al., 1998; Jones and Lucki, 2005; Mayorga et al., 2001; Pattij et al., 2003). These mice are also insensitive to the antidepressant-like effects of SRIs in the tail suspension test implying the receptor may be an important mechanism in these effects (Knobelman et al., 2001a, 2001b; Mayorga et al., 2001).
In terms of anxiety, 5-HT1A-R knockout mice exhibit increased spontaneous anxiety-like behaviors on the standard exploration-based tests (Bailey and Toth, 2004; Gross et al., 2000; Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998; Zhuang et al., 1999). In addition, Olivier and colleagues have demonstrated exaggerated autonomic (e.g., hyperthermia, tachycardia) responses to acute stress exposure in these mice (Pattij et al., 2001, 2002a, 2002b) (see also Gross et al., 2000). Finally, consistent with a loss of the inhibitory actions at 5-HT1A-R's in the mPFC and the excitatory effects of 5-HT1A-Rs on the HPA-axis, respectively, both effects are diminished by knockout of the receptor (Bruening et al., 2006; Gross et al., 2000).
Further extending the phenotypic profile of the 5-HT1A-R knockout mouse, Gross and colleagues have recently demonstrated that these mice have a fear bias towards environmental cues that are ambiguous predictors of trauma and which produce little fear in non-mutant mice (Klemenhagen et al., 2006; Tsetsenis et al., 2007). 5-HT1A-Rs are expressed on pyramidal neurons in the hippocampus (Chalmers and Watson, 1991; Clement et al., 1996; Khawaja, 1995; Kia et al., 1996a, 1996b; Pazos and Palacios, 1985; Raurich et al., 1999; Wright et al., 1995) where they exert an inhibitory action (Corradetti et al., 1998). 5-HT1A-R knockout mice display signs of hippocampal hyperexcitability (Sibille et al., 2000) and Tsetsenis et al. found that inhibition of cells within the dentate gyrus subregion of the hippocampus was sufficient to normalize the abnormal fear bias in the knockouts (Tsetsenis et al., 2007). While the hippocampus is not a focus of the current review, these findings are consonant with the idea that the hippocampus works in concert with the PFC and amygdala to gate environmental information regarding the precise stimulus features of threatening stimuli (Maren and Quirk, 2004; McHugh et al., 2007) (see also Gray and McNaughton, 1996). The data in knockout mice identify hippocampal 5-HT1A-Rs as a mechanism subserving this process. It is worth pointing out, therefore, that stress exposure likely impacts this mechanism via changes in 5-HT1A-R expression and function in the hippocampus. An increase in hippocampal 5-HT1A-R binding or mRNA is seen in rodents exposed to glucocorticoids, unpredictable stress, amygdala-kindling, and, in some studies at least, maternal separation (Arborelius et al., 2004; Harvey et al., 2003; Kalynchuk et al., 2006; Lopez et al., 1999; Neumaier et al., 2002; Vazquez et al., 2000, 2002).
Both the fear-related and anxiety-like phenotypes in 5-HT1A-R knockout mice appear to have their origins in development. The most compelling evidence of this comes from the finding that restricting genetic loss of 5-HT1A-R to the second and third postnatal weeks was sufficient to recapitulate the pro-anxiety-like effects of constitutive gene knockout (Gross et al., 2002). Contrariwise, deleting the 5-HT1A-R only in adulthood had no effect on anxiety-like behavior. Using a simple pharmacological approach, the fear-bias to ambiguity was mimicked in non-mutant mice by treatment with a 5-HT1A-R antagonist (WAY 100635) during the third and fourth postnatal weeks (Tsetsenis et al., 2007). These data again speak to the importance of serotonin as a factor driving the development of the corticolimbic systems subserving the stress response later in life (for further discussion, see Ansorge et al., 2007; Gross and Hen, 2004; Hariri and Holmes, 2006).
The rodent data strongly support variation in the human HTR1A gene as a candidate for stress-related disorders. A SNP (−1019C/G) in the promoter region of the human HTR1A gene is associated with depression, suicide and panic (Huang et al., 2004; Lemonde et al., 2003). There is also an interesting fMRI report that relatively low levels of 5-HT1A-R autoreceptor binding in the midbrain predict increased amygdala reactivity to threatening faces (Fisher et al., 2006), which extends positron emission tomography studies indicating lesser PFC 5-HT1A-R binding and blunted neuroendocrine responses to the 5-HT1A-R partial agonist ipsapirone in patients with panic disorder and depression (Lesch et al., 1992; Lopez-Figueroa et al., 2004; Neumeister et al., 2004b; Sullivan et al., 2005). However, it was not clear in these cases whether this apparent loss of 5-HT1A-R was genetic in origin or a consequence of a stress phenotype. We still await real insight into this genetic contribution of this receptor in human stress-related disease.
8. 5-HT1B receptors
5-HT1B-Rs serve as terminal presynaptic autoreceptors regulating serotonin release and as heteroreceptors on non-serotonin neurons (Moret and Briley, 1997; Morikawa et al., 2000). The autoreceptor function of 5-HT1B-Rs is exemplified by the augmentation of stimulated-increases in ECF serotonin levels in forebrain regions by pharmacological antagonism or gene knockout of the receptor (Ase et al., 2000; de Groote et al., 2002a, 2002b; Knobelman et al., 2001a, 2001b; Malagie et al., 2001; Trillat et al., 1997). Virus-induced overexpression of 5-HT1B-R autoreceptors has been shown to increase basal, but attenuate stress-induced, anxiety-like behavior in rats (Clark et al., 2002, 2004); which is generally consistent with the pro-anxiety effects of 5-HT1B-R agonist such as CP 94,253 (Lin and Parsons, 2002; Moret and Briley, 2000). On the other hand, normal variation in levels of 5-HT1B-R mRNA in the DRN of the rat has also been correlated with differences in anxiety-like behaviors, such that higher 5-HT1B-R levels were associated with relatively low levels of anxiety-like behavior in various tests (Clark et al., 2004; Kaiyala et al., 2003; Neumaier et al., 2002) (cf. Edwards et al., 1991). However, this relationship was lost in rats that were exposed to forced swim, restraint or shock stress, possibly due to the downregulation of 5-HT1B-Rs caused by the stress exposure (Bolanos-Jimenez et al., 1995).
Gene knockout of mouse Htr1b is notable for the hyper-aggressive, locomotor hyperactivity and impaired impulse control phenotype that results (Bouwknecht et al., 2001; Malleret et al., 1999; Saudou et al., 1994; Zhuang et al., 1999). This, in and of itself, is pertinent to stress in view of the high incidence of suicide, a form of self-directed impulsive aggression, in stress-related disorders such as depression. 5-HT1B-R knockout mice do not exhibit any clear change in anxiety-like behavior. However, these mice do show an exaggerated autonomic reaction to acute stress and depressive-like enhancement of paradoxical sleep and an antidepressant-like response in forced swim and tail suspension test that is normalized by SRI treatment or PCPA depletion of central serotonin (Boutrel et al., 1999; Brunner et al., 1999; de Boer and Koolhaas, 2005; Dirks et al., 2001; Groenink et al., 2003; Jones and Lucki, 2005; Lopez-Rubalcava et al., 2000; Mayorga et al., 2001; Olivier and van Oorschot, 2005; Pattij et al., 2003; Ramboz et al., 1996; Sibille et al., 2007). On the face of it the antidepressant-like phenotype is congruent with the antidepressant-like activity of the selective 5-HT1B-R antagonist SB-616234-A (Dawson et al., 2006).
Providing insight into the signaling mechanisms underlying the 5-HT1B-Rs role in stress, Svenningsson et al. recently found that the activity of p11 (also known as S100A10), an intracellular signaling molecule which localizes 5-HT1B-R to the cell surface, is increased in mouse brain following antidepressant treatment, and is downregulated in postmortem brain samples from human depressives (Svenningsson et al., 2006). These authors were also able to demonstrate that gene knockout of p11 (causing loss of 5-HT1B-R function) caused increased ‘depression-related’ behavior in mouse tail suspension and sucrose preference tests, while transgenic overexpression of p11 produced the opposite pheno-type. The ‘pro-depressive’ phenotype of p11 knockout contrasts from the ‘antidepressant-like’ phenotype of knockout of 5-HT1B-R itself, but the reason for this is not clear.
Any link between genetic variation in the human HT1BR gene and stress is unlikely to be any less straightforward, but the available data are meager. Perhaps most notable is that a G816C SNP in the human HT1BR gene, which could encode for lesser 5-HT1B-R binding (Duan et al., 2003; Huang et al., 1999), is significantly more frequent in depression (Huang et al., 2003).
9. 5-HT2A receptors
Although distributed in various corticolimbic regions, there is accumulating evidence that 5-HT2A-Rs play a particularly important role in mediating serotonin's action in the mPFC during stress. 5-HT2A-R are located on the dendrites of glutamatergic pyramidal neurons and interneurons in the rodent mPFC (Aghajanian and Marek, 1997; Cornea-Hebert et al., 1999; Hamada et al., 1998; Jansson et al., 2001; Miner et al., 2003; Willins et al., 1997; Xu and Pandey, 2000). Electrophysiological studies have demonstrated that selectively stimulating 5-HT2A-Rs (typically by co-applying a mixed 5-HT2A-R/5-HT2C-R agonist and a 5-HT2C-R antagonist to isolate 5-HT2A-Rs) can either excite or inhibit the activity of neurons in the rat mPFC, although an excitatory action appears to predominate under most conditions (Aghajanian and Marek, 1997, 1999; Amargos-Bosch et al., 2007; Araneda and Andrade, 1991; Arvanov et al., 1999; Ashby et al., 1990, 1994; Liu and Aghajanian, 2008; Marek et al., 2006; Puig et al., 2003; Zhou and Hablitz, 1999). On the basis of what appears to be an extrasynaptic localization of 5-HT2A-Rs on mPFC neurons (Jansson et al., 2001), some authors have proposed that the excitatory action of this receptor may be particularly prominent under conditions of high serotonin release (Sharp et al., 2007).
There is strong evidence that 5-HT2A-Rs work in close concert with the glutamate system to modulate mPFC activity (for reviews, see Beique et al., 2007; Celada et al., 2004). 5-HT2A-R activation augments glutamatergic thalamic input to the mPFC, and synergizes with AMPA and metabotropic mGluR2/3 glutamate receptors to excite mPFC neurons (Aghajanian and Marek, 1999; Ashby et al., 1990; Beique et al., 2007; Lambe and Aghajanian, 2001; Lopez-Gil et al., 2007; Marek et al., 2001, 2006; Martin-Ruiz et al., 2001; Muschamp et al., 2004; Puig et al., 2003; Scruggs et al., 2000, 2003). In addition, 5-HT2A-Rs positively modulate stress-induced release of dopamine in the mPFC (Pehek et al., 2006). As such, changes in 5-HT2A-R function in mPFC would be expected to have a broad impact on the overall activation of the mPFC following stress. In this context, exposure to chronic unpredictable stress, isolation rearing or chronic corticosterone treatment have been found to increase 5-HT2A-R binding in the mPFC (and amygdala) and alter 5-HT2-mediated head shakes (Chaouloff et al., 1994; Izumi et al., 2002; Katagiri et al., 2001; Ossowska et al., 2001; Preece et al., 2004; Schiller et al., 2003; Takao et al., 1997). It is difficult to posit whether this upregulation is the result of either stress-induced elevation or diminution of serotonin in the mPFC. 5-HT2A-Rs are atypical amongst 5-HT receptors in exhibiting desensitization and downregulation not only following chronic agonism but also in response to chronic antagonism and serotonin depletion; thus upregulation could potentially occur through various stress-related changes in serotonergic neurotransmission (Blackshear et al., 1986; Blackshear and Sanders-Bush, 1982; Buckholtz et al., 1985; Conn and Sanders-Bush, 1986; Gray and Roth, 2001; Marek et al., 2001; McKenna et al., 1989).
As already noted, pharmacological studies indicate that 5-HT2A-Rs can sometimes exert inhibitory actions in the mPFC. This likely occurs via local γ-aminobutyric acid (GABA)ergic interneurons that synapse onto projection neurons (Abi-Saab et al., 1999; Amargos-Bosch et al., 2004; Blue et al., 1988; Feng et al., 2001a; Jansson et al., 2001; Martin-Ruiz et al., 2001; Pompeiano et al., 1994; Roth et al., 1998; Santana et al., 2004; Willins et al., 1997; Zhou and Hablitz, 1999). These inhibitory actions provide an additional mechanism by which 5-HT2A-Rs can modulate mPFC activity and speak to the highly nuanced nature of 5-HT2A-R function in this key brain region. Defining the physiological conditions under which inhibitory versus excitatory actions of 5-HT2A-Rs in mPFC predominate, and how this balance is shifted by stress and genetic variation, are major issues that are not yet answered. This balance will not only determine the intrinsic functionality of the mPFC but also the level of negative feedback control over the DRN.
We discussed earlier how 5-HT1A-Rs in the mPFC control DRN activity via a subcortical projection. Selective stimulation of PFC 5-HT2A-Rs decreases (but in some cases increases) the activity of DRN neurons to affect a consequent change in ECF serotonin concentrations in mPFC and other forebrain targets (Boothman et al., 2003; Bortolozzi et al., 2003; Celada et al., 2001; Martin-Ruiz et al., 2001). As in the aforementioned 5-HT1A-R model of DRN regulation, 5-HT2A-R-mediated excitation of the mPFC likely inhibits DRN neuronal activity via stimulation of local GABAergic interneurons (Boothman et al., 2006; Boothman and Sharp, 2005; Liu et al., 2000). Although activation of 5-HT1A-Rs and 5-HT2A-Rs in the mPFC can both exert an inhibitory influence on the DRN, there is evidence that co-application of agonists for the two subtypes can nullify these subcortical effects (Amargos-Bosch et al., 2004; Araneda and Andrade, 1991; Martin-Ruiz et al., 2001). Notwithstanding the complexity of these interactions, the functional localization of 5-HT2A-Rs in the mPFC positions this receptor subtype to exert a profound influence over the serotonergic response to stress.
Beyond the PFC, most amygdala nuclei show low to moderate levels of 5-HT2A-R binding and mRNA (Pazos et al., 1985; Pompeiano et al., 1994; Wright et al., 1995). (Tangentially, 5-HT2A-R-immunoreactive neurons in the BLA project to the mediodorsal thalamus; thereby providing another link to the mPFC (McDonald and Mascagni, 2007).) Immunocytochemical analyses indicate 5-HT2A-R expression on the dendrites of pyramidal neurons in the BLA, and more intense straining on parvalbumin-containing interneurons in this area (Cornea-Hebert et al., 1999; McDonald and Mascagni, 2007; Morilak and Ciaranello, 1993; Xu and Pandey, 2000). The presence of 5-HT2A-Rs on these GABAergic interneurons likely accounts for the inhibitory actions of the receptor in the amygdala. These effects are inferred from the observation that serotonin or mixed 5-HT2A-R/5-HT2C-R agonists such as DOI inhibit BLA neuronal firing in a manner that can be mimicked by 5-HT2A-R (not 5-HT1A-R) agonists and be blocked by GABA receptor antagonists (Rainnie, 1999; Stein et al., 2000; Stutzmann and LeDoux, 1999).
For a number of years, a lack of agonists with specificity for 5-HT2A-Rs over 5-HT2C-Rs meant that pharmacological dissociation of the role of these subtypes in stress-related behaviors had to be inferred from the ability of subtype-selective antagonists to block the behavioral effects of mixed 5-HT2A-R/5-HT2C-R agonists (e.g., DOI). This approach has shown that systemic antagonism of 5-HT2A-Rs, but not 5-HT2C-Rs, is sufficient to block the anxiolytic-like effects of DOI or SRIs, even though selective 5-HT2A-R antagonists such as MDL 100,907 have weak inherent anxiolytic-like activity (Griebel, 1995; Griebel et al., 1997; Kehne et al., 1996; Millan, 2003; Mora et al., 1997; Nic Dhonnchadha et al., 2003; Ripoll et al., 2006; Schreiber et al., 1998). Systemic treatment with selective 5-HT2A-R antagonists such as MDL 100,907 is also capable of blunting the corticosterone release and activation of CRH-positive PVN hypothalamic neurons produced by mixed 5-HT2A-R/5-HT2C-R agonists like DOI (e.g., Hemrick-Luecke and Evans, 2002; Van de Kar et al., 2001).
The pharmacological data are extended by the finding that gene knockout of the mouse Htr2a causes a decrease in anxiety-like behavior in various tasks (Weisstaub et al., 2006). Weisstaub et al. were able to demonstrate that expressing 5-HT2A-Rs in the cortex of these knockout mice was sufficient to normalize the anxiety-like behavior, suggesting that cortical 5-HT2A-Rs normally serve to promote anxiety-like behavior. Precisely how this relates to the regulation of mPFC neuronal activity by 5-HT2A-Rs is not yet clear. Furthermore, although 5-HT2A-R knockout mice showed normal ‘depression-related’ behavior in the forced swim test, and a normal HPA-axis response to this stressor (Weisstaub et al., 2006) further examination of these mice for other stress-related behaviors would be informative. Such studies are encouraged by the increased PFC 5-HT2A-R binding found in depression (Meyer et al., 1999; Yatham et al., 2000), and the antidepressant efficacy of drugs with 5-HT2A-R antagonist properties (e.g., nefazodone, trazodone, mirtazapine) (Celada et al., 2004; Millan, 2003).
Genetic associations between stress-related disorders and variants in the human HTR2A gene, including the more well-studied 102 T/C and Tyr452 variants, remain preliminary (Norton and Owen, 2005; Serretti et al., 2007a). There has in fact been more interest in HTR2A gene variation as a risk factor for psychosis given the role of the 5-HT2A-R subtype in mechanism of action of both psychotomimetics and antipsychotics (Kroeze and Roth, 1998; Meltzer, 1999; Sanders-Bush et al., 2003). This is nonetheless potentially very salient to stress. First, executive dysfunction is not limited to schizophrenia, and is prominent in affective disorders such as major depression (Taylor Tavares et al., 2007). Second, 5-HT2A-Rs localized in the mPFC have been found to be important modulators of executive functions that are known to be impaired in affective as well as psychotic illness, such as working memory, cognitive flexibility and impulse control (Clarke et al., 2004; Stein et al., 2007; Williams et al., 2002; Winstanley et al., 2004). Third, exposure to stress causes dendritic retraction of mPFC pyramidal neurons and blunts 5-HT2A-R-mediated modulation of these neurons (Liu and Aghajanian, 2008), and produces associated deficits in PFC-mediated behaviors such as fear extinction and attentional set-shifting (Izquierdo et al., 2006; Liston et al., 2006). Thus, loss of 5-HT2A-R function in the mPFC could be a mechanism linking stress with some of the cognitive and executive symptoms found in stress-related disease.
10. 5-HT2C receptors
5-HT2C-Rs are highly expressed in the mPFC and amygdala (Abramowski et al., 1995; Clemett et al., 2000; Li et al., 2003; Lopez-Gimenez et al., 2002; Molineaux et al., 1989; Pompeiano et al., 1994; Sharma et al., 1997; Wright et al., 1995). Pharmacological studies point to a role for this receptor subtype in mediating serotonin's pro-anxiety effects at the level of the amygdala. Treatment with the non-specific serotonin receptor agonist m-chlorophenylpiperazine (mCPP) has potent anxiogenic effects in rodent and man alike (Griebel, 1995; Menard and Treit, 1999; Millan, 2003; Murphy et al., 1986). Selective blockade of 5-HT2C-Rs with compounds such as SB 242084, either systemically or in the amygdala, is sufficient to prevent mCPP-induced (and in some cases, spontaneous or stress-induced) rodent anxiety-like behavior (Campbell and Merchant, 2003; Cornelio and Nunes-de-Souza, 2007; Graeff et al., 1996; Griebel, 1995; Hackler et al., 2007; Harada et al., 2006, 2007; Kennett et al., 1996; Martin et al., 2002; Millan, 2003). Systemic treatment with SB 242084 also blocks the enhancement of conditioned fear produced by acute SRI treatment (Burghardt et al., 2004, 2007) (cf. Santos et al., 2006). The ability of SB 242084 to antagonize the anxiogenic-like effects of mCPP were associated with a significant attenuation of neural activation in the amygdala (as well as the hippocampus and hypothalamus, but not mPFC) (Hackler et al., 2007). Finally, the aforementioned anxiety-like phenotype in SERT knockout mice is associated with a significant increase in 5-HT2C-R binding density in the amygdala (Li et al., 2003). Collectively, these findings indicate that serotonin's ability to promote anxiety is mediated in part by actions at 5-HT2C-Rs in the amygdala. This presumably occurs by exciting projection neurons therein, although there is surprisingly little electrophysiological verification of such an action.
The pharmacological literature on 5-HT2C-R and stress-related behaviors has been extended by a mouse model of gene knockout of the X-linked Htr2c gene. Gene knockout of 5-HT2C-R leads to a decrease in anxiety-like behavior on a variety of assays (Heisler et al., 2007a). These mice also exhibit significantly lesser DOI- or mCPP-induced activation of CRH-positive cells in the central nucleus of the amygdala and bed nucleus of the stria terminalis, and signs of CRH downregulation in the PVN of the hypothalamus (Heisler et al., 2007a, 2007b). Serotonin normally stimulates glucocorticoid release in part via an action on CRH neurons in the PVN (Calogero et al., 1990, 1993; Fuller and Snoddy, 1980; Heisler et al., 2007b; Jorgensen et al., 1998, 2002), but Heisler et al. showed that this effect is lost in the 5-HT2C-R knockout mice and ablated in non-mutant mice systemically treated with the selective 5-HT2C-R antagonist RS 102221. Whether 5-HT2C-R inactivation would also inhibit stress-induced HPA-axis activation has not been reported; although one study showed a greater decrease in body weight in response to acute restraint stress in aged 5-HT2C-R knockout mice (Chou-Green et al., 2003).
5-HT2C-R knockout mice do not show abnormalities in tests for ‘depression-related’ behaviors such as the tail suspension test. However, either 5-HT2C-R gene knockout or systemic treatment with the selective 5-HT2C-R antagonists SB 242084 or RS 102221 has been shown to both potentiate the antidepressant-related effects of SRIs in the tail suspension test and augment the increases in cortical and hippocampal ECF serotonin levels produced by SRI treatment (Cremers et al., 2004, 2007). On the other hand, systemic treatment with the novel 5-HT2C-R agonists WAY 163909, RO 600175 also exert antidepressant-like effects in a range of rodent assays (Cryan and Lucki, 2000; Martin et al., 1998; Rosenzweig-Lipson et al., 2007). These data have not been reconciled but they imply that the influence of genetically driven variation in human HTR2C function on risk for depression in humans may be complex.
An interesting feature of both the rodent and human 5-HT2C-R is that the receptor undergoes post-transcriptional RNA editing to an isoform with reduced constitutive and agonist-stimulated signaling activity (Burns et al., 1997; Sanders-Bush et al., 2003). The BALB/cJ and DBA/2J inbred mouse strains have been found to exhibit differences in basal rates of 5-HT2C-R editing in the mPFC and amygdala relative to other strains such as C57BL/6J (Englander et al., 2005; Hackler et al., 2006). Schmauss and colleagues have shown that either exposure to swim stress in adulthood or chronic maternal separation early in development increases 5-HT2C-R editing in the PFC of the stress-reactive BALB/cJ strain but not stress-resilient C57BL/6J strain, and that the increase in BALB/cJ can be normalized by SRI treatment (Bhansali et al., 2007; Englander et al., 2005). This has led to the idea that trait variation in 5-HT2C-R editing could contribute to differences in stress reactivity in the mouse and, by extension, increase risk for stress-related disorders in the human (Schmauss, 2003). Intriguingly, a number of studies have found increased 5-HT2C-R editing in the mPFC of schizophrenics and depressive suicides (Gurevich et al., 2002; Sodhi et al., 2001). There is also preliminary evidence that structural variants in the human HTR2C gene, including a Cys23Ser SNP, are associated with bipolar disorder and major depression (Lappalainen et al., 1999; Lerer et al., 2001; Massat et al., 2007).
11. 5-HT3 receptors
The 5-HT3-R distinguishes itself from other members of the 5-HT receptor family by being a ligand-gated ion channel (as opposed to a G-protein-coupled receptor) (Derkach et al., 1989) and by being largely expressed in the rodent brain as a presynaptic heteroreceptor on non-serotonergic neurons, including GABA interneurons in the mPFC and amygdala (Kilpatrick et al., 1987; Morales et al., 1998; Morales and Bloom, 1997; Tecott et al., 1993). Systemic administration of 5-HT3-R antagonists such ondansetron and tropisetron have anxiolytic-like effects in rodent assays, although this is not always consistently seen (reviewed in Costall and Naylor, 2004; Griebel, 1995; Millan, 2003). There are 5-HT3A-R and 5-HT3B-R subtypes of this receptor, but despite its presence in the human amygdala (Davies et al., 1999) little is known about the contribution of 5-HT3B-R to corticolimbic function and stress. By contrast, the contribution of 5-HT3A-R has been demonstrated by the finding that gene knockout of Htr3A in mouse is associated with a reduction in anxiety-like behavior on various tasks (Bhatnagar et al., 2004b; Kelley et al., 2003). 5-HT3A-R knockout mice also show exaggerated amygdala-mediated conditioned fear (Bhatnagar et al., 2004b), which might reflect the reported pro-cognitive effects of 5-HT3-R antagonists in rats (Costall and Naylor, 2004).
5-HT3A-Rs are located in the PVN of the hypothalamus (Tecott et al., 1993). 5-HT3A-R knockout mice show an attenuated adrenocorticotropin-releasing hormone response to restraint stress (Bhatnagar et al., 2004b; Bhatnagar and Vining, 2004) similar to the attenuated restraint stress-induced prolactin response produced by the 5-HT3-R antagonist ondansetron in rats (Jorgensen et al., 1992; Nonaka, 1999) (but see Jorgensen et al., 1998). By extension the elevated levels of CRH mRNA in the central nucleus of the amygdala of 5-HT3A-R knockout mice may be an upstream compensation for a chronically depressed HPA-axis (Bhatnagar et al., 2004b), although it could also reflect a loss of normal GABAergic facilitation mediated by this receptor in the amygdala (Koyama et al., 2002; Turner et al., 2004). Systemic treatment with 5-HT3-R antagonists including ondansetron and ramosetron also inhibit CRH and restraint stress-induced colonic propulsion (Hirata et al., 2007; Miyata et al., 1993, 1998; Yamamoto et al., 1998). This is one reason why 5-HT3-R antagonists have garnered interest as potential treatments for Irritable Bowel Syndrome, a condition frequently found in stress-related conditions (Gershon, 2005).
Recent evidence indicates that SRIs and other antidepressants have 5-HT3-R antagonist properties (Eisensamer et al., 2003, 2005). Systemic treatment with the 5-HT3-R antagonist ondansetron can augment the antidepressant-like actions of SRIs in the forced swim and tail suspension tests, while the 5-HT3-R agonist 1-(m-chlorophenyl)-biguanide (mCPBG) has the opposite effect (Nakagawa et al., 1998; Redrobe and Bourin, 1997) (cf. Akagawa et al., 1999; Millan et al., 2003). 5-HT3A-R knockout mice do not display changes in baseline behaviors in these tests with the exception of increased ‘depression-related’ behavior in female knockouts with repeated forced swim exposure (Bhatnagar et al., 2004a). Whether these mice respond abnormally to antidepressants is not known.
There is emerging evidence of a link between 5-HT3-R, stress and alcohol abuse. There is a high degree of comorbidity between stress-related disorders and alcohol abuse and ondansetron has efficacy in the treatment of certain types of alcoholism (Johnson, 2004). In the rodent, systemic treatment with 5-HT3 antagonists including ondansetron, tropisetron and LY-278,584 reduces stress-induced reinstatement of alcohol self-administration and reward-related place learning (Le et al., 2006; Matsuzawa et al., 1999). 5-HT3A-R knockout mice fail to show the normal reduction in voluntary ethanol consumption produced by 5-HT3-R antagonist LY-278,584, but otherwise show normal baseline consumption and sensitivity to the acute behavioral effects of ethanol (Hodge et al., 2004). A different phenotype is produced by post-developmental transgenic overexpression of 5-HT3-R in forebrain regions including the mPFC and amygdala (Engel et al., 1998; Harrell and Allan, 2003). These transgenic mice show increased sensitivity to the locomotor stimulant effects of ethanol and reduced voluntary ethanol self-administration (but normal ethanol discriminative stimulus capabilities) (Engel and Allan, 1999; Engel et al., 1998; Shelton et al., 2004). Interestingly, this phenotype is mimicked by exposure to chronic swim stress in certain inbred strains of mice (Boyce-Rustay et al., 2007a, 2007b). It also similar to the ethanol-related phenotype found in SERT knockout mice (Boyce-Rustay et al., 2006; Daws et al., 2006), which display increased 5-HT3-R binding and mRNA levels in various brain regions, including a near 50% increase in the mPFC (Mossner et al., 2004). Collectively, these data suggest that the 5-HT3-R might be an important link between stress and alcohol-related behaviors, including the propensity to abuse alcohol.
Variation in the human HTR3A thus represents an attractive candidate for affective illness and alcoholism. An allelic variant of a SNP in the human HTR3A gene, C178T, is associated with increased 5-HT3-R expression in vitro (Niesler et al., 2001). Individuals carrying this variant exhibit lower scores for anxiety-related traits such as harm avoidance (Melke et al., 2003) and reduced amygdala and PFC activity in response to threatening stimuli as measured by fMRI (Iidaka et al., 2005). This interesting source of gene variation in the serotonin system warrants further study.
12. 5-HT4, 5-HT5A, 5-HT6, and 5-HT7 receptors
This review has largely focused on the 5-HT1, 5-HT2 and 5-HT3 receptor classes because other receptor subtypes have been relatively less well studied due to their recent cloning or a lack of well-defined research tools such as subtype-selective ligands or gene mutant mice. Of course, this does not necessarily imply that these subtypes play any less of a role in modulating serotonin's stress-related actions. In fact there are promising leads for a number of subtypes expressed in the DRN-mPFC-amygdala circuit.
Both 5-HT4-Rs and 5-HT5A-Rs have been shown to exert negative feedback control over the DRN, probably via an indirect, mPFC-mediated, and a direct, autoreceptor-mediated, mechanism respectively (Lucas et al., 2005; Lucas and Debonnel, 2002; Thomas et al., 2006). Genetic deletion of the mouse Htr5a has minimal effect on anxiety-like behavior (Grailhe et al., 1999). Systemic treatment with 5-HT4-R-selective doses of the agonists RS 67333 and prucalopride has demonstrated an antidepressant-like profile across a range of assays (forced swim test, olfactory bulbectomy, chronic mild stress) in rats (Lucas et al., 2007). Pharmacological antagonism or gene knockout of 5-HT4-R in the mouse reduces anxiety-like behaviors, and also attenuates certain stress-induced changes in gut motility and feeding (Compan et al., 2004; Conductier et al., 2006; Kennett et al., 1997; Smriga and Torii, 2003). These data hint at a contribution of 5-HT4-Rs to the gastrointestinal and eating-related symptoms that are prominent in stress-related disorders.
Pharmacological antagonism of 5-HT6-R produces cognitive improvement in conjunction with anxiolytic-like and antidepressant-like activity, and a blockade of certain neural, molecular and behavioral effects of SRIs (Mitchell and Neumaier, 2005; Svenningsson et al., 2007; Wesolowska, 2008; Wesolowska and Nikiforuk, 2007, 2008; Woolley et al., 2004). This contrasts with the pro-anxiety effects of antisense knockdown of 5-HT6-R (Hamon et al., 1999) and a report in abstract form that mouse Htr6 knockout leads to increased anxiety-like behavior (Tecott et al., 1998). Finally, antagonism of 5-HT7-R, via systemic SB-258719 or SB-269970 treatment, also produces antidepressant-like effects, and there is preliminary evidence that this receptor is upregulated by stress (Guscott et al., 2005; Hedlund et al., 2005; Wesolowska et al., 2006; Yau et al., 2001). Along similar lines, Htr7 knockout gene knockout produces an antidepressant-like (and in some studies anxiolytic-like) phenotype and decreased paradoxical sleep (Guscott et al., 2005; Hedlund et al., 2005; Hedlund and Sutcliffe, 2007).
These emerging preclinical data encourage further investigation of these various serotonin receptor subtypes. Not surprisingly however, we still know very little about how genetic variation in any of these receptors might affect risk for stress-related psychiatric disorders in man.
13. Summary and concluding remarks
The large literature from preclinical studies in rodents indicates that genetically driven functional disruption at all levels of the serotonin system – from prenatal development to neurotransmission in the adult brain – can influence the behavioral, physiological and neuroendocrinological response to stress. While the field is still at an early stage and there is much yet to be clarified, advances have been made in defining the contributions of key molecules within the serotonin system.At the risk of oversimplifying a hugely complex literature, some of the main findings and avenues for future work can be summarized.
It is increasingly evident that the influence of serotonin gene variation begins during ontogeny with the developmental formation of the neural systems mediating the stress response including, but certainly not limited to, the pathway interconnecting the DRN, mPFC and amygdala. By acting during development, functional alterations of Pet-1, Tph1, MAOA, SERT, and 5-HT1A-R function have all been found to produce lasting changes in rodent stress-related phenotypes and/or the corticolimbic systems subserving the stress response. Defining the putative developmental influence of natural gene variation in these molecules is critical for future work. There are also good grounds for predicting that genetically driven loss of the key molecules controlling serotonin synthesis (Tph2), packaging (VMAT2), reuptake (SERT) and degradation (MAOA) would cause an imbalance in serotonin availability and, in doing so, significantly limit the plasticity of the adult serotonin system's adaptation to stress.
Serotonergic neurotransmission in the DRN-mPFC-DRN pathway occurs through a myriad of receptors with complimentary and opposing actions (see Fig. 3). Pharmacological and electrophysiological studies in the adult brain imply that variation in 5-HT1A-R function in the mPFC and amygdala will determine the extent of serotonin's inhibitory postsynaptic actions in these regions, as well as mPFC-mediated negative feedback to serotonin neurons. 5-HT1A-Rs as well as 5-HT1B-Rs, also directly regulate serotonergic neuronal firing or 5-HT release in their capacity as presynaptic autoreceptors. Variation in 5-HT2A-R is expected to have a major influence on serotonin's actions in the mPFC with implications not only for stress-responsivity but also stress-induced cognitive-executive dysfunction. 5-HT2C-Rs are a critical component of the amygdala and HPA-axis response to stress and there is already evidence that genetic and epigenetic (e.g., mRNA editing) variation in this receptor impacts stress-related phenotypes. The 5-HT3A-R is also distributed in the mPFC and amygdala, and this subtype appears to have a distinct role in linking stress and alcohol-related behaviors. Of the other 14-plus serotonin receptor subtypes, there is already data encouraging the further exploration of the role of 5-HT4-R, 5-HT5A-R, 5-HT6-R, and 5-HT7-R in mediating serotonergic modulation of the response to stress.
Fig. 3.
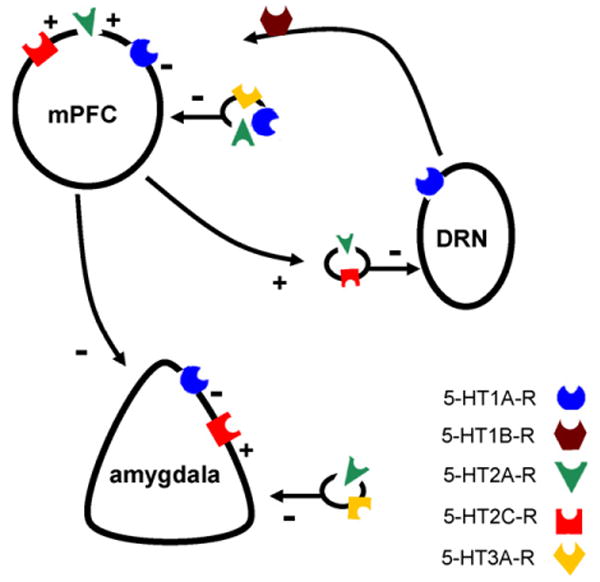
Localization of 5-HT1A-R, 5-HT1B-R, 5-HT2A-R, 5-HT2C-R, and 5-HT3A-R serotonin receptor subunits in the DRN-mPFC-amygdala circuit. These receptor subtypes exhibit a complex and often overlapping pattern of localization within the circuit. DRN = dorsal raphe nucleus, mPFC = medial prefrontal cortex.
This impressive corpus of data supports the contribution of molecular nodes throughout the serotonin system to the stress response. It is unlikely, however, that a gene variant in any one node within this complex system would in and of itself act in isolation to promote risk for stress-related disease. The serotonin system is by its nature modulatory and extremely plastic. A genetic variant in one component would be expected to lead to compensatory changes at both the molecular and system level. For example, a loss of function variant in TPH2-mediated synthesis could be mitigated by a compensatory downregulation in SERT-mediated reuptake, or a genetically driven increase in 5-HT2A-R drive of PFC neurons could be balanced by a parallel increase in 5-HT1A-R inhibition on the same neurons. Likewise, the reciprocal nature of interconnected regions within the DRN-mPFC-amygdala circuit allows for a functional alteration in one brain region in response to an abnormal level of activity in another.
From this perspective, stress-related pathology would be expected to manifest only when the net effect of multiple gene variants causes a system-level failure to maintain homeostasis in the face of persistent environmental challenge (Caspi and Moffitt, 2006; Holmes and Hariri, 2003; Jabbi et al., 2007; Murphy et al., 2003). Disentangling these epistatic interactions will be an enormous task, but there is every reason to be optimistic that major advances toward this goal can be made in the coming years.
Acknowledgments
I would like to thank Ahmad Hariri, John F. Cryan, Patrick Fisher, and David Goldman for valuable discussion and comments during the preparation of this article. Supported by the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program.
References
- Abi-Saab WM, Bubser M, Roth RH, Deutch AY. 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology. 1999;20:92–96. doi: 10.1016/S0893-133X(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Abramowski D, Rigo M, Duc D, Hoyer D, Staufenbiel M. Localization of the 5-hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology. 1995;34:1635–1645. doi: 10.1016/0028-3908(95)00138-7. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann NY Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res. 2006;170:126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Akagawa Y, Masuda Y, Maruyama A, Shimizu T, Hishikawa Y. Effects of repeated selective serotonin reuptake inhibitor paroxetine treatments on mouse forced swimming. Methods Find Exp Clin Pharmacol. 1999;21:599–601. [PubMed] [Google Scholar]
- Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, Hamon M, Adrien J. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci. 2006;26:5554–5564. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargos-Bosch M, Adell A, Artigas F. Antipsychotic drugs reverse the AMPA receptor-stimulated release of 5-HT in the medial prefrontal cortex. J Neurochem. 2007;102:550–561. doi: 10.1111/j.1471-4159.2007.04532.x. [DOI] [PubMed] [Google Scholar]
- Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Differences in behavior and monoamine laterality following neonatal clomipramine treatment. Dev Psychobiol. 2002;41:50–57. doi: 10.1002/dev.10055. [DOI] [PubMed] [Google Scholar]
- Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter. II. Suicidal behavior. Mol Psychiatry. 2003a;8:646–653. doi: 10.1038/sj.mp.4001336. [DOI] [PubMed] [Google Scholar]
- Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter. I. Affective disorders. Mol Psychiatry. 2003b;8:574–591. doi: 10.1038/sj.mp.4001328. [DOI] [PubMed] [Google Scholar]
- Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav Neurosci. 2001;115:443–454. [PubMed] [Google Scholar]
- Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol. 2007;7:8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Hawks BW, Owens MJ, Plotsky PM, Nemeroff CB. Increased responsiveness of presumed 5-HT cells to citalopram in adult rats subjected to prolonged maternal separation relative to brief separation. Psychopharmacology (Berl) 2004;176:248–255. doi: 10.1007/s00213-004-1883-x. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Magro P, Roberts R, Wang RY. A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci. 1999;11:2917–2934. doi: 10.1046/j.1460-9568.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT(1A) or 5-HT(1B) receptor knockout mice. J Neurochem. 2000;75:2415–2426. doi: 10.1046/j.1471-4159.2000.0752415.x. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Edwards E, Wang RY. Electrophysiological evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in the rat medial prefrontal cortex: an iontophoretic study. Synapse. 1994;17:173–181. doi: 10.1002/syn.890170306. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Jiang LH, Kasser RJ, Wang RY. Electrophysiological characterization of 5-hydroxytryptamine2 receptors in the rat medial prefrontal cortex. J Pharmacol Exp Ther. 1990;252:171–178. [PubMed] [Google Scholar]
- Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res. 2003;959:58–67. doi: 10.1016/s0006-8993(02)03727-7. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Toth M. Variability in the benzodiazepine response of serotonin 5-HT1A receptor null mice displaying anxiety-like phenotype: evidence for genetic modifiers in the 5-HT-mediated regulation of GABA(A) receptors. J Neurosci. 2004;24:6343–6351. doi: 10.1523/JNEUROSCI.0563-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protein G q. J Neurosci. 2007;27:1467–1473. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Pituitary-adrenal activity in acute and chronically stressed male and female mice lacking the 5-HT-3A receptor. Stress. 2004;7:251–256. doi: 10.1080/10253890500044422. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Nowak N, Babich L, Bok L. Deletion of the 5-HT3 receptor differentially affects behavior of males and females in the Porsolt forced swim and defensive withdrawal tests. Behav Brain Res. 2004a;153:527–535. doi: 10.1016/j.bbr.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Sun LM, Raber J, Maren S, Julius D, Dallman MF. Changes in anxiety-related behaviors and hypothalamic–pituitary–adrenal activity in mice lacking the 5-HT-3A receptor. Physiol Behav. 2004b;81:545–555. doi: 10.1016/j.physbeh.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Blackshear MA, Sanders-Bush E. Serotonin receptor sensitivity after acute and chronic treatment with mianserin. J Pharmacol Exp Ther. 1982;221:303–308. [PubMed] [Google Scholar]
- Blackshear MA, Martin LL, Sanders-Bush E. Adaptive changes in the 5-HT2 binding site after chronic administration of agonists and antagonists. Neuropharmacology. 1986;25:1267–1271. doi: 10.1016/0028-3908(86)90146-2. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Blue ME, Yagaloff KA, Mamounas LA, Hartig PR, Molliver ME. Correspondence between 5-HT2 receptors and serotonergic axons in rat neocortex. Brain Res. 1988;453:315–328. doi: 10.1016/0006-8993(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Bolanos-Jimenez F, Manhaes de Castro RM, Seguin L, Cloez-Tayarani I, Monneret V, Drieu K, Fillion G. Effects of stress on the functional properties of pre- and postsynaptic 5-HT1B receptors in the rat brain. Eur J Pharmacol. 1995;294:531–540. doi: 10.1016/0014-2999(95)00590-0. [DOI] [PubMed] [Google Scholar]
- Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT(2C) receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br J Pharmacol. 2006;149:861–869. doi: 10.1038/sj.bjp.0706935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothman LJ, Sharp T. A role for midbrain raphe gamma aminobutyric acid neurons in 5-hydroxytryptamine feedback control. Neuroreport. 2005;16:891–896. doi: 10.1097/00001756-200506210-00004. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Allers KA, Rasmussen K, Sharp T. Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br J Pharmacol. 2003;139:998–1004. doi: 10.1038/sj.bjp.0705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F, Ceci A, Bietti G, Donetti A. BIMT 17, a 5-HT1A receptor agonist/5-HT2A receptor antagonist, directly activates postsynaptic 5-HT inhibitory responses in the rat cerebral cortex. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:283–290. doi: 10.1007/BF00168558. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Amargos-Bosch M, Toth M, Artigas F, Adell A. In vivo efflux of serotonin in the dorsal raphe nucleus of 5-HT1A receptor knockout mice. J Neurochem. 2004;88:1373–1379. doi: 10.1046/j.1471-4159.2003.02267.x. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Amargos-Bosch M, Adell A, Diaz-Mataix L, Serrats J, Pons S, Artigas F. In vivo modulation of 5-hydroxytryptamine release in mouse prefrontal cortex by local 5-HT(2A) receptors: effect of antipsychotic drugs. Eur J Neurosci. 2003;18:1235–1246. doi: 10.1046/j.1460-9568.2003.02829.x. [DOI] [PubMed] [Google Scholar]
- Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–2212. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- Bourin M. Is it possible to predict the activity of a new antidepressant in animals with simple psychopharmacological tests? Fundam Clin Pharmacol. 1990;4:49–64. doi: 10.1111/j.1472-8206.1990.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Franc B, Hen R, Hamon M, Adrien J. Key role of 5-HT1B receptors in the regulation of paradoxical sleep as evidenced in 5-HT1B knock-out mice. J Neurosci. 1999;19:3204–3212. doi: 10.1523/JNEUROSCI.19-08-03204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Monaca C, Hen R, Hamon M, Adrien J. Involvement of 5-HT1A receptors in homeostatic and stress-induced adaptive regulations of paradoxical sleep: studies in 5-HT1A knock-out mice. J Neurosci. 2002;22:4686–4692. doi: 10.1523/JNEUROSCI.22-11-04686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der Gugten J, Maes RA, Hen R, Olivier B. Absence of 5-HT(1B) receptors is associated with impaired impulse control in male 5-HT(1B) knockout mice. Biol Psychiatry. 2001;49:557–568. doi: 10.1016/s0006-3223(00)01018-0. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007a;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Janos AL, Holmes A. Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav Brain Res. 2007b doi: 10.1016/j.bbr.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, Hariri AR. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatry. 2005;10:884–888. 805. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Bruening S, Oh E, Hetzenauer A, Escobar-Alvarez S, Westphalen RI, Hemmings HC, Jr, Singewald N, Shippenberg T, Toth M. The anxiety-like phenotype of 5-HT receptor null mice is associated with genetic background-specific perturbations in the prefrontal cortex GABA-glutamate system. J Neurochem. 2006;99:892–899. doi: 10.1111/j.1471-4159.2006.04129.x. [DOI] [PubMed] [Google Scholar]
- Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, Egan MF, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry. 2007;13:313–324. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- Buckholtz NS, Freedman DX, Middaugh LD. Daily LSD administration selectively decreases serotonin2 receptor binding in rat brain. Eur J Pharmacol. 1985;109:421–425. doi: 10.1016/0014-2999(85)90407-8. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bush DE, McEwen BS, Ledoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem. 2002;277:36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Bagdy G, Moncada ML, D'Agata R. Effect of selective serotonin agonists on basal, corticotrophin-releasing hormone- and vasopressin-induced ACTH release in vitro from rat pituitary cells. J Endocrinol. 1993;136:381–387. doi: 10.1677/joe.0.1360381. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Bagdy G, Szemeredi K, Tartaglia ME, Gold PW, Chrousos GP. Mechanisms of serotonin receptor agonist-induced activation of the hypothalamic-pituitary-adrenal axis in the rat. Endocrinology. 1990;126:1888–1894. doi: 10.1210/endo-126-4-1888. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Carola V, Frazzetto G, Pascucci T, Audero E, Puglisi-Allegra S, Cabib S, Lesch KP, Gross C. Identifying molecular substrates in a mouse model of the serotonin transporter × environment risk factor for anxiety and depression. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.08.013. PMID: 17949690. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A. Effects of mild early life stress on abnormal emotion-related behaviors in 5-HTT knockout mice. Behav Genet. 2007;37:214–222. doi: 10.1007/s10519-006-9129-9. [DOI] [PubMed] [Google Scholar]
- Casanovas JM, Hervas I, Artigas F. Postsynaptic 5-HT1A receptors control 5-HT release in the rat medial prefrontal cortex. Neuroreport. 1999;10:1441–1445. doi: 10.1097/00001756-199905140-00010. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargos-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain—a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Baudrie V, Coupry I. Effects of chlorisondamine and restraint on cortical [3H]ketanserin binding, 5-HT2A receptor-mediated head shakes, and behaviours in models of anxiety. Neuropharmacology. 1994;33:449–456. doi: 10.1016/0028-3908(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Cheng LL, Wang SJ, Gean PW. Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci. 1998;10:2163–2172. doi: 10.1046/j.1460-9568.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- Chou-Green JM, Holscher TD, Dallman MF, Akana SF. Repeated stress in young and old 5-HT(2C) receptor knockout mice. Physiol Behav. 2003;79:217–226. doi: 10.1016/s0031-9384(03)00096-9. [DOI] [PubMed] [Google Scholar]
- Christiansen L, Tan Q, Iachina M, Bathum L, Kruse TA, McGue M, Christensen K. Candidate gene polymorphisms in the serotonergic pathway: influence on depression symptomatology in an elderly population. Biol Psychiatry. 2007;61:223–230. doi: 10.1016/j.biopsych.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Clark JA, Pai LY, Flick RB, Rohrer SP. Differential hormonal regulation of tryptophan hydroxylase-2 mRNA in the murine dorsal raphe nucleus. Biol Psychiatry. 2005;57:943–946. doi: 10.1016/j.biopsych.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Clark JA, Flick RB, Pai LY, Szalayova I, Key S, Conley RK, Deutch AY, Hutson PH, Mezey E. Glucocorticoid modulation of tryptophan hydroxylase-2 protein in raphe nuclei and 5-hydroxytryptophan concentrations in frontal cortex of C57/Bl6 mice. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002041. PMID: 17622221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Vincow ES, Sexton TJ, Neumaier JF. Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res. 2004;1007:86–97. doi: 10.1016/j.brainres.2004.01.070. [DOI] [PubMed] [Google Scholar]
- Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clement Y, Kia KH, Daval G, Verge D. An autoradiographic study of serotonergic receptors in a murine genetic model of anxiety-related behaviors. Brain Res. 1996;709:229–242. doi: 10.1016/0006-8993(95)01297-4. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Clifford EM, Gartside SE, Umbers V, Cowen PJ, Hajos M, Sharp T. Electrophysiological and neurochemical evidence that pindolol has agonist properties at the 5-HT1A autoreceptor in vivo. Br J Pharmacol. 1998;124:206–212. doi: 10.1038/sj.bjp.0701796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compaan JC, Groenink L, van der Gugten J, Maes RA, Olivier B. 5-HT1A receptor agonist flesinoxan enhances Fos immunoreactivity in rat central amygdala, bed nucleus of the stria terminalis and hypothalamus. Eur J Neurosci. 1996;8:2340–2347. doi: 10.1111/j.1460-9568.1996.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Compaan JC, Groenink L, Van der Gugten J, Maes RA, Olivier B. Pretreatment with 5-HT1A receptor agonist flesinoxan attenuates Fos protein in rat hypothalamus. Eur J Pharmacol. 1997;324:161–168. doi: 10.1016/s0014-2999(97)00071-x. [DOI] [PubMed] [Google Scholar]
- Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R. Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J Neurosci. 2004;24:412–419. doi: 10.1523/JNEUROSCI.2806-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conductier G, Dusticier N, Lucas G, Cote F, Debonnel G, Daszuta A, Dumuis A, Nieoullon A, Hen R, Bockaert J, Compan V. Adaptive changes in serotonin neurons of the raphe nuclei in 5-HT(4) receptor knock-out mouse. Eur J Neurosci. 2006;24:1053–1062. doi: 10.1111/j.1460-9568.2006.04943.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. Regulation of serotonin-stimulated phosphoinositide hydrolysis: relation to the serotonin 5-HT-2 binding site. J Neurosci. 1986;6:3669–3675. doi: 10.1523/JNEUROSCI.06-12-03669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cornelio AM, Nunes-de-Souza RL. Anxiogenic-like effects of mCPP microinfusions into the amygdala (but not dorsal or ventral hippocampus) in mice exposed to elevated plus-maze. Behav Brain Res. 2007;178:82–89. doi: 10.1016/j.bbr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Corradetti R, Laaris N, Hanoun N, Laporte AM, Le Poul E, Hamon M, Lanfumey L. Antagonist properties of (—)-pindolol and WAY 100635 at somatodendritic and postsynaptic 5-HT1A receptors in the rat brain. Br J Pharmacol. 1998;123:449–462. doi: 10.1038/sj.bjp.0701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. 5-HT3 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:27–37. doi: 10.2174/1568007043482624. [DOI] [PubMed] [Google Scholar]
- Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci USA. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crayton JW, Joshi I, Gulati A, Arora RC, Wolf WA. Effect of corticosterone on serotonin and catecholamine receptors and uptake sites in rat frontal cortex. Brain Res. 1996;728:260–262. doi: 10.1016/0006-8993(96)00189-8. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Rea K, Bosker FJ, Wikstrom HV, Hogg S, Mork A, Westerink BH. Augmentation of SSRI effects on serotonin by 5-HT2C antagonists: mechanistic studies. Neuropsychopharmacology. 2007;32:1550–1557. doi: 10.1038/sj.npp.1301287. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mork A, Honig G, Bogeso KP, Westerink BH, den Boer H, Wikstrom HV, Tecott LH. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Brodkin ES, Blendy JA, Berrettini WH, Lucki I. Pharmacogenomic evaluation of the antidepressant citalopram in the mouse tail suspension test. Neuropsychopharmacology. 2006;31:2433–2442. doi: 10.1038/sj.npp.1301065. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther. 2000;295:1120–1126. [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, Jacob R, Walton RT, Grasby PM. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci. 2005;25:2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LA, Hughes ZA, Starr KR, Storey JD, Bettelini L, Bacchi F, Arban R, Poffe A, Melotto S, Hagan JJ, Price GW. Characterisation of the selective 5-HT1B receptor antagonist SB-616234-A (1-[6-(cis-3,5-dimethylpiperazin-1-yl)-2,3-dihydro-5-methoxyindol-1-yl]-1-[2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methanone hydrochloride): in vivo neurochemical and behavioural evidence of anxiolytic/antidepressant activity. Neuropharmacology. 2006;50:975–983. doi: 10.1016/j.neuropharm.2006.01.010. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- de Groote L, Olivier B, Westenberg HG. Extracellular serotonin in the prefrontal cortex is limited through terminal 5-HT(1B) autoreceptors: a microdialysis study in knockout mice. Psychopharmacology (Berl) 2002a;162:419–424. doi: 10.1007/s00213-002-1117-z. [DOI] [PubMed] [Google Scholar]
- de Groote L, Olivier B, Westenberg HG. The effects of selective serotonin reuptake inhibitors on extracellular 5-HT levels in the hippocampus of 5-HT(1B) receptor knockout mice. Eur J Pharmacol. 2002b;439:93–100. doi: 10.1016/s0014-2999(02)01417-6. [DOI] [PubMed] [Google Scholar]
- Delorme R, Durand CM, Betancur C, Wagner M, Ruhrmann S, Grabe HJ, Nygren G, Gillberg C, Leboyer M, Bourgeron T, Courtet P, Jollant F, Buresi C, Aubry JM, Baud P, Bondolfi G, Bertschy G, Perroud N, Malafosse A. No human tryptophan hydroxylase-2 gene R441H mutation in a large cohort of psychiatric patients and control subjects. Biol Psychiatry. 2006;60:202–203. doi: 10.1016/j.biopsych.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Dirks A, Pattij T, Bouwknecht JA, Westphal TT, Hijzen TH, Groenink L, van der Gugten J, Oosting RS, Hen R, Geyer MA, Olivier B. 5-HT1B receptor knockout, but not 5-HT1A receptor knockout mice, show reduced startle reactivity and footshock-induced sensitization, as measured with the acoustic startle response. Behav Brain Res. 2001;118:169–178. doi: 10.1016/s0166-4328(00)00326-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Duan J, Sanders AR, Molen JE, Martinolich L, Mowry BJ, Levinson DF, Crowe RR, Silverman JM, Gejman PV. Polymorphisms in the 5′-untranslated region of the human serotonin receptor 1B (HTR1B) gene affect gene expression. Mol Psychiatry. 2003;8:901–910. doi: 10.1038/sj.mp.4001403. [DOI] [PubMed] [Google Scholar]
- Dubrovina NI, Popova NK, Gilinskii MA, Tomilenko RA, Seif I. Acquisition and extinction of a conditioned passive avoidance reflex in mice with genetic knockout of monoamine oxidase A. Neurosci Behav Physiol. 2006;36:335–339. doi: 10.1007/s11055-006-0022-z. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Edwards E, Harkins K, Wright G, Henn FA. 5-HT1b receptors in an animal model of depression. Neuropharmacology. 1991;30:101–105. doi: 10.1016/0028-3908(91)90050-l. [DOI] [PubMed] [Google Scholar]
- Eisensamer B, Uhr M, Meyr S, Gimpl G, Deiml T, Rammes G, Lambert JJ, Zieglgansberger W, Holsboer F, Rupprecht R. Antidepressants and antipsychotic drugs colocalize with 5-HT3 receptors in raft-like domains. J Neurosci. 2005;25:10198–10206. doi: 10.1523/JNEUROSCI.2460-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisensamer B, Rammes G, Gimpl G, Shapa M, Ferrari U, Hapfelmeier G, Bondy B, Parsons C, Gilling K, Zieglgansberger W, Holsboer F, Rupprecht R. Antidepressants are functional antagonists at the serotonin type 3 (5-HT3) receptor. Mol Psychiatry. 2003;8:994–1007. doi: 10.1038/sj.mp.4001314. [DOI] [PubMed] [Google Scholar]
- Engel SR, Allan AM. 5-HT3 receptor over-expression enhances ethanol sensitivity in mice. Psychopharmacology (Berl) 1999;144:411–415. doi: 10.1007/s002130051025. [DOI] [PubMed] [Google Scholar]
- Engel SR, Lyons CR, Allan AM. 5-HT3 receptor over-expression decreases ethanol self administration in transgenic mice. Psychopharmacology (Berl) 1998;140:243–248. doi: 10.1007/s002130050763. [DOI] [PubMed] [Google Scholar]
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci USA. 2005;102:5582–5587. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard A, Laporte AM, Chastanet M, Hen R, Hamon M, Adrien J. 5-HT1A and 5-HT1B receptors control the firing of serotoninergic neurons in the dorsal raphe nucleus of the mouse: studies in 5-HT1B knock-out mice. Eur J Neurosci. 1999;11:3823–3831. doi: 10.1046/j.1460-9568.1999.00800.x. [DOI] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001a;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Ma Y, Vogel GW. The critical window of brain development from susceptive to insusceptive. Effects of clomipramine neonatal treatment on sexual behavior. Brain Res Dev Brain Res. 2001b;129:107–110. doi: 10.1016/s0165-3806(01)00158-4. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Fox MA, Andrews AM, Wendland JR, Lesch KP, Holmes A, Murphy DL. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 2007;195:147–166. doi: 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- Fratta W, Biggio G, Mercurio G, Di Vittorio P, Tagliamonte A, Gessa GL. Letter: the effect of d- and l-p-chlorophenylalanine on the metabolism of 5-hydroxytryptamine in brain. J Pharm Pharmacol. 1973;25:908–909. doi: 10.1111/j.2042-7158.1973.tb09972.x. [DOI] [PubMed] [Google Scholar]
- Freis ED. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N Engl J Med. 1954;251:1006–1008. doi: 10.1056/NEJM195412162512504. [DOI] [PubMed] [Google Scholar]
- Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, Dutriez-Casteloot I, Enache M, Maccari S, Barden N, Cohen-Salmon C, Hamon M, Lanfumey L. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci. 2004;24:2787–2796. doi: 10.1523/JNEUROSCI.4132-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, Wetsel WC. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci. 2007;27:10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Snoddy HD. Effect of serotonin-releasing drugs on serum corticosterone concentration in rats. Neuroendocrinology. 1980;31:96–100. doi: 10.1159/000123057. [DOI] [PubMed] [Google Scholar]
- Funada M, Hara C. Differential effects of psychological stress on activation of the 5-hydroxytryptamine- and dopamine-containing neurons in the brain of freely moving rats. Brain Res. 2001;901:247–251. doi: 10.1016/s0006-8993(01)02160-6. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Johnson DA, Leitch MM, Troakes C, Ingram CD. Early life adversity programs changes in central 5-HT neuronal function in adulthood. Eur J Neurosci. 2003;17:2401–2408. doi: 10.1046/j.1460-9568.2003.02668.x. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Hajos-Korcsok E, Bagdy E, Harsing LG, Jr, Sharp T, Hajos M. Neurochemical and electrophysiological studies on the functional significance of burst firing in serotonergic neurons. Neuroscience. 2000;98:295–300. doi: 10.1016/s0306-4522(00)00060-9. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39:S184–S193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- Gilbert F, Brazell C, Tricklebank MD, Stahl SM. Activation of the 5-HT1A receptor subtype increases rat plasma ACTH concentration. Eur J Pharmacol. 1988;147:431–439. doi: 10.1016/0014-2999(88)90178-1. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Murphy DL, Lesch K, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther. 2001;296:987–995. [PubMed] [Google Scholar]
- Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- Graeff FG. Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, Hen R. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron. 1999;22:581–591. doi: 10.1016/s0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: reprise. Nebr Symp Motiv. 1996;43:61–134. [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995;65:319–395. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. A comparative study of the effects of selective and non-selective 5-HT2 receptor subtype antagonists in rat and mouse models of anxiety. Neuropharmacology. 1997;36:793–802. doi: 10.1016/s0028-3908(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Groenink L, van Bogaert MJ, van der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behav Pharmacol. 2003;14:369–383. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Alves SE, Clark JA, Pai LY, Schaeffer JM, Rohrer SP. Estrogen receptor-beta regulates tryptophan hydroxylase-1 expression in the murine midbrain raphe. Biol Psychiatry. 2005;57:938–942. doi: 10.1016/j.biopsych.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J, Rupniak NM, Patel S, Whiting PJ, Hutson PH, Fone KC, Biello SM, Kulagowski JJ, McAllister G. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Hackler EA, Airey DC, Shannon CC, Sodhi MS, Sanders-Bush E. 5-HT(2C) receptor RNA editing in the amygdala of C57BL/6J, DBA/2J, and BALB/cJ mice. Neurosci Res. 2006;55:96–104. doi: 10.1016/j.neures.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hackler EA, Turner GH, Gresch PJ, Sengupta S, Deutch AY, Avison MJ, Gore JC, Sanders-Bush E. 5-Hydroxytryptamine2C receptor contribution to m-chlorophenylpiperazine and N-methyl-beta-carboline-3-carboxamide-induced anxiety-like behavior and limbic brain activation. J Pharmacol Exp Ther. 2007;320:1023–1029. doi: 10.1124/jpet.106.113357. [DOI] [PubMed] [Google Scholar]
- Hajos-Korcsok E, Robinson DD, Yu JH, Fitch CS, Walker E, Merchant KM. Rapid habituation of hippocampal serotonin and norepinephrine release and anxiety-related behaviors, but not plasma corticosterone levels, to repeated footshock stress in rats. Pharmacol Biochem Behav. 2003;74:609–616. doi: 10.1016/s0091-3057(02)01047-x. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hajos-Korcsok E, Sharp T. Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity in the rat. Br J Pharmacol. 1999;126:1741–1750. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Hajos M, Gartside SE, Varga V, Sharp T. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology. 2003;45:72–81. doi: 10.1016/s0028-3908(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Tetko IV, Hyland B, Sharp T, Villa AE. Different tonic regulation of neuronal activity in the rat dorsal raphe and medial prefrontal cortex via 5-HT(1A) receptors. Neurosci Lett. 2001;304:129–132. doi: 10.1016/s0304-3940(01)01751-7. [DOI] [PubMed] [Google Scholar]
- Hamada S, Senzaki K, Hamaguchi-Hamada K, Tabuchi K, Yamamoto H, Yamamoto T, Yoshikawa S, Okano H, Okado N. Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Brain Res Mol Brain Res. 1998;54:199–211. doi: 10.1016/s0169-328x(97)00322-7. [DOI] [PubMed] [Google Scholar]
- Hamon M, Doucet E, Lefevre K, Miquel MC, Lanfumey L, Insausti R, Frechilla D, Del Rio J, Verge D. Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology. 1999;21:68S–76S. doi: 10.1016/S0893-133X(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Harada K, Yamaji T, Matsuoka N. Activation of the serotonin 5-HT(2C) receptor is involved in the enhanced anxiety in rats after single-prolonged stress. Pharmacol Biochem Behav. 2007;89:11–16. doi: 10.1016/j.pbb.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Harada K, Aota M, Inoue T, Matsuda R, Mihara T, Yamaji T, Ishibashi K, Matsuoka N. Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. Eur J Pharmacol. 2006;553:171–184. doi: 10.1016/j.ejphar.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harrell AV, Allan AM. Improvements in hippocampal-dependent learning and decremental attention in 5-HT(3) receptor overexpressing mice. Learn Mem. 2003;10:410–419. doi: 10.1101/lm.56103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BH, Naciti C, Brand L, Stein DJ. Endocrine, cognitive and hippocampal/cortical 5HT 1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res. 2003;983:97–107. doi: 10.1016/s0006-8993(03)03033-6. [DOI] [PubMed] [Google Scholar]
- He M, Sibille E, Benjamin D, Toth M, Shippenberg T. Differential effects of 5-HT1A receptor deletion upon basal and fluoxetine-evoked 5-HT concentrations as revealed by in vivo microdialysis. Brain Res. 2001;902:11–17. doi: 10.1016/s0006-8993(01)02271-5. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Sutcliffe JG. The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci Lett. 2007;414:247–251. doi: 10.1016/j.neulet.2006.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007a;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GS, O'Rahilly S, Colmers WF, Elmquist JK, Tecott LH. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci. 2007b;27:6956–6964. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemrick-Luecke SK, Evans DC. Comparison of the potency of MDL 100,907 and SB 242084 in blocking the serotonin (5-HT)(2) receptor agonist-induced increases in rat serum corticosterone concentrations: evidence for 5-HT(2A) receptor mediation of the HPA axis. Neuropharmacology. 2002;42:162–169. doi: 10.1016/s0028-3908(01)00166-6. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo–pituitary–adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, Hilakivi I. Increased adult behavioral ‘despair’ in rats neonatally exposed to desipramine or zimeldine: an animal model of depression? Pharmacol Biochem Behav. 1987;28:367–369. doi: 10.1016/0091-3057(87)90454-0. [DOI] [PubMed] [Google Scholar]
- Hirata T, Keto Y, Funatsu T, Akuzawa S, Sasamata M. Evaluation of the pharmacological profile of ramosetron, a novel therapeutic agent for irritable bowel syndrome. J Pharmacol Sci. 2007;104:263–273. doi: 10.1254/jphs.fp0070620. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Kelley SP, Bratt AM, Iller K, Schroeder JP, Besheer J. 5-HT(3A) receptor subunit is required for 5-HT3 antagonist-induced reductions in alcohol drinking. Neuropsychopharmacology. 2004;29:1807–1813. doi: 10.1038/sj.npp.1300498. [DOI] [PubMed] [Google Scholar]
- Hodges H, Green S, Glenn B. Evidence that the amygdala is involved in benzodiazepine and serotonergic effects on punished responding but not on discrimination. Psychopharmacology (Berl) 1987;92:491–504. doi: 10.1007/BF00176484. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 1998;19:187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- Holmes A. The pharmacology of anxiety. In: Blanchard RJ, Blanchard DC, Nutt DJ, editors. Handbook of Anxiety and Fear. Elsevier; London: 2008. [Google Scholar]
- Holmes A, Hariri AR. The serotonin transporter gene-linked polymorphism and negative emotionality: placing single gene effects in the context of genetic background and environment. Genes Brain Behav. 2003;2:332–335. doi: 10.1046/j.1601-1848.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ. Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol. 2003;459:221–230. doi: 10.1016/s0014-2999(02)02874-1. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl) 2002a;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003a;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002b;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003b;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003c;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Huang YY, Grailhe R, Arango V, Hen R, Mann JJ. Relationship of psychopathology to the human serotonin1B genotype and receptor binding kinetics in postmortem brain tissue. Neuropsychopharmacology. 1999;21:238–246. doi: 10.1016/S0893-133X(99)00030-5. [DOI] [PubMed] [Google Scholar]
- Huang YY, Oquendo MA, Friedman JM, Greenhill LL, Brodsky B, Malone KM, Khait V, Mann JJ. Substance abuse disorder and major depression are associated with the human 5-HT1B receptor gene (HTR1B) G861C polymorphism. Neuropsychopharmacology. 2003;28:163–169. doi: 10.1038/sj.npp.1300000. [DOI] [PubMed] [Google Scholar]
- Huang YY, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, Zalsman G, Brodsky B, Arango V, Brent DA, Mann JJ. Human 5-HT1A receptor C(-1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol. 2004;7:441–451. doi: 10.1017/S1461145704004663. [DOI] [PubMed] [Google Scholar]
- Huizinga D, Haberstick BC, Smolen A, Menard S, Young SE, Corley RP, Stallings MC, Grotpeter J, Hewitt JK. Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotype. Biol Psychiatry. 2006;60:677–683. doi: 10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Ozaki N, Matsumoto A, Nogawa J, Kinoshita Y, Suzuki T, Iwata N, Yamamoto Y, Okada T, Sadato N. A variant C178T in the regulatory region of the serotonin receptor gene HTR3A modulates neural activation in the human amygdala. J Neurosci. 2005;25:6460–6466. doi: 10.1523/JNEUROSCI.5261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Suzuki K, Inoue T, Li XB, Maki Y, Muraki I, Kitaichi Y, Hashimoto S, Koyama T. Long-lasting change in 5-HT2A receptor-mediated behavior in rats after a single footshock. Eur J Pharmacol. 2002;452:199–204. doi: 10.1016/s0014-2999(02)02302-6. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, Ormel J, den Boer JA. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol Psychiatry. 2007;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jansson A, Tinner B, Bancila M, Verge D, Steinbusch HW, Agnati LF, Fuxe K. Relationships of 5-hydroxytryptamine immunoreactive terminal-like varicosities to 5-hydroxytryptamine-2A receptor-immunoreactive neuronal processes in the rat forebrain. J Chem Neuroanat. 2001;22:185–203. doi: 10.1016/s0891-0618(01)00133-8. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Loder MK, Sheward WJ, Pei Q, Deacon RM, Benson MA, Olverman HJ, Hastie ND, Harmar AJ, Shen S, Sharp T. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci. 2006;26:8955–8964. doi: 10.1523/JNEUROSCI.5356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Role of the serotonergic system in the neurobiology of alcoholism: implications for treatment. CNS Drugs. 2004;18:1105–1118. doi: 10.2165/00023210-200418150-00005. [DOI] [PubMed] [Google Scholar]
- Jones MD, Lucki I. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology. 2005;30:1039–1047. doi: 10.1038/sj.npp.1300664. [DOI] [PubMed] [Google Scholar]
- Jordan S, Kramer GL, Zukas PK, Petty F. Previous stress increases in vivo biogenic amine response to swim stress. Neurochem Res. 1994;19:1521–1525. doi: 10.1007/BF00969000. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Knigge U, Warberg J. Effect of serotonin 5-HT1, 5-HT2, and 5-HT3 receptor antagonists on the prolactin response to restraint and ether stress. Neuroendocrinology. 1992;56:371–377. doi: 10.1159/000126251. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Knigge U, Kjaer A, Vadsholt T, Warberg J. Serotonergic involvement in stress-induced ACTH release. Brain Res. 1998;811:10–20. doi: 10.1016/s0006-8993(98)00901-9. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Knigge U, Kjaer A, Moller M, Warberg J. Serotonergic stimulation of corticotropin-releasing hormone and pro-opiomelanocortin gene expression. J Neuroendocrinol. 2002;14:788–795. doi: 10.1046/j.1365-2826.2002.00839.x. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Vincow ES, Sexton TJ, Neumaier JF. 5-HT1B receptor mRNA levels in dorsal raphe nucleus: inverse association with anxiety behavior in the elevated plus maze. Pharmacol Biochem Behav. 2003;75:769–776. doi: 10.1016/s0091-3057(03)00152-7. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kalynchuk LE, Pinel JP, Meaney MJ. Serotonin receptor binding and mRNA expression in the hippocampus of fearful amygdala-kindled rats. Neurosci Lett. 2006;396:38–43. doi: 10.1016/j.neulet.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Kang M, Pyun KH, Jang CG, Kim H, Bae H, Shim I. Nelumbinis Semen reverses a decrease in hippocampal 5-HT release induced by chronic mild stress in rats. J Pharm Pharmacol. 2005;57:651–656. doi: 10.1211/0022357056055. [DOI] [PubMed] [Google Scholar]
- Katagiri H, Kagaya A, Nakae S, Morinobu S, Yamawaki S. Modulation of serotonin2A receptor function in rats after repeated treatment with dexamethasone and L-type calcium channel antagonist nimodipine. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1269–1281. doi: 10.1016/s0278-5846(01)00179-8. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M. Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett. 1993;162:81–84. doi: 10.1016/0304-3940(93)90565-3. [DOI] [PubMed] [Google Scholar]
- Keck ME, Sartori SB, Welt T, Muller MB, Ohl F, Holsboer F, Landgraf R, Singewald N. Differences in serotonergic neurotransmission between rats displaying high or low anxiety/depression-like behaviour: effects of chronic paroxetine treatment. J Neurochem. 2005;92:1170–1179. doi: 10.1111/j.1471-4159.2004.02953.x. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic–pituitary–adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- Kehr J, Yoshitake T, Wang FH, Wynick D, Holmberg K, Lendahl U, Bartfai T, Yamaguchi M, Hokfelt T, Ogren SO. Microdialysis in freely moving mice: determination of acetylcholine, serotonin and noradrenaline release in galanin transgenic mice. J Neurosci Methods. 2001;109:71–80. doi: 10.1016/s0165-0270(01)00403-4. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Bratt AM, Hodge CW. Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol. 2003;461:19–25. doi: 10.1016/s0014-2999(02)02960-6. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Bright F, Trail B, Blackburn TP, Sanger GJ. Anxiolytic-like actions of the selective 5-HT4 receptor antagonists SB 204070A and SB 207266A in rats. Neuropharmacology. 1997;36:707–712. doi: 10.1016/s0028-3908(97)00037-3. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, Gager T, Thomas D, Baxter GS, Forbes IT, Ham P, Blackburn TP. In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br J Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja X. Quantitative autoradiographic characterisation of the binding of [3H]WAY-100635, a selective 5-HT1A receptor antagonist. Brain Res. 1995;673:217–225. doi: 10.1016/0006-8993(94)01416-f. [DOI] [PubMed] [Google Scholar]
- Kia HK, Brisorgueil MJ, Hamon M, Calas A, Verge D. Ultrastructural localization of 5-hydroxytryptamine1A receptors in the rat brain. J Neurosci Res. 1996a;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996b;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Jones BJ, Tyers MB. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Shih JC, Chen K, Chen L, Bao S, Maren S, Anagnostaras SG, Fanselow MS, De Maeyer E, Seif I, Thompson RF. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci USA. 1997;94:5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. Stress. 1998;2:251–263. doi: 10.3109/10253899809167289. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682:189–196. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- Klein DF, Fink M. Psychiatric reaction patterns to imipramine. Am J Psychiatry. 1962;119:432–438. doi: 10.1176/ajp.119.5.432. [DOI] [PubMed] [Google Scholar]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- Knobelman DA, Hen R, Lucki I. Genetic regulation of extracellular serotonin by 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) autoreceptors in different brain regions of the mouse. J Pharmacol Exp Ther. 2001a;298:1083–1091. [PubMed] [Google Scholar]
- Knobelman DA, Hen R, Blendy JA, Lucki I. Regional patterns of compensation following genetic deletion of either 5-hydroxytryptamine(1A) or 5-hydroxytryptamine(1B) receptor in the mouse. J Pharmacol Exp Ther. 2001b;298:1092–1100. [PubMed] [Google Scholar]
- Koyama S, Kubo C, Rhee JS, Akaike N. Presynaptic serotonergic inhibition of GABAergic synaptic transmission in mechanically dissociated rat basolateral amygdala neurons. J Physiol. 1999;518(Pt 2):525–538. doi: 10.1111/j.1469-7793.1999.0525p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Murakami N, Kubo C, Nabekura J, Akaike N. Role of presynaptic 5-HT1A and 5-HT3 receptors in modulation of synaptic GABA transmission in dissociated rat basolateral amygdala neurons. Life Sci. 2002;72:375–387. doi: 10.1016/s0024-3205(02)02280-4. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Roth BL. The molecular biology of serotonin receptors: therapeutic implications for the interface of mood and psychosis. Biol Psychiatry. 1998;44:1128–1142. doi: 10.1016/s0006-3223(98)00132-2. [DOI] [PubMed] [Google Scholar]
- Krysiak R, Obuchowicz E, Herman ZS. Conditioned fear-induced changes in neuropeptide Y-like immunoreactivity in rats: the effect of diazepam and buspirone. Neuropeptides. 2000;34:148–157. doi: 10.1054/npep.2000.0804. [DOI] [PubMed] [Google Scholar]
- Laaris N, Haj-Dahmane S, Hamon M, Lanfumey L. Glucocorticoid receptor-mediated inhibition by corticosterone of 5-HT1A autoreceptor functioning in the rat dorsal raphe nucleus. Neuropharmacology. 1995;34:1201–1210. doi: 10.1016/0028-3908(95)00095-n. [DOI] [PubMed] [Google Scholar]
- Laaris N, Le Poul E, Hamon M, Lanfumey L. Stress-induced alterations of somatodendritic 5-HT1A autoreceptor sensitivity in the rat dorsal raphe nucleus–in vitro electrophysiological evidence. Fundam Clin Pharmacol. 1997;11:206–214. doi: 10.1111/j.1472-8206.1997.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci. 2001;21:9955–9963. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M, Di Paolo T. Effect of chronic estradiol, tamoxifen or raloxifene treatment on serotonin 5-HT1A receptor. Brain Res Mol Brain Res. 2003;112:82–89. doi: 10.1016/s0169-328x(03)00049-4. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Pardon MC, Laaris N, Joubert C, Hanoun N, Hamon M, Cohen-Salmon C. 5-HT1A autoreceptor desensitization by chronic ultramild stress in mice. Neuroreport. 1999;10:3369–3374. doi: 10.1097/00001756-199911080-00021. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Long JC, Virkkunen M, Ozaki N, Goldman D, Linnoila M. HTR2C Cys23Ser polymorphism in relation to CSF monoamine metabolite concentrations and DSM-III-R psychiatric diagnoses. Biol Psychiatry. 1999;46:821–826. doi: 10.1016/s0006-3223(98)00361-8. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. Effects of dexfenfluramine and 5-HT3 receptor antagonists on stress-induced reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2006;186:82–92. doi: 10.1007/s00213-006-0346-y. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Lee M, Bailer UF, Frank GK, Henry SE, Meltzer CC, Price JC, Mathis CA, Putnam KT, Ferrell RE, Hariri AR, Kaye WH. Relationship of a 5-HT transporter functional polymorphism to 5-HT1A receptor binding in healthy women. Mol Psychiatry. 2005;10:715–716. doi: 10.1038/sj.mp.4001680. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer B, Macciardi F, Segman RH, Adolfsson R, Blackwood D, Blairy S, Del Favero J, Dikeos DG, Kaneva R, Lilli R, Massat I, Milanova V, Muir W, Noethen M, Oruc L, Petrova T, Papadimitriou GN, Rietschel M, Serretti A, Souery D, Van Gestel S, Van Broeckhoven C, Mendlewicz J. Variability of 5-HT2C receptor cys23ser polymorphism among European populations and vulnerability to affective disorder. Mol Psychiatry. 2001;6:579–585. doi: 10.1038/sj.mp.4000883. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Wiesmann M, Hoh A, Muller T, Disselkamp-Tietze J, Osterheider M, Schulte HM. 5-HT1A receptor-effector system responsivity in panic disorder. Psychopharmacology (Berl) 1992;106:111–117. doi: 10.1007/BF02253597. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knock-out mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- Li Q, Wichems CH, Ma L, Van de Kar LD, Garcia F, Murphy DL. Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J Neurochem. 2003;84:1256–1265. doi: 10.1046/j.1471-4159.2003.01607.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: application of recombinant adenovirus containing 5-HT1A sequences. J Neurosci. 2004;24:10868–10877. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Inoue T, Abekawa T, Weng S, Nakagawa S, Izumi T, Koyama T. 5-HT1A receptor agonist affects fear conditioning through stimulations of the postsynaptic 5-HT1A receptors in the hippocampus and amygdala. Eur J Pharmacol. 2006;532:74–80. doi: 10.1016/j.ejphar.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Lin D, Parsons LH. Anxiogenic-like effect of serotonin(1B) receptor stimulation in the rat elevated plus-maze. Pharmacol Biochem Behav. 2002;71:581–587. doi: 10.1016/s0091-3057(01)00712-2. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, O'Callaghan MJ, Butterworth AR, Wilson J, Cole J, Watson WP. Low alcohol preference among the “high alcohol preference” C57 strain of mice; preference increased by saline injections. Psychopharmacology (Berl) 1999;147:182–189. doi: 10.1007/s002130051159. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT(2) receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, Lopez JF, Watson SJ. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lopez-Gil X, Babot Z, Amargos-Bosch M, Sunol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Tecott LH, Palacios JM, Mengod G, Vilaro MT. Serotonin 5-HT (2C) receptor knockout mice: autoradiographic analysis of multiple serotonin receptors. J Neurosci Res. 2002;67:69–85. doi: 10.1002/jnr.10072. [DOI] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Hen R, Cruz SL. Anxiolytic-like actions of toluene in the burying behavior and plus-maze tests: differences in sensitivity between 5-HT(1B) knockout and wild-type mice. Behav Brain Res. 2000;115:85–94. doi: 10.1016/s0166-4328(00)00241-2. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Liberzon I, Vazquez DM, Young EA, Watson SJ. Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biol Psychiatry. 1999;45:934–937. doi: 10.1016/s0006-3223(98)00224-8. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG. Meta-analysis of serotonin transporter polymorphisms and affective disorders. Psychiatr Genet. 2004;14:121–129. doi: 10.1097/00041444-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lucas G, Debonnel G. 5-HT4 receptors exert a frequency-related facilitatory control on dorsal raphe nucleus 5-HT neuronal activity. Eur J Neurosci. 2002;16:817–822. doi: 10.1046/j.1460-9568.2002.02150.x. [DOI] [PubMed] [Google Scholar]
- Lucas G, Compan V, Charnay Y, Neve RL, Nestler EJ, Bockaert J, Barrot M, Debonnel G. Frontocortical 5-HT4 receptors exert positive feedback on serotonergic activity: viral transfections, subacute and chronic treatments with 5-HT4 agonists. Biol Psychiatry. 2005;57:918–925. doi: 10.1016/j.biopsych.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, Lambas-Senas L, Wiborg O, Haddjeri N, Pineyro G, Sadikot AF, Debonnel G. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55:712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Ma QP, Yin GF, Ai MK, Han JS. Serotonergic projections from the nucleus raphe dorsalis to the amygdala in the rat. Neurosci Lett. 1991;134:21–24. doi: 10.1016/0304-3940(91)90499-j. [DOI] [PubMed] [Google Scholar]
- Maciag D, Williams L, Coppinger D, Paul IA. Neonatal citalopram exposure produces lasting changes in behavior which are reversed by adult imipramine treatment. Eur J Pharmacol. 2006a;532:265–269. doi: 10.1016/j.ejphar.2005.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006b;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagie I, Trillat AC, Bourin M, Jacquot C, Hen R, Gardier AM. 5-HT1B Autoreceptors limit the effects of selective serotonin re-uptake inhibitors in mouse hippocampus and frontal cortex. J Neurochem. 2001;76:865–871. doi: 10.1046/j.1471-4159.2001.00083.x. [DOI] [PubMed] [Google Scholar]
- Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD. 5-Hydroxytryptamine2A (5-HT2A) receptor regulation in rat prefrontal cortex: interaction of a phenethylamine hallucinogen and the metabotropic glutamate2/3 receptor agonist LY354740. Neurosci Lett. 2006;403:256–260. doi: 10.1016/j.neulet.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105:379–392. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R, Ugedo L. Electrophysiological evidence for postsynaptic 5-HT(1A) receptor control of dorsal raphe 5-HT neurones. Neuropharmacology. 2001;41:72–78. doi: 10.1016/s0028-3908(01)00050-8. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Duclos M, Mormede P, Manier G, Chaouloff F. Hippocampal and striatal [(3)H]5-HT reuptake under acute stressors in two rat strains differing for their emotivity. Neurosci Lett. 2000;288:246–248. doi: 10.1016/s0304-3940(00)01246-5. [DOI] [PubMed] [Google Scholar]
- Martin JR, Ballard TM, Higgins GA. Influence of the 5-HT2C receptor antagonist, SB-242084, in tests of anxiety. Pharmacol Biochem Behav. 2002;71:615–625. doi: 10.1016/s0091-3057(01)00713-4. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bos M, Jenck F, Moreau J, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HH, Broekkamp CL, Ruigt GS, Kohler C, Delft AM. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- Massat I, Lerer B, Souery D, Blackwood D, Muir W, Kaneva R, Nothen MM, Oruc L, Papadimitriou GN, Dikeos D, Serretti A, Bellivier F, Golmard JL, Milanova V, Del-Favero J, Van Broeckhoven C, Mendlewicz J. HTR2C (cys23ser) polymorphism influences early onset in bipolar patients in a large European multicenter association study. Mol Psychiatry. 2007;12:797–798. doi: 10.1038/sj.mp.4002018. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Roles of 5-HT3 and opioid receptors in the ethanol-induced place preference in rats exposed to conditioned fear stress. Life Sci. 1999;64:PL241–PL249. doi: 10.1016/s0024-3205(99)00144-7. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther. 2001;298:1101–1107. [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience. 2007;146:306–320. doi: 10.1016/j.neuroscience.2007.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Neurotransmitter synthetic enzymes. Prog Neurobiol. 1973;2:69–117. doi: 10.1016/0301-0082(73)90007-5. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Nazarali AJ, Himeno A, Saavedra JM. Chronic treatment with (+/−)DOI, a psychotomimetic 5-HT2 agonist, downregulates 5-HT2 receptors in rat brain. Neuropsychopharmacology. 1989;2:81–87. doi: 10.1016/0893-133x(89)90010-9. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Melke J, Westberg L, Nilsson S, Landen M, Soderstrom H, Baghaei F, Rosmond R, Holm G, Bjorntorp P, Nilsson LG, Adolfsson R, Eriksson E. A polymorphism in the serotonin receptor 3A (HTR3A) gene and its association with harm avoidance in women. Arch Gen Psychiatry. 2003;60:1017–1023. doi: 10.1001/archpsyc.60.10.1017. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Menard J, Treit D. Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev. 1999;23:591–613. doi: 10.1016/s0149-7634(98)00056-6. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Kapur S, Houle S, DaSilva J, Owczarek B, Brown GM, Wilson AA, Kennedy SH. Prefrontal cortex 5-HT2 receptors in depression: an [18F]setoperone PET imaging study. Am J Psychiatry. 1999;156:1029–1034. doi: 10.1176/ajp.156.7.1029. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Veiga S, Girardon S, Brocco M. Blockade of serotonin 5-HT1B and 5-HT2A receptors suppresses the induction of locomotor activity by 5-HT reuptake inhibitors, citalopram and fluvoxamine, in NMRI mice exposed to a novel environment: a comparison to other 5-HT receptor subtypes. Psychopharmacology (Berl) 2003;168:397–409. doi: 10.1007/s00213-003-1389-y. [DOI] [PubMed] [Google Scholar]
- Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther. 2005;108:320–333. doi: 10.1016/j.pharmthera.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. Am J Physiol. 1998;274:G827–G831. doi: 10.1152/ajpgi.1998.274.5.G827. [DOI] [PubMed] [Google Scholar]
- Miyata K, Ito H, Yamano M, Hidaka K, Kamato T, Nishida A, Yuki H. Comparison of the effects of trimebutine and YM114 (KAE-393), a novel 5-HT3 receptor antagonist, on stress-induced defecation. Eur J Pharmacol. 1993;250:303–310. doi: 10.1016/0014-2999(93)90395-x. [DOI] [PubMed] [Google Scholar]
- Miyata S, Yamada N, Hirano S, Tanaka S, Kamei J. Diabetes attenuates psychological stress-elicited 5-HT secretion in the prefrontal cortex but not in the amygdala of mice. Brain Res. 2007;1147:233–239. doi: 10.1016/j.brainres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Torres OI, Galler JR, Morgane PJ. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally malnourished rats. Brain Res. 2007;1148:226–233. doi: 10.1016/j.brainres.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineaux SM, Jessell TM, Axel R, Julius D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci USA. 1989;86:6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2003;86:210–219. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Mora PO, Netto CF, Graeff FG. Role of 5-HT2A and 5-HT2C receptor subtypes in the two types of fear generated by the elevated T-maze. Pharmacol Biochem Behav. 1997;58:1051–1057. doi: 10.1016/s0091-3057(97)00057-9. [DOI] [PubMed] [Google Scholar]
- Morales M, Bloom FE. The 5-HT3 receptor is present in different sub-populations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- Moret C, Briley M. 5-HT autoreceptors in the regulation of 5-HT release from guinea pig raphe nucleus and hypothalamus. Neuropharmacology. 1997;36:1713–1723. doi: 10.1016/s0028-3908(97)00145-7. [DOI] [PubMed] [Google Scholar]
- Moret C, Briley M. The possible role of 5-HT(1B/D) receptors in psychiatric disorders and their potential as a target for therapy. Eur J Pharmacol. 2000;404:1–12. doi: 10.1016/s0014-2999(00)00581-1. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol Pharmacol. 2000;58:1271–1278. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Ciaranello RD. Ontogeny of 5-hydroxytryptamine2 receptor immunoreactivity in the developing rat brain. Neuroscience. 1993;55:869–880. doi: 10.1016/0306-4522(93)90447-n. [DOI] [PubMed] [Google Scholar]
- Mossner R, Schmitt A, Hennig T, Benninghoff J, Gerlach M, Riederer P, Deckert J, Lesch KP. Quantitation of 5HT3 receptors in forebrain of serotonin transporter deficient mice. J Neural Transm. 2004;111:27–35. doi: 10.1007/s00702-003-0074-y. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.08.016. PMID: 17949693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Mueller EA, Garrick NA, Aulakh CS. Use of serotonergic agents in the clinical assessment of central serotonin function. J Clin Psychiatry. 1986;47(Suppl):9–15. [PubMed] [Google Scholar]
- Murphy DL, Uhl GR, Holmes A, Ren-Patterson R, Hall FS, Sora I, Detera-Wadleigh S, Lesch KP. Experimental gene interaction studies with SERT mutant mice as models for human polygenic and epistatic traits and disorders. Genes Brain Behav. 2003;2:350–364. doi: 10.1046/j.1601-1848.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA. Lysergic acid diethylamide and [—]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 2004;1023:134–140. doi: 10.1016/j.brainres.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Ishima T, Takashima T. The 5-HT3 receptor agonist attenuates the action of antidepressants in the forced swim test in rats. Brain Res. 1998;786:189–193. doi: 10.1016/s0006-8993(97)01459-5. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Edwards E, Plotsky PM. 5-HT(1B) mrna regulation in two animal models of altered stress reactivity. Biol Psychiatry. 2002;51:902–908. doi: 10.1016/s0006-3223(01)01371-3. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Young T, Stastny J. Implications of genetic research on the role of the serotonin in depression: emphasis on the serotonin type 1A receptor and the serotonin transporter. Psychopharmacology (Berl) 2004a;174:512–524. doi: 10.1007/s00213-004-1950-3. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004b;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Hascoet M, Jolliet P, Bourin M. Evidence for a 5-HT2A receptor mode of action in the anxiolytic-like properties of DOI in mice. Behav Brain Res. 2003;147:175–184. doi: 10.1016/s0166-4328(03)00179-7. [DOI] [PubMed] [Google Scholar]
- Niesler B, Flohr T, Nothen MM, Fischer C, Rietschel M, Franzek E, Albus M, Propping P, Rappold GA. Association between the 5′ UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics. 2001;11:471–475. doi: 10.1097/00008571-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Nonaka KO. Involvement of 5-HT3 receptors in the prolactin release induced by immobilization stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:497–503. doi: 10.1016/s0278-5846(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Norton N, Owen MJ. HTR2A: association and expression studies in neuropsychiatric genetics. Ann Med. 2005;37:121–129. doi: 10.1080/07853890510037347. [DOI] [PubMed] [Google Scholar]
- Olivier B, van Oorschot R. 5-HT1B receptors and aggression: a review. Eur J Pharmacol. 2005;526:207–217. doi: 10.1016/j.ejphar.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, Goldman D, Arango V, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 2007;64:201–208. doi: 10.1001/archpsyc.64.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowska G, Nowa G, Kata R, Klenk-Majewska B, Danilczuk Z, Zebrowska-Lupina I. Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm. 2001;108:311–319. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Pan L, Gilbert F. Activation of 5-HT1A receptor subtype in the paraventricular nuclei of the hypothalamus induces CRH and ACTH release in the rat. Neuroendocrinology. 1992;56:797–802. doi: 10.1159/000126332. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:52–58. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- Pattij T, Groenink L, Oosting RS, van der Gugten J, Maes RA, Olivier B. GABA(A)-benzodiazepine receptor complex sensitivity in 5-HT(1A) receptor knockout mice on a 129/Sv background. Eur J Pharmacol. 2002a;447:67–74. doi: 10.1016/s0014-2999(02)01893-9. [DOI] [PubMed] [Google Scholar]
- Pattij T, Groenink L, Hijzen TH, Oosting RS, Maes RA, van der Gugten J, Olivier B. Autonomic changes associated with enhanced anxiety in 5-HT(1A) receptor knockout mice. Neuropsychopharmacology. 2002b;27:380–390. doi: 10.1016/S0893-133X(02)00317-2. [DOI] [PubMed] [Google Scholar]
- Pattij T, Broersen LM, van der Linde J, Groenink L, van der Gugten J, Maes RA, Olivier B. Operant learning and differential-reinforcement-of-low-rate 36-s responding in 5-HT1A and 5-HT1B receptor knockout mice. Behav Brain Res. 2003;141:137–145. doi: 10.1016/s0166-4328(02)00345-5. [DOI] [PubMed] [Google Scholar]
- Pattij T, Hijzen TH, Groenink L, Oosting RS, van der Gugten J, Maes RA, Hen R, Olivier B. Stress-induced hyperthermia in the 5-HT(1A) receptor knockout mouse is normal. Biol Psychiatry. 2001;49:569–574. doi: 10.1016/s0006-3223(00)01022-2. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Pazos A, Engel G, Palacios JM. beta-Adrenoceptor blocking agents recognize a subpopulation of serotonin receptors in brain. Brain Res. 1985;343:403–408. doi: 10.1016/0006-8993(85)90766-8. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31:265–277. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Petty F, Kramer G, Wilson L, Jordan S. In vivo serotonin release and learned helplessness. Psychiatry Res. 1994;52:285–293. doi: 10.1016/0165-1781(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Popa D, El Yacoubi M, Vaugeois JM, Hamon M, Adrien J. Homeostatic regulation of sleep in a genetic model of depression in the mouse: effects of muscarinic and 5-HT1A receptor activation. Neuropsychopharmacology. 2006;31:1637–1646. doi: 10.1038/sj.npp.1300948. [DOI] [PubMed] [Google Scholar]
- Popova NK, Vishnivetskaya GB, Ivanova EA, Skrinskaya JA, Seif I. Altered behavior and alcohol tolerance in transgenic mice lacking MAO A: a comparison with effects of MAO A inhibitor clorgyline. Pharmacol Biochem Behav. 2000;67:719–727. doi: 10.1016/s0091-3057(00)00417-2. [DOI] [PubMed] [Google Scholar]
- Popova NK, Maslova LN, Morosova EA, Bulygina VV, Seif I. MAO A knockout attenuates adrenocortical response to various kinds of stress. Psychoneuroendocrinology. 2006;31:179–186. doi: 10.1016/j.psyneuen.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Preece MA, Dalley JW, Theobald DE, Robbins TW, Reynolds GP. Region specific changes in forebrain 5-hydroxytryptamine1A and 5-hydroxytryptamine2A receptors in isolation-reared rats: an in vitro autoradiography study. Neuroscience. 2004;123:725–732. doi: 10.1016/j.neuroscience.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl) 2002;162:406–414. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Puig MV, Celada P, Diaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Saudou F, Amara DA, Belzung C, Dierich A, LeMeur M, Segu L, Misslin R, Buhot MC, Hen R. Behavioral characterization of mice packing the 5-HT1B receptor. NIDA Res Monogr. 1996;161:39–57. [PubMed] [Google Scholar]
- Raurich A, Mengod G, Artigas F, Cortes R. Displacement of the binding of 5-HT(1A) receptor ligands to pre- and postsynaptic receptors by (—)pindolol. A comparative study in rodent, primate and human brain. Synapse. 1999;34:68–76. doi: 10.1002/(SICI)1098-2396(199910)34:1<68::AID-SYN8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M. Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol. 1997;325:129–135. doi: 10.1016/s0014-2999(97)00115-5. [DOI] [PubMed] [Google Scholar]
- Rees M, Norton N, Jones I, McCandless F, Scourfield J, Holmans P, Moorhead S, Feldman E, Sadler S, Cole T, Redman K, Farmer A, McGuffin P, Owen MJ, Craddock N. Association studies of bipolar disorder at the human serotonin transporter gene (hSERT; 5HTT) Mol Psychiatry. 1997;2:398–402. doi: 10.1038/sj.mp.4000256. [DOI] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Lesch KP, Lu B, Murphy DL. Gender-dependent modulation of brain monoamines and anxiety-like behaviors in mice with genetic serotonin transporter and BDNF deficiencies. Cell Mol Neurobiol. 2006;26:755–780. doi: 10.1007/s10571-006-9048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Sherrill S, Huang SJ, Tolliver T, Lesch KP, Lu B, Murphy DL. Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. J Neurosci Res. 2005;79:756–771. doi: 10.1002/jnr.20410. [DOI] [PubMed] [Google Scholar]
- Renard CE, Dailly E, David DJ, Hascoet M, Bourin M. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam Clin Pharmacol. 2003;17:449–455. doi: 10.1046/j.1472-8206.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Fink H. Anxiety but not arousal increases 5-hydroxytryptamine release in the rat ventral hippocampus in vivo. Eur J Neurosci. 2005;22:1185–1189. doi: 10.1111/j.1460-9568.2005.04251.x. [DOI] [PubMed] [Google Scholar]
- Ripoll N, Hascoet M, Bourin M. Implication of 5-HT2A subtype receptors in DOI activity in the four-plates test-retest paradigm in mice. Behav Brain Res. 2006;166:131–139. doi: 10.1016/j.bbr.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, Bakkum EA, O'Connor PA, Carnes M, Bethea CL, van de Kar LD. Comparison of neuroendocrine and behavioral effects of ipsapirone, a 5-HT1A agonist, in three stress paradigms: immobilization, forced swim and conditioned fear. Brain Res. 1992;580:205–214. doi: 10.1016/0006-8993(92)90946-7. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, Lucki I, Malberg JE, Grauer S, Brennan J, Cryan JF, Sukoff Rizzo SJ, Dunlop J, Barrett JE, Marquis KL. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology (Berl) 2007;192:159–170. doi: 10.1007/s00213-007-0710-6. [DOI] [PubMed] [Google Scholar]
- Roth BL, Willins DL, Kristiansen K, Kroeze WK. 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): where structure meets function. Pharmacol Ther. 1998;79:231–257. doi: 10.1016/s0163-7258(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 1996;739:57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Rusnak M, Kvetnansky R, Jelokova J, Palkovits M. Effect of novel stressors on gene expression of tyrosine hydroxylase and monoamine transporters in brainstem noradrenergic neurons of long-term repeatedly immobilized rats. Brain Res. 2001;899:20–35. doi: 10.1016/s0006-8993(01)02126-6. [DOI] [PubMed] [Google Scholar]
- Salchner P, Singewald N. Neuroanatomical substrates involved in the anxiogenic-like effect of acute fluoxetine treatment. Neuropharmacology. 2002;43:1238–1248. doi: 10.1016/s0028-3908(02)00329-5. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Fentress H, Hazelwood L. Serotonin 5-ht2 receptors: molecular and genomic diversity. Mol Interv. 2003;3:319–330. doi: 10.1124/mi.3.6.319. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Santos JM, Martinez RC, Brandao ML. Effects of acute and subchronic treatments with fluoxetine and desipramine on the memory of fear in moderate and high-intensity contextual conditioning. Eur J Pharmacol. 2006;542:121–128. doi: 10.1016/j.ejphar.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Schiller L, Jahkel M, Kretzschmar M, Brust P, Oehler J. Autoradiographic analyses of 5-HT1A and 5-HT2A receptors after social isolation in mice. Brain Res. 2003;980:169–178. doi: 10.1016/s0006-8993(03)02832-4. [DOI] [PubMed] [Google Scholar]
- Schmauss C. Serotonin 2C receptors: suicide, serotonin, and runaway RNA editing. Neuroscientist. 2003;9:237–242. doi: 10.1177/1073858403253669. [DOI] [PubMed] [Google Scholar]
- Schreiber R, De Vry J. Neuronal circuits involved in the anxiolytic effects of the 5-HT1A receptor agonists 8-OH-DPAT ipsapirone and buspirone in the rat. Eur J Pharmacol. 1993;249:341–351. doi: 10.1016/0014-2999(93)90531-l. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Melon C, De Vry J. The role of 5-HT receptor subtypes in the anxiolytic effects of selective serotonin reuptake inhibitors in the rat ultrasonic vocalization test. Psychopharmacology (Berl) 1998;135:383–391. doi: 10.1007/s002130050526. [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett. 2003;346:137–140. doi: 10.1016/s0304-3940(03)00547-0. [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci. 2000;20:8846–8852. doi: 10.1523/JNEUROSCI.20-23-08846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME. Learned helplessness. Annu Rev Med. 1972;23:407–412. doi: 10.1146/annurev.me.23.020172.002203. [DOI] [PubMed] [Google Scholar]
- Serretti A, Drago A, De Ronchi D. HTR2A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies. Curr Med Chem. 2007a;14:2053–2069. doi: 10.2174/092986707781368450. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007b;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- Sharma A, Punhani T, Fone KC. Distribution of the 5-hydroxytryptamine2C receptor protein in adult rat brain and spinal cord determined using a receptor-directed antibody: effect of 5,7-dihydroxytryptamine. Synapse. 1997;27:45–56. doi: 10.1002/(SICI)1098-2396(199709)27:1<45::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman LJ, Raley J, Queree P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Dukat M, Allan AM. Effect of 5-HT3 receptor over-expression on the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2004;28:1161–1171. doi: 10.1097/01.alc.0000138687.27452.e2. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the Serotonin(1A) receptor in mice results in downregulation of major GABA(A) receptor alpha subunits, reduction of GABA(A) receptor binding, and benzodiazepine-resistant anxiety. J Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Su J, Leman S, Le Guisquet AM, Ibarguen-Vargas Y, Joeyen-Waldorf J, Glorioso C, Tseng GC, Pezzone M, Hen R, Belzung C. Lack of serotonin(1B) receptor expression leads to age-related motor dysfunction, early onset of brain molecular aging and reduced longevity. Mol Psychiatry. 2007;12:1042–1056. doi: 10.1038/sj.mp.4001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RC, Brandao ML. Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: an ethological analysis. Pharmacol Biochem Behav. 2000;65:209–216. doi: 10.1016/s0091-3057(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Silva RC, Cruz AP, Avanzi V, Landeira-Fernandez J, Brandao ML. Distinct contributions of median raphe nucleus to contextual fear conditioning and fear-potentiated startle. Neural Plast. 2002;9:233–247. doi: 10.1155/NP.2002.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smriga M, Torii K. L-Lysine acts like a partial serotonin receptor 4 antagonist and inhibits serotonin-mediated intestinal pathologies and anxiety in rats. Proc Natl Acad Sci USA. 2003;100:15370–15375. doi: 10.1073/pnas.2436556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, Burnet PW, Makoff AJ, Kerwin RW, Harrison PJ. RNA editing of the 5-HT(2C) receptor is reduced in schizophrenia. Mol Psychiatry. 2001;6:373–379. doi: 10.1038/sj.mp.4000920. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M. Direct Immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci. 1990;2:1144–1154. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. (—)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur J Pharmacol. 1986;128:295–298. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- Stein C, Davidowa H, Albrecht D. 5-HT(1A) receptor-mediated inhibition and 5-HT(2) as well as 5-HT(3) receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse. 2000;38:328–337. doi: 10.1002/1098-2396(20001201)38:3<328::AID-SYN12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Hemmings S, Moolman-Smook H, Audenaert K. 5-HT2A: its role in frontally mediated executive function and related psychopathology. CNS Spectr. 2007;12:512–516. doi: 10.1017/s1092852900021246. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Storey JD, Robertson DA, Beattie JE, Reid IC, Mitchell SN, Balfour DJ. Behavioural and neurochemical responses evoked by repeated exposure to an elevated open platform. Behav Brain Res. 2006;166:220–229. doi: 10.1016/j.bbr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19:RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Oquendo MA, Simpson N, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin1A receptor binding in major depression is related to psychic and somatic anxiety. Biol Psychiatry. 2005;58:947–954. doi: 10.1016/j.biopsych.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Qi H, Carruthers R, Witkin JM, Nomikos GG, Greengard P. Biochemical and behavioral evidence for antidepressant-like effects of 5-HT6 receptor stimulation. J Neurosci. 2007;27:4201–4209. doi: 10.1523/JNEUROSCI.3110-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Takada Y, Nagai N, Urano T, Takada A. Previous exposure to footshock stress attenuates nicotine-induced serotonin release in rat striatum during the subsequent stress. Brain Res Bull. 2000;52:285–290. doi: 10.1016/s0361-9230(00)00267-7. [DOI] [PubMed] [Google Scholar]
- Takao K, Nagatani T, Kitamura Y, Yamawaki S. Effects of corticosterone on 5-HT1A and 5-HT2 receptor binding and on the receptor-mediated behavioral responses of rats. Eur J Pharmacol. 1997;333:123–128. doi: 10.1016/s0014-2999(97)01126-6. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI, Baratta M, Bland ST, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Inescapable shock activates serotonergic neurons in all raphe nuclei of rat. Behav Brain Res. 2004;153:233–239. doi: 10.1016/j.bbr.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Tanaka E, North RA. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol. 1993;69:1749–1757. doi: 10.1152/jn.1993.69.5.1749. [DOI] [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biol Psychiatry. 2007;62:917–924. doi: 10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci USA. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Chu HM, Brennan TJ. Neurobehavioral analysis of 5-HT6 receptor null mutant mice. Proceedings of the Fourth IUPHAR Satellite Meeting on Serotonin; Rotterdam. 1998. [Google Scholar]
- Temel Y, Boothman LJ, Blokland A, Magill PJ, Steinbusch HW, Visser-Vandewalle V, Sharp T. Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci USA. 2007;104:17087–17092. doi: 10.1073/pnas.0704144104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Soffin EM, Roberts C, Kew JN, de la Flor RM, Dawson LA, Fry VA, Coggon SA, Faedo S, Hayes PD, Corbett DF, Davies CH, Hagan JJ. SB-699551-A (3-cyclopentyl-N-[2-(dimethylamino)ethyl]-N-[(4′-{[(2-phenylethyl)amino]methyl}-4-biphenylyl)methyl]propanamide dihydrochloride), a novel 5-ht5A receptor-selective antagonist, enhances 5-HT neuronal function: Evidence for an autoreceptor role for the 5-ht5A receptor in guinea pig brain. Neuropharmacology. 2006;51:566–577. doi: 10.1016/j.neuropharm.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Thorre K, Chaouloff F, Sarre S, Meeusen R, Ebinger G, Michotte Y. Differential effects of restraint stress on hippocampal 5-HT metabolism and extracellular levels of 5-HT in streptozotocin-diabetic rats. Brain Res. 1997;772:209–216. doi: 10.1016/s0006-8993(97)00841-x. [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Armando I, Saavedra JM, Li Q, Murphy DL. Life-long serotonin reuptake deficiency results in complex alterations in adrenomedullary responses to stress. Ann NY Acad Sci. 2004;1018:99–104. doi: 10.1196/annals.1296.011. [DOI] [PubMed] [Google Scholar]
- Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Trillat AC, Malagie I, Scearce K, Pons D, Anmella MC, Jacquot C, Hen R, Gardier AM. Regulation of serotonin release in the frontal cortex and ventral hippocampus of homozygous mice lacking 5-HT1B receptors: in vivo microdialysis studies. J Neurochem. 1997;69:2019–2025. doi: 10.1046/j.1471-4159.1997.69052019.x. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TJ, Mokler DJ, Luebke JI. Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies. Neuroscience. 2004;129:703–718. doi: 10.1016/j.neuroscience.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2007;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang Y, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez DM, Lopez JF, Van Hoers H, Watson SJ, Levine S. Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res. 2000;855:76–82. doi: 10.1016/s0006-8993(99)02307-0. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Eskandari R, Zimmer CA, Levine S, Lopez JF. Brain 5-HT receptor system in the stressed infant rat: implications for vulnerability to substance abuse. Psychoneuroendocrinology. 2002;27:245–272. doi: 10.1016/s0306-4530(01)00048-8. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, Harley J, Kuhar MJ. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140:355–365. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Cheng LL, Gean PW. Cross-modulation of synaptic plasticity by beta-adrenergic and 5-HT1A receptors in the rat basolateral amygdala. J Neurosci. 1999;19:570–577. doi: 10.1523/JNEUROSCI.19-02-00570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward HE, Johnson EA, Goodman IJ, Birkle DL, Cottrell DJ, Azzaro AJ. Corticotropin-releasing factor and defensive withdrawal: inhibition of monoamine oxidase prevents habituation to chronic stress. Pharmacol Biochem Behav. 1998;60:209–215. doi: 10.1016/s0091-3057(97)00580-7. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garret JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Deguzman TB, McMahon F, Rudnick G, Detera-Wadleigh SD, Murphy DL. SERT Ileu425Val in autism, Asperger syndrome and obsessive-compulsive disorder. Psychiatr Genet. 2008;18:31–39. doi: 10.1097/YPG.0b013e3282f08a06. [DOI] [PubMed] [Google Scholar]
- Wesolowska A. The anxiolytic-like effect of the selective 5-HT(6) receptor antagonist SB-399885: The impact of benzodiazepine receptors. Eur J Pharmacol. 2008;580:355–360. doi: 10.1016/j.ejphar.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A. Effects of the brain-penetrant and selective 5-HT6 receptor antagonist SB-399885 in animal models of anxiety and depression. Neuropharmacology. 2007;52:1274–1283. doi: 10.1016/j.neuropharm.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A. The selective 5-HT(6) receptor antagonist SB-399885 enhances anti-immobility action of antidepressants in rats. Eur J Pharmacol. 2008;582:88–93. doi: 10.1016/j.ejphar.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006;51:578–586. doi: 10.1016/j.neuropharm.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Wurts SW, Hall FS, Lesch KP, Murphy DL, Uhl GR, Edgar DM. Altered rapid eye movement sleep timing in serotonin transporter knockout mice. Neuroreport. 2003;14:233–238. doi: 10.1097/00001756-200302100-00015. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Marsden CA, Fone KC. 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Xu T, Pandey SC. Cellular localization of serotonin(2A) (5HT(2A)) receptors in the rat brain. Brain Res Bull. 2000;51:499–505. doi: 10.1016/s0361-9230(99)00278-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto O, Niida H, Tajima K, Shirouchi Y, Masui Y, Ueda F, Kise M, Kimura K. Inhibition of stress-stimulated colonic propulsion by alpha 2-adrenoceptor antagonists in rats. Neurogastroenterol Motil. 1998;10:523–532. doi: 10.1046/j.1365-2982.1998.00127.x. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, Zis AP, Ruth TJ. Brain serotonin2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiatry. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Seckl JR. Acute restraint stress increases 5-HT7 receptor mRNA expression in the rat hippocampus. Neurosci Lett. 2001;309:141–144. doi: 10.1016/s0304-3940(01)02054-7. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Matsumoto M, Togashi H, Saito H. Effects of conditioned fear stress on 5-HT release in the rat prefrontal cortex. Pharmacol Biochem Behav. 1995;51:515–519. doi: 10.1016/0091-3057(95)00045-x. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Wang FH, Kuteeva E, Holmberg K, Yamaguchi M, Crawley JN, Steiner R, Bartfai T, Ogren SO, Hokfelt T, Kehr J. Enhanced hippocampal noradrenaline and serotonin release in galanin-overexpressing mice after repeated forced swimming test. Proc Natl Acad Sci USA. 2004;101:354–359. doi: 10.1073/pnas.0307042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Junior, Graeff FG. Behavioral effects of intra-amygdala injections of GABA and 5-HT acting drugs in the elevated plus-maze. Braz J Med Biol Res. 1994;27:2453–2456. [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Gainetdinov RR, Caron MG. Functional polymorphisms of the brain serotonin synthesizing enzyme tryptophan hydroxylase-2. Cell Mol Life Sci. 2006;63:6–11. doi: 10.1007/s00018-005-5417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein RA, Jaing C, Murphy DL, Lanthorn TH, Holmes A. Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience. 2006a;140:321–334. doi: 10.1016/j.neuroscience.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RWt, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006b;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology. 1999;21:52S–60S. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]
- Ziabreva I, Poeggel G, Schnabel R, Braun K. Separation-induced receptor changes in the hippocampus and amygdala of Octodon degus: influence of maternal vocalizations. J Neurosci. 2003;23:5329–5336. doi: 10.1523/JNEUROSCI.23-12-05329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, Moller HJ, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
- Zucker M, Weizman A, Rehavi M. Repeated swim stress leads to downregulation of vesicular monoamine transporter 2 in rat brain nucleus accumbens and striatum. Eur Neuropsychopharmacol. 2005;15:199–201. doi: 10.1016/j.euroneuro.2004.08.009. [DOI] [PubMed] [Google Scholar]


