Abstract
Background
The glutamate system plays a major role in mediating EtOH’s effects on brain and behavior, and is implicated in the pathophysiology of alcohol-related disorders. N-methyl-D-aspartate receptor (NMDAR) antagonists such as MK-801 (dizocilpine) interact with EtOH at the behavioral level, but the molecular basis of this interaction is unclear.
Methods
We first characterized the effects of MK-801 treatment on responses to the ataxic (accelerating rotarod), hypothermic and sedative/hypnotic effects of acute EtOH administration in C57BL/6J and 129/SvImJ inbred mice. Effects of another NMDAR antagonist, phencyclidine, on EtOH-induced sedation/hypnosis were also assessed. Gene knockout of the NMDAR subunit NR2A or L-alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate GluR1 or pharmacological antagonism of the NMDAR subunit NR2B (via Ro 25-6981) was employed to examine whether inactivating any one of these glutamate signaling molecules modified MK-801’s effect on EtOH-related behaviors.
Results
MK-801 markedly potentiated the ataxic effects of 1.75 g/kg EtOH and the sedative/hypnotic effects of 3.0 g/kg EtOH, but not the hypothermic effects of 3.0 g/kg EtOH, in C57BL/6J and 129/SvImJ mice. Phencyclidine potentiated EtOH-induced sedation/hypnosis in both inbred strains. Neither NR2A nor GluR1 KO significantly altered basal EtOH-induced ataxia, hypothermia, or sedation/hypnosis. Ro 25-6981 modestly increased EtOH-induced sedation/hypnosis. The ability of MK-801 to potentiate EtOH-induced ataxia and sedation/hypnosis was unaffected by GluR1 KO or NR2B antagonism. NR2A KO partially reduced MK-801 + EtOH-induced sedation/hypnosis, but not ataxia or hypothermia.
Conclusions
Data confirm a robust and response-specific potentiating effect of MK-801 on sensitivity to EtOH’s intoxicating effects. Inactivation of three major components of glutamate signaling had no or only partial impact on the ability of MK-801 to potentiate behavioral sensitivity to EtOH. Further work to elucidate the mechanisms underlying NMDAR × EtOH interactions could ultimately provide novel insight into the role of NMDARs in alcoholism and its treatment.
Keywords: Alcohol, Glutamate, NMDA, AMPA, Mouse, MK-801, Ethanol, Rotarod, Hypothermia, Ataxia, Sedation
Ethanol (ETOH) has potent effects on manifold behaviors. These effects are mediated by complex changes in neural function that are not yet clear. A growing corpus of data points to a major role for the glutamate system in mediating EtOH’s effects on brain and behavior, and implicates glutamate dysfunction in the pathophysiology of alcohol- related disorders.
In vitro, EtOH acts as an allosteric inhibitor of the N-methyl-D-aspartate (NMDAR) class of ionotropic glutamate receptors at doses that induce behavioral intoxication (Lovinger et al., 1989;Masood et al., 1994). Chronic exposure to EtOH in vitro and in vivo leads to up-regulation of NMDAR (Carpenter-Hyland et al., 2004; Crabbe et al., 1991; Kumari and Ticku, 2000; Roberto et al., 2006; Smothers et al., 1997). Behaviorally, uncompetitive NMDAR antagonists such as ( + )-5-methyl-10,11-dihydro-SH-dibenzo[a,d]cyclohepten- 5,10-imine maleate (MK-801, dizocilpine) and ketamine substitute for relatively high doses of EtOH in rodent and monkey drug discrimination tests and mimic the subjective feeling of EtOH intoxication in humans (Grant, 1999; Hodge et al., 2006; Krystal et al., 2003; Rassnick et al., 1992). Furthermore, acute administration of NMDAR antagonists decreases EtOH self-administration and reward-related responses to EtOH in rats and mice (Boyce-Rustay and Cunningham, 2004; Krystal et al., 2003; McMillen et al., 2004; Rassnick et al., 1992). On the other hand, NMDAR antagonist treatment potentiates the acute depressant (sedative/hypnotic) effects of EtOH in rodents (Beleslin et al., 1997; Boyce-Rustay and Holmes, 2005; Silveri and Spear, 2002; Wilson et al., 1990). This latter finding is congruent with evidence from some but not all studies that the Long Sleep line of mice selected for relatively high sensitivity to EtOH’s depressant effects exhibit low MK-801-binding, diminished locomotor stimulant effects to MK-801 and attenuated NMDAR-mediated responses in hippocampus and striatum, as compared to Short Sleep mice (Daniell and Phillips, 1994; Hanania and Zahniser, 2002; Hanania et al., 2000, 2002; Musleh et al., 1996; Velardo et al., 1998; Wilson and Collins, 1996; Zahniser et al., 1999).
The molecular basis of these complex interactions between the NMDAR and EtOH is still not well understood. NMDAR are heteromeric complexes composed of an obligatory NR1 subunit together with NR2 (NR2A-D) and sometimes NR3 subunits (Schorge and Colquhoun, 2003). NMDAR subunit composition affects sensitivity to EtOH in vitro. Mutagenesis of NR1 can bidirectionally alter sensitivity to EtOH (Jin and Woodward, 2006; Ronald et al., 2001). NR2A- and NR2B-containing NMDAR are more sensitive to EtOH than NR2C- or NR2D-containing NMDAR (Masood et al., 1994; Mirshahi and Woodward, 1995). Previous studies have shown that NR2B-selective antagonists such as ifenprodil and Ro 25-6981 potentiate the depressant effects of EtOH in mice (Boyce-Rustay and Holmes, 2005; Malinowska et al., 1999; Yaka et al., 2003), while NR2A knockout (KO) mice exhibit normal acute responses to EtOH (Boyce-Rustay and Holmes, 2006a; Sato et al., 2006). Beyond these data, little is known about the relative contribution of specific NMDAR subunits to EtOH’s behavioral effects and interactions between NMDAR antagonists and EtOH on behavior (Woodward, 1999).
NMDAR signaling is closely linked to the L-alphaamino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) GluR1 receptor subtype, particularly in the context of NMDAR-mediated neuroadaptation to (psychostimulant) drugs of abuse (Chao and Nestler, 2004; Wolf et al., 2004). Moreover, EtOH inhibits AMPA-related currents in cultured rat cortical and medial septal neurons (Fischer et al., 2003; Frye and Fincher, 2000) [although AMPA receptors appear to be less sensitive to EtOH than NMDARs (Costa et al., 2000; Lovinger et al., 1989)]. Recent studies have shown that KO mice lacking GluR1 display normal basal responses to EtOH (Cowen et al., 2003) but fail to respond to the locomotor hyperactivity-inducing effects of MK-801 (Wiedholz et al., 2008). The potential role of GluR1 in mediating MK-801 effects on EtOH intoxication has not been studied.
The aim of the present study was to further characterize the behavioral, genetic and molecular basis of NMDAR × EtOH interactions in vivo. We first characterized the effects of the uncompetitive NMDAR antagonist MK-801 on multiple measures of acute EtOH treatment (accelerating rotarod ataxia, hypothermia and sedation/hypnosis) in two genetically distinct inbred strains, C57BL/6J and 129/SvImJ (Payseur and Place, 2007). For comparison, the effects of another compound with NMDAR antagonist properties, phencyclidine, was also assessed. Next we tested whether functional inactivation of the NMDAR NR2A, NR2B or AMPA GluR1 subunits would alter MK-801’s effects on EtOH behaviors by testing whether these effects were altered following gene deletion of the NR2A or GluR1, or pharmacological blockade of NR2B. Results demonstrate that NMDA antagonism strongly potentiates acute sensitivity to EtOH in a behavior-specific manner, but does so in a manner that is either unaffected or, in one case, partially attenuated by loss of function of any one of three of the major glutamate receptor subunits.
MATERIAL AND METHODS
Subjects
Experiments in non-mutant mice were conducted in C57BL/6J and 129/SvImJ (129S1) mice obtained from The Jackson Laboratory (Bar Harbor, ME). These inbred strains were chosen because they are commonly used as genetic background strains for mutant lines (including the knockout lines tested in the current study) and are “group A” priority strains in the Mouse Phenome Project, an international effort to provide the biomedical research community with phenotypic data on the most commonly used mouse strains (http://www.jax.org/phenome).
NR2A KO mice were generated as previously described (Sakimura et al., 1995) and backcrossed to produce a ~99% congenic C57BL/6J genetic background as confirmed by genome scan (Boyce-Rustay and Holmes, 2006b). GluR1 KO mice were generated as previously described (Zamanillo et al., 1999) and backcrossed to produce a ~75% congenic C57BL/6J genetic background as confirmed by genome scan (Weidholz et al., 2008). To avoid potential phenotypic abnormalities resulting from genotypic differences in maternal behavior and early life environment (Millstein and Holmes, 2007), WT, HET, and KO of mutants KO lines were generated from HET × HET matings. NR2A KO and GluR1 KO were bred at The Jackson Laboratory, shipped to the NIH at ~8 weeks of age, and housed in a temperature and humidity controlled vivarium under a 12 hours light/dark cycle (lights on 06:00 hours). The experimenter remained blind to genotype during experimentation (mice were identified by subcutaneous microchips or ear notches). Males and females were used. All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee and strictly followed the NIH guidelines “Using Animals in Intramural Research.”
General Procedures
Sensitivity to EtOH effects was examined using 3 separate assays: EtOH-induced ataxia, hypothermia, and sedation/hypnosis. Because these assays provide 3 distinct measures of EtOH’s acute behavioral effects that likely recruit overlapping but dissociable neural and genetic mechanisms (Crabbe, 2007; Crabbe et al., 2006), they provide a broad phenotypic assessment of MK-801 × EtOH interactions and are well established in our laboratory (Boyce-Rustay and Holmes, 2006a; Boyce-Rustay et al., 2007). For all experiments (except for the experiment assessingMK-801 effects on loss vs. recovery of the righting reflex, and the experiment testing the effects of phencyclidine), a single cohort of mice was tested on each assay with the assay involving the lowest dose (i.e., ataxia) first, followed by hypothermia and sedation/hypnosis, with an interval of at least 1 week between tests. This regimen has been employed in previous studies (Boyce-Rustay and Holmes, 2006a; Boyce-Rustay et al., 2006) and is not expected to produce long-term tolerance to EtOH effects (Crabbe, 2007).
EtOH-Induced Ataxia
EtOH-induced ataxia was assessed using the accelerating rotarod as previously described (Boyce-Rustay and Holmes, 2005; Rustay et al., 2003b). The apparatus was a Med Associates rotarod typically used for testing rats (model ENV-577). The 7-cm-diameter dowel was covered with 320 grit sandpaper to provide a uniform surface that prevented mice gripping the rubberized dowel. Mice were placed onto the rotarod dowel which was then accelerated at a constant rate of 8 rpm/min up to 40 rpm. The latency to fall to the floor 10.5 cm below was automatically recorded by photocell beams, with a maximum cutoff latency of 5 minutes.
Mice first received 10 consecutive training trials separated by a 30-seconds inter-trial interval. Twenty-four hours later, there was a baseline acclimation trial followed by 2 more baseline trials (average = pre-drug performance). Mice were then injected with MK-801 (doses as described below), then, 30 minutes later, with 1.75 g/kg EtOH. Thirty minutes thereafter, there was 1 acclimation trial followed by 2 test trials (average = post-drug performance). The dependent measure was the difference in pre- versus post-drug performance (=delta latency).
A previous study showed that 0.3 mg/kg MK-801 alone caused ataxia in CD-1 mice (Grant et al., 1992). Therefore, a pilot experiment was conducted in an independent cohort of EtOH-naïve C57BL/6J mice to determine whether treatment with MK-801 alone produced ataxic effects in C57BL/6J at the dose range employed. Mice were trained and tested as described above under general procedures with the exception that they were not injected with EtOH. Results showed that there was a significant increase in latency to fall during training (F9,540 = 21.75, p < 0.01; data not shown), and that during testing, MK-801 treatment produced a significant effect on delta latency to fall (F3,34 = 12.15, p < 0.01, n = 9 to 10/dose). Post hoc tests showed that the highest dose (0.3 mg/kg) of MK-801 produced modest ataxia relative to vehicle, in the absence of EtOH, whereas the lower doses were without effect (0 mg/kg = 4.22 ± 6.11 delta latency to fall in seconds, 0.1 mg/kg = 6.40 ± 6.75, 0.2 mg/kg = 5.33 ± 8.93, 0.3 mg/kg = 52.60 ± 10.84).
EtOH-Induced Hypothermia
EtOH-induced hypothermia was tested as previously described (Boyce-Rustay and Holmes, 2006a). Basal core body temperature was first measured by inserting a Thermalert TH-5 thermometer (Physitemp, Clifton, NJ) 2 cm into the rectum until a stable reading was obtained. Mice were then injected with MK-801 (doses as described below), then, 30 minutes later, with 3.0 g/kg EtOH. Temperature was measured 30, 60, 90, and 120 minutes later to provide an average post-EtOH measure. The difference between pre-EtOH/- post-MK-801 versus post-EtOH temperature was taken as the dependent measure (=delta temperature). Ambient room temperature was 23°C. Pilot observations showed that the dose range of MK-801 employed does not produce hypothermia in the absence of EtOH.
EtOH-Induced Sedation/Hypnosis
EtOH-induced sedation/hypnosis was assessed as previously described (Daws et al., 2006). Mice were then injected with MK-801 (doses detailed below), then, 30 minutes later, with 3.0 g/kg EtOH and placed into the supine position in a “V”-shaped chamber. Sleep time was measured as the time from injection to recovery of the righting reflex (turning onto all 4 paws twice in 30 seconds after initial self-righting). Pilot observations showed that the dose range of MK-801 employed does not produce loss of righting in the absence of EtOH.
MK-801 × EtOH Interactions in C57BL/6J and 129S1 Mice
Using the behavioral procedures described above, we first assessed the effects of treatment with 0, 0.1, 0.2, or 0.3 mg/kg MK-801 on EtOH-induced ataxia, hypothermia and sedation/hypnosis in EtOH-naïve C57BL/6J and 129S1 mice.
MK-801 × EtOH Interactions on Latency to Lose Righting Reflex and Blood EtOH Concentrations at Recovery of the Righting Reflex in C57BL/6J Mice
The results of our first experiment demonstrated that MK-801 significantly potentiated the sedative/hypnotic effects of EtOH in C57BL/6J and 129S1 mice as measured by prolongation of the latency to regain the righting reflex. We next extended our analysis by testing whether MK-801 also affected: (1) the latency to lose the righting reflex, and (2) EtOH metabolism, as measured by blood EtOH concentrations (BECs) at loss and recovery of the righting reflex. EtOH-naïve C57BL/6J mice were pretreated with 0, 0.1, 0.2, or 0.3 mg/kg MK-801 and, 30 minutes later, 3.0 g/kg EtOH and immediately placed into the supine position in “V”-shaped chambers as above. Mice were then gently turned every 2 seconds until they remained supine for 5 seconds ( = “loss of righting reflex”) as previously described (Ponomarev and Crabbe, 2004). Once the righting reflex was lost, a subset of mice was killed via cervical dislocation and rapid decapitation and trunk blood was taken for BEC analysis using the Analox AM1 Alcohol Analyzer (Analox Instruments USA, Inc., Lunenburg, MA). The remaining mice were left until recovery of the righting reflex and killed on awakening for BEC analysis.
MK-801 × EtOH Interactions Following NR2A KO
After characterizing MK-801 × EtOH interactions in non-mutant inbred mice, the next series of experiments sought to identify the molecular components underlying this interaction. We first examined whether MK-801-potentiation of EtOH sensitivity was altered following KO of NR2A. We employed a KO approach to probe the role of NR2A because there are no compounds with good selectivity for NR2A over NR2B (Kash and Winder, 2007; Neyton and Paoletti, 2006). Basal rotarod-ataxic, sedative/hypnotic and hypothermic effects to EtOH per se have been previously shown to be normal in NR2A KO mice (Boyce-Rustay and Holmes, 2005, 2006a). NR2A KO, HET, andWT mice were tested for EtOH-induced ataxia, hypothermia and sedation/hypnosis following pretreatment with 0 or 0.2 mg/kg MK-801. To assess BEC at recovery of the righting reflex, mice were killed for trunk blood collection.
MK-801 × EtOH Interactions Following NR2B Antagonism
While constitutive KO of NR2B is lethal in mice, the pharmacological compound Ro 25-6981 is a highly selective antagonist for NR2B over NR2A (Kash and Winder, 2007; Neyton and Paoletti, 2006). Therefore, we used Ro 25-6981 to test whether NR2B antagonism affected MK-801’s effects on EtOH behaviors. These experiments were conducted in C57BL/6J mice.
We first examined the effects of Ro 25-6981 per se on sensitivity to EtOH. Mice were injected with 0, 1, 3, or 10 mg/kg Ro 25-6981 [doses chosen based on (Boyce-Rustay and Holmes, 2005)] 60 minutes (to mimic the interval employed in the Ro 25-6981 × MK-801 × EtOH interaction experiments below) prior to testing for EtOH-induced ataxia, hypothermia or sedation/hypnosis.
Next we tested the effects of Ro 25-6981 on the MK-801 × EtOH interaction. Mice were injected with 0, 3 or 10 mg/kg Ro 25-6981 30 minutes prior to 0 or 0.2 mg/kg MK-801, and then assessed for EtOH-induced ataxia, hypothermia and sedation/hypnosis.
MK-801 × EtOH Interactions Following GluR1 KO
The final experiment examined the impact of loss of GluR1 on MK-801-potentiation of EtOH sensitivity. There are no GluR1-selective antagonists and so we again employed a KO approach for these experiments. Basal ataxic, sedative/hypnotic and hypothermic effects of EtOH have previously been shown to be normal in GluR1 KO mice (Cowen et al., 2003). GluR1 KO, HET, and WT mice were tested using the same procedure as described for NR2A KO mice.
Drugs
EtOH (200 proof) was prepared in 0.9% saline to produce 20% v/v solutions and injected intraperitoneally (i.p.). EtOH dose was altered by manipulating the volume of injection. MK-801 (( + )-5- methyl-10,11-dihydro-SH-dibenzo[a,d]cyclohepten-5,10-imine maleate) (Sigma, St. Louis, MO) and Ro 25-6981 (R-(R*,S*)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propranolol hydrochloride) (Tocris Cookson, St. Louis, MO) were injected i.p. in a volume of 10 mL/kg body weight.
Statistical Analysis
The effects of drug, genotype, and sex on behavioral responses to EtOH were first analyzed by use of 3-factor analysis of variance (ANOVA). No main effects of sex, or 2-way or 3-way interactions between sex and the other 2 factors were found and data were collapsed across sex. The effects of drug and genotype were analyzed by use of 2-factor ANOVA and Newman–Keuls post hoc tests where appropriate. The effect of trial and genotype during rotarod training was measured by 2-factor ANOVA with repeated measures for trial. The threshold for statistical significance was p < 0.05.
RESULTS
MK-801 × EtOH Interactions in C57BL/6J Mice
EtOH-Induced Ataxia
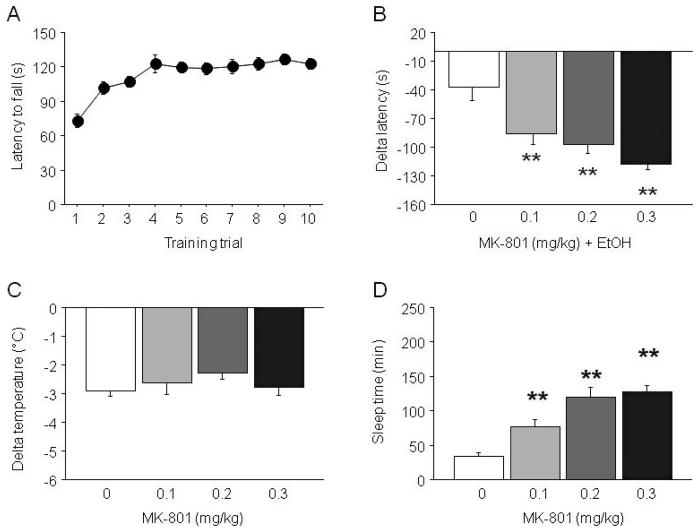
During rotarod training C57BL/6J mice showed significantly longer latencies to fall across trials (F9,387 = 13.16, p < 0.01) (Fig. 1A). Groups subsequently assigned to different MK-801 doses did not differ during training. During testing, drug treatment produced a significant effect on delta latency to fall (F3,29 = 11.59, p < 0.01). Post hoc analysis showed that all doses of MK- 801 pretreated mice exhibited greater ataxia than treatment with EtOH alone (Fig. 1B).
Fig. 1.
Effects of MK-801 on sensitivity to the ataxic, hypothermic and sedative/hypnotic effects of EtOH in C57BL/6J mice. (A) Rotarod learning. (B) MK-801 dose-dependently potentiated EtOH-induced ataxia. (C) MK-801 did not affect EtOH-induced hypothermia. (D) MK-801 dose-dependently potentiated EtOH-induced sedation/hypnosis. n = 7 to 12/dose. **p < 0.01 versus 0. Data in Figs 1–7 are means ± SEM.
EtOH-Induced Hypothermia
MK-801 treatment did not significantly affect the hypothermic effects of EtOH (Fig. 1C).
EtOH-Induced Sedation/Hypnosis
MK-801 treatment significantly affected EtOH-induced sleep time (F3,41=14.28, p < 0.01). Post hoc analysis showed that MK-801 dose-dependently increased sleep time as compared to EtOH alone (Fig. 1D).
MK-801 × EtOH Interactions in 129S1 Mice
EtOH-Induced Ataxia
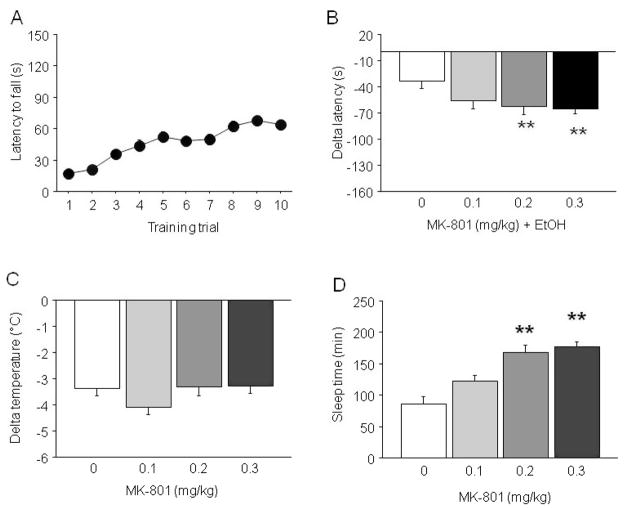
During training 129S1 showed significantly longer latencies across trials (F9,288 = 26.10, p < 0.01) (Fig. 2A), although performance was noticeably poorer than for C57BL/6J. Mice subsequently assigned to different MK-801 doses did not differ in performance during training. Drug treatment produced a significant effect on delta latency to fall (F3,29 = 3.17, p < 0.05). Post hoc analysis showed that 0.2 and 0.3 mg/kg MK-801 produced significantly greater ataxia than treatment with EtOH alone (Fig. 2B).
Fig. 2.
Effects of MK-801 on sensitivity to the ataxic, hypothermic and sedative/hypnotic effects of EtOH in 129S1 mice. (A) Rotarod learning. (B) MK-801 dose-dependently potentiated EtOH-induced ataxia. (C) MK-801 did not affect EtOH-induced hypothermia. (D) MK-801 dose-dependently potentiated EtOH-induced sedation/hypnosis. n = 8 to 11/dose. **p < 0.01 versus 0.
EtOH-Induced Hypothermia
MK-801 did not alter the hypothermic effects of EtOH in 129S1 (Fig. 2C).
EtOH-Induced Sedation/Hypnosis
MK-801 produced a significant effect on EtOH-induced sleep time (F3,37 = 19.07, p < 0.01). Post hoc analysis showed that MK-801 dosedependently increased sleep time as compared to EtOH alone (Fig. 2D).
Phencyclidine × EtOH Interactions in C57BL/6J and 129S1 Mice
We next went on to test another compound with NMDAR antagonist properties, phencyclidine, in C57BL/6J mice. Phencyclidine produced a significant effect on EtOH-induced sleep time (F3,32 = 6.54, p < 0.01). Post hoc analysis showed that 6 and 9 mg/kg phencyclidine increased EtOHinduced sleep time as compared to EtOH alone (vehicle = 36.1 ± 3.8 minutes, 3 mg/kg = 39.2 ± 2.7, 6 mg/kg = 60.2 ± 8.2, 9 mg/kg = 67.8 ± 7.7, n = 9/dose).
Phencyclidine also produced a significant effect on EtOHinduced sleep time in 129S1 mice (F3,42 = 8.13, p < 0.01). Post hoc analysis showed that 6 and 9 mg/kg phencyclidine increased EtOH-induced sleep time as compared to EtOH alone (vehicle = 114.9 ± 11.2 minutes, 3 mg/kg = 110.6 ± 13.0, 6 mg/kg = 168.3 ± 3.8, 9 mg/kg = 147.9 ± 7.1, n = 11 to 12/dose).
MK-801 × EtOH Interactions on Latency to Lose Righting Reflex and Blood EtOH Concentrations at Recovery of the Righting Reflex in C57BL/6J Mice
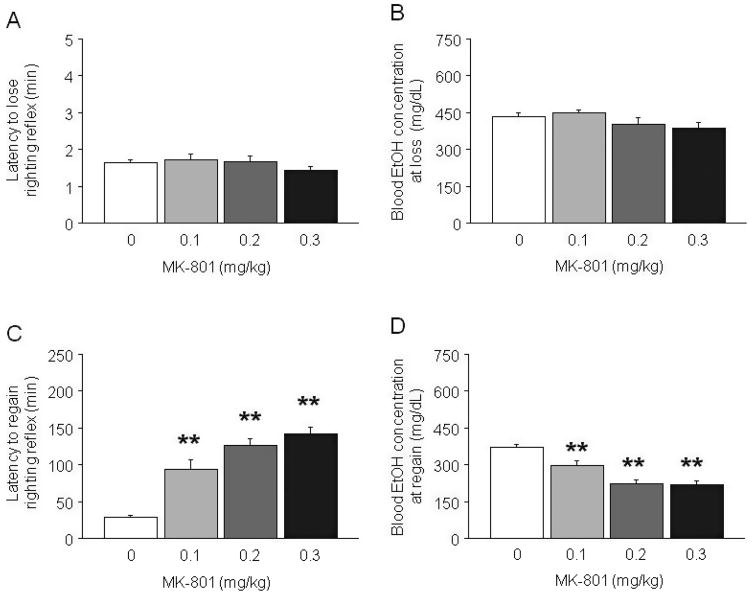
MK-801 treatment failed to alter either the latency to lose the righting reflex or BECs at loss of righting (Fig. 3A and B). MK-801 significantly affected the latency to recover the reflex (i.e., sleep time) (F3,20 = 25.13, p < 0.01) and BECs at recovery (F3,20 = 19.69, p < 0.01). Post hoc analysis showed that all doses of MK-801 increased the latency to recover relative to EtOH alone (Fig. 3C), and that BECs were lower at recovery for all MK-801 doses relative to EtOH alone (F3,20 = 19.69, p < 0.01) (Fig. 3D).
Fig. 3.
Effects of MK-801 on latency to lose and recover the righting reflex, and blood EtOH concentrations (BECs) at loss and recovery in C57BL/6J mice. MK-801 did not affect (A) the latency to lose the righting reflex, or (B) BECs at loss of the reflex. (C) MK-801 dose-dependently increased the latency to recover the righting reflex (sleep time). (D) BECs at recovery of the reflex were lower in MK-801-treated mice relative to EtOH-treated controls. n = 7 to 9/dose. **p < 0.01 versus 0.
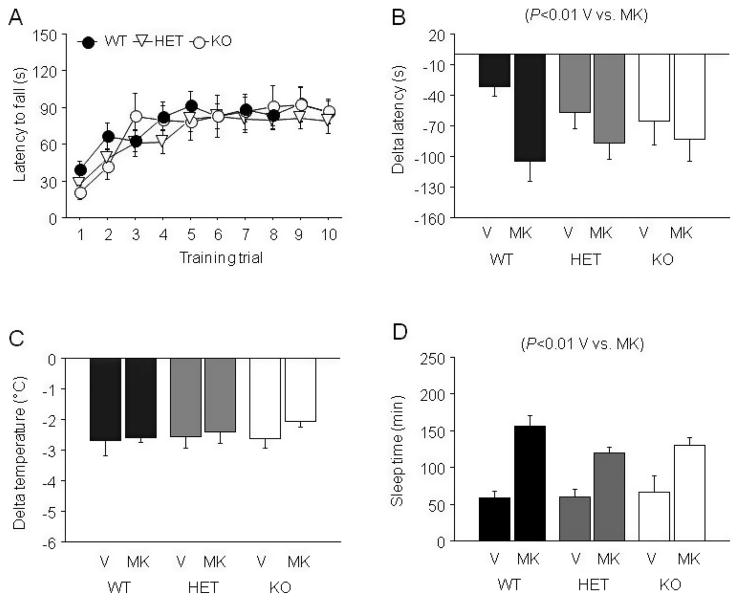
MK-801 × EtOH Interactions Following NR2A KO
EtOH-Induced Ataxia
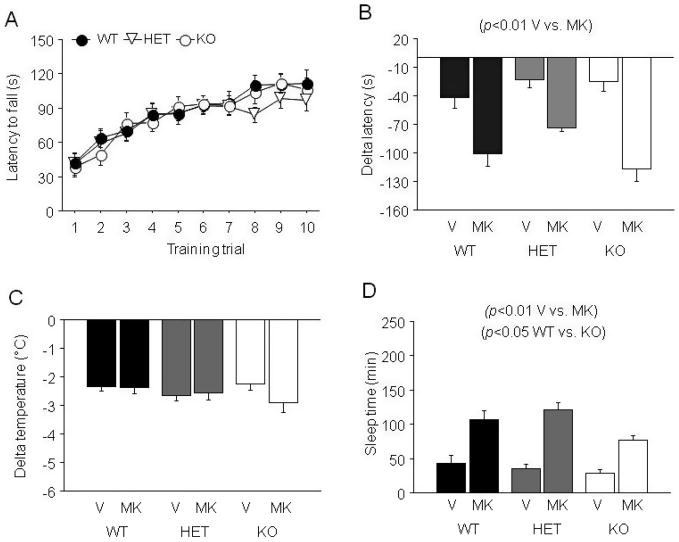
During training there was a significant effect of trial on latency to fall (F9,522 = 33.48, p < 0.01), but no effect of genotype and no genotype × trial interaction; indicating that genotypes improved with trials at a similar rate (Fig. 4A). During testing there was a significant effect of MK-801 (F1,53 = 63.95, p < 0.01) and genotype (F2,53 = 3.52, p < 0.05) but no genotype × drug interaction for delta latency to fall. Post hoc tests on data collapsed across MK-801 treatment found no significant genotype differences. MK-801 caused significantly greater ataxia than EtOH alone (Fig. 4B).
Fig. 4.
Effects of MK-801 on sensitivity to the ataxic, hypothermic and sedative/hypnotic effects of EtOH in NR2A HET and KO mice. (A) Rotarod learning. (B) MK-801 potentiated EtOH-induced ataxia regardless of NR2A genotype. (C) MK-801 failed to alter EtOH-induced hypothermia regardless of NR2A genotype. (D) MK-801 potentiated sedation/hypnosis regardless of NR2A genotype, but sleep time duration was, overall, shorter in NR2A KO. n = 6 to 11/genotype/dose. V = vehicle, MK = MK-801, WT = wild-type, HET = heterozygous, KO = knockout.
EtOH-Induced Hypothermia
Neither NR2A genotype nor MK-801 treatment had a significant effect on the hypothermic effects of EtOH (Fig. 4C).
EtOH-Induced Sedation/Hypnosis
There was a significant effect of MK-801 treatment (F1,49 = 72.82, p < 0.01) and genotype (F2,49 = 4.30, p < 0.01) but no MK- 801 × genotype interaction for sleep time. MK-801 significantly increased sleep time (Fig. 4D). Data collapsed across MK-801 treatment and subject to post hoc analysis showed that sleep time was significantly shorter in NR2A KO as compared to WT.
There was a significant effect of MK-801 treatment (F1,40 = 29.77, p < 0.01) but not genotype and no MK-801 × genotype interaction for BECs at recovery. MK-801- treated mice had lower BECs at recovery than mice treated with EtOH alone, paralleling their longer sleep times (Table 1).
Table 1.
Blood EtOH Concentrations (BECs) at Recovery of the Righting Reflex Following NR2A KO, NR2B Antagonism or GluR1 KO
| EtOH
|
EtOH + MK
|
EtOH
|
EtOH + MK
|
EtOH
|
EtOH + MK
|
|
|---|---|---|---|---|---|---|
| Genotype
|
||||||
| WT | HET | KO | ||||
| NR2A KO | 334 ± 17 | 292 ± 14 | 358 ± 14 | 272 ± 12 | 367 ± 16 | 311 ± 8 |
| GluR1 KO | 335 ± 10 | 254 ± 5 | 382 ± 17 | 290 ± 10 | 362 ± 16 | 297 ± 13 |
| Ro 25-6981 dose
|
||||||
| 1 mg/kg
|
3 mg/kg
|
10 mg/kg
|
||||
| EtOH | EtOH + MK | EtOH | EtOH + MK | EtOH | EtOH + MK | |
| Ro 25-6981 | 375 ± 16 | 328 ± 26 | 393 ± 17 | 313 ± 21 | 371 ± 10 | 311 ± 12 |
Mice treated with 0.2 mg/kg MK-801 (EtOH + MK) showed significantly lower BECs at recovery, relative to mice treated with EtOH alone, regardless of NR2A or GluR1 genotype or Ro 25-6981 treatment. This paralleled the longer sleep time responses to MK-801 in all three experiments. n = 5 to 9/group. Data are mean + SEM mg/dL.
EtOH Effects Following NR2B Antagonism
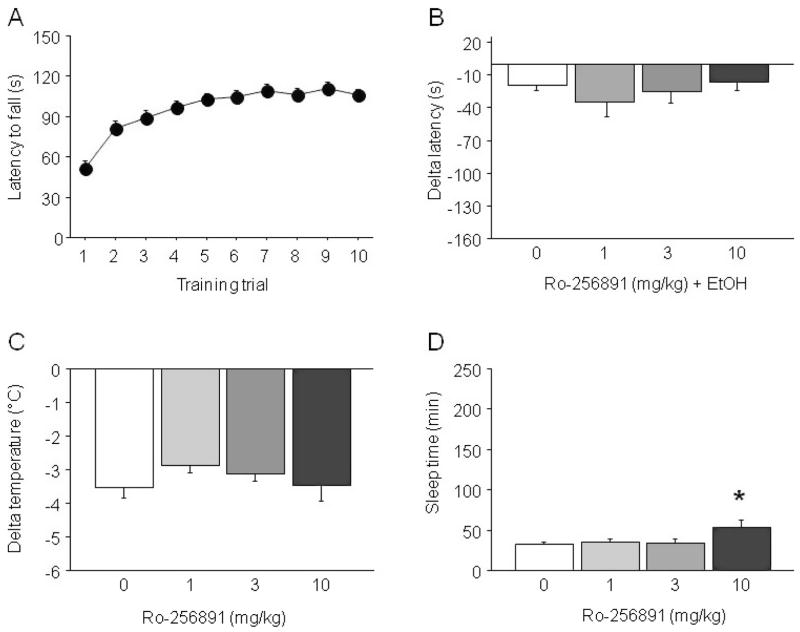
Prior to assessing effects of pretreatment with Ro 25-6981 on MK-801 × EtOH interactions, we examined the effects of Ro 25-6981 on sensitivity to EtOH per se.
EtOH-Induced Ataxia
During training there was a significant reduction in latency to fall across trials (F9,459 = 25.05, p < 0.01) (Fig. 5A). Groups subsequently assigned to different Ro 25-6981 doses did not differ during training. During testing there was a significant effect of treatment for delta latency to fall (F4,47 = 2.98, p < 0.05). However, post hoc analysis found no significant difference between any one Ro 25-6981 dose and EtOH alone (Fig. 5B).
Fig. 5.
Effects of Ro 25-6981 on sensitivity to the ataxic, hypothermic, and sedative/hypnotic effects of EtOH in C57BL/6J mice. (A) Rotarod learning. (B) Ro 25-6981 did not potentiate EtOH-induced ataxia. (C) Ro 25-6981 failed to alter EtOH-induced hypothermia. (D) The highest dose of Ro 25-6981 produced a modest potentiation of sedation/hypnosis. n = 8 to 14/dose. V = vehicle.
EtOH-Induced Hypothermia
Ro 25-6981 did not affect the hypothermic effects of EtOH (Fig. 5C).
EtOH-Induced Sedation/Hypnosis
Ro 25-6981 treatment had a significant effect on the sedative/hypnotic effects of EtOH (F3,49 = 3.22, p < 0.05). Post hoc analysis showed that 10 mg/kg Ro 25-6981 significantly increased sleep time relative to EtOH alone (Fig. 5D).
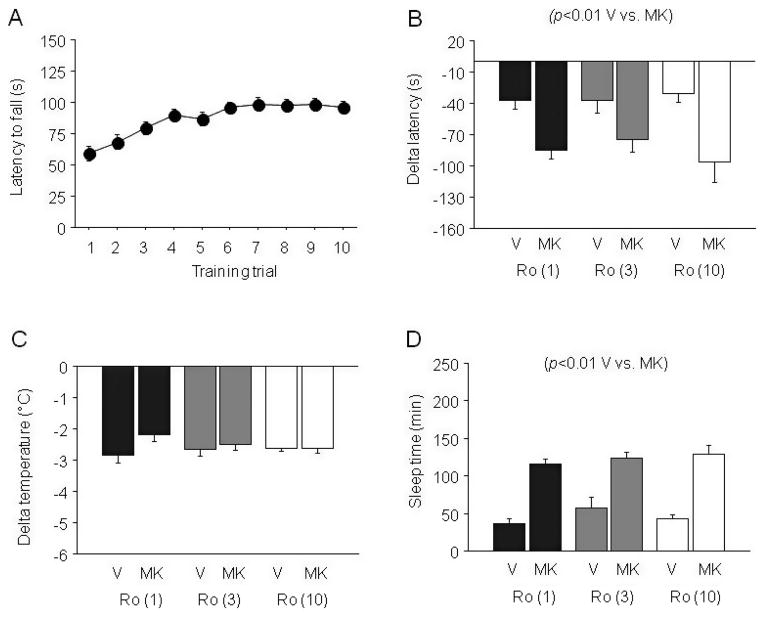
MK-801 × EtOH Interactions Following NR2B Antagonism
EtOH-Induced Ataxia
During training there was a significant effect of trial for latency to fall (F9,360 = 13.03, p < 0.01) (Fig. 6A). Groups subsequently assigned to different drug doses did not differ during training. During testing there was a significant effect of MK-801 (F1,29 = 3.17, p < 0.05) but not of Ro 25-6981 and no MK-801 × Ro 25-6981 interaction for delta latency to fall. MK-801 caused significantly greater EtOH-induced ataxia than EtOH alone (Fig. 6B).
Fig. 6.
Effects of MK-801 on sensitivity to the ataxic, hypothermic and sedative/hypnotic effects of EtOH following pretreatment with Ro 25-6981 in C57BL/6J mice. (A) Rotarod learning. (B) MK-801 dose-dependently potentiated EtOH-induced ataxia regardless of Ro 25-6981 pretreatment. (C) MK-801 did not alter EtOH-induced hypothermia regardless of Ro 25-6981 pretreatment. (D) MK-801 dose-dependently potentiated EtOH-induced sedation/hypnosis regardless of Ro 25-6981 pretreatment. n = 5 to 8/genotype/treatment. V = vehicle, MK = MK-801, Ro (1) = 1 mg/kg Ro 25-6981, Ro (3) = 3 mg/kg Ro 25-6981, Ro (10) = 10 mg/kg Ro 25-6981.
EtOH-Induced Hypothermia
Neither Ro 25-6981 nor MK-801 treatment had a significant effect on the hypothermic effects of EtOH (Fig. 6B).
EtOH-Induced Sedation/Hypnosis
There was a significant effect of MK-801 treatment (F1,34 = 118.37, p < 0.01) but no effect of Ro 25-6981 and no MK-801 × Ro 25-6981 interaction for sleep time. MK-801 significantly increased sleep time relative to EtOH alone (Fig. 6D).
There was a significant effect of MK-801 treatment (F1,34 = 15.39, p < 0.01) but not Ro 25-6981 and no MK-801 × Ro 25-6981 interaction for BECs at recovery. MK-801-treated mice had significantly lower BECs than non-MK-801-treated controls at recovery, paralleling their longer sleep times (Table 1).
MK-801 × EtOH Interactions Following GluR1 KO
EtOH-Induced Ataxia
During training there was a significant effect of trial for latency to fall (F9,360 = 20.20, p < 0.01), but no effect of genotype and no genotype × trial interaction. Genotypes improved with trials at a similar rate (Fig. 7A). During testing there was a significant effect of MK-801 (F1,37 = 7.54, p < 0.01) but not genotype and no genotype × drug interaction for delta latency to fall. MK-801 significantly increased EtOH-induced ataxia relative to EtOH alone (Fig. 7B).
Fig. 7.
Effects of MK-801 on sensitivity to the ataxic, hypothermic and sedative/hypnotic effects of EtOH in GluR1 HET and KO mice. (A) Rotarod learning. (B) MK-801 potentiated EtOH-induced ataxia regardless of GluR1 genotype. (C) MK-801 did not alter EtOH-induced hypothermia regardless of GluR1 genotype. (D) MK-801 potentiated sedation/hypnosis regardless of GluR1 genotype. n = 6 to 8/genotype/treatment. V = vehicle, MK = MK-801, WT = wild-type, HET = heterozygous, KO = knockout.
EtOH-Induced Hypothermia
Neither GluR1 genotype nor MK-801 treatment had a significant effect on the hypothermic effects of EtOH (Fig. 7C).
EtOH-Induced Sedation
Hypnosis. There was a significant effect of MK-801 treatment (F1,29 = 46.84, p < 0.01) but no effect of genotype and no MK-801 × genotype interaction for sleep time. MK-801 significantly increased sleep time relative to EtOH alone (Fig. 7D).
There was a significant effect of MK-801 treatment (F1,29 = 73.04, p < 0.01) and genotype (F2,29 = 3.87, p < 0.05) but no MK-801 × genotype interaction for BECs at recovery. MK-801-treated mice had significantly lower BECs at recovery than mice treated with EtOH alone. Post hoc analysis collapsed across MK-801 treatment found no significant BEC differences between genotypes (Table 1).
DISCUSSION
The main findings of the present study were first, that the uncompetitive NMDAR antagonist MK-801 markedly potentiated EtOH’s acute intoxicating effects in two inbred strains in an assay-specific manner, and second, that these effects were unaltered or, in one case, partially attenuated, by functional inactivation of the NR2A or NR2B NMDAR subunits, or the AMPA GluR1 subunit.
In non-mutant mice, pretreatment with MK-801 markedly and dose-dependently increased both the sedative/hypnotic (sleep time) effects of 3 g/kg EtOH and the ataxic effects of 1.75 g/kg EtOH measured in the accelerating rotarod. BECs at recovery from the sedative/hypnotic effects of EtOH paralleled the prolonged sleep time produced by MK-801 treatment; such that BECs were lower at awakening in the longer-sleep MK-801-treated mice relative to mice treated with EtOH alone. (The same pattern of BECs was seen in the NR2A KO, NR2B antagonist and GluR1 KO experiments discussed below). This implies that the EtOH-potentiating effects of MK-801 were not simply an artifact of the drug interfering with EtOH clearance. Further discounting effects of MK-801 on EtOH metabolism, MK-801 treatment had no affect on the hypothermic effects of EtOH even though these effects were measured with the same dose and over the same timeframe as sedation/hypnosis. Thus, the first conclusion of the present study is that MK-801’s effects on EtOH were responsive-selective, with an effect on two behavioral measures of “intoxication” but no effect on a physiological measure.
MK-801 potentiation of EtOH’s depressant and ataxic effects was replicated in two genetically distinction inbred strains, C57BL/6J and 129S1. Replication across strains indicates that the MK-801 and EtOH interaction generalizable across diverse genetic backgrounds (although a fuller strain comparison would be needed to substantiate this). Moreover, given the greater sensitivity to the ataxic and depressant effects of EtOH per se in 129S1 relative to C57BL/6J, present data indicate that MK-801 is able to potentiate EtOH’s effects even against a high-sensitive baseline phenotype. Finally, both C57BL/6J and 129S1 mice showed increased sensitivity to EtOH’s sedative/hypnotic effects following treatment with another drug with NMDAR antagonist actions, phencycline. Although the magnitude of the interaction was less pronounced than for MK-801, as reported previously (Daniell, 1990), this shows that the potentiating effects of MK-801 extend to other NMDAR antagonists.
Present data confirm and extend earlier studies showing that treatment with MK-801, phencyclidine or with other NMDAR antagonists, including memantine, ketamine or 2-amino-5-phosphonovaleric acid, potentiates EtOH’s locomotor sedating and sedative/hypnotic but not hypothermic effects in rats (Beleslin et al., 1997; Brunet et al., 1985;Daniell, 1990; Danysz et al., 1992; Poch et al., 1990; Silveri and Spear, 2002, 2004), and various mouse strains including C57BL/6J, DBA/2J and the Long Sleep and Short Sleep lines (Goldberg et al., 1983; Kuribara, 1994; Maas et al., 2005; Meyer and Phillips, 2003a,b; Shen and Phillips, 1998; Wilson et al., 1990). Our findings are also consistent with reports that NMDAR antagonists, includingMK-801, augment the ataxic effect of EtOH in rats tested on the rotarod (McMillen et al., 2004), NSA mice tested on the horizontal wire test (Vanover, 1999) and DBA/2J mice tested on the grid test (Meyer and Phillips, 2003a). This consistency across different measures of ataxia is itself notable given evidence that these assays measure dissociable behaviors mediated by distinct molecular and genetic factors (Boyce-Rustay and Holmes, 2006a; Rustay et al., 2003a). Finally, although not tested in our study, MK-801 has also been shown to accentuate the locomotorstimulant effects of EtOH in DBA/2J mice (Meyer and Phillips, 2003a; Shen and Phillips, 1998). Thus, the present data further strengthen the literature demonstrating a marked potentiating effect of MK-801 and other NMDAR antagonists on a variety (but not all) of measures of EtOH’s acute intoxicating actions.
Behavioral indices of the magnitude of acute EtOH intoxication such as ataxia and sleep time are composite measures of initial sensitivity to EtOH’s effects and acute functional tolerance (AFT) to these effects (Erwin and Deitrich, 1996; Mellanby, 1919; Ponomarev and Crabbe, 2004; Tabakoff and Ritzmann, 1979). As with other forms of drug tolerance, AFT to EtOH’s behavioral actions is a neuroadaptive process. NMDARs are known to play a critical role in the neuroadaptive processes associated with exposure to other drugs of abuse (Hyman, 2005) and, more generally, to various forms of neural plasticity, including those thought to underlie learning (Malenka and Bear, 2004). MK-801 and other NMDAR antagonists (e.g., CGP 39551, ketamine) can block the development of tolerance to EtOH’s sedative/hypnotic and ataxic effects in rats and C57BL/6J mice measured over days (i.e., not AFT) (Karcz-Kubicha and Liljequist, 1995; Khanna et al., 1991, 1993, 1997; Szabo et al., 1994; Wu et al., 1993) [but see grid test ataxia in DBA/2J mice (Meyer and Phillips, 2003a)]. MK-801 also blocks the development of locomotorsensitization to EtOH in DBA/2J and Swiss mice, another form of neuroadaptation (Broadbent et al., 2003; Kotlinska et al., 2006; Meyer and Phillips, 2003a, 2007). Two studies that have explicitly examined AFT, have found that MK-801 and ketamine both inhibit the development AFT to EtOH’s ataxic (tilt test) or sedative/hypnotic effects in rats (Khanna et al., 2002; Silveri and Spear, 2004). We found that MK-801 treatment did not affect the initial latency to lose the righting reflex nor BECs at loss. While this could be construed as support for a lack of MK-801 on initial sensitivity and therefore more probably an effect on AFT, it is weak evidence of such a dissociation. Thus, the potential influence of MK-801 on AFT is not yet fully clear and this remains an important question for future study.
As noted in the Introduction, EtOH acts as an allosteric inhibitor of NMDAR-mediated neuronal responses at intoxicating doses in vitro (Lovinger et al., 1989; Masood et al., 1994). Woodward and colleagues among others have shown that, in vitro, the functional state of the NR1 subunit modulates EtOH effects on the NMDAR (Jin and Woodward, 2006; Ronald et al., 2001), while both the NR2A and NR2B subunits, but less so NR2C and NR2D, also determine sensitivity to EtOH (Masood et al., 1994; Mirshahi and Woodward, 1995). Replicating earlier results (Boyce-Rustay and Holmes, 2005, 2006a; Sato et al., 2006), gene knockout of NR2A did not affect the (rotarod) ataxic, sedative/hypnotic or hypothermic effects of EtOH on the same measures and EtOH doses that were clearly potentiated by MK-801. Pharmacological antagonism of NR2B with Ro 25-6981 in C57BL/6J mice produced only a modest increase in the sedative/hypnotic response to EtOH in one experiment (Ro 25-6981 alone) but not another (Ro 25-6981 × MK-801), and was without effect on EtOH’s ataxic and hypothermic effects. These relatively modest effects are similar to those obtained in earlier studies of Ro 25-6981 and other NR2B-selective antagonists in mice (Boyce-Rustay and Holmes, 2005; Malinowska et al., 1999; Yaka et al., 2003). Thus, taken together, these data demonstrate that functional inactivation of either NR2A- or NR2B-containing NMDARs alone is insufficient to mimic the potent EtOH-potentiating action of the subunit-non-selective NMDAR antagonist MK-801.
Inactivation of NR2A- or NR2B-containing NMDARs could have conceivably altered MK-801’s effects in various ways. If MK-801’s antagonist effects were dependent upon NR2A- or NR2B-containing NMDARs then inactivation of either subunit would have been predicted to diminish MK- 801’s effect on EtOH responses. Contrariwise, if MK-801’s antagonist action was independent of NR2A or NR2B then the combination of MK-801 blockade of NMDARs with inactivation of NR2A- or NR2B-containing receptors could have further potentiated EtOH’s behavioral effects. Data showed that NR2B antagonism via Ro 25-6981 treatment failed to affect MK-801’s EtOH-potentiating effects on any behavioral measure. On the other hand, although KO of NR2A also failed to alter MK-801’s effects on EtOH-induced ataxia or hypothermia, NR2A KO mice showed a general reduction in the sleep time response relative to WT controls. Because NR2A KO mice show normal sedative/hypnotic response to EtOH per se (this experiment, (Boyce-Rustay and Holmes, 2005, 2006a), this reduction appears to be most parsimoniously attributed to a partial reduction in the response to MK-801s potentiating effects. As such, this suggests that the NR2A subunit contributes to MK-801’s effects on at least this one specific measure of EtOH intoxication, and provide novel insight into the molecular basis of the MK-801 × EtOH interaction.
Notwithstanding this one difference, the overarching conclusion is that functional inactivation of NR2A nor NR2B is not sufficient to ablate the ability of a NMDAR antagonist to potentiate EtOH intoxication. There are a number of possible explanations for this. One is that NR2A- and NR2Bcontaining NMDARs both contribute to MK-801’s effects on EtOH behaviors, but that loss of any one is insufficient to markedly affect responses other than sedation/hypnosis. Alternatively, other NMDAR subunits may be more important than NR2A and NR2B for these effects. In this context, NR2C-, NR2D- and NR3A/NR3B-containing NMDAR all to some extent interact with EtOH in vitro (Masood et al., 1994; Mirshahi and Woodward, 1995; Smothers and Woodward, 2007) and represent possible candidates. However, their contribution to either MK-801’s or EtOH’s in vivo actions remains unclear (see Woodward (1999)). Another possibility is that MK-801s effects on EtOH sensitivity are in fact unrelated to the drug’s NMDAR antagonistic properties. Although seemingly unlikely, this cannot be fully excluded given the drug’s known effects on for example norepinephrine (Snell et al., 1988), dopamine (Seeman et al., 2005) and acetylcholine (Ramoa et al., 1990) function.
The present data show that MK-801’s effects on EtOH responses were normal in GluR1 KO mice [as were baseline EtOH behaviors, replicating (Cowen et al., 2003)]. NMDARs act in concert with AMPA receptors to mediate certain forms of behavior, notably learning and neuroadaptation to drugs of abuse (Chao and Nestler, 2004; Malenka and Bear, 2004; Wolf et al., 2004). Our data demonstrate that the MK- 801 × EtOH interaction is independent of functioning AMPA GluR1 subunits. Interestingly, this contrasts markedly with the recent demonstration that the same line of GluR1 KO mice fail to respond to the locomotor hyperactivity-inducing effects of MK-801 at doses similar to those tested in the current study (Wiedholz et al., 2008). Thus the contribution of GluR1 to MK-801’s effects on locomotor activity and EtOH behaviors are dissociable; likely reflecting the recruitment of different neural circuitry. More generally, the lack of GluR1 KO effects in the present study adds to the somewhat surprising conclusion that MK-801’s ability to potentiate EtOH responses is insensitive to loss of key molecular components mediating glutamate signaling. Thus, the mechanism by which MK-801 interacts with EtOH to affect behavioral intoxication remains to be elucidated. On a final note, while we found no evidence of statistical interactions between sex, genotype and MK-801 treatment, the possibility that larger sample sizes would reveal sex differences in the manner in which either GluR1 KO mice, or the other inbred or mutant lines tested, respond to MK-801-potentiation of EtOH’s effects should not be ruled out.
In summary, the current study demonstrates that the uncompetitive NMDAR antagonist MK-801 strongly potentiates the sedative/hypnotic and ataxic, but not the hypothermic, effects of acute EtOH in mice. However, the EtOH-potentiating effects of MK-801 were largely unaltered by gene KO of NR2A or GluR1 or pharmacological antagonism of NR2B, with the exception of slightly attenuated sedative/hypnotic response in NR2A KO mice. Individuals at-risk for alcoholism show lesser sensitivity/tolerance to EtOH-induced intoxication (Newlin and Thomson, 1991; Schuckit, 1994) and are also less sensitive to the negative effects of NMDAR antagonists (Petrakis et al., 2004). These observations support a role for NMDAR in the pathophysiology and treatment of alcoholism (Carpenter-Hyland and Chandler, 2007; Krystal et al., 2003). A better understanding of the molecular basis of MK-801’s potent effects on EtOH intoxication could serve to further elucidate this role.
Acknowledgments
We are grateful to Dr. Rolf Sprengel for providing the original breeding stock of GluR1 null mutant mice and to Dr. David Lovinger for comments on an earlier version of the manuscript. Research supported by the Intramural Research Programs of the National Institute of Alcohol Abuse and Alcoholism (Z01-AA000411).
Footnotes
No claim to original U.S. government works.
References
- Beleslin DB, Djokanovic N, Jovanovic-Micic D, Samardzic R. Opposite effects of GABAA and NMDA receptor antagonists on ethanol-induced behavioral sleep in rats. Alcohol. 1997;14:167–173. doi: 10.1016/s0741-8329(96)00140-1. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav Neurosci. 2004;118:822–834. doi: 10.1037/0735-7044.118.4.822. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Functional roles of NMDA receptor NR2A and NR2B subunits in the acute intoxicating effects of ethanol in mice. Synapse. 2005;56:222–225. doi: 10.1002/syn.20143. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 2006a;187:455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Genetic Inactivation of the NMDA Receptor NR2A Subunit has Anxiolytic- and Antidepressant-Like Effects in Mice. Neuropsychopharmacology. 2006b;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Janos AL, Holmes A. Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav Brain Res. 2007;186:133–137. doi: 10.1016/j.bbr.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Kampmueller KM, Koonse SA. Expression of behavioral sensitization to ethanol by DBA/2J mice: the role of NMDA and non-NMDA glutamate receptors. Psychopharmacology (Berl) 2003;167:225–234. doi: 10.1007/s00213-003-1404-3. [DOI] [PubMed] [Google Scholar]
- Brunet BL, Reiffenstein RJ, Williams T, Wong L. Toxicity of phencyclidine and ethanol in combination. Alcohol Drug Res. 1985;6:341–349. [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav. 2007;86:200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu Rev Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- Costa ET, Soto EE, Cardoso RA, Olivera DS, Valenzuela CF. Acute effects of ethanol on kainate receptors in cultured hippocampal neurons. Alcohol Clin Exp Res. 2000;24:220–225. [PubMed] [Google Scholar]
- Cowen MS, Schroff KC, Gass P, Sprengel R, Spanagel R. Neurobehavioral effects of alcohol in AMPA receptor subunit (GluR1) deficient mice. Neuropharmacology. 2003;45:325–333. doi: 10.1016/s0028-3908(03)00174-6. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Current Protocols in Neuroscience. Wiley; New York: 2007. Overview of Mouse Assays of Ethanol Intoxication. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK. Effects of convulsants on handling-induced convulsions in mice selected for ethanol withdrawal severity. Brain Res. 1991;550:1–6. doi: 10.1016/0006-8993(91)90397-e. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Daniell LC. The noncompetitive N-methyl-D-aspartate antagonists, MK-801, phencyclidine and ketamine, increase the potency of general anesthetics. Pharmacol Biochem Behav. 1990;36:111–115. doi: 10.1016/0091-3057(90)90134-4. [DOI] [PubMed] [Google Scholar]
- Daniell LC, Phillips TJ. Differences in ethanol sensitivity of brain NMDA receptors of long-sleep and short-sleep mice. Alcohol Clin Exp Res. 1994;18:1482–1490. doi: 10.1111/j.1530-0277.1994.tb01454.x. [DOI] [PubMed] [Google Scholar]
- Danysz W, Dyr W, Jankowska E, Glazewski S, Kostowski W. The involvement of NMDA receptors in acute and chronic effects of ethanol. Alcohol Clin Exp Res. 1992;16:499–504. doi: 10.1111/j.1530-0277.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owen AM, Baganz NL, Boyce-Rustay J, Millstein RA, Wiedholz L, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin VG, Deitrich RA. Genetic selection and characterization of mouse lines for acute functional tolerance to ethanol. J Pharmacol Exp Ther. 1996;279:1310–1317. [PubMed] [Google Scholar]
- Fischer W, Franke H, Illes P. Effects of acute ethanol on the Ca2+ response to AMPA in cultured rat cortical GABAergic nonpyramidal neurons. Alcohol Alcohol. 2003;38:394–399. doi: 10.1093/alcalc/agg108. [DOI] [PubMed] [Google Scholar]
- Frye GD, Fincher A. Sustained ethanol inhibition of native AMPA receptors on medial septum/diagonal band (MS/DB) neurons. Br J Pharmacol. 2000;129:87–94. doi: 10.1038/sj.bjp.0703039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Salama AI, Patel JB, Malick JB. Novel non-benzodiazepine anxiolytics. Neuropharmacology. 1983;22(12B):1499–1504. doi: 10.1016/0028-3908(83)90118-1. [DOI] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Snell LD, Rogawski MA, Thurkauf A, Tabakoff B. Comparison of the effects of the uncompetitive N-methyl-D-aspartate antagonist (+−)-5-aminocarbonyl-10,11-dihydro-5H-dibenzo[a,d] cyclohepten-5,10-imine (ADCI) with its structural analogs dizocilpine (MK-801) and carbamazepine on ethanol withdrawal seizures. J Pharmacol Exp Ther. 1992;260:1017–1022. [PubMed] [Google Scholar]
- Hanania T, McCreary AC, Haughey HM, Salaz DO, Zahniser NR. MK-801- and ethanol-induced activity in inbred long-sleep and short-sleep mice: dopamine and serotonin systems. Eur J Pharmacol. 2002;457:125–135. doi: 10.1016/s0014-2999(02)02685-7. [DOI] [PubMed] [Google Scholar]
- Hanania T, Negri CA, Dunwiddie TV, Zahniser NR. N-methyl-D-aspartate receptor responses are differentially modulated by noncompetitive receptor antagonists and ethanol in inbred long-sleep and short-sleep mice: behavior and electrophysiology. Alcohol Clin Exp Res. 2000;24:1750–1758. [PubMed] [Google Scholar]
- Hanania T, Zahniser NR. Locomotor activity induced by noncompetitive NMDA receptor antagonists versus dopamine transporter inhibitors: opposite strain differences in inbred long-sleep and short-sleep mice. Alcohol Clin Exp Res. 2002;26:431–440. [PubMed] [Google Scholar]
- Hodge CW, Grant KA, Becker HC, Besheer J, Crissman AM, Platt DM, Shannon EE, Shelton KL. Understanding how the brain perceives alcohol: neurobiological basis of ethanol discrimination. Alcohol Clin Exp Res. 2006;30:203–213. doi: 10.1111/j.1530-0277.2006.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Jin C, Woodward JJ. Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcohol Clin Exp Res. 2006;30:673–679. doi: 10.1111/j.1530-0277.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Liljequist S. Effects of post-ethanol administration of NMDA and non-NMDA receptor antagonists on the development of ethanol tolerance in C57B1 mice. Psychopharmacology (Berl) 1995;120:49–56. doi: 10.1007/BF02246144. [DOI] [PubMed] [Google Scholar]
- Kash T, Winder DG. NMDAR LTP and LTD induction: 2B or Not 2B…is that the question? Debates in Neuroscience. 2007;1:79–84. [Google Scholar]
- Khanna JM, Morato GS, Kalant H. Effect of NMDA antagonists, an NMDA agonist, and serotonin depletion on acute tolerance to ethanol. Pharmacol Biochem Behav. 2002;72:291–298. doi: 10.1016/s0091-3057(01)00773-0. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Shah G, Chau A. Effect of NMDA antagonists on rapid tolerance to ethanol under two different testing paradigms. Pharmacol Biochem Behav. 1997;57:693–697. doi: 10.1016/s0091-3057(96)00390-5. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Shah G, Weiner J, Wu PH, Kalant H. Effect of NMDA receptor antagonists on rapid tolerance to ethanol. Eur J Pharmacol. 1993;230:23–31. doi: 10.1016/0014-2999(93)90405-7. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Wu PH, Weiner J, Kalant H. NMDA antagonist inhibits rapid tolerance to ethanol. Brain Res Bull. 1991;26:643–645. doi: 10.1016/0361-9230(91)90109-w. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M, Danysz W. N-methyl-D-aspartate and group I metabotropic glutamate receptors are involved in the expression of ethanol-induced sensitization in mice. Behav Pharmacol. 2006;17:1–8. doi: 10.1097/01.fbp.0000181600.95405.c7. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Kumari M, Ticku MK. Regulation of NMDA receptors by ethanol. Prog Drug Res. 2000;54:152–189. [PubMed] [Google Scholar]
- Kuribara H. Potentiation of the ambulation-increasing effect induced by combined administration of MK-801 with ethanol in mice. Psychopharmacology (Berl) 1994;113:453–456. doi: 10.1007/BF02245222. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Maas JW, Jr, Vogt SK, Chan GC, Pineda VV, Storm DR, Muglia LJ. Calcium-stimulated adenylyl cyclases are critical modulators of neuronal ethanol sensitivity. J Neurosci. 2005;25:4118–4126. doi: 10.1523/JNEUROSCI.4273-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Napiorkowska-Pawlak D, Pawlak R, Buczko W, Gothert M. Ifenprodil influences changes in mouse behaviour related to acute and chronic ethanol administration. Eur J Pharmacol. 1999;377:13–19. doi: 10.1016/s0014-2999(99)00393-3. [DOI] [PubMed] [Google Scholar]
- Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol Pharmacol. 1994;45:324–329. [PubMed] [Google Scholar]
- McMillen BA, Joyner PW, Parmar CA, Tyer WE, Williams HL. Effects of NMDA glutamate receptor antagonist drugs on the volitional consumption of ethanol by a genetic drinking rat. Brain Res Bull. 2004;64:279–284. doi: 10.1016/j.brainresbull.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mellanby E. Alcohol: its absorption into and disappearance from the blood under different conditions. Paper presented at the Medical Research Committee; London. 1919. [Google Scholar]
- Meyer PJ, Phillips TJ. Bivalent effects of MK-801 on ethanol-induced sensitization do not parallel its effects on ethanol-induced tolerance. Behav Neurosci. 2003a;117:641–649. doi: 10.1037/0735-7044.117.3.641. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Sensitivity to ketamine, alone or in combination with ethanol, is altered in mice selectively bred for sensitivity to ethanol’s locomotor effects. Alcohol Clin Exp Res. 2003b;27:1701–1709. doi: 10.1097/01.ALC.0000093602.00193.39. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Behavioral sensitization to ethanol does not result in cross-sensitization to NMDA receptor antagonists. Psychopharmacology (Berl) 2007;195:103–115. doi: 10.1007/s00213-007-0871-3. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Mirshahi T, Woodward JJ. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg(2+)-insensitive mutants. Neuropharmacology. 1995;34:347–355. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- Musleh W, Alvarez S, Baudry M, Alkana RL. Effects of ethanol and temperature on NMDA receptor function in different mouse genotypes. Alcohol Clin Exp Res. 1996;20:1299–1304. doi: 10.1111/j.1530-0277.1996.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics. Alcohol Clin Exp Res. 1991;15:399–405. doi: 10.1111/j.1530-0277.1991.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payseur BA, Place M. Prospects for association mapping in classical inbred mouse strains. Genetics. 2007;175:1999–2008. doi: 10.1534/genetics.106.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Poch G, Dittrich P, Reiffenstein RJ, Lenk W, Schuster A. Evaluation of experimental combined toxicity by use of dose-frequency curves: comparison with theoretical additivity as well as independence. Can J Physiol Pharmacol. 1990;68:1338–1345. doi: 10.1139/y90-202. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. Characterization of acute functional tolerance to the hypnotic effects of ethanol in mice. Alcohol Clin Exp Res. 2004;28:991–997. doi: 10.1097/01.alc.0000131978.79857.5e. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Alkondon M, Aracava Y, Irons J, Lunt GG, Deshpande SS, Wonnacott S, Aronstam RS, Albuquerque EX. The anticonvulsant MK-801 interacts with peripheral and central nicotinic acetylcholine receptor ion channels. J Pharmacol Exp Ther. 1990;254:71–82. [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem. 2001;276:44729–44735. doi: 10.1074/jbc.M102800200. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci U S A. 2003a;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Influence of task parameters on rotarod performance and sensitivity to ethanol in mice. Behav Brain Res. 2003b;141:237–249. doi: 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Sato Y, Seo N, Kobayashi E. Ethanol-induced hypnotic tolerance is absent in N-methyl-D-aspartate receptor [varepsilon] 1 subunit knockout mice. Anesth Analg. 2006;103:117–120. doi: 10.1213/01.ane.0000220944.27963.b1. table of contents. [DOI] [PubMed] [Google Scholar]
- Schorge S, Colquhoun D. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci. 2003;23:1151–1158. doi: 10.1523/JNEUROSCI.23-04-01151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Seeman P, Ko F, Tallerico T. Dopamine receptor contribution to the action of PCP, LSD and ketamine psychotomimetics. Mol Psychiatry. 2005;10:877–883. doi: 10.1038/sj.mp.4001682. [DOI] [PubMed] [Google Scholar]
- Shen EH, Phillips TJ. MK-801 potentiates ethanol’s effects on locomotor activity in mice. Pharmacol Biochem Behav. 1998;59:135–143. doi: 10.1016/s0091-3057(97)00389-4. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals. Alcohol Clin Exp Res. 2002;26:449–456. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on acute and rapid tolerance to ethanol during ontogeny. Alcohol Clin Exp Res. 2004;28:884–894. doi: 10.1097/01.alc.0000128221.68382.ba. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Mrotek JJ, Lovinger DM. Chronic ethanol exposure leads to a selective enhancement of N-methyl-D-aspartate receptor function in cultured hippocampal neurons. J Pharmacol Exp Ther. 1997;283:1214–1222. [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-D-aspartate NR1 and NR3 subunits. J Pharmacol Exp Ther. 2007;322:739–748. doi: 10.1124/jpet.107.123836. [DOI] [PubMed] [Google Scholar]
- Snell LD, Yi SJ, Johnson KM. Comparison of the effects of MK-801 and phencyclidine on catecholamine uptake and NMDA-induced norepinephrine release. Eur J Pharmacol. 1988;145:223–226. doi: 10.1016/0014-2999(88)90235-x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Tabakoff B, Hoffman PL. The NMDA receptor antagonist dizocilpine differentially affects environment-dependent and environment-independent ethanol tolerance. Psychopharmacology (Berl) 1994;113:511–517. doi: 10.1007/BF02245231. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Ritzmann RF. Acute tolerance in inbred and selected lines of mice. Drug Alcohol Depend. 1979;4:87–90. doi: 10.1016/0376-8716(79)90043-7. [DOI] [PubMed] [Google Scholar]
- Vanover KE. Interaction of ethanol with excitatory amino acid receptor antagonists in mice. Eur J Pharmacol. 1999;368:137–142. doi: 10.1016/s0014-2999(99)00030-8. [DOI] [PubMed] [Google Scholar]
- Velardo MJ, Simpson VJ, Zahniser NR. Differences in NMDA receptor antagonist-induced locomotor activity and [3H]MK-801 binding sites in short-sleep and long-sleep mice. Alcohol Clin Exp Res. 1998;22:1509–1515. [PubMed] [Google Scholar]
- Weidholz LM, Owen AM, Horton RE, Feyder M, Karlsson RM, Hefner K, Sprengel R, Ceikel T, Daws LC, Holmes A. Mice lacking the AMPA GluR1 receptor exhibit hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry. 2008;13:631–640. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Bosy TZ, Ruth JA. NMDA agonists and antagonists alter the hypnotic response to ethanol in LS and SS mice. Alcohol. 1990;7:389–395. doi: 10.1016/0741-8329(90)90021-4. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Collins AC. Different levels of [3H]MK-801 binding in long-sleep and short-sleep lines of mice. Alcohol. 1996;13:315–320. doi: 10.1016/0741-8329(95)02113-2. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Woodward JJ. Ionotropic glutamate receptors as sites of action for ethanol in the brain. Neurochem Int. 1999;35:107–113. [PubMed] [Google Scholar]
- Wu PH, Mihic SJ, Liu JF, Le AD, Kalant H. Blockade of chronic tolerance to ethanol by the NMDA antagonist, (+)-MK-801. Eur J Pharmacol. 1993;231:157–164. doi: 10.1016/0014-2999(93)90444-m. [DOI] [PubMed] [Google Scholar]
- Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003;27:1736–1742. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Negri CA, Hanania T, Gehle VM. MK-801-induced locomotor activity in long-sleep × short-sleep recombinant inbred mouse strains: correlational analysis with low-dose ethanol and provisional quantitative trait loci. Alcohol Clin Exp Res. 1999;23:1721–1729. [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]