Abstract
Beside chemical properties and topographical features, mechanical properties of gels have been recently demonstrated to play an important role in various cellular processes, including cell attachment, proliferation, and differentiation. In this work, we used multilayer films made of poly(L-lysine)/Hyaluronan (PLL/HA) of controlled stiffness to investigate the effects of mechanical properties of thin films on skeletal muscle cells (C2C12 cells) differentiation. Prior to differentiation, cells need to adhere and proliferate in growth medium. Stiff films (E0 > 320 kPa) promoted formation of focal adhesions and organization of the cytoskeleton as well as an enhanced proliferation, whereas soft films were not favorable for cell anchoring, spreading or proliferation. Then C2C12 cells were switched to a low serum containing medium to induce cell differentiation, which was also greatly dependent on film stiffness. Although myogenin and troponin T expressions were only moderately affected by film stiffness, the morphology of the myotubes exhibited striking stiffness-dependent differences. Soft films allowed differentiation only for few days and the myotubes were very short and thick. Cell clumping followed by aggregates detachment could be observed after ~2 to 4 days. On stiffer films, significantly more elongated and thinner myotubes were observed for up to ~ 2 weeks. Myotube striation was also observed but only for the stiffer films. These results demonstrate that film stiffness modulates deeply adhesion, proliferation and differentiation, each of these processes having its own stiffness requirement.
Keywords: self-assembled films, layer-by-layer, hyaluronan, cell differentiation, myoblasts, adhesion
1. Introduction
In the field of biomaterials, controlling the surface properties of the materials is of crucial importance as these properties can influence cell behavior including recolonization, adhesion, migration and possibly differentiation [1]. Cell/material interactions are influenced by a large number of parameters including surface chemistry [2, 3] and topography [4, 5]. More recently, the role of the mechanical properties of the matrix has began to be unravelled [6, 7]. Matrix stiffness was first found to have an effect on cell morphology, adhesion, proliferation and migration [6, 8, 9, 10, 11]. Very different cell types have been shown to be sensitive to matrix stiffness [6, 12, 13, 14] although the particular responses appear to be cell-type specific [9]. Most of these studies deal with “model” 3D gels of polyacrylamide (PA) or polydimethylsiloxane (PDMS), which can be prepared easily and whose elasticity can be tuned by varying the cross-linker concentration [6, 14]. Biodegradable alginate gels were also used to investigate the relation between chondrocyte and myoblast adhesion and gel elasticity [12, 15].
Recently, a new focus has emerged since the rigidity of a matrix was also found to influence higher order cellular processes such as cell differentiation. This is nicely exemplified by two examples. The first is the differentiation of breast epithelial cells into tubules when cultured in floating 3D collagen gels but not when cultured on gels attached to the culture dish [16]. The second is the recent finding that polyacrylamide (PA) gels of controlled stiffness can direct stem cell lineage specification. Soft matrices that mimic brain are neurogenic, stiffer matrices that mimic muscles are myogenic and comparatively rigid matrices that mimic collagenous bone prove osteogenic [17]. PA gels were also found to influence C2C12 skeletal muscle cell differentiation [10]. These studies on cell differentiation are important for understanding how cells are responding to their microenvironment. In particular, there are now several evidences that cardiac, vascular and muscle pathologies, such as as cardiomyopathies, atherosclerosis and muscular dystrophies are associated with alterations in the mechanical properties of the matrix. Thus, it is important for applications in the field of the regenerative medicine to characterize the sensitivity of the cells of interest to substrate mechanical properties.
An important question is whether this “mechano-sensitivity” effect remains valid on 2D substrates like films (thickness ranging from nanometer to micrometer thicknesses). The layer-by-layer technique that allows to prepare polyelectrolyte multilayer films (PEM) [18] seems to be an appropriate tool to answer such questions. These surface coatings can be deposited easily on biomaterial surfaces and have versatile properties. First, their bulk and surface chemistry can be modulated by carefully choosing the type of polymer used in the buildup and the film end-layer [19] [20] [21]. Second, their “bulk” mechanical properties can be modulated, either by incorporation of nanocolloids in the films [22], building films at different pH for a given polycation/polyanion pair [11], or covalently cross-linking the films using [23]. Using the carbodiimide chemistry, we recently prepared a wide range of films of increased stiffness by simply varying the cross-linker concentration [24]. Cross-linking was found to have only minor effects on film roughness and film wettability in the range where important differences in cell adhesion were observed [24]. Recently, we found that chondrosarcoma cell adhesion and spreading gradually increases when film cross-linking increases [24]. Also, Thompson et al recently showed that the attachment and proliferation of human microvascular endothelial cells (MVECs) can be regulated through independent changes in the rigidity and terminal polyion layers of PEM films made of synthetic polyelectrolytes [11].
Studies on cell differentiation in contact with PEM films have only begun to emerge due to the versatile properties of PEM films and in particular, to the possibility to embed in the films growth factors affecting cell fate [25]. An important question that remains unanswered, independently of growth factor insertion in the film, is whether thin film mechanical properties can affect cell differentiation. Poly(L-lysine)/Hyaluronan (PLL/HA) films represent a new way to address this question as they can be cross-linked at varying extents. To our knowledge, such type of film is the only one available at this date for tuning film Young’s modulus in a stepwise fashion.
In this study, we use (PLL/HA) cross-linked films at various degrees to investigate skeletal muscle cell differentiation. The C2C12 cells were chosen as model cells as this cell line is already widely used for studies of muscle biology and regeneration [26]. Myogenic differentiation on control tissue culture polystyrene surfaces is a well understood process in terms of the sequence of events and protein expression [27] [28]. In vitro and in vivo during muscle regeneration, the process of new muscle formation requires that quiescent mononucleated muscle precursor cells become activated, proliferate and differentiate. Thus, we first studied adhesion and proliferation of these cells, a prerequisite to cell differentiation. In vitro, differentiation is achieved by decreasing serum concentration, which inhibits proliferation and induces expression of myogenic-specific proteins associated with the contractile machine, including Troponin T and sarcomeric myosin.
To our knowledge, this is the first study aimed at elucidating the role of thin film mechanical properties (with film thickness in the micrometer range) on cell differentiation over a large range of film stiffness. Technical advances in the design of films with nanometer scale control of the properties (including mechanical properties, internal structure, roughness…) open thus a totally new field of research at the interface between material science and cell biology. Second, this study can help in the design of biocompatible artificial devices with controlled surface properties for a specific cell response. As PEM are more and more employed to coat implant surfaces [29], it is important to control the cellular events induced by the films. Third, skeletal muscle tissue engineering holds promise for the treatment of a variety of muscle diseases. Engineering muscle constructs in vitro provides an alternative to autologous transfer for tissue replacement.
2. Results
2.1. Film properties upon cross-linking
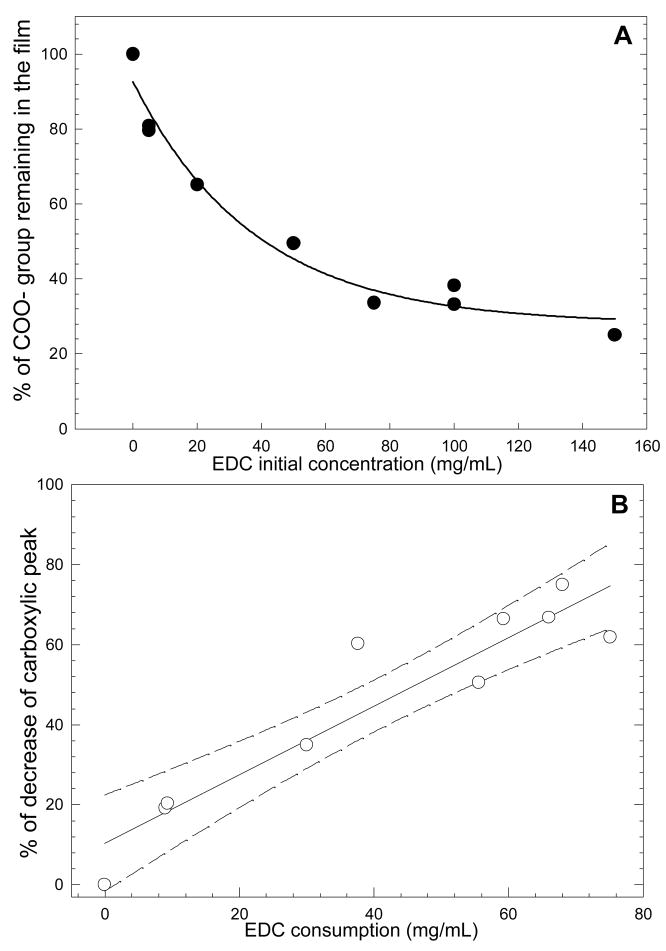
(PLL/HA)12 polyelectrolyte multilayer films of ~1μmin thickness [30] were used as a model for thin films of tunable rigidity. Their physico-chemical properties including film stiffness, as measured by their apparent surface elastic modulus E0, film roughness and serum protein adsorption are summarized in Table 1. Using the EDC cross-linking protocol, we were able to prepare films spanning two orders of magnitude in Young’s moduli (from ~3 kPa for native films, ~100 kPa for low cross-linked films to ~ 400 kPa for the highly cross-linked films) [24] with only minor variations in films roughness (from ~1 to ~7 nm). In addition, film thickness was found to vary by no more than 5% for the different cross-linking degrees (data not shown). Interestingly, the percentage of carboxylic groups engaged in cross-linking could be estimated by semi-quantitative infrared spectroscopy (Figure 1A). It steadily decreased when EDC concentration was increased. In addition, it correlated well with the consumption of EDC molecules (Figure 1B). As one carboxylic group forms a covalent amide bond with one amine group, the overall charge density in the film bulk should decrease, whereas the film surface should remain negative (due to the remaining free carboxylic groups). This was indeed verified by zeta potential measurements [23].
Table 1.
Summary of the physico-chemical properties of cross-linked (PLL/HA)12 films for EDC varied between 5 and 100 mg/mL (EDCx means that EDC final concentration is x mg/mL). Young’s modulus was determined by AFM nano-indentation experiments [31], film roughness was determined from AFM images of film topography. Serum adsorption was measured by quartz crystal microbalance. Film thickness remained constant at ~1μm for all conditions.
| Type of film | Native | EDC5 | EDC10 | EDC30 | EDC50 | EDC100 |
|---|---|---|---|---|---|---|
| Young’s modulus E0 (kPa) * | 3 | 99 | 162 | 301 | 354 | 382 |
| Fold increase | 1 | ~ × 33 | ~ × 54 | ~ × 100 | ~ × 118 | ~ × 127 |
| Roughness (nm) * | 1.1 ± 0.3 | NA | 3.8 ± 0.5 | 5.8 ± 0.4 | 7.1 ± 1.4 | 7.4 ± 0.2 |
| Serum adsorption (ng/cm2) | 1947 | NA | 88 | 62 | 44 | 177 |
Values taken from ref [24].
FIGURE 1.
(A) Percentage of carboxylic groups remaining in the film after the cross-linking procedure, as a function of the initial EDC concentration. (B) Percentage of decrease of the carboxylic peak (band at 1610 cm−1) plotted as a function of the EDC consumption (band at 1700 cm−1). Also shown is a regression line with the error interval (95%, dashed lines).
Film wettability was assessed prior and after contact with complete medium (medium containing 10% foetal bovine serum). The native films are moderately hydrophobic but all the cross-linked films were very hydrophilic, independently of the cross-linker concentration, with water contact angles being systematically below 10° (prior to contact with serum) and below 20° (after contact with serum) (Figure 2). Also, the amount of proteins adsorbed from the serum was quantified by quartz crystal microbalance (Table 1). It is much lower for all the cross-linked films (~100 ng/cm2 ) than for the native one (~20 times higher).
FIGURE 2.
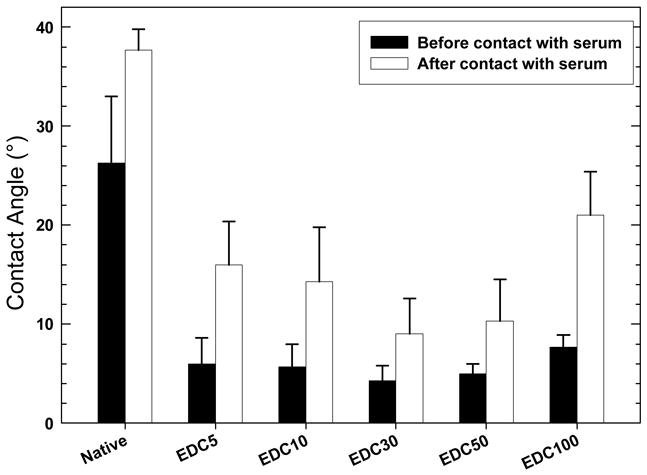
Water contact angle for native and cross-linked films at increasing EDC concentrations, prior (black bars) and after contact with growth medium containing 10% FBS (white bars) (data are means ± standard deviation, SD of three independent slides).
2.1. Film cross-linking modulates myoblast attachment, proliferation and cytoskeletal organization
Before investigating C2C12 skeletal myoblast differentiation, cells need to adhere and proliferate on the films. C2C12 cells were thus cultured in growth medium (GM) on (PLL/HA)12 films cross-linked at various EDC concentrations. Few cells attached on the native (e.g. un-cross-linked) films (Figure S1), whereas the cells cultured on the cross-linked films adhered and spread (Figure S1–S2). The cell number increased with cross-linking and significant differences exist between low and high cross-linked films. However, for EDC concentrations higher than 50 mg/mL, all the seeded cells attached as well as on plastic (Fig. S1A). Cell proliferation measured by direct cell counting and by a BrdU proliferation assay was also affected by cross-linking (Figure S1, B, C). For the native films, cell number decreased as a function of time. Cells cultured on EDC2 and EDC5 films show little proliferation. Cells grown on EDC10 cross-linked films exhibit a slightly higher proliferation rate, but still significantly lower than for stiffer films, for which the proliferation rate levels off for EDC concentration equal or greater than 50 mg/mL (Figure S1B). These results were confirmed by the BrdU quantification test (Figure S1C).
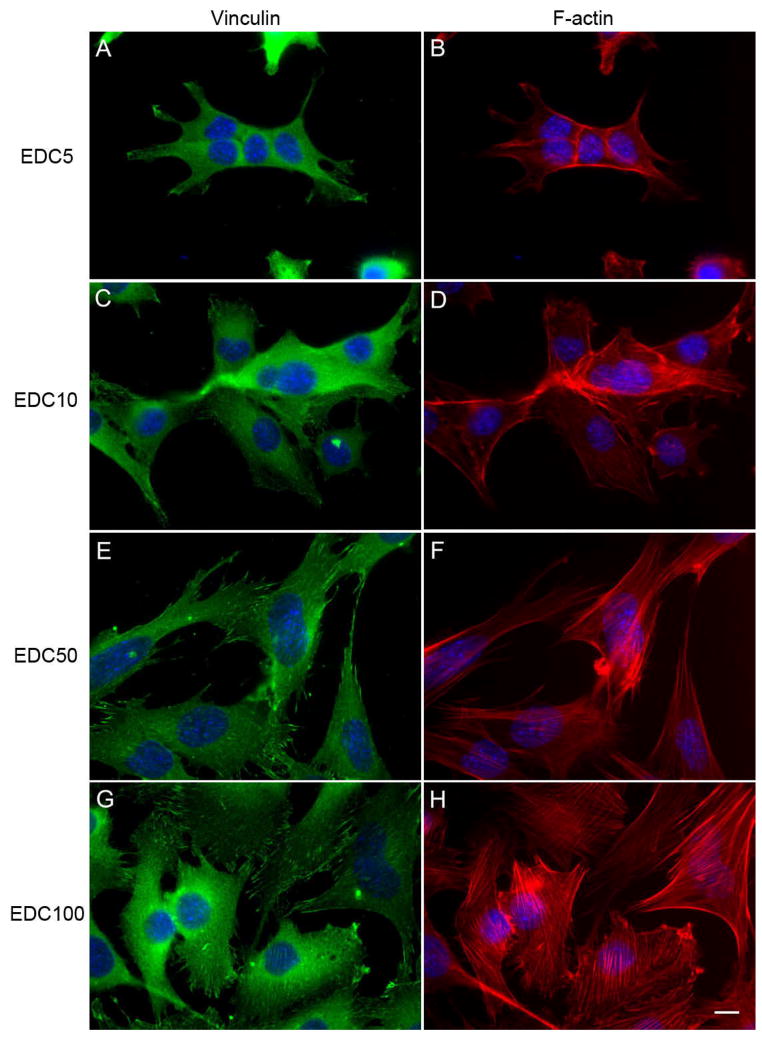
To determine whether focal adhesions and actin are affected by film stiffness, C2C12 cells on cross-linked films were stained by immuno-fluorescence for vinculin, a component of focal adhesions (Figure 3, A, C, E, G) and F-actin (Figure 3, B, D, F, H) after one day in GM. One can observe that cells cultured on the EDC5 and EDC10 films show no clear vinculin plaques nor F-actin stress fibers (Figure 3, A–D). In contrary, cells cultured on the EDC50 and EDC100 films show numerous, large, elongated focal adhesions and well organized F-actin stress fibers. Vinculin is clearly localized at the tip of elongated structures. The number density of focal adhesions is also significantly increased on EDC50 and EDC100 films as compared to the low cross-linked films (Figure 4). Obviously the cells developed more numerous and better organized adhesion structures on the more cross-linked films.
FIGURE 3.
Film cross-linking induces organization of focal adhesion proteins and of the cytoskeleton. Vinculin (left images) and cytoskeletal actin (right images) were observed for cells cultured on (PLL/HA)12 films cross-linked at various EDC concentrations after one day in growth medium: EDC5 (A, B), EDC10 (C, D), EDC50 (E, F), and EDC100 (G, H) (Scale bar is 10 μm).
FIGURE 4.
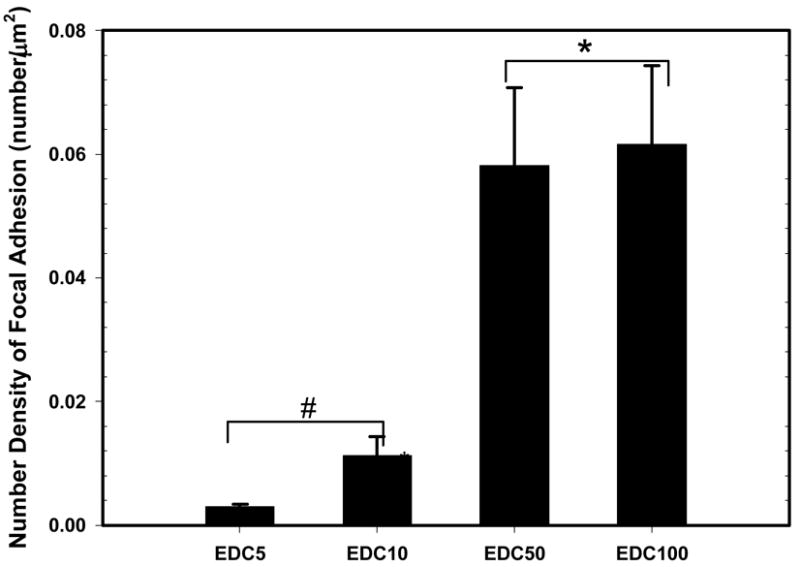
Quantification of the number density of focal adhesions based on the images of Figure 3 (data are means ± standard deviation, SD of three slides). Conditions, which are statistically different can be divided in two groups: (EDC5 and EDC10, #) and (EDC50 and EDC100, *) (p < 0.05).
We then focused on the observation of these cell/film contacts using the SEM technique at high resolution (Figure S3). Depending on film cross-linking, the cell/film contacts, although numerous for all conditions, exhibit very different morphologies. On the softest films (EDC5 and EDC10), short and small microspikes or filopodia are visible (Figure S3A), some of them being as long as 5μm on EDC10 films (Figure S3B). Cells cultured on the stiffest films (EDC50 and EDC100) show numerous, very thin and long filopodia and/or cell extensions, whose length can reach about ten micrometers (Figure S3 C,D). Lamellipodia are visible [32] and dorsal microvilli can also be observed on the stiffest films.
All these observations suggest that it is difficult for the cells to anchor and spread on the softest films whereas the highly cross-linked films are much more favorable for cell attachment and anchoring. Based on the results obtained on attachment and proliferation, several cross-linking conditions reported in Table 1 were chosen for investigating cell differentiation.
2.2 Film cross-linking influences the myogenic differentiation process morphologically but not the expression of muscle-specific proteins
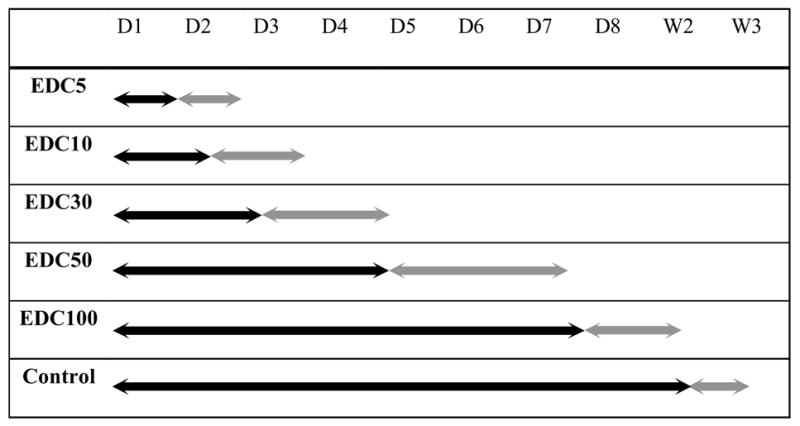
The development of skeletal muscle is a multistep process, including initial cell proliferation, subsequent withdrawal from the cell cycle and differentiation into multinucleated myotubes [28]. To determine the effect of film stiffness on C2C12 cells differentiation, cells were first allowed to proliferate close to confluence in GM and then switched to differentiation medium (DM) to induce differentiation. We took care that cell density was the same for all films when switching to DM. We first followed by phase-contrast microscopy over several days the overall behavior of cells on films cross-linked at different EDC concentrations. During differentiation, C2C12 cells align and fuse together to form myotubes, when cultured on control tissue culture polystyrene surfaces (Figure 5, J–L), as usually observed [33] (plastic was always used as control surface as C2C12 cells are known to not differentiate optimally on bare glass [10]). We qualitatively observed cell differentiation, i.e. cell alignment, fusion and formation of myotubes, on all types of films and noticed important differences in the fate of the cells and in the kinetics of the events (Table 2 and Figure 5). For very soft films (EDC5, 10), few cells fused and cells began to detach after two to three days in DM. Thus, progressively, cells formed large aggregates or “clumps” and started to loose connectivity with the films. Finally, they detached within 2–3 days (Table 2, gray arrow). This phenomenon could be observed for stiffer films, although it was greatly delayed. For EDC30, cells differentiated and formed myotubes after one to two days in DM (Figure 5B). Later on, they also started to detach from the films (beginning between the third and fourth day) as can be observed on Figure 5C. The more cross-linked the films are, the longer is the time period over which differentiation progresses and myotubes could remain adherent on the films. Thus, cells grown on EDC50 films form myotubes (visible at day 3 in Figure 5E) that begin to clump between day 5 and day 7. This aggregation begins to be observable in Figure 5F. For films grown on EDC100 films, myotubes that formed after two days orientate, elongate and thicken over the course of the experiment (Figure 5H, I). They remain adherent until they also begin to clump and detach between day 8 and day 10 (data not shown). Noticeably, we also observed a spontaneous detachment for cells differentiated on the plastic surface after 12 to 15 days in culture (Table 2). Thus, the kinetics of detachment occurred on time periods from days to weeks depending on the film stiffness. We also noticed that the largest variability from one experiment to the other one was observed for the soft films (EDC ≤10 mg/mL).
FIGURE 5.
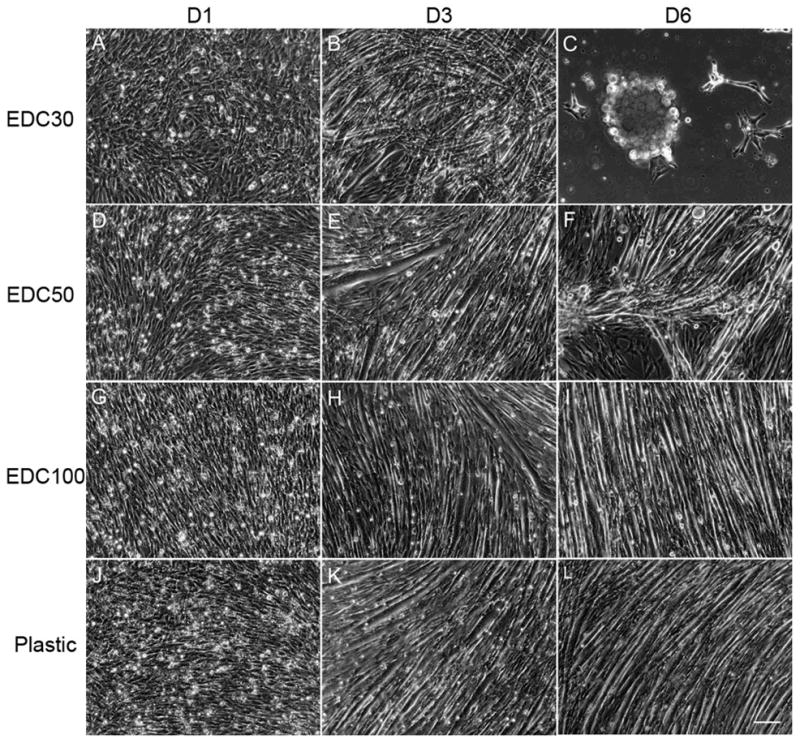
Film cross-linking affects cell morphology during differentiation. Phase contrast microscopy observations of C2C12 cells differentiation cultured in DM on (PLL/HA)12 films cross-linked at various EDC concentrations at day 1 (left), day 3 (middle) and day 6 (right), respectively: EDC30, 300 kPa (A, B, C), EDC50, 354 kPa (D, E, F), EDC100, 382 kPa (G, H, I) and tissue culture polystyrene, P (J, K, L) (Scale bar is 100 μm).
Table 2.
Summary of the phase-contrast microscopy observations for cells cultured on cross-linked films (CL) and on tissue culture polystyrene for several days (D) or weeks (W) in DM. Black bars indicate the time period over which cell can stably attach and differentiate on the films; gray bars indicate the time period over which the cells began to aggregate (prior to detachment from the films). The detachment could occur over several days (for low and medium CL films) or even weeks (in the case of EDC 100 and control surfaces). (results from at least four independent experiments for each condition, three slides per condition for each experiment).
|
Myoblast differentiation onto the cross-linked (PLL/HA)12 films was then followed by analysis of the expression of muscle-specific proteins like myogenin and troponin T. For control surfaces, these proteins are known to be expressed after about 1–2 days in DM [33, 34] and until at least day 6. Immunoblots of myogenin and troponin T expression at day 3 and day 6 in DM, for cells cultured on EDC30, EDC50 and EDC100 films are shown in Figure 6. One can observe that cells on cross-linked films express similar levels of both markers as compared to the control, except for the EDC30 film at day 6 for which protein expression decreased. This may be attributed to cell detachment that is almost completed at day 6 on this film type and may affect markers expression. From these immunoblots, one can thus conclude that both early myogenin and later troponin markers of C2C12 differentiation stages are not affected by film cross-linking for the highest EDC concentrations.
FIGURE 6.
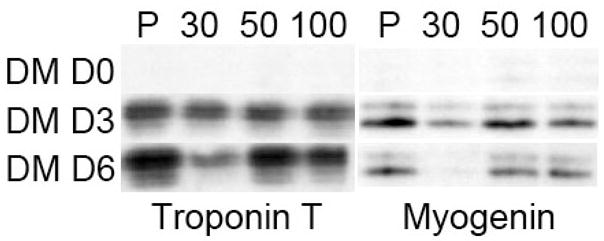
Myogenin and troponin T expressions for C2C12 cells cultured on (PLL/HA)12 films cross-linked at various EDC concentrations (EDC30, EDC50 and EDC100) as compared to tissue-culture plastic (P). The expressions were assessed by immunoblot analysis of 20 μg protein of cell lysates collected before differentiation (D0), after 3 and 6 days (D3, D6) of culture in differentiation medium (DM).
2.3 Quantification of myotubes morphology and striation
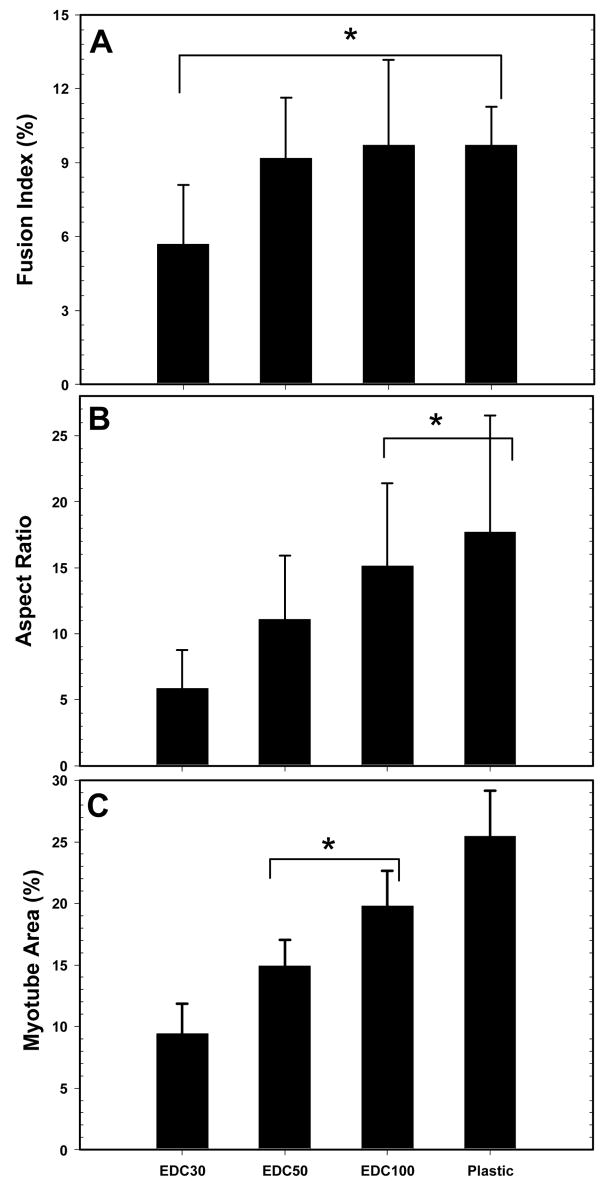
To further quantify the differentiation process, immuno-staining for troponin T, myf5 and myogenin (data not shown) were performed after three days in DM onto the different films (Figure 7). Whereas film cross-linking had no striking effect on the expression of muscle-specific proteins, it had a significant effect on myotubes morphology and size. On EDC30 films, myotubes are relatively short but very thick and nuclei tend to cluster and not to be well aligned (Figure 7A). On EDC50 films, myotubes exhibit longer and thinner shapes (Figure 7B). They are even longer and thinner on EDC100 films and on the control surface (Figure 7C and D). From these images, several parameters could be calculated: the fusion index, the aspect ratio and the surface areas of the myotubes (Figure 8).
FIGURE 7.
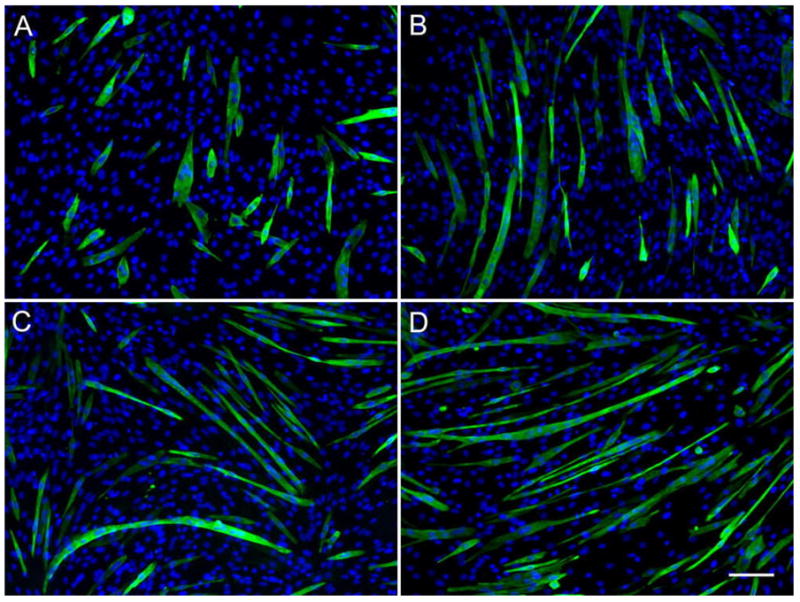
Film cross-linking affects myotubes morphology. Immuno-fluorescence images of troponin T (green) and DNA (blue) of C2C12 cells cultured on (PLL/HA)12 films cross-linked at various EDC concentration after 3 days in DM: EDC30 (A), EDC50 (B), EDC100 (C), as compared to tissue-culture plastic (D). (Scale bar is 100 μm)
FIGURE 8.
Quantification of myotubes fusion and morphology at Day 3 in DM: fusion index (A), aspect ratio of myotubes (B), total myotubes area over a given surface expressed in percentage (five different fields were measured for each condition) (C). Data are means ± SD. The conditions linked by (*) are statistically similar, the other one being all different (p < 0.05).
The fusion index is for EDC30 films about half that of the other films and control surfaces (5.6 % as compared to about 10%), which are very similar (Figure 8A). However, differences were not statistically significant. Thus, the fusion index is a parameter which does not appear to be very sensitive to film stiffness. However, when one looks at the aspect ratio of the myotubes and at their surface areas, striking differences begin to emerge (Figure 8B and C). Both parameters significantly increase with film cross-linking. The highest values for both parameters are always obtained on the control tissue culture polystyrene surface. Thus, even if myotubes can form on all cross-linked films, their morphology appears to be very different. From relatively thick and short on the softest films (EDC30), they begin to elongate and to be thinner when EDC concentration is increased, the maximum of elongation and of surface area being reached at EDC100 (E0 ~400 kPa).
It is worth noting that under conditions where myotubes area are similar (EDC50 and EDC100), the aspect ratio is higher for EDC100. After the initial fusion into nascent myotubes, the myotubes should further grow by fusion and subsequently express myosin heavy chain (MHC) leading to a striated pattern [28]. Thus, C2C12 cells grown for 7 days on the more cross-linked films (EDC50 and EDC100) and on plastic surface were stained for MHC (Figure 9). In fact, lower EDC concentrations could not be investigated in this later part of the differentiation process due to premature cell detachment. One can first notice that myotubes show clear MHC staining on both film and control surfaces. The number of striated myotubes was quantified for the different surfaces and showed great differences depending on the film stiffness (Figure 9D). For EDC50 films, the low percentage of striation (~14%) was related to a diffuse myosin organization. The percentage of striation increased for stiffer EDC100 films (~43%) and was maximal for the control surface (~69%). These results further suggest that film cross-linking not only affects the morphology of myotubes, as already observed when comparing the myotube area and the aspect ratio, but also the progression of differentiation.
FIGURE 9.
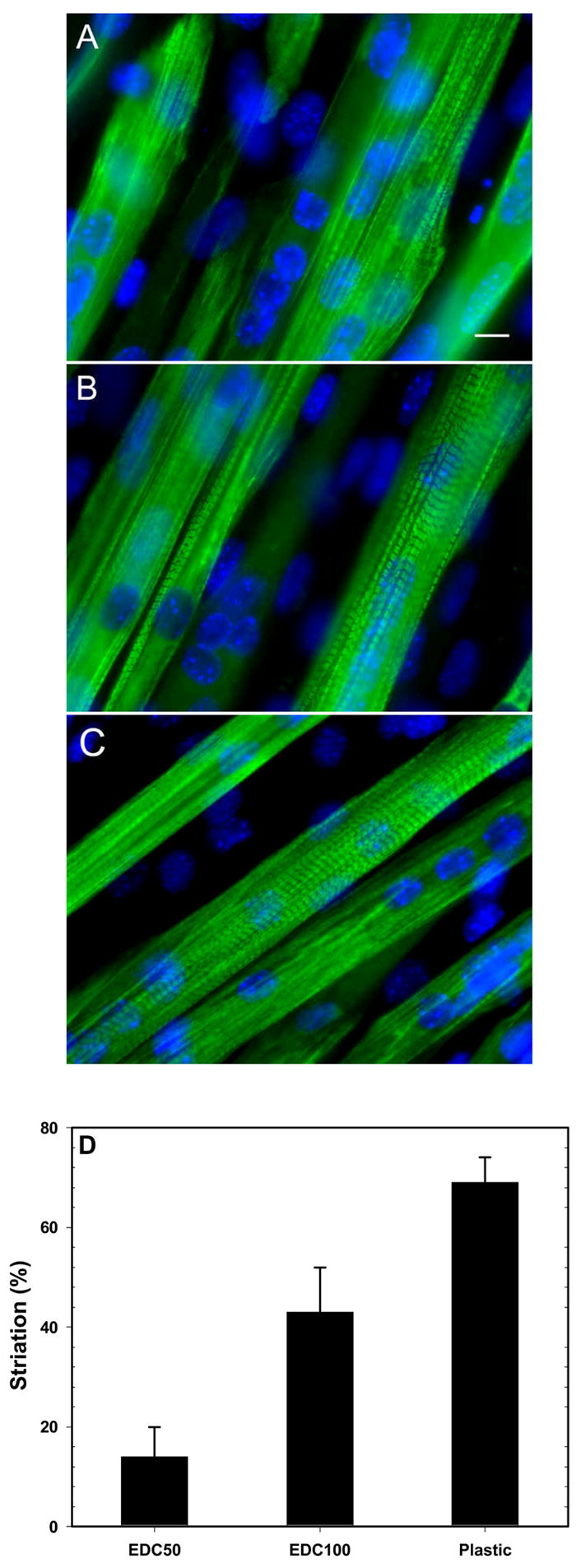
Film cross-linking affects the striation of myotubes. Immuno-fluorescence images of myosin heavy chain (MHC) for C2C12 cells cultured on (PLL/HA)12 films cross-linked at various EDC concentration after 7 days in DM: EDC50 (A), EDC100 (B) and plastic (C) (Scale bar is 10 μm). The percentage of striated myotubes was also calculated for the different surfaces (D). Data are means from at least 100 myotubes (means ± SD).
3. Discussion
3.1. Relative importance of film stiffness, roughness and wettability upon film cross-linking
By looking at the data from Table 1, it appears clearly that not only E0 increased as a function of film cross-linking, but that also film roughness is increasing (from ~4 nm to 7.5 nm for cross-linked films). It may thus be argued that it is difficult to pinpoint the causing effects of the mechanics from those resulting from the topography (surface roughness) and from the chemistry (nature of chemical groups, hydrophilicity). However, useful information can be gained from previous studies that investigated either the role of surface roughness or wettability. At relatively high roughness (submicrometer down to ~100 nm), it is clear that substrate topography influences cell response, the cells responding to roughness by a higher cell thickness and a delayed apparition of the focal contacts [5, 35]. At the nanometer scale, Washburn et al. [36] showed that the rate of proliferation on the smooth regions of a poly(L-lactic acid) film (below 1 nm) is much greater than on the rough regions (around 12 nm). The observed variations in proliferation were however weak in the 3–7nm range. Similarly, Cai et al showed that the titanium nanometer scale surface roughness (from 2 to 21 nm) does not significantly affect protein adsorption nor osteoblast proliferation or cell viability [37]. For (PLL/HA) cross-linked films, the change in roughness is very small (from ~ 4 to 7.5 nm for EDC10 and 100, respectively) and the films are not structured topographically. Also, the low serum protein adsorption for all cross-linked films did not correlate with the cross-linking degree. Thus, film roughness and serum protein adsorption are unlikely to contribute to the important changes observed in C2C12 adhesion, proliferation and differentiation.
With respect to film chemistry and, as a consequence, water contact angle, it is important to point out that the nature of the functional groups at the film surface remains unchanged (carboxylic groups), even if the overall charge density in the film decreases due to cross-linking. Indeed, all the (PLL/HA) cross-linked films are hydrophilic and exhibit only minor variations in the wettability with contact angles lower than 10° for cross-linked films before contact with serum (resp. below 20° after contact with serum). This is very different from other types of surfaces, such as self-assembled monolayers (SAMs) presenting very different functional groups (such as methyl, vinyl, amine, carboxyl, hydroxyl [38]), for which water contact angles span a much larger range (15°– 90°). In the case of SAMs, differences in cell attachment [39] as well as differences in myoblast differentiation [2] could be attributed to film chemistry and/or wettability. The nature of the functional groups was indeed found to be the most important factor [40]. For all these reasons, small changes in (PLL/HA) film chemistry and wettability upon cross-linking are likely to play a minor role in the observed differences in cell behavior.
As the highest changes in cell behaviors correlate with the highest changes in Young’s moduli (E0 varying from ~100 kPa for EDC5 to ~400 kPa for EDC100 in a monotonic way), it is likely that film stiffness is the most important parameter.
3.2. Mechano-sensitivity on various types of substrates: effect on adhesion and proliferation
We demonstrate that film mechanical properties modulate myoblast proliferation and anchorage. As an almost general rule, most cell types tend to adhere and spread better onto a stiff matrix than onto a soft one [6]. As already observed for C2C12 cells on polyacrylamide matrices [10, 17], F-actin and stress fibers in cells are also increasingly organized when film rigidity is increased. Similarly, the number and length of motile structures (like microspikes, filopodia and lamellipodia) are also related to film stiffness (Figure S3). Importantly, it has to be noticed that the previous studies were always performed on model synthetic matrices (PA [6], poly(dimethylsiloxane)[41] and N-isopropylacrylamide (NiPAM)[42]) or alginate gels [12] that all required the grafting of adhesive ligands or the adsorption of matrix proteins (collagen [43], fibronectin [2], or laminin [44]) to allow for cell adhesion. The surface chemical modification of these gel matrices was indeed an absolute prerequisite for making cells to adhere in contrary to the data presented here for the thin (PLL/HA) cross-linked films. Very interestingly, we provide for the first time a new type of surface modification, a polyelectrolyte multilayer film of controlled thickness containing biocompatible polymers (PLL and HA) that has the ability to allow cell adhesion without the need for an additional adhesive protein pre-coating of the surface. Once attached, cells further proliferate. However, adhesion and proliferation rate are not equally sensitive to film stiffness (Figure S1). In the present case, for (PLL/HA)12 films, cells develop well organized adhesive structures and proliferate well when E0 is higher than 350 kPa (corresponding to a EDC50 film). All together, our data suggest that adhesion is the weakest for the soft films, which is consistent with the recent data obtained for C2C12 on PA gels [10, 17].
3.3 Effect of film cross-linking on early differentiation
Notably, our results demonstrate that the effects of film stiffness influence a high order cellular process, such as differentiation. During normal myogenesis, myoblasts must exit the cell cycle and follow an ordered set of cellular events, including cell adhesion, migration and membrane fusion [45]. Mammalian myoblast fusion occurs in two phases [46]. Initially, myoblasts fuse with one another to form small, nascent myotubes. Additional myoblasts subsequently fuse with nascent myotubes, leading to the formation of large, mature myotubes. Myoblast differentiation requires much more than just adhesion and proliferation and the material surface appears extremely important. Thus, C1C12 cells can adhere but not proliferate nor differentiate on crosslinked chitosan gels [47] and they even differentiate into osteoblasts (instead of differentiating into myotubes) when cultured on N-isopropylacrylamide (NiPAM) polymers [42]. In the present case, an important finding is that all cross-linked films allow myoblast differentiation. Furthermore, our results show that early stages of the differentiation process most likely depend on the (PLL/HA)12 film stiffness. For soft films, cells begin to fuse and then form clumps that will prematurely detach from the films (after few days in DM). For stiff films (like EDC100 films), cell detachment will also occur but is greatly delayed in time. In this latter case however, long and thin myotubes have reached a late stage and are already clearly striated (Figure 7 and 9). An intermediate case is observed for moderately cross-linked films (EDC30, E0 ~ 300 kPa) for which myotubes form but detached prior to being striated. Importantly, it has to be noticed that this detachment was not attributed to a film rupture, as was checked by confocal laser scanning microscopy using fluorescently labeled PLL for visualizing the film [48] (data not shown). Such aggregate formation and spontaneous detachment after 2–3 days has indeed already been observed for C2C12 cells cultured on alginate matrices of various compositions [15]. In this case, the cells that tended to aggregate were apparently the non-fused ones. C2C12 detachment was also evidenced for cells plated on a Sylgard substrate [49] or when they were plated at a high density on wavy micropatterned silicone substrates [44]. In this latter study, the detachment was attributed to cell overgrowth from the initial high plating density and mentioned that the balance between cell/cell interactions and cell/substrate interactions is particularly important.
Interestingly, the expression of myogenic differentiation markers like Troponin T and myogenin at D3 (Figure 6) is very similar for all film types, as well as the fusion index, which is only slightly lower for EDC30 films. For the cross-linked (PLL/HA) films, the aspect ratio and the myotubes area are however significantly lower for the medium cross-linking condition (Figure 8). Thus, the differentiation process is engaged but the morphological consequences of the differentiation greatly depend on the film properties. In our case, the short, thick myotubes (with no nuclei alignment) observed on EDC30 films as well as their subsequent detachment may be related to cell/film contacts. For soft films, the absence of well organized adhesion structures may lead to a deregulated myofibril assembly, which may hinder further myotubes growth. Myosin II, a molecular motor known to play an important role in the generation of traction forces and in contractility [17, 50] might also be involved in the cell clumping observed on soft films. Another hypothesis is that film stiffness might affect myoblast motility, a parameter that was recently shown to be very important in the growth of myotubes after their initial formation [51] and which is currently under study.
3.4 Later stages of differentiation also depends on film cross-linking
Noticeably, the morphological differences between the films are even increased at a later stage in cell differentiation, when myotubes are striated (Figure 9). At this stage only reached by the stiffest films (EDC ≥ 50, E0 ~ 350 kPa), a relatively small difference in film stiffness induces an appreciable effect on the percentage of striated myotubes, the tissue culture polystyrene surface remaining however the most favorable for myotube striation. We thus observed a correlation between films stiffness and myotubes striation, increased stiffness leading to a higher striation percentage. This is somewhat different from the results obtained on patterned PA gels for which striation was optimal at an intermediate Young’s modulus of 12 kPa [10]. As myotubes are exerting increasing forces upon formation, while they are contracting, the cell-film adhesion has to be strong enough to support the whole differentiation process [52]. The contractile force caused by myotubes could thus be the main reason for the late detachment observed on the stiffest films. The more mature the myotubes are, the higher force they will produce [53]. In fact, Engler et al showed that the adhesion force of isolated mature myotubes cultured on micropatterned polyacrylamide gels increases exponentially with substrate stiffness [10].
The hypotheses regarding the role of film stiffness in the early stage fusion process and the late growth of striated myotubes will have to be further checked at a molecular level. Which molecules or complexes are sensitive to film stiffness are presently unclear and remain to be elucidated in the future.
4. Conclusions
We demonstrate that film stiffness modulates deeply not only initial myoblast adhesion, proliferation but also myoblast differentiation into myotubes. Very importantly, cross-linked (PLL/HA) films do not require a specific protein or ligand pre-coating for being adhesive and they adsorb only low amounts of serum proteins, as compared to the native films. In addition, early steps of myoblast fusion into myotubes occurred for a wide range of film stiffness. However, myotubes morphology (aspect ratio, surface area) and striation exhibited significant film-stiffness dependent differences. On soft films, myotubes are initially short and thick before clumping and detaching collectively. On tissue culture polystyrene and on stiff films, cells form elongated and thin striated myotubes. Differences in cell motility, in cell/film or cell/cell molecular contacts may be the reasons for these observations. Further studies will be conducted to fully elucidate this point. The present findings highlight, in particular, the important role of the surface mechanical properties in cell/material interactions. As the polyelectrolyte multilayer films of controlled stiffness can also be loaded with bioactive molecules [54] and present the advantages of being non-toxic, biocompatible and biodegradable [55], they could be further be employed as “multi-functional” films to investigate the respective roles of stiffness versus bioactivity and to control several key cellular processes. These PEM films could also find applications in the fields of regenerative medicine and muscle/cardiac tissue engineering, where controlling the cell/material interactions is crucial for guiding the cellular response. On a more fundamental point of view, these nanoscale films are particularly relevant to cell studies, as many different parameters, and among which mechanical properties, can be varied. Future investigations of subcellular responses to mechanical cues are thus foreseen. (cette parti reprend la phrase surligné en vert)
6. Materials and Methods
6.1. Film preparation and cross-linking procedure
HA (sodium hyaluronate, 3.5×105 g/mol) was purchased from Medipol (Switzerland) and PLL (2.6×104 g/mol) was purchased from Sigma (France). PLL (0.5 mg/mL) and HA (1 mg/mL) were dissolved in a Hepes buffer (20 mM Hepes and 0.15 M NaCl at pH 7.4). During (PLL/HA)12 film construction, all of the rinsing steps were performed with 0.15 M NaCl aqueous solution at pH ~6. The films were prepared as previously described [24] with a dipping machine (Dipping Robot DR3, Kierstein GmbH, Germany) on 14 mm diameter glass slides (VWR Scientific, France) for microscopy and immuno-cytochemistry and on 24×24 mm square glass slides for Western Blot.
For film cross-linking (CL), we followed a protocol using the water soluble carbodiimide, 1-Ethyl-3-(3-Dimethylamino-propyl)Carbodiimide (EDC) and N-Hydrosulfosuccinimide (sulfo-NHS) (both purchased from Sigma, St Quentin-Fallavier, France) [23] (EDC varying from 2 to 100 mg/mL and sulfo-NHS being fixed at 11 mg/mL).
6.2. Film characterization
Several techniques were used to characterize the film properties including atomic force microsocpy (AFM) imaging, Fourier transform infrared spectroscopy measurements in attenuated total reflection (ATR-FTIR), quart crystal microbalance (QCM-D) and wettability measurements. These experimental methods have been extensively described in our previous publications [24] [56]. For the determination of the percentage of carboxylic groups remaining in the film, values of the absorbance corresponding to the –COO− asymmetric stretch from HA (1610 cm−1) were taken at the end of the film buildup and after cross-linking (after the final rinsing step). For the wettability measurements, films in the NaCl solution or films that have been in contact with the serum containing medium (10% FBS) for 2 h were first rinsed with the NaCl solution, then with pure water, dried under a nitrogen flow for 5 min, let at room temperature overnight prior to being assayed. For QCM-D measurements of the adsorbed protein amounts, the (PLL/HA)12 films were built ex-situ on the SiO2 coated QCM crystals using the automatic dipping machine and were subsequently cross-linked overnight. Each film coated crystal was introduced in the QCM chamber, equilibrated at 25°C first with 0.15 M NaCl (pH ~6) and then with the medium for ~4 h, after which the serum containing medium was flown over the film for ~1 h. The frequency shifts were measured at four frequencies and the third overtone (ν = corresponding to the 15-MHz resonance frequency) was used to calculate the adsorbed mass according to Sauerbrey equation, m=−CΔf/ν, where C is a constant characteristic of the crystal used (C= 17.7 ng.cm−2.Hz−1).
6.3. Cell Culture
C2C12 cells (< 20 passages, kindly provided by Cécile Gauthier-Rouvière, CRBM, Montpellier) were maintained in polystyrene flasks in a 37 °C, 5% CO2 incubator, and cultured in a 1:1 Dulbecco’s Modified Eagle Medium (DMEM)/Ham’s F12 medium (growth medium, GM, Gibco, Invitrogen, Cergy-Pontoise, France) supplemented with 10% foetal bovine serum (FBS, PAA Laboratories, Les Mureaux, France), containing 100 U/mL penicillin G and 100 μg/mL streptomycin (Gibco, Invitrogen, Cergy-Pontoise, France). Cells were subcultured prior to reaching 60–70% confluence (approximately every 2 days). For differentiation experiments, C2C12 cells seeded on films at 15 000 cells/cm2 in GM were allowed to grow for two days and were then switched to the differentiation medium (DM, 1:1 DMEM/F12) supplemented with 2% horse serum (HS, PAA Laboratories, Les Mureaux, France), containing 10 U/mL penicillin G and 10 μg/mL streptomycin. Tissue culture polystyrene was always used as control.
6.4. Attachment and Proliferation Assays
For the cell counting test, (PLL/HA)12 films were directly fabricated into 96-well plates. Briefly, 50 μL of PLL were introduced into each well and let adsorbed for 8 min. Wells were then washed twice with the rinsing solution and 50 μL of HA were introduced in each well, let adsorbed for 8 min and subsequently rinsed. The process was repeated until 12 layer pairs films have been deposited. Then, films were cross-linked prior to cell seeding at 7 500 cells/cm2 in GM. Cell numbers were assessed after different time periods in culture using a cell counting kit (CyQUANT, Molecular Probes, Invitrogen, Cergy-Pontoise, France). In brief, cells were washed three times with phosphate buffered saline (PBS) and frozen at −80 °C overnight. After thawing the cells at room temperature (RT), a mixture of the cyQUANT GR dye and cell-lysis buffer was introduced and fluorescence of the plates was directly measured using a fluorescence microplate reader (Twinkle LB 970, Berthold, Germany) with the excitation filter set at 485 ± 7 nm and emission filter at 535 ± 12 nm.
Cell incorporation of BrdU (5-bromo-2′-deoxyuridine) was determined using a cell proliferation kit (GE Healthcare Biosciences, Orsay, France) [57] according to the provider’s instructions.
6.5. Immunofluorescence
Cells were fixed in 3.7% formaldehyde in PBS followed by 4 min of permeabilization in 0.2% Triton X-100 in PBS. Antibodies were incubated for 60 min in PBS containing 0.1% bovine serum albumin (BSA, Roche, Germany). Nuclei were stained with Hoechst 33342 (Molecular Probes, Invitrogen, France) at 5 μg/mL for 10 min at RT. For the attachment experiments, cells were labeled with rhodamine-phalloidin (1:800, Sigma, France) and monoclonal mouse anti-vinculin (1:400, Sigma, France). The number density of focal adhesions was calculated as the number of focal adhesions divided by the total cell area [50]. For the differentiation experiments, cells were labeled with monoclonal mouse anti-troponin T (1:100), mouse anti-myosin heavy chain (MHC, 1:500) (both from Sigma, France), rabbit anti-myogenin (1:60), or rabbit anti-Myf-5 (1:300) (both from Santa Cruz Biotechnology, Tebu-Bio, France) [58]. Primary antibodies were revealed using Alexa Fluor 488 or Alexa Fluor 572-conjugated goat anti-mouse or anti-rabbit antibodies (1:1000, Molecular Probes-Invitrogen, Cergy-Pontoise, France) as secondary antibodies. The samples were mounted onto coverslips with antifade reagent (ProLong Gold, Molecular Probes-Invitrogen, France) and viewed under fluorescence microscopy (Axiovert 200M, Zeiss, Germany) using a 10X and 63X apochromat objective. Images were obtained using a CoolSNAP EZ CCD camera and acquired with Metavue software (both from Ropper Scientific, Evry, France).
6.6. Scanning Electron Microscopy
Cells grown on CL films for 24 h were fixed in 2.5% glutaraldehyde/0.1 M sodium cacodylate/0.1 M sucrose, pH 7.2. Samples were then gradually dehydrated using increasing concentrations of ethanol and processed for SEM as previously described [59] [60], followed by critical point drying with CO2 and gold sputtering. The samples were observed in a Hitachi S4000 scanning electron microscope (Tokyo, Japan) at 15 kV.
6.7. Differentiation assay
For differentiation experiments, C2C12 cells seeded on the CL films at 15 000 cells/cm2 in GM were allowed to grow for 2 days and were then switched to DM. Cells were imaged over several days or even weeks by phase-contrast microscopy (10× objective). At least four different time-lapse observations were performed for each film type. Immuno-fluorescence labeling of myogenic proteins were performed as described above. All image quantifications were performed using Image J software v1.38 (NIH, Bethesda) on cells labeled with anti-troponin T or MHC. The fusion index (FI) was determined by dividing the total number of nuclei in myotubes (≥ 2 nuclei) by the total number of nuclei counted [33]. The aspect ratio (AR) was determined by dividing the length of myotubes by their width. The total areas of differentiated cells were measured over an entire image. The percentage of striation of myotubes was determined by dividing the number of striated myotubes by the total number of myotubes. More than 100 myotubes were analyzed for each slide (two slides per condition, experiments performed in triplicate).
6.8. Gel Electrophoresis and Immunoblotting
Cells cultured on 24×24 mm slides in 6-well plates were scraped and rinsed in PBS. Cells were then lysed in Laemmli sample buffer (40 mM Tris-HCl, pH 6.8, 5 mM DTT, 1% SDS, 7.5% glycerol, 0.01% bromophenol blue). After boiling, 20 μg of protein samples were loaded on a 15% polyacrylamide gel and transferred onto Immobilon-P membranes (Millipore, Molsheim, France). Membranes were saturated in 5% milk in PBS (pH 7.4) containing 0.5% Tween 20 (PBST) for 1 h and subsequently incubated with monoclonal antibodies against myogenin (1:500, BD Biosciences, Pont-de-Claix, France), troponin T (1:5000, Sigma, France). Membranes were washed and incubated with a peroxidase-conjugated anti-mouse secondary antibody (1:10000, Amersham Biosciences, USA). After washing, membranes were incubated with chemiluminescence reagents and detected by scanning autoradiographs (enhanced chemiluminescence, HyperFilm™ ECL, GE Healthcare, Orsay, France).
6.9. Statistics
Data are reported as mean ± standard deviation (SD) of the mean. Statistical comparisons using XLSTAT software were based on an analysis of variance (ANOVA) and Tukey’s test for pairwise comparisons (p < 0.05 was considered significant). They generated groups of statistically identical results. Statistically identical values are reported on the figures, all other values are different one from another.
Supplementary Material
Acknowledgments
This work has been supported by the “Association Française contre les Myopathies” (AFM, grant n°12671), by the « Association pour la Recherche sur la Cancer” (grant n° 7918), by the “Fondation Recherche Médicale” (grant n°INE20061108297), by “Agence Nationale pour la Recherche” (grant ANR-06-NANO-006) and by the NIH (R21 grant) via a subcontract to C.P. (no. 544168A). We thank Cécile Gauthier-Rouvière (CRBM, Montpellier) for providing C2C12 cells and for fruitful discussions, Fabrice Mérézègue and Philippe Montcourrier (CRBM, Montpellier) for their technical help with the SEM experiments. Dennis Discher, Adam Engler (University of Pennsylvania, Philadelphia) and Michel Pucéat (Généthon, Evry, France) are acknowledged for their fruitful advices. CP is a Junior Member of the “Institut Universitaire de France” whose support is gratefully acknowledged. KR is indebted to the CNRS for providing a post-doctoral fellowship and TC thanks the AFM for a PhD fellowship.
References
- 1.Shin H, Jo S, Mikos AG. Biomaterials. 2003;24:4353. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]; a) Stevens MM, George JH. Science. 2005;310:1135. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]; a) Puleo DA, Nanci A. Biomaterials. 1999;20:2311. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]; a) Castner DG, Ratner BD. Surf Sci. 2002;500:28. [Google Scholar]
- 2.Lan MA, Gersbach CA, Michael KE, Keselowsky BG, Garcia AJ. Biomaterials. 2005;26:4523. doi: 10.1016/j.biomaterials.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 4.Dalby MJ, Yarwood SJ, Riehle MO, Johnstone HJH, Affrossman S, Curtis ASG. Exp Cell Res. 2002;276:1. doi: 10.1006/excr.2002.5498. [DOI] [PubMed] [Google Scholar]
- 5.Dalby MJ, Riehle MO, Sutherland DS, Agheli H, Curtis AS. European Journal of Cell Biology. 2004;83:159. doi: 10.1078/0171-9335-00369. [DOI] [PubMed] [Google Scholar]
- 6.Pelham RJ, Wang YL. Proc Natl Acad Sci. 1997;94:13661. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo CM, Wang HB, Dembo M, Wang YL. Biophys J. 2000;79:144. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; a) Discher DE, Janmey P, Wang YL. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 8.Wong J, Leach J, Brown X. Surf Sci. 2004;570:119. [Google Scholar]; a) Richert L, Engler AJ, Discher DE, Picart C. Biomacromolecules. 2004;5:1908. doi: 10.1021/bm0498023. [DOI] [PubMed] [Google Scholar]
- 9.Georges PC, Janmey PA. J Appl Physiol. 2005;98:1547. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- 10.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. J Cell Biol. 2004;166:877. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson MT, Berg MC, Tobias IS, Rubner MF, Van Vliet KJ. Biomaterials. 2005;26:6836. doi: 10.1016/j.biomaterials.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Genes NG, Rowley JA, Mooney DJ, Bonassar LJ. Arch Biochem Biophys. 2004;422:161. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Cell Motil Cytoskeleton. 2005;60:24. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]; a) Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neuroreport. 2002;13:2411. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JY, Velasco A, Rajagopalan P, Pham Q. Langmuir. 2003;19:1908. [Google Scholar]
- 15.Rowley JA, Mooney DJ. J Biomed Mater Res. 2002;60:217. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 16.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. J Cell Biol. 2003;163:583. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Decher G. Science. 1997;277:1232. [Google Scholar]; (a) Lvov YM. Thin film nanofabrication by alternate adsorption of polyions, nanoparticles, and proteins. In: Nalwa HS, editor. Handbook of Surfaces and Interfaces of Materials. Vol. 3. Nanostructures Materials, Micelles, and Colloids: Academic Press; 2001. [Google Scholar]
- 19.Serizawa T, Yamaguchi M, Akashi M. Biomacromolecules. 2002;3:724. doi: 10.1021/bm0200027. [DOI] [PubMed] [Google Scholar]
- 20.Salloum DS, Olenych SG, Keller TC, Schlenoff JB. Biomacromolecules. 2005;6:161. doi: 10.1021/bm0497015. [DOI] [PubMed] [Google Scholar]
- 21.Berg MC, Yang SY, Hammond PT, Rubner MF. Langmuir. 2004;20:1362. doi: 10.1021/la0355489. [DOI] [PubMed] [Google Scholar]; a) Picart C, Elkaim R, Richert L, Audoin F, Da Silva Cardoso M, Schaaf P, Voegel J-C, Frisch B. Adv Funct Mater. 2005;15:83. [Google Scholar]
- 22.Koktysh DS, Liang X, Yun BG, Pastoriza-Santos I, Matts RL, Giersig M, Serra-Rodríguez C, Liz-Marzán LM, Kotov NA. Adv Funct Mater. 2002;12:255. [Google Scholar]
- 23.Richert L, Boulmedais F, Lavalle P, Mutterer J, Ferreux E, Decher G, Schaaf P, Voegel JC, Picart C. Biomacromolecules. 2004;5:284. doi: 10.1021/bm0342281. [DOI] [PubMed] [Google Scholar]
- 24.Schneider A, Francius G, Obeid R, Schwinté P, Frisch B, Schaaf P, Voegel JC, Senger B, Picart C. Langmuir. 2006;7:2882. doi: 10.1021/la0521802. [DOI] [PubMed] [Google Scholar]
- 25.Dierich A, Le Guen E, Messaddeq N, Stoltz S, Netter P, Schaaf P, Voegel JC, Benkirane-Jessel N. Adv Mater. 2007;19:693. [Google Scholar]; a) Nadiri A, Kuchler-Bopp S, Mjahed H, Hu B, Haikel Y, Schaaf P, Voegel JC, Benkirane-Jessel N. Small. 2007;3:1577. doi: 10.1002/smll.200700115. [DOI] [PubMed] [Google Scholar]
- 26.Bach AD, Beier JP, Stern-Staeter J, Horch RE. J Cell Mol Med. 2004;8:413. doi: 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabourin LA, Rudnicki MA. clin Genet. 2000;57:16. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- 28.Andres V, Walsh K. J Cell Biol. 1996;132:657. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinckenbach S, Hemmerle J, Dieval F, Arntz Y, Kretz JG, Durand B, Chakfe N, Schaaf P, Voegel JC, Vautier D. Journal of Biomedical Materials Res A. 2007 doi: 10.1002/jbm.a.31333. Epub 6 july. [DOI] [PubMed] [Google Scholar]
- 30.Picart C, Lavalle P, Hubert P, Cuisinier FJG, Decher G, Schaaf P, Voegel JC. Langmuir. 2001;17:7414. [Google Scholar]
- 31.Francius G, Hemmerle J, Ohayon J, Schaaf P, Voegel JC, Picart C, Senger B. Micros Res Techniq. 2006;69:84. doi: 10.1002/jemt.20275. [DOI] [PubMed] [Google Scholar]
- 32.Adams JC. J Cell Sci. 2002;115:257. doi: 10.1242/jcs.115.2.257. [DOI] [PubMed] [Google Scholar]
- 33.Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouviere C. Mol Biol Cell. 2007;18:1734. doi: 10.1091/mbc.E06-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dedieu S, Mazeres G, Cottin P, Brustis JJ. International Journal of Developmental Biology. 2002;46:235. [PubMed] [Google Scholar]
- 35.Zinger O, Anselme K, Denzer A, Habersetzer P, Wieland M, Jeanfils J, Hardouin P, Landolt D. Biomaterials. 2004;25:2695. doi: 10.1016/j.biomaterials.2003.09.111. [DOI] [PubMed] [Google Scholar]; a) Dalby MJ, Childs S, Riehle MO, Johnstone HJ, Affrossman S, Curtis AS. Biomaterials. 2003;24:927. doi: 10.1016/s0142-9612(02)00427-1. [DOI] [PubMed] [Google Scholar]
- 36.Washburn NR, Yamada KM, Simon CG, Jr, Kennedy SB, Amis EJ. Biomaterials. 2004;25:1215. doi: 10.1016/j.biomaterials.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Cai K, Bossert J, Jandt KD. Colloids Surf B Biointerfaces. 2006;49:136. doi: 10.1016/j.colsurfb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Tzoneva R, Faucheux N, Groth T. Biochim Biophys A. 2007;1770:1538. doi: 10.1016/j.bbagen.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Faucheux N, Tzoneva R, Nagel MD, Groth T. Biomaterials. 2006;27:234. doi: 10.1016/j.biomaterials.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 40.Webb K, Hlady V, Tresco PA. J Biomed Mater Res. 2000;49:362. doi: 10.1002/(sici)1097-4636(20000305)49:3<362::aid-jbm9>3.0.co;2-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray DS, Tien J, Chen CS. J Biomed Mat Res A. 2003;66:605. doi: 10.1002/jbm.a.10585. [DOI] [PubMed] [Google Scholar]
- 42.Smith E, Yang J, McGann L, Sebald W, Uludag H. Biomaterials. 2005;26:7329. doi: 10.1016/j.biomaterials.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 43.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher DE. Biophys J. 2004;86:617. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam MT, Sim S, Zhu X, Takayama S. Biomaterials. 2006;27:4340. doi: 10.1016/j.biomaterials.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Knudsen KA, Horwitz AF. Develop Biol. 1977;58:328. doi: 10.1016/0012-1606(77)90095-1. [DOI] [PubMed] [Google Scholar]
- 46.Horsley V, Pavlath GK. Cells Tissues Organs. 2004;176:67. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- 47.Yeo Y, Geng W, Ito T, Kohane DS, Burdick JA, Radisic M. Journal of Biomedical Materials Research B. 2007;81:312. doi: 10.1002/jbm.b.30667. [DOI] [PubMed] [Google Scholar]
- 48.Picart C, Mutterer J, Richert L, Luo Y, Prestwich GD, Schaaf P, Voegel JC, Lavalle P. Proc Natl Acad Sci. 2002;99:12531. doi: 10.1073/pnas.202486099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennis RG, Kosnik PE, Gilbert ME, Faulkner JA. American Journal of Physiology Cell Physiology. 2001;280:C288. doi: 10.1152/ajpcell.2001.280.2.C288. [DOI] [PubMed] [Google Scholar]
- 50.Guo WH, Frey MT, Burnham NA, Wang YL. Biophys J. 2006;90:2213. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jansen KM, Pavlath GK. J Cell Biol. 2006;174:403. doi: 10.1083/jcb.200601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neumann T, Hauschka SD, Sanders JE. Tissue Eng. 2003;9:995. doi: 10.1089/107632703322495637. [DOI] [PubMed] [Google Scholar]
- 53.Clemmens EW, Regnier M. J Muscle Res Cell Motil. 2004;25:515. doi: 10.1007/s10974-004-3787-0. [DOI] [PubMed] [Google Scholar]
- 54.Schneider A, Vodouhê A, Richert L, Francius G, Le Guen E, Schaaf P, Voegel JC, Frisch F, Picart C. Biomacromolecules. 2007;8:139. doi: 10.1021/bm060765k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richert L, Schneider A, Vautier D, Jessel N, Payan E, Schaaf P, Voegel JC, Picart C. Cell Biochem Biophys. 2006;44:273. doi: 10.1385/CBB:44:2:273. [DOI] [PubMed] [Google Scholar]
- 56.Schneider A, Picart C, Senger B, Schaaf P, Voegel JC, Frisch B. Langmuir. 2007;23:2655. doi: 10.1021/la062163s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gratzner HG, Leif RC, Ingram DJ, Castro A. Exp Cell Res. 1975;95:88. doi: 10.1016/0014-4827(75)90612-6. [DOI] [PubMed] [Google Scholar]
- 58.Meriane M, Roux P, Primig M, Fort P, Gauthier-Rouviere C. Mol Biol Cell. 2000;11:2513. doi: 10.1091/mbc.11.8.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guillemot JC, Montcourrier P, Vivier E, Davoust J, Chavrier P. J Cell Sci. 1997;110:2215. doi: 10.1242/jcs.110.18.2215. [DOI] [PubMed] [Google Scholar]
- 60.Brunk U, Collins VP, Arro E. Journal of Microscopy. 1981;123:121. doi: 10.1111/j.1365-2818.1981.tb01288.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.