Abstract
Earlier studies of antigen-receptor loci implicated directed monoallelic association with peri-centromeric heterochromatin in the initiation or maintenance of allelic exclusion. Here we provide evidence for a fundamentally different basis for Tcrb allelic exclusion. Using 3D Immuno-FISH we found that germline Tcrb alleles associated stochastically and at high frequency with the nuclear lamina or with peri-centromeric heterochromatin in developing thymocytes, and that such interactions inhibited Vβ-to-DβJβ recombination prior to β-selection. Introduction of an ectopic enhancer into Tcrb reduced these interactions and impaired allelic exclusion. We propose that initial Vβ-to-DβJβ recombination events are generally monoallelic in developing thymocytes due to frequent stochastic, rather than directed, interactions of Tcrb alleles with repressive nuclear compartments. Such interactions may be essential for Tcrb allelic exclusion.
INTRODUCTION
Lymphocytes create diverse antigen receptor repertoires by recombination of variable (V), diversity (D) and joining (J) gene segments1. T lymphocytes bearing an αβ T cell receptor (TCR) develop as a consequence of V(D)J recombination events that occur during two discrete stages of thymocyte development. The Tcrb gene is assembled in CD4-CD8- double negative (DN) thymocytes. Those DN thymocytes that successfully rearrange the Tcrb gene are selected to proliferate and to differentiate into CD4+CD8+ double positive (DP) thymocytes in a process called β-selection2. DP thymocytes then undergo Vα-to-Jα recombination, leading to the expression of αβ TCR complexes.
An important but poorly understood aspect of V(D)J recombination is the phenomenon of allelic exclusion, which limits some antigen receptor loci to produce a functional protein from only a single allele3-6. In developing αβ T lymphocytes, Tcrb gene rearrangement is subject to allelic exclusion, whereas Tcra gene rearrangement is not. Tcrb gene rearrangement occurs in two steps, first Dβ-to-Jβ, and then Vβ-to-DβJβ 3, 5. Tcrb allelic exclusion is manifested at the Vβ-to-DβJβ step, and is thought to be mediated in two phases3, 5. First, it is highly unlikely that both Tcrb alleles will undergo Vβ-to-DβJβ recombination simultaneously. Second, productive recombination on one allele is thought to provoke a feedback signal that suppresses further Vβ-to-DβJβ recombination on the other allele. These two constraints permit Vβ-to-DβJβ recombination on the second allele only if nonproductive recombination occurs on the first allele.
Feedback inhibition of Tcrb gene recombination is initiated by pre-TCR signaling and is linked to DN to DP differentiation2, 3, 5. Previous studies have indicated that suppression of further Tcrb gene recombination in DP thymocytes is associated with changes in Vβ chromatin structure that limit access of the recombinase to chromosomal recombination signal sequences, as well as with a locus conformational change (decontraction) that increases the physical distance between Vβ and DβJβ segments7-10.
The intiation of Tcrb allelic exclusion is less well understood. Two types of mechanisms have been invoked to explain asynchronous recombination of Tcrb alleles in DN thymocytes3-6. In deterministic models, the two Tcrb alleles in each cell would represent nonequivalent substrates for the recombinase and this initial allelic choice would dictate the order of subsequent recombination events. In this regard, it was suggested, based on replication timing, that the two immunoglobulin Igk alleles are differentially marked and the early replicating allele undergoes DNA demethylation in pre-B cells and is predisposed to rearrange first11, 12. By contrast, stochastic models invoke mechanisms that reduce the efficiency of recombination on both alleles and thereby diminish the likelihood that the two alleles initiate recombination simultaneously13. It was suggested that limiting regulators randomly and infrequently activate Igk alleles in pre-B cells, thereby contributing to Igk allelic exclusion. In addition, inefficient Vβ and VH recombination signal sequences were suggested to be rate limiting for recombination and to contribute to Tcrb and Igh allelic exclusion, respectively14,15. Whether asynchronous recombination of Tcrb alleles is established through a deterministic or a stochastic mechanism remains an important but unresolved question.
The association of antigen receptor loci with two distinct nuclear compartments has been suggested to regulate recombination. One such compartment is the nuclear periphery. This compartment is comprised of two sub-domains, the inner nuclear membrane-nuclear lamina and the nuclear pore complexes, that appear to divergently regulate gene activity16. Positioning of genes at nuclear pore complexes is thought to promote gene activity, whereas association of genes at the inner nuclear membrane-nuclear lamina is considered to repress their activity17-20. Several genes have been documented to reposition away from the nuclear periphery in conjunction with their activation21-23. Moreover, inducible tethering of active genes to the mammalian inner nuclear membrane was shown to inhibit their transcription24. Igh and Igk alleles were found to preferentially localize to the nuclear periphery in hematopoietic progenitors and T lineage cells. However, these loci reposition away from the nuclear periphery in pro-B cells, suggesting that repositioning is an important step in their activation24-27. A second such compartment is peri-centromeric heterochromatin 17, 28, 29. The silencing of developmentally regulated genes is often associated with their juxtaposition in trans to foci of peri-centromeric heterochromatin30, 31. Association of Igh and Igk alleles with peri-centromeric heterochromatin has been linked to allelic exclusion. Igh alleles display minimal association with peri-centromeric foci at the pro-B cell stage, during which Igh recombination occurs. However, the nonrearranged Igh allele is recruited to peri-centromeric foci in pre-B cells, implicating this relocalization in feedback inhibition of further Igh recombination32, 33. In addition, the recruitment of one (late replicating) Igk allele to peri-centromeric foci in pre-B cells is thought to bias initial Igk recombination in these cells to the nonassociated allele34.
A recent study provided evidence for predominantly monoallelic association of Tcrb alleles with peri-centromeric heterochromatin throughout T cell development10. Analysis of DP thymocytes indicated that the allele associated with peri-centromeric heterochromatin in a given thymocyte was decontracted, suggesting that it had not undergone rearrangement. Based on these observations the authors inferred that recruitment of one Tcrb allele to peri-centromeric heterochromatin in DN thymocytes may inhibit its recombination, thus biasing the other allele to rearrange first. However, the recombination status of the two alleles was not directly determined but inferred from their states of contraction. Moreover, the relationship between subnuclear localization and contraction was only addressed in thymocytes that already had received a signal for feedback inhibition. Thus, whether association of Tcrb alleles with peri-centromeric heterochromatin was relevant for asynchronous recombination in DN thymocytes or for feedback inhibition of recombination in DP thymocytes could not be resolved.
To address the aforementioned unresolved issues, here we examined the relationship between subnuclear localization and recombination of Tcrb alleles. We found that Tcrb alleles associated at high frequency with either the nuclear lamina or peri-centromeric heterochromatin. However, in contrast with previous work, we found these associations to be stochastic rather than directed, and not strictly monoallelic. By analyzing DN thymocytes deficient in feedback signaling, we established a direct role for subnuclear localization in the asynchronous rearrangement of Tcrb alleles. Moreover, we obtained evidence that reduced interaction of Tcrb alleles with the nuclear lamina or peri-centromeric heterochromatin is associated with a loss of allelic exclusion. We propose a stochastic rather than a deterministic model by which subnuclear localization contributes to the initiation of Tcrb allelic exclusion.
Results
Tcrb alleles frequently associate with repressive compartments
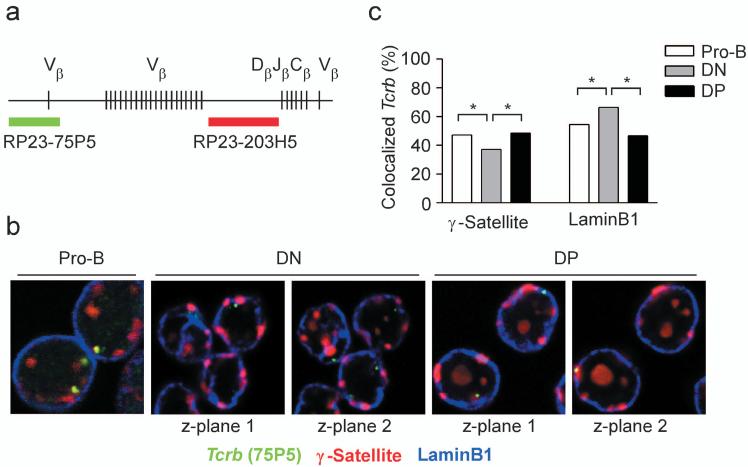
We studied association of Tcrb alleles with peri-centromeric heterochromatin and the nuclear lamina in nuclei of developing thymocytes. Locus behavior prior to β-selection was assessed in DN thymocytes obtained from Rag2-/- mice, whereas locus behavior subsequent to β-selection was assessed in DP thymocytes of wild-type mice. As a control, we analyzed locus behavior in Rag2-/- pro-B cells. We identified the nuclear location of Tcrb alleles independent of their rearrangement status by probing with BAC clone RP23-75P5, which identifies the Cβ-distal end of the locus (Fig. 1a,b). Peri-centromeric heterochromatin was identified using a probe that recognizes γ-satellite repeats, whereas immunostaining with an antibody specific for LaminB1 was used to identify the nuclear lamina (Fig. 1b). We found that Tcrb alleles frequently colocalized with γ-satellite repeats (47.2% of alleles) and laminB1 (54.5% of alleles) in pro-B cells (Fig. 1b,c). Tcrb alleles associated with peri-centromeric heterochromatin in DN thymocytes at a reduced frequency (37.1% of alleles) as compared to pro-B cells, but their association with the nuclear lamina (66.4% of alleles) was substantially higher than in pro-B cells. DP thymocytes displayed associations with peri-centromeric heterochromatin (48.4%) and the nuclear lamina (46.6%) that were similar to those in pro-B cells.
Figure 1.
Subnuclear localization of Tcrb alleles in Rag2-/- pro-B cells, Rag2-/- DN thymocytes, and sorted wild-type DP thymocytes. (a) Tcrb locus, including the relative positions of the BAC clones used to visualize its subnuclear localization. (b) 3D Immuno-FISH, showing both Tcrb alleles in representative nuclei of each of three cell types. Nuclei were hybridized with BAC RP23-75P5 to identify the distal end of the Tcrb locus (green) and a plasmid containing γ-satellite repeats to identify peri-centromeric heterochromatin (red), and were stained with an antibody specific for laminB1 to identify the nuclear lamina (blue). (c) Colocalization of Tcrb alleles with either γ-satellite repeats or laminB1. γ-satellite data, 604 pro-B, 432 DN and 862 DP alleles (two to three slides each); laminB1 data, 578 pro-B, 232 DN and 972 DP alleles (two to three slides each). *, P < 0.05.
For comparison, we analyzed subnuclear positioning of the constitutively active gene Actb (Supplementary Fig. 1, online). Only 10% of Actb alleles co-localized with γ-satellite repeats and 20% with LaminB1 in all cell types examined. Thus this highly transcribed gene only infrequently associates with peri-centromeric heterochromatin and the nuclear lamina.
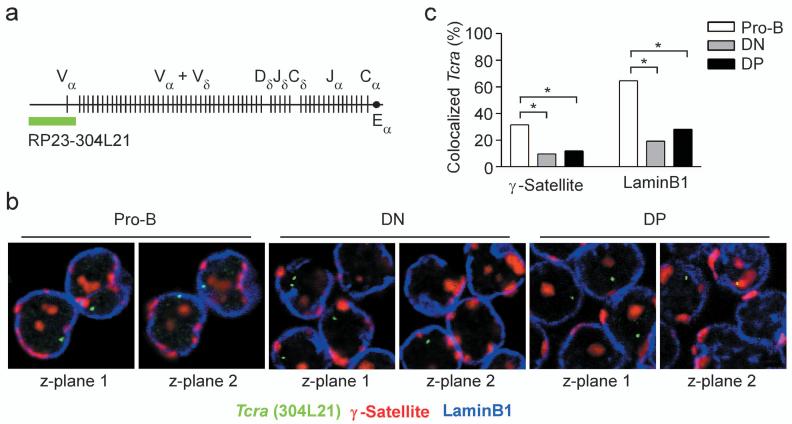
We also analyzed subnuclear positioning of Tcra alleles (Fig. 2a). Like Tcrb alleles, Tcra alleles frequently colocalized with γ-satellite (31.5%) or laminB1 (64.6%) in pro-B cells (Fig. 2b,c). In contrast to Tcrb alleles, Tcra alleles only infrequently colocalized with γ-satellite or laminB1 in DN (9.6% and 19.2% respectively) and DP (11.8% and 28.1% respectively) thymocytes. The Tcra locus is active in both DN and DP thymocytes due to the activation of Tcrd gene segments in DN thymocytes and Tcra gene segments in DP thymocytes35. Thus, the Tcra locus is associated with peri-centromeric heterochromatin or the nuclear lamina when inactive (in pro-B cells) and dissociates from these compartments when active (in DN and DP thymocytes).
Figure 2.
Subnuclear localization of Tcra alleles in Rag2-/- pro-B cells, Rag2-/- DN thymocytes, and sorted wild-type DP thymocytes. (a) Tcra locus, including the position of the BAC clone used to visualize its subnuclear localization. (b) 3D Immuno-FISH, showing Tcra alleles in representative nuclei of each of the three cell types. Nuclei were hybridized with BAC RP23-304L21 to identify the Tcra locus (green) and a plasmid containing γ-satellite repeats to identify peri-centromeric heterochromatin (red), and were stained with an antibody specific for laminB1 to identify the nuclear lamina (blue). (c) Colocalization of Tcra alleles with either γ-satellite repeats or laminB1. γ-satellite data, 302 pro-B, 52 DN and 178 DP alleles (one slide each); laminB1 data, 302 pro-B, 52 DN and 178 DP alleles (one slide each). *, P < 0.05.
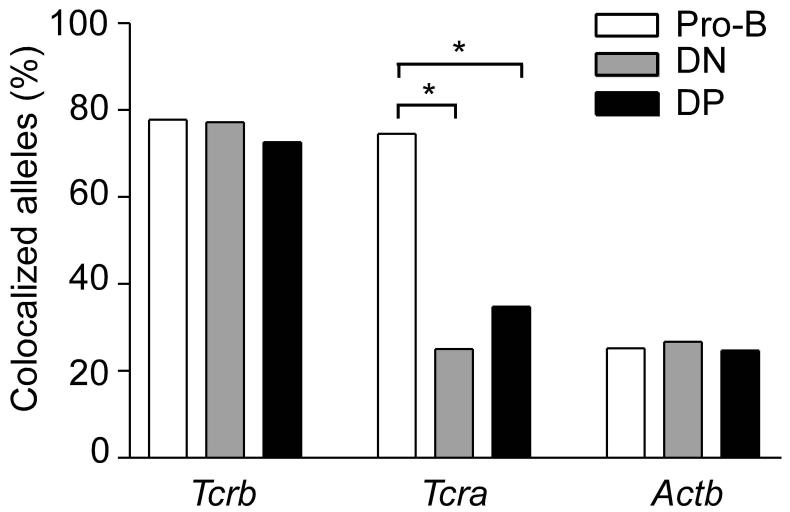
The nuclear compartments defined by γ-satellite hybridization and laminB1 staining partially overlap, as γ-satellite repeats were found both centrally in the nucleus and at the nuclear periphery. Colocalization of Tcrb alleles with γ-satellite, laminB1 or both was extremely high (77.8% in pro-B cells, 77.2% in DN thymocytes, and 72.6% in DP thymocytes) (Fig. 3). By contrast, although 74.5% of Tcra alleles were associated with these compartments in pro-B cells, only 25.0% and 34.8% were associated in DN and DP thymocytes, respectively. Tcra association rates in DN and DP thymocytes were similar to those of Actb, which associated only 25% of the time in all cell types (Fig. 3). It is striking that Tcrb alleles so frequently associate with peri-centromeric heterochromatin or the nuclear lamina in DN and DP thymocytes even though the Tcrb locus is expressed at both developmental stages.
Figure 3.
Colocalization of Tcrb, Tcra, and Actb alleles with γ-satellite or laminB1. Alleles colocalizing with γ-satellite alone, laminB1 alone, or simultaneously with γ-satellite and laminB1 were summed. Tcrb data, 424 pro-B, 232 DN and 390 DP alleles (one slide each); Tcra data, 302 pro-B, 52 DN and 178 DP alleles (one slide each); Actb data, 246 pro-B, 232 DN and 162 DP alleles (one slide each). *, P < 0.05.
Tcrb alleles independently associate with repressive compartments
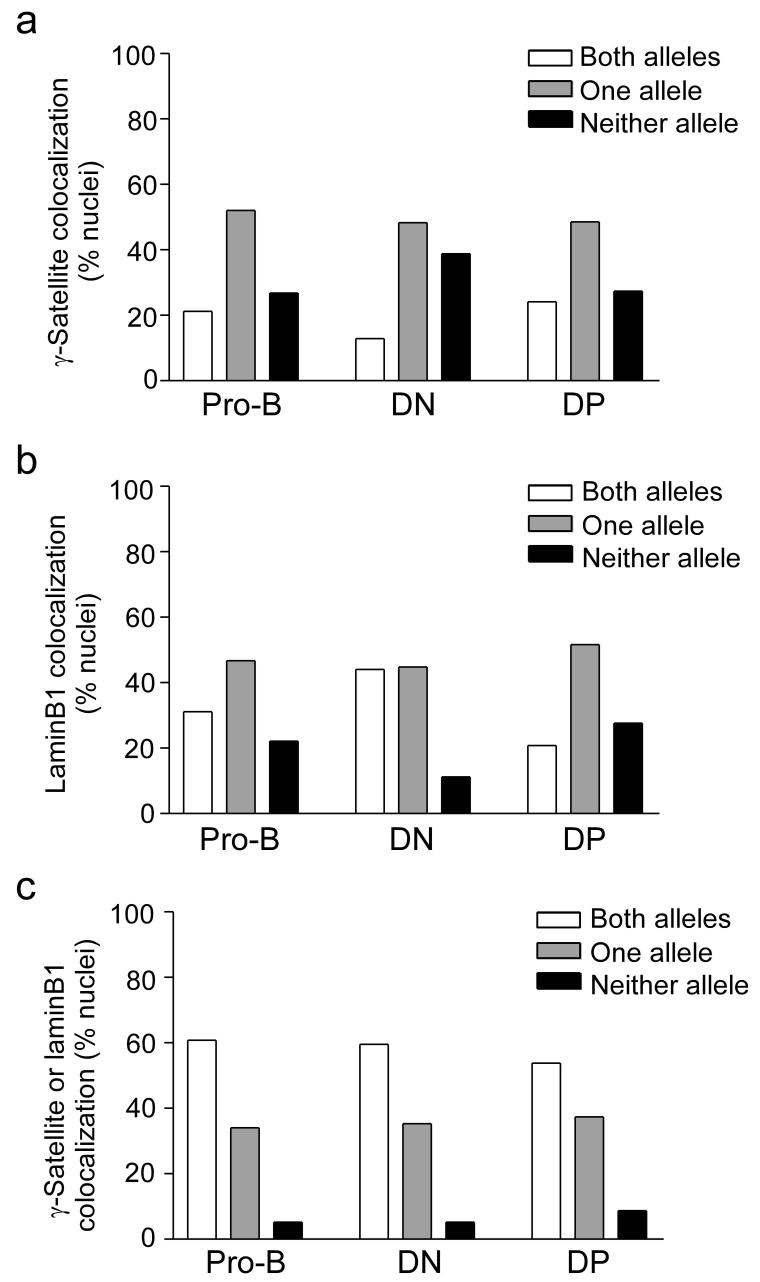
Skok et al.10 reported monoallelelic Tcrb association with peri-centromeric heterochromatin in about 75% of DN and DP thymocyte nuclei. Their data imply that the two alleles in any nucleus are intrinsically different and have distinct probabilities to associate with peri-centromeric heterochromatin. This difference might then bias Tcrb gene recombination to one allele and contribute to the initiation of allelic exclusion. However, our data do not support strict monoallelic association of the Tcrb gene with peri-centromeric heterochromatin or the nuclear lamina. In DN thymocytes, 37.1% of Tcrb alleles colocalized with γ-satellite (Fig. 1c). If the two Tcrb alleles in DN thymocytes were equivalent and associated independently with peri-centromeric heterochromatin, 13.7% of DN nuclei should have two associated alleles (37.1% × 37.1%), 46.7% should have one associated allele (2 × 37.1% × 62.9%) and 39.6% should have neither allele associated (62.9% × 62.9%) (Table 1). This is very close to the observed allelic distribution in DN thymocytes (12.9%, 48.3%, and 38.8%, respectively) (Table 1, Fig. 4a). Likewise, the observed distribution of DN thymocyte nuclei having both, one, or no alleles associated with the nuclear lamina was predicted almost exactly from calculations based on an overall association frequency of 66.4% (Fig. 1c, Table 1, Fig. 4b). Tcrb alleles behaved similarly in recombinase-sufficient DN thymocytes (Supplementary Table 1, online), and independently segregated to both nuclear compartments in nuclei of all cell types examined (Table 1). Similar observations were made for the Actb and Tcra genes in all three cell types (Supplementary Table 2,3, online).
Table 1.
Independent segregation of Tcrb alleles with laminB1 or γ-satellite
| Experimental distribution (%) | Theoretical distribution of nuclei (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nuclear compartment | Cell type | Number of nuclei | Allelic association rate (%) | Neither allele | One allele | Both alleles | Neither allele | One allele | Both alleles |
| p | (1-p)2 | 2p(1-p) | p2 | ||||||
| laminB1 | pro-B | 289 | 54.5 | 22.1 | 46.7 | 31.1 | 20.7 | 49.6 | 29.7 |
| DN | 116 | 66.4 | 11.2 | 44.8 | 44.0 | 11.3 | 44.6 | 44.1 | |
| DP | 486 | 46.6 | 27.6 | 51.6 | 20.8 | 28.5 | 49.8 | 21.7 | |
| γ-satellite | pro-B | 302 | 47.2 | 26.8 | 52.0 | 21.2 | 27.9 | 49.8 | 22.3 |
| DN | 116 | 37.1 | 38.8 | 48.3 | 12.9 | 39.6 | 46.7 | 13.7 | |
| DP | 431 | 48.4 | 27.4 | 48.5 | 24.1 | 26.7 | 49.9 | 23.4 | |
| laminB1, γ-satellite, or both | pro-B | 212 | 77.8 | 5.60 | 36.9 | 66.2 | 4.9 | 34.5 | 60.5 |
| DN | 116 | 77.2 | 5.20 | 35.3 | 59.5 | 5.2 | 35.5 | 59.4 | |
| DP | 195 | 72.6 | 8.70 | 37.4 | 53.8 | 7.5 | 39.8 | 52.7 | |
Figure 4.
Distribution of nuclei with zero, one, or two Tcrb alleles colocalized with γ-satellite or laminB1. (a) Colocalization with γ-satellite, in 289 pro-B, 116 DN and 486 DP nuclei (b) Colocalization with laminB1, in 302 pro-B, 116 DN and 431 DP nuclei. (c) Colocalization with either γ-satellite or laminB1, in 289 pro-B, 116 DN and 431 DP nuclei. Data were accumulated from two to three independently prepared slides per cell type.
Notably, Immuno-FISH analysis with probes for the Tcrb locus, γ-satellite repeats and laminB1 revealed that only 5.3% of DN thymocyte nuclei had two Tcrb alleles “free” of both compartments (Fig. 4c). In contrast, 35.5% had one allele colocalized with either γ-satellite or laminB1 and 59.5% had both alleles colocalized with either γ-satellite or laminB1. These experimentally determined values match closely the values calculated from rates of association with the individual compartments (Table 1).
We conclude that although the Tcrb locus is monoallelically associated with peri-centromeric heterochromatin and with the nuclear lamina in subsets of thymocyte nuclei, these subsets arise stochastically as a result of two indistinguishable alleles independently associating with these nuclear compartments. Thus, there is no biological mechanism directing monoallelic association. We further conclude that fully 95% of DN thymocytes have at least one Tcrb allele associated with peri-centromeric heterochromatin or the nuclear lamina.
Biased localization of unrearranged Tcrb alleles
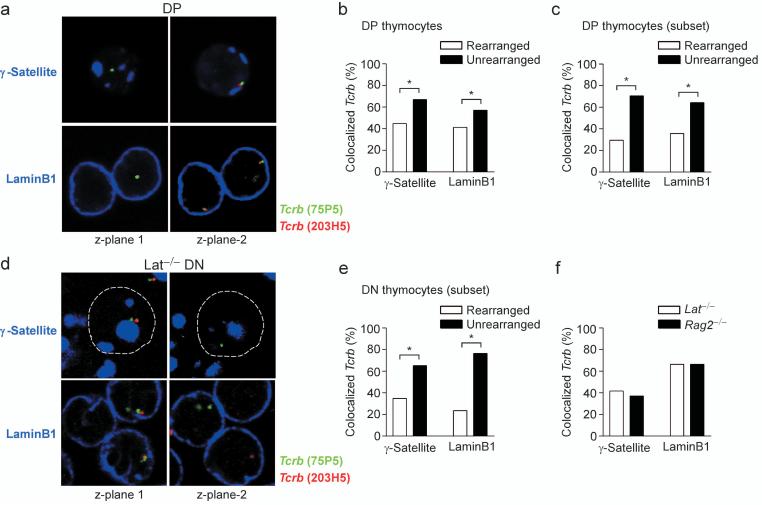
Given that Tcrb alleles associate at high frequency with pericentromeric heterochromatin and the nuclear lamina in DN and DP thymocytes, we hypothesized that these associations may inhibit Vβ-to-DβJβ recombination and promote either the initiation or the maintenance of Tcrb allelic exclusion. To address this hypothesis, we first asked whether rearranged and unrearranged Tcrb alleles were differentially associated with pericentromeric heterochromatin or the nuclear lamina in DP thymocytes. Unrearranged Tcrb alleles were identified by their hybridization with both the Cβ-distal Tcrb probe (RP23-75P5) and a Cβ-proximal probe (RP23-203H5) that identifies the region situated between Vβ and Dβ gene segments. Rearranged Tcrb alleles were identified by their hybridization to only the distal probe (RP23-75P5), as the region detected by the proximal probe (RP23-203H5) is deleted by Vβ-to-DβJβ recombination (Fig. 1a).
We found that unrearranged Tcrb alleles more frequently colocalized with γ-satellite (66.9%) as compared to rearranged alleles (44.8%) (Fig. 5a,b). Similarly, unrearranged Tcrb alleles more frequently colocalized with laminB1 (57.0%) as compared to rearranged alleles (41.3%). Of note, this analysis includes data from two non-informative subsets of nuclei that may cause the relationship between localization and recombination to be underestimated. In one subset, both alleles are rearranged; in the other, both alleles are associated with a repressive compartment. For a more informative analysis, we focused on nuclei that had rearranged only one allele and in which the Tcrb gene monoallelically colocalized with either γ-satellite or laminB1. In this subset of nuclei the unrearranged allele colocalized with γ-satellite 70.5% of the time, whereas the rearranged allele colocalized only 29.5% of the time (Fig. 5c). Similarly the unrearranged allele colocalized with laminB1 64.3% of the time and the rearranged allele colocalized 35.7% of the time. The strong bias for the unassociated allele to be the rearranged allele suggests that association with pericentromeric heterochromatin and association with the nuclear lamina may each inhibit Vβ-to-DβJβ recombination.
Figure 5.
Subnuclear localization of rearranged and unrearranged Tcrb alleles. (a) 3D Immuno-FISH, showing Tcrb alleles in DP nuclei using distal (75P5, green) and proximal (203H5, red) probes. Peri-centromeric heterochromatin (γ-satellite, blue, upper panels). Nuclear lamina (laminB1, blue, lower panels). (b) Colocalization of rearranged and unrearranged alleles with γ-satellite or laminB1 in DP nuclei. γ-satellite, 145 rearranged and 327 unrearranged alleles (one slide); laminB1, 179 rearranged and 403 unrearranged alleles (two slides). (c) Colocalization of rearranged and unrearranged alleles with γ-satellite or laminB1 in the subset of DP cells with nuclei having monoallelic Tcrb locus association and one rearranged Tcrb allele. γ-satellite, 145 nuclei (one slide); laminB1, 179 nuclei (two slides). (d) 3D Immuno-FISH, showing Tcrb alleles in nuclei of Lat-/- thymocytes. Probe strategy was identical to (a). (e) Colocalization of rearranged and unrearranged alleles with γ-satellite or laminB1 in the subset of Lat-/- cells with nuclei having monoallelic Tcrb locus association and one rearranged allele. γ-satellite, 161 nuclei (one slide); laminB1, 54 nuclei (one slide). (f) Colocalization of Tcrb alleles with γ-satellite or laminB1 in Rag2-/- DN nuclei and in Lat-/- DN nuclei containing two rearranged alleles. γ-satellite, 232 Rag2-/- and 36 Lat-/- alleles (two and one slides, respectively); laminB1, 232 Rag2-/- and 220 Lat-/- alleles (two slides each). *, P < 0.05.
Subnuclear localization biases initial Tcrb recombination
Data from DP thymocytes cannot distinguish whether a relationship between rearrangement status and subnuclear location reflects a post-β-selection sorting of alleles linked to feedback inhibition or a pre-β-selection relationship. The former possibility suggests that unrearranged alleles are moved to pericentromeric heterochromatin or the nuclear periphery as part of feedback inhibition of recombination in DP thymocytes. The latter suggests that association with these compartments influences which allele initially undergoes recombination in DN thymocytes. To distinguish these possibilities, we examined the relationship between rearrangement and subnuclear positioning in DN thymocytes that had yet to receive an allelic exclusion signal.
Thymocytes from Lat-deficient mice cannot signal through their pre-TCR and thus do not generate a feedback signal and do not differentiate to the DP stage36, 37. We identified rearranged and unrearranged Tcrb alleles in DN thymocytes of Lat-/- mice using the distal and proximal Tcrb probes (Fig. 5d), as described above. We also identified Tcrb excision circles that selectively hybridized with the proximal probe (Fig. 5d, lower right panel). In the informative subsets of Lat-deficient nuclei that contain only one rearranged allele and one allele colocalized with either γ-satellite or laminB1, we noted a strong bias for the rearranged allele to be the “free” allele (Fig. 5d,e). Rearranged and unrearranged Tcrb alleles colocalized with γ-satellite in 34.8% and 65.2% of nuclei, respectively, and with laminB1 in 23.5% and 76.5% of nuclei, respectively. We conclude that association with peri-centromeric heterochromatin or the nuclear lamina reduces the efficiency of recombination, such that in DN thymocytes with one associated and one non-associated allele, the non-associated allele is more likely to rearrange first.
An alternative interpretation of these data is that recombination reduces the probability that an allele remains associated with peri-centromeric heterochromatin or the nuclear lamina. To address this possibility we compared the positioning of unrearranged Tcrb alleles in Rag2-/- DN thymocyte nuclei to that of rearranged Tcrb alleles in the subset of Lat-/- DN thymocyte nuclei containing two rearranged alleles (Fig. 5f). Notably, we detected no significant difference in colocalization of Tcrb alleles with γ-satellite repeats or laminB1 in the two populations. Thus recombination does not influence the association of Tcrb alleles with peri-centromeric heterochromatin or the nuclear lamina.
The two probe strategy for analysis of Tcrb alleles also allowed evaluation of the orientation of unrearranged Tcrb alleles relative to peri-centromeric heterochromatin and the nuclear lamina (Fig. 5a,d). For those instances in which a clear orientation could be discerned, the Cβ-distal portion of the Tcrb locus was usually closest to peri-centromeric heterochromatin or the nuclear lamina (Supplementary Fig. 2, online). This orientation might provide the basis for preferential inhibition of Vβ-to-DβJβ recombination. Previous studies had similarly shown that peripheral Igh loci are oriented with V gene segments nearer the nuclear periphery than C gene segments24-27.
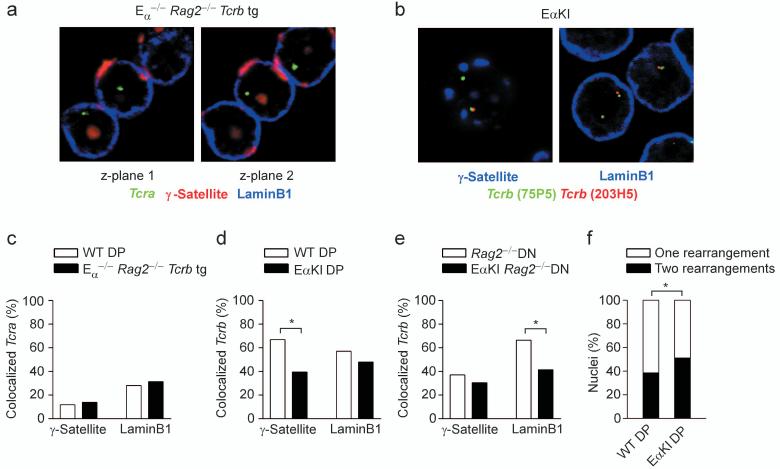
An ectopic enhancer perturbs Tcrb localization and recombination
Studies of the β-globin locus have shown that subnuclear positioning can be influenced by enhancers and locus control regions38-40. We asked whether TCR locus enhancers function similarly by comparing Tcra locus positioning in DP thymocytes of Tcra enhancer-deleted (Eα-/- ) and wild-type mice. Surprisingly, we detected no effect of Eα-deletion on positioning of the Tcra locus relative to peri-centromeric heterochromatin or the nuclear lamina (Fig. 6a, c). Thus, either Eα plays no role in subnuclear positioning of the Tcra locus, or it is functionally redundant with other Tcra locus regulatory elements in this regard. To distinguish these possibilities, we analyzed DP thymocytes of mice carrying an ectopic insertion of Eα into the Vβ portion of the Tcrb locus (EαKI mice)8. Because some but not all Vβ-to-DβJβ recombination events would delete the inserted Eα, we focused specifically on unrearranged alleles in EαKI DP thymocytes. We found that unrearranged EαKI alleles colocalized with γ-satellite at a frequency that was reduced as compared to unrearranged wild-type alleles (39.5% vs. 66.9%) (Fig. 6b, d). We also detected a reduction in colocalization of unrearranged EαKI alleles with laminB1 as compared to unrearranged wild-type alleles (47.9% vs. 57.0%), although this difference fell just short of achieving statistical significance (Fig. 6d). Therefore Eα can inhibit associations with peri-centromeric heterochromatin and the nuclear lamina in DP thymocytes.
Figure 6.
Influence of Eα on subnuclear localization and allelic exclusion. (a) 3D Immuno-FISH, showing Tcra alleles in nuclei of Eα-/- Rag2-/- Tcrb transgenic thymocytes. Tcra (304L21, green), peri-centromeric heterochromatin (γ-satellite, red), nuclear lamina (laminB1, blue). (b) 3D Immuno-FISH, showing Tcrb alleles in nuclei of EαKI thymocytes. Distal Tcrb (75P5, green), proximal Tcrb (203H5, red), peri-centromeric heterochromatin (γ-satellite, blue, left panel), nuclear lamina (laminB1, blue, right panel). (c) Tcra colocalization with γ-satellite or laminB1 in wild-type DP and Eα-/-Rag2-/- Tcrb transgenic thymocytes. WT DP, 178 alleles; Eα-/-Rag2-/- Tcrb tg DP, 210 alleles (one slide each). (d) Colocalization of unrearranged Tcrb alleles with γ-satellite or laminB1 in wild-type and EαKI DP thymocytes. γ-satellite, 145 WT and 114 EαKI alleles (one slide each); laminB1, 179 WT and 48 EαKI alleles (one slide each). (e) Colocalization of unrearranged Tcrb alleles with γ-satellite or laminB1 in DN thymocytes of Rag2-/- and EαKI Rag2-/- mice. γ-satellite, 145 Rag2-/- and 128 EαKI Rag2-/- alleles (two slides each); laminB1, 316 Rag2-/- and 128 EαKI Rag2-/- alleles (two slides each). (f) Quantification of Vβ-to-DβJβ recombination in wild-type and EαKI DP nuclei. WT, 527 nuclei; EαKI 129 nuclei (two slides each). *, P < 0.05.
We compared EαKI to wild-type alleles in DN thymocytes (both on a Rag2-/- background) to determine if the influence of Eα on subnuclear positioning was developmentally regulated. Surprisingly, EαKI alleles colocalized with laminB1 at a frequency that was significantly reduced as compared to wild-type (41.4% vs. 66.4%) (Fig. 6e). We also noted reduced colocalization with γ-satellite (30.5% vs. 37.1%), although the difference was not statistically significant. Therefore Eα can influence locus compartmentalization in DN thymocytes, even though it cannot activate transcription in these cells.
The disruption of Tcrb nuclear localization in EαKI thymocytes prompted us to ask if relocalization was accompanied by a measurable perturbation in Tcrb gene recombination. Efficient allelic exclusion predicts that 60% of DP nuclei should have one allele rearranged while 40% should have both alleles rearranged4, 41. Our data from wild-type DP thymocytes is consistent with these predictions, as 61.5% (324 of 527) of nuclei had one allele rearranged while 38.5% (203 of 527) had both alleles rearranged (Fig. 6f). The latter likely represents a slight underestimate of nuclei with two rearranged alleles, as Vβ14 rearrangements are undetectable by FISH. Notably, in EαKI DP thymocytes, 48.8% (63 of 129) of nuclei had one allele rearranged whereas 51.2 % (66 of 129) had both alleles rearranged. Thus, addition of an ectopic enhancer into the Tcrb locus increased the number of nuclei with two rearranged alleles, implying a disruption of allelic exclusion.
Discussion
Previous studies of the Igh, Igk and Tcrb loci implicated regulated monoallelic association with peri-centromeric heterchromatin in the initiation or maintenance of allelic exclusion10, 32-34. Here we have addressed in detail the nuclear localization of Tcrb alleles during T lymphocyte development and provide a fundamentally different view based on the following observations. First, we found that Tcrb alleles associate with peri-centromeric heterochromatin and with the nuclear lamina at high frequencies throughout T cell development. Second, we found that these associations occur stochastically rather than in a directed fashion, with the two Tcrb alleles in a cell associating independently and with equal probability with the two compartments. Third, we found that associated Tcrb alleles were less likely to have undergone Vβ-to-DβJβ recombination, and that this bias was established prior to β-selection and independent of feedback inhibition. Fourth, we found that an ectopic enhancer inhibited Tcrb locus association with peri-centromeric heterochromatin and the nuclear lamina and impaired allelic exclusion. We propose that stochastic, high frequency associations of Tcrb alleles with peri-centromeric heterochromatin and with the nuclear lamina promote asynchronous Vβ-to-DβJβ recombination and are critical for the initiation of Tcrb allelic exclusion.
Our data suggest that the nuclear lamina and peri-centromeric heterochromatin are both repressive for Vβ-to-DβJβ recombination and distinguish three subsets of DN thymocytes based on the number of Tcrb alleles that are associated with these compartments. Approximately 35% of DN thymocytes have one allele associated with either the nuclear lamina or peri-centromeric heterochromatin. Asynchronous recombination in this subset can be readily understood based on the observed bias for the nonassociated allele to be the first to undergo Vβ-to-DβJβ recombination. In approximately 60% of nuclei, both Tcrb alleles are associated with a repressive compartment. We propose that asynchronous recombination is promoted in this subset by inefficient recombination on both alleles. This will create an expanded time window for Vβ-to-DβJβ recombination, thus diminishing the likelihood that such rearrangements will be initiated simultaneously on both alleles. Only 5% of nuclei display two alleles that are free of both the nuclear lamina and peri-centromeric heterochromatin. Thus, stochastic association of Tcrb alleles with repressive nuclear compartments may diminish the likelihood that Vβ-to-DβJβ recombination is initiated biallelically in fully 95% of developing thymocytes. This asynchrony would allow thymocytes sufficient time to test the quality of recombination on the first allele and to initiate feedback inhibition of recombination on the second allele.
Our data suggest that Vβ-to-DβJβ recombination can occur on both associated and nonassociated alleles but that it occurs more efficiently on nonassociated alleles. Nevertheless our analysis may underestimate the extent to which association with repressive nuclear compartments impairs recombination, because three color Immuno-FISH does not allow concurrent analysis of rearrangement status and colocalization with both γ-satellite and laminB1. Thus, in the informative subset of cells with one free and one associated allele, the allele judged to be free of one compartment may have been associated with the undetected repressive compartment. Such biallelically associated cells are intrinsically uninformative in assessing a recombination bias and their inclusion would suppress any measured bias. Thus the preference to undergo recombination on a free as compared to an associated allele may be greater than the measured 65:35 (peri-centromeric) and 76:24 (nuclear lamina) ratios would suggest. Regardless, because it is unclear whether associated Tcrb alleles interact stably with the nuclear lamina and pericentromeric heterochromatin or transiently associate and dissociate from these compartments, we cannot determine the extent to which Vβ-to-DβJβ recombination is inhibited by molecular interactions with these compartments.
Our previous study of EαKI Tcrb alleles indicated that the introduced Eα caused a three-fold increase in Vβ12 recombination in DN thymocytes8. We also detected a disruption of Vβ12 allelic exclusion that we attributed to a loss of allelic asynchrony in DN thymocytes, as we found no evidence for disruption of feedback inhibition in EαKI DP thymocytes8. Herein we show that EαKI Tcrb alleles less frequently colocalized with γ-satellite in DP thymocytes and less frequently colocalized with laminB1 in DN thymocytes. The latter resulted in an increased frequency of DN thymocyte nuclei with two free alleles (from 5.2% to 18.8%). We propose that this change in nuclear configuration increases the probability of near-simultaneous recombination of both alleles in DN thymocytes; the detected increase in DP thymocytes with two rearranged alleles would follow as a direct consequence.
Our FISH analysis indicates a 12% increase in thymocytes with two Tcrb rearrangements. Because only 1/3 of the additional rearrangements should be in-frame, these data argue that 4% of EαKI DP thymocytes may be phenotypically allelically included. Previous dual staining analyses of EαKI T lymphocytes identified an allelically included Vβ12+ population that could represent, in total, about 0.5-1.0% of all T lymphocytes8. The apparent underestimation of allelic inclusion by Jackson et al.8 may reflect the fact that only two Vβ segments were examined in that study. Additionally, some cells with two in-frame rearrangements may not detectably express two cell surface TCRs. Regardless, the two studies, taken together, identify relocalization away from repressive nuclear compartments and allelic inclusion as two linked consequences of ectopic insertion of Eα.
Several aspects of our observations differ markedly from those in a previous study10. First, the authors of that study noted substantially higher frequencies of association of Tcra alleles with the nuclear periphery in all cell populations. Second, although they found Tcrb alleles to associate with peri-centromeric heterochromatin at frequencies similar to those reported here, they observed substantially higher frequencies of monoallelic association. A potential explanation for the difference in peripheral localization is that we scored colocalization by overlap between the TCR loci and laminB1 signals, wheras Skok et al.10 did not use a specific marker to define the nuclear periphery. Differences in monoallelic Tcrb gene association with peri-centromeric heterochromatin are more difficult to explain. In addition, we provided direct measures of Tcrb allelic recombination status and assessed the significance of repressive compartment association for Tcrb gene recombination prior to β-selection. We conclude that Tcrb alleles associate stochastically and at high frequency with both the nuclear lamina and pericentromeric heterochromatin and that such associations inhibit Vβ-to-DβJβ recombination prior to β-selection. Our data suggests a fundamentally different basis for the intiation of Tcrb allelic exclusion in which asynchronous Vβ-to-DβJβ recombination is achieved stochastically rather than through strictly regulated allelic choice.
Materials and Methods
Mice
Rag2-/- mice42, Lat-/- mice36, Eα-/-Rag2-/- × Tcrb transgenic mice43, EαKI mice8, and EαKI Rag2-/- mice8 were described previously. All mice were used in accordance with protocols approved by the Duke University and University of Chicago Institutional Animal Care and Use Committees.
Cell isolation
Rag2-/- pro-B cells were isolated and cultured as described previously44. DP thymocytes were sorted to 98 % (wild-type) or 95% (EαKI) purity as described37.
FISH probes and antibodies
BAC clones RP23-75P5 (Cβ-distal end of the Tcrb locus), RP23-203H5 (Cβ-proximal region of the Tcrb locus), RP23-304L21 (Cα-distal end of the Tcra locus), and RP23-97O1 (Actb locus) were used to prepare locus-specific DNA probes. The BAC clones were labeled using a Biotin or Digoxigenin (DIG) nick-translation (NT) kit (Roche). Foci were then detected using either FITC-conjugated streptavidin (Jackson Immunoresearch Laboratories) to detect biotin-incorporate probes or Cy3- or Cy5-labeled anti-DIG antibody (Jackson Immunoresearch Laboratories) to detect DIG-incorporated probes. The probe used to detect peri-centromeric heterochromatin was composed of eight tandem copies of the major γ-satellite repeat sequence and was labeled by direct incorporation of dUTP-alexa488 or dUTP-alexa56832. The nuclear lamina was detected using a polyclonal goat anti-laminB1(sc-6217, Santa Cruz Biotech) antibody followed by Cy5-labeled anti-goat (Jackson Immunoresearch Laboratories).
3D DNA Immuno-FISH and confocal imaging
Methods for cell fixation and FISH that preserve three-dimensional structure were derived from Solovei et al45. One million thymocytes in 300 μl PBS were attached to poly-L-lysine slides for 30 min at 4°C (each slide representing an independent cell isolation). Cells were then fixed in 4% paraformaldehyde, permeabilized with 0.5% saponin and 0.5% Triton-X, treated with 0.1N HCl, and freeze-thawed 4 to 5 times in 20% glycerol. Slides were stored at this point for up to 1 month at -80°C. Slides were denatured by submerging in 70% formamide 2X SSC at 76°C for 3 mins, followed by 1 min treatment in 50% formamide 2X SSC. Conjugated probes were precipitated in ethanol with blocking DNA (consisting of mouse C0t DNA, human placental DNA, and salmon sperm DNA), resuspended in 50% deionized formamide containing 2X SSC and 10% Dextran sulfate, boiled for 5 min, and pre-annealed at 37°C for at least 1 hour prior to slide hybridization. Probes were then added to the slide for hybridization under a rubber cement sealed coverslip in a humid chamber for 48-96h at 37°C. Following hybridization, the slides were washed 3 times in 50% formamide 2X SSC at 42°C followed by 3 washes in 0.2X SSC at 63°C. The slides were blocked with 4% BSA, 2X SSC and then incubated with the appropriate antibodies in 4% BSA, 2X SSC. Excess antibody was removed by washing 3 times in 0.1% Triton-X in 2X SSC. Secondary antibodies, if required, were incubated and washed in the same manner. Slides were mounted in VectaShield (Vector Laboratories) and imaged on an Olympus confocal microscope. Images from the confocal microscope were saved as stacks of TIFFs and processed with ImageJ software46. The images were passed through a Kalman stack filter and then segmented such that foci of gene loci were of uniform size, that the nuclear lamina was the thinnest contiguous circle possible, and that centromeric heterochromatin regions had distinct edges (see example in Supplementary Fig. 3, online). Gene foci were then considered to be colocalized with γ-satellite or laminB1 if at least two adjacent pixels between the two signals were positive in both channels. A Tcrb allele was considered unrearranged if the distal and proximal foci overlapped or were within 2 pixels of each other and was considered rearranged if the distal focus was at least 5 pixels away from the nearest proximal focus. Foci separated by a distance of 2 to 5 pixels were considered non-informative and were dropped from the data set. Only nuclei with two detectable alleles were evaluated.
Statistical Analysis
Statistical differences between populations were determined by using Fisher’s exact two-tailed contingency table.
Supplementary Material
Acknowledgements
We thank B. Sleckman (Washington University School of Medicine, St. Louis, MO) and Y. Zhuang (Duke University Medical Center, Durham, NC) for comments on the manuscript. Supported by the N.I.H (GM41052 and AI49934 to M.S.K.) and the Howard Hughes Medical Institute (to H.S.)
References
- 1.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 2.Michie AM, Zuniga-Pflucker JC. Regulation of thymocyte differentiation: pre-TCR signals and β-selection. Semin Immunol. 2002;14:311–323. doi: 10.1016/s1044-5323(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 3.Khor B, Sleckman BP. Allelic exclusion at the TCRβ locus. Curr Opin Immunol. 2002;14:230–234. doi: 10.1016/s0952-7915(02)00326-6. [DOI] [PubMed] [Google Scholar]
- 4.Mostoslavsky R, Alt FW, Rajewsky K. The lingering enigma of the allelic exclusion mechanism. Cell. 2004;118:539–544. doi: 10.1016/j.cell.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Jackson AM, Krangel MS. Turning T-cell receptor β recombination on and off: more questions than answers. Immunol Rev. 2006;209:129–141. doi: 10.1111/j.0105-2896.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 6.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 7.Tripathi R, Jackson A, Krangel MS. A change in the structure of Vβ chromatin associated with TCR β allelic exclusion. J Immunol. 2002;168:2316–2324. doi: 10.4049/jimmunol.168.5.2316. [DOI] [PubMed] [Google Scholar]
- 8.Jackson A, Kondilis HD, Khor B, Sleckman BP, Krangel MS. Regulation of T cell receptor β allelic exclusion at a level beyond accessibility. Nat Immunol. 2005;6:189–197. doi: 10.1038/ni1157. [DOI] [PubMed] [Google Scholar]
- 9.Agata Y, et al. Regulation of T cell receptor β gene rearrangements and allelic exclusion by the helix-loop-helix protein, E47. Immunity. 2007;27:871–884. doi: 10.1016/j.immuni.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Skok JA, et al. Reversible contraction by looping of the Tcra and Tcrb loci in rearranging thymocytes. Nat Immunol. 2007;8:378–387. doi: 10.1038/ni1448. [DOI] [PubMed] [Google Scholar]
- 11.Mostoslavsky R, et al. κ chain monoallelic demethylation and the establishment of allelic exclusion. Genes Dev. 1998;12:1801–1811. doi: 10.1101/gad.12.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mostoslavsky R, et al. Asynchronous replication and allelic exclusion in the immune system. Nature. 2001;414:221–225. doi: 10.1038/35102606. [DOI] [PubMed] [Google Scholar]
- 13.Schlissel M. Allelic exclusion of immunoglobulin gene rearrangement and expression: why and how? Semin Immunol. 2002;14:207–212. doi: 10.1016/s1044-5323(02)00044-1. discussion 225-206. [DOI] [PubMed] [Google Scholar]
- 14.Liang HE, Hsu LY, Cado D, Schlissel MS. Variegated transcriptional activation of the immunoglobulin κ locus in pre-B cells contributes to the allelic exclusion of light-chain expression. Cell. 2004;118:19–29. doi: 10.1016/j.cell.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Liang HE, et al. The “dispensable” portion of RAG2 is necessary for efficient V-to-DJ rearrangement during B and T cell development. Immunity. 2002;17:639–651. doi: 10.1016/s1074-7613(02)00448-x. [DOI] [PubMed] [Google Scholar]
- 16.Brown CR, Silver PA. Pore-ing the right dose. Nat Cell Biol. 2006;8:430–431. doi: 10.1038/ncb0506-430. [DOI] [PubMed] [Google Scholar]
- 17.Kosak ST, Groudine M. Form follows function: The genomic organization of cellular differentiation. Genes Dev. 2004;18:1371–1384. doi: 10.1101/gad.1209304. [DOI] [PubMed] [Google Scholar]
- 18.Pickersgill H, et al. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet. 2006;38:1005–1014. doi: 10.1038/ng1852. [DOI] [PubMed] [Google Scholar]
- 19.Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Schneider R, Grosschedl R. Dynamics and interplay of nuclear architecture, genome organization, and gene expression. Genes Dev. 2007;21:3027–3043. doi: 10.1101/gad.1604607. [DOI] [PubMed] [Google Scholar]
- 21.Hewitt SL, High FA, Reiner SL, Fisher AG, Merkenschlager M. Nuclear repositioning marks the selective exclusion of lineage-inappropriate transcription factor loci during T helper cell differentiation. Eur J Immunol. 2004;34:3604–3613. doi: 10.1002/eji.200425469. [DOI] [PubMed] [Google Scholar]
- 22.Zink D, et al. Transcription-dependent spatial arrangements of CFTR and adjacent genes in human cell nuclei. J Cell Biol. 2004;166:815–825. doi: 10.1083/jcb.200404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams RR, et al. Neural induction promotes large-scale chromatin reorganisation of the Mash1 locus. J Cell Sci. 2006;119:132–140. doi: 10.1242/jcs.02727. [DOI] [PubMed] [Google Scholar]
- 24.Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- 25.Kosak ST, et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 26.Fuxa M, et al. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Q, Riblet R, Schildkraut CL. Sites that direct nuclear compartmentalization are near the 5′ end of the mouse immunoglobulin heavy-chain locus. Mol Cell Biol. 2005;25:6021–6030. doi: 10.1128/MCB.25.14.6021-6030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher AG, Merkenschlager M. Gene silencing, cell fate and nuclear organisation. Curr Opin Genet Dev. 2002;12:193–197. doi: 10.1016/s0959-437x(02)00286-1. [DOI] [PubMed] [Google Scholar]
- 29.Su RC, Sridharan R, Smale ST. Assembly of silent chromatin during thymocyte development. Semin Immunol. 2005;17:129–140. doi: 10.1016/j.smim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Brown KE, et al. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91:845–854. doi: 10.1016/s0092-8674(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 31.Brown KE, Baxter J, Graf D, Merkenschlager M, Fisher AG. Dynamic repositioning of genes in the nucleus of lymphocytes preparing for cell division. Mol Cell. 1999;3:207–217. doi: 10.1016/s1097-2765(00)80311-1. [DOI] [PubMed] [Google Scholar]
- 32.Skok JA, et al. Nonequivalent nuclear location of immunoglobulin alleles in B lymphocytes. Nat Immunol. 2001;2:848–854. doi: 10.1038/ni0901-848. [DOI] [PubMed] [Google Scholar]
- 33.Roldan E, et al. Locus ‘decontraction’ and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldmit M, et al. Epigenetic ontogeny of the Igk locus during B cell development. Nat Immunol. 2005;6:198–203. doi: 10.1038/ni1154. [DOI] [PubMed] [Google Scholar]
- 35.Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor α/δ locus. Immunol Rev. 2004;200:224–232. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 37.Jackson AM, Krangel MS. A role for MAPK in feedback inhibition of Tcrb recombination. J Immunol. 2006;176:6824–6830. doi: 10.4049/jimmunol.176.11.6824. [DOI] [PubMed] [Google Scholar]
- 38.Francastel C, Walters MC, Groudine M, Martin DI. A functional enhancer suppresses silencing of a transgene and prevents its localization close to centrometric heterochromatin. Cell. 1999;99:259–269. doi: 10.1016/s0092-8674(00)81657-8. [DOI] [PubMed] [Google Scholar]
- 39.Ragoczy T, Telling A, Sawado T, Groudine M, Kosak ST. A genetic analysis of chromosome territory looping: diverse roles for distal regulatory elements. Chromosome Res. 2003;11:513–525. doi: 10.1023/a:1024939130361. [DOI] [PubMed] [Google Scholar]
- 40.Ragoczy T, Bender MA, Telling A, Byron R, Groudine M. The locus control region is required for association of the murine β-globin locus with engaged transcription factories during erythroid maturation. Genes Dev. 2006;20:1447–1457. doi: 10.1101/gad.1419506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khor B, Sleckman BP. Intra- and inter-allelic ordering of T cell receptor β-chain gene assembly. Eur J Immunol. 2005;35:964–970. doi: 10.1002/eji.200425806. [DOI] [PubMed] [Google Scholar]
- 42.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 43.Sleckman BP, Bardon CG, Ferrini R, Davidson L, Alt FW. Function of the TCR α enhancer in αβ and γδ T cells. Immunity. 1997;7:505–515. doi: 10.1016/s1074-7613(00)80372-6. [DOI] [PubMed] [Google Scholar]
- 44.Smithson G, Medina K, Ponting I, Kincade PW. Estrogen suppresses stromal cell-dependent lymphopoiesis in culture. J Immunol. 1995;155:3409–3417. [PubMed] [Google Scholar]
- 45.Solovei I, et al. Spatial preservation of nuclear chromatin architecture during three-dimensional fluorescence in situ hybridization (3D-FISH) Exp Cell Res. 2002;276:10–23. doi: 10.1006/excr.2002.5513. [DOI] [PubMed] [Google Scholar]
- 46.Bertolino E, et al. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nat Immunol. 2005;6:836–843. doi: 10.1038/ni1226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.