Abstract
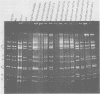
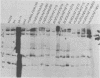
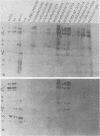
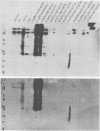
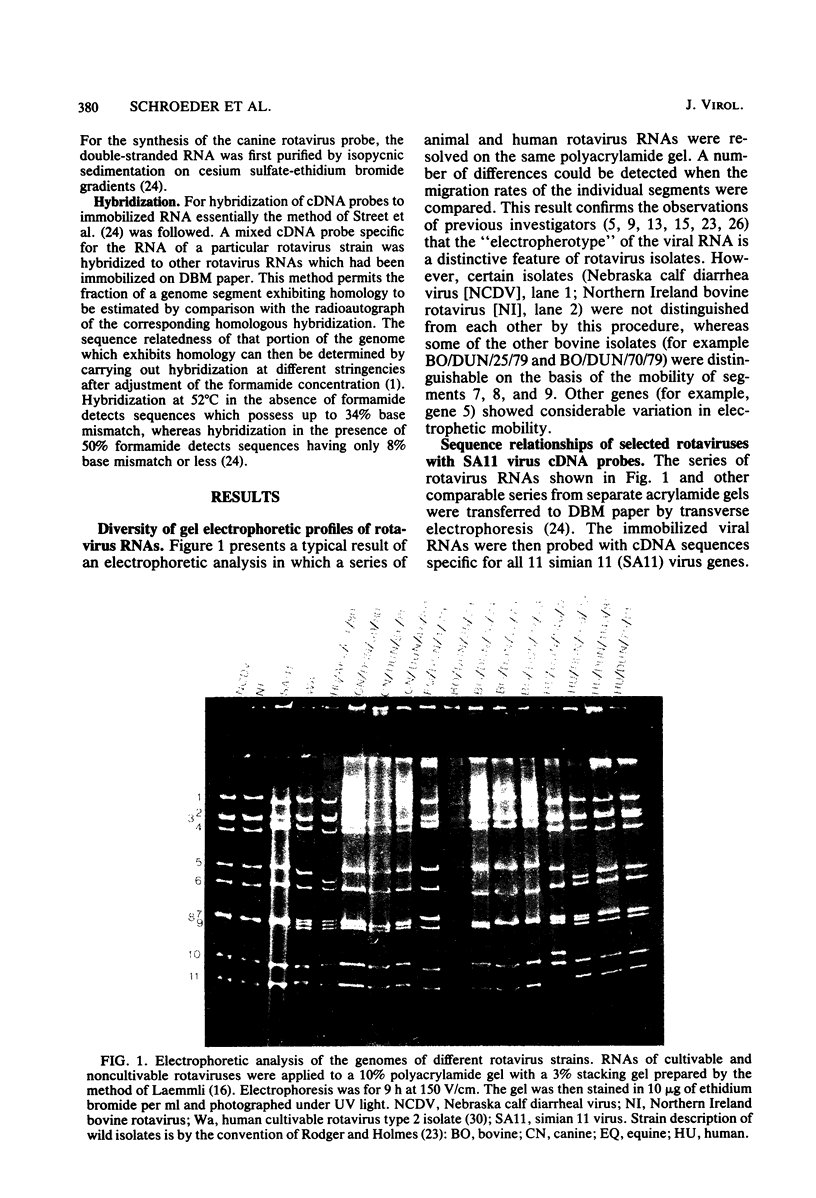
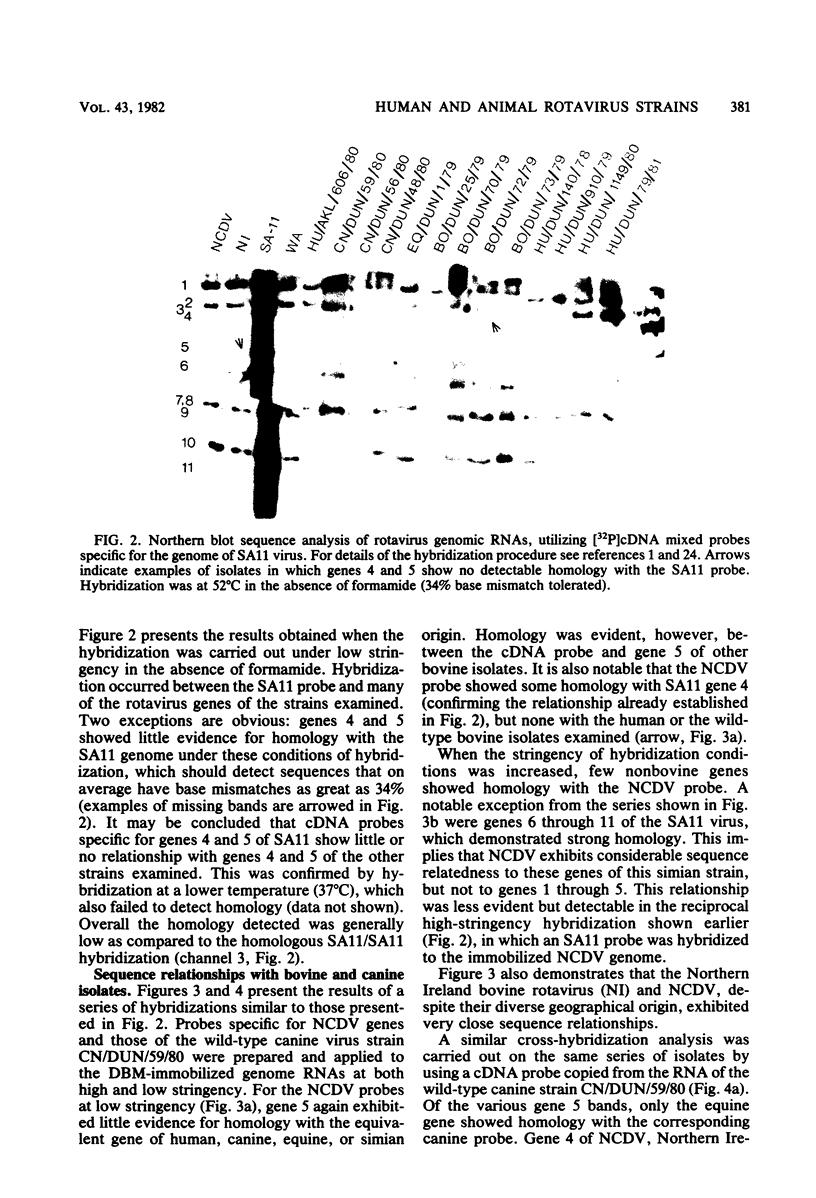
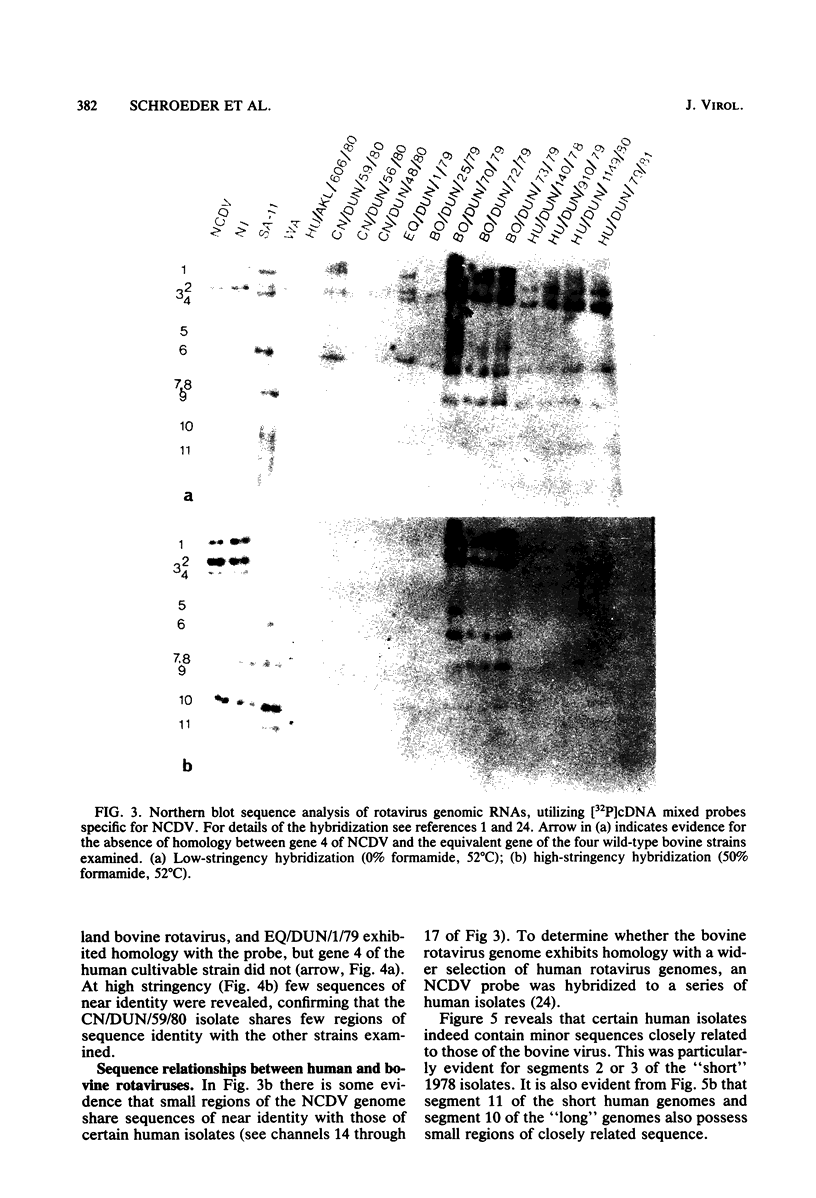
The sequence relationships of a range of cultivable and noncultivable human and animal rotaviruses were investigated by hybridization of rotavirus cDNA probes to genomic RNAs immobilized on diazobenzyloxymethyl paper. Under conditions of low stringency (34% base mismatch tolerated) most genome segments exhibited partial homology except for genes 4 and 5. In contrast, under more stringent conditions of hybridization in which no more than 8% base mismatch was tolerated, few segments exhibited homology. Generally the human and animal rotaviruses were found to possess distinct nucleic acid sequences that exhibit only a low order of sequence relatedness. These results are consistent with the notion that both cumulative changes in nucleic acid sequences and the interchange of segments may be involved in the evolution of distinct rotavirus strains.
Full text
PDF

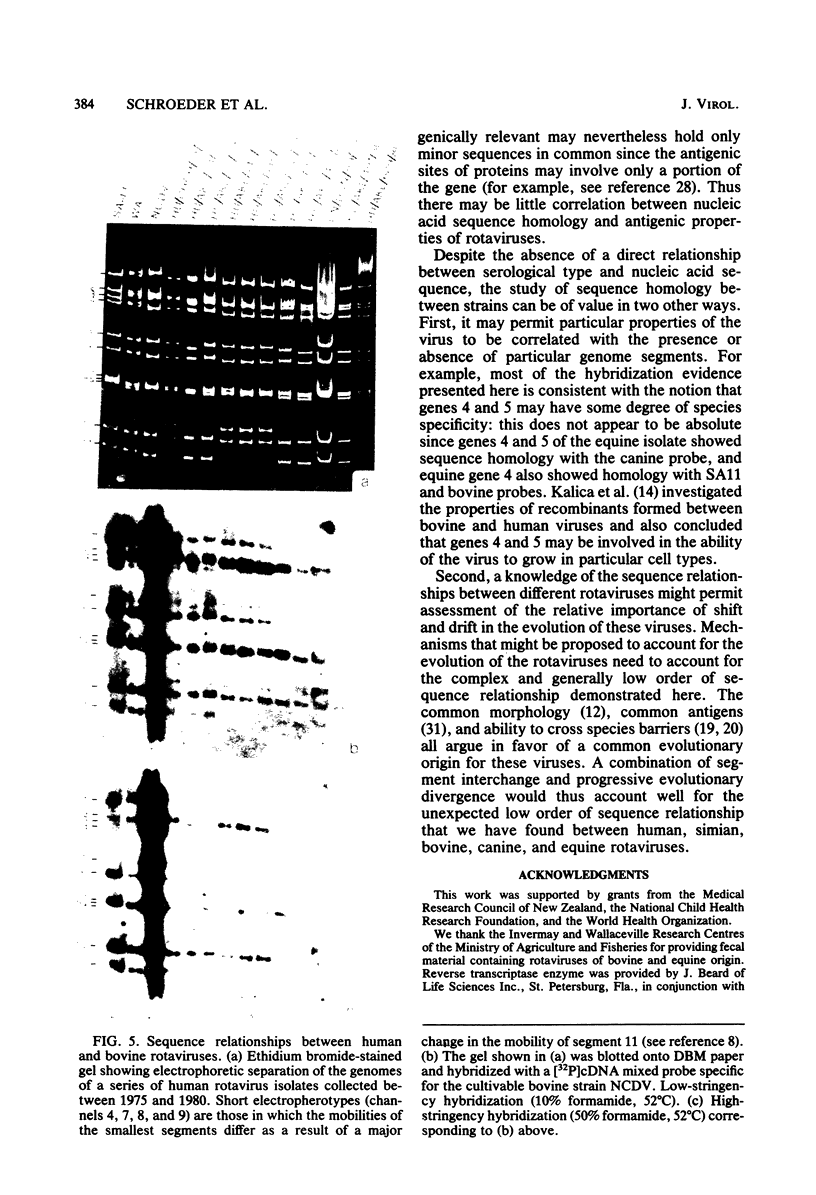

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Beards G. M., Pilfold J. N., Thouless M. E., Flewett T. H. Rotavirus serotypes by serum neutralisation. J Med Virol. 1980;5(3):231–237. doi: 10.1002/jmv.1890050307. [DOI] [PubMed] [Google Scholar]
- Croxson M. C., Bellamy A. R. Extraction of rotavirus from human feces by treatment with lithium dodecyl sulfate. Appl Environ Microbiol. 1981 Jan;41(1):255–260. doi: 10.1128/aem.41.1.255-260.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson M. C., Bellamy A. R. Two strains of human rotavirus in Auckland. N Z Med J. 1979 Sep 26;90(644):235–237. [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Gene-coding assignments of rotavirus double-stranded RNA segments 10 and 11. J Virol. 1981 Jun;38(3):1099–1103. doi: 10.1128/jvi.38.3.1099-1103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Muñz O., Serafin F., Romero P. Shift in the prevalent human rotavirus detected by ribonucleic acid segment differences. Infect Immun. 1980 Feb;27(2):351–354. doi: 10.1128/iai.27.2.351-354.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman B. M. Variation in orbiviruses. J Gen Virol. 1979 Jul;44(1):1–15. doi: 10.1099/0022-1317-44-1-1. [DOI] [PubMed] [Google Scholar]
- Holmes I. H. Viral gastroenteritis. Prog Med Virol. 1979;25:1–36. [PubMed] [Google Scholar]
- Kalica A. R., Garon C. F., Wyatt R. G., Mebus C. A., van Kirk D. H., Chanock R. M., Kapikian A. Z. Differentiation of human and calf reoviruslike agents associated with diarrhea using polyacrylamide gel electrophoresis of RNA. Virology. 1976 Oct 1;74(1):86–92. doi: 10.1016/0042-6822(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Sereno M. M., Wyatt R. G., Mebus C. A., Chanock R. M., Kapikian A. Z. Comparison of human and animal rotavirus strains by gel electrophoresis of viral RNA. Virology. 1978 Jun 15;87(2):247–255. doi: 10.1016/0042-6822(78)90130-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Studies on the origin of pandemic influenza. 3. Evidence implicating duck and equine influenza viruses as possible progenitors of the Hong Kong strain of human influenza. Virology. 1973 Feb;51(2):383–391. doi: 10.1016/0042-6822(73)90437-6. [DOI] [PubMed] [Google Scholar]
- Mebus C. A., Wyatt R. G., Sharpee R. L., Sereno M. M., Kalica A. R., Kapikian A. Z., Twiehaus M. J. Diarrhea in gnotobiotic calves caused by the reovirus-like agent of human infantile gastroenteritis. Infect Immun. 1976 Aug;14(2):471–474. doi: 10.1128/iai.14.2.471-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton P. J., Petric M., Szymanski M. T. Propagation of infantile gastroenteritis virus (orbi-group) in conventional and germfree piglets. Infect Immun. 1975 Dec;12(6):1276–1280. doi: 10.1128/iai.12.6.1276-1280.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. S. Global surveillance of influenza. Br Med Bull. 1979 Jan;35(1):9–14. doi: 10.1093/oxfordjournals.bmb.a071548. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Craven J. A., Williams I. Letter: Demonstration of reovirus-like particles in intestinal contents of piglets with diarrhoea. Aust Vet J. 1975 Nov;51(11):536–536. doi: 10.1111/j.1751-0813.1975.tb06917.x. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Holmes I. H. Comparison of the genomes of simian, bovine, and human rotaviruses by gel electrophoresis and detection of genomic variation among bovine isolates. J Virol. 1979 Jun;30(3):839–846. doi: 10.1128/jvi.30.3.839-846.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street J. E., Croxson M. C., Chadderton W. F., Bellamy A. R. Sequence diversity of human rotavirus strains investigated by northern blot hybridization analysis. J Virol. 1982 Aug;43(2):369–378. doi: 10.1128/jvi.43.2.369-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S. Human rotavirus in young dogs. Med J Aust. 1976 Dec 11;2(24):922–923. doi: 10.5694/j.1326-5377.1976.tb115512.x. [DOI] [PubMed] [Google Scholar]
- Verly E., Cohen J. Demonstration of size variation of RNA segments between different isolates of calf rotavirus. J Gen Virol. 1977 Jun;35(3):583–586. doi: 10.1099/0022-1317-35-3-583. [DOI] [PubMed] [Google Scholar]
- Walker P. J., Mansbridge J. N., Gorman B. M. Genetic Analysis of Orbiviruses by Using RNase T(1) Oligonucleotide Fingerprints. J Virol. 1980 Jun;34(3):583–591. doi: 10.1128/jvi.34.3.583-591.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Bridger J., Hall G. A., Jones J. M., Jackson G. The isolation of reovirus-like agents (rota-viruses) from acute gastroenteritis of piglets. J Med Microbiol. 1976 May;9(2):203–209. doi: 10.1099/00222615-9-2-203. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Barbour B., Wyatt R. G., Kalica A. R., Kapikian A. Z., Chanock R. M. Enzyme-linked immunosorbent assay for identification of rotaviruses from different animal species. Science. 1978 Jul 21;201(4352):259–262. doi: 10.1126/science.208150. [DOI] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P., Kapsenberg J. G., Enders G., Mutanda L. N. Human rotavirus serotypes. Lancet. 1981 Apr 25;1(8226):944–945. doi: 10.1016/s0140-6736(81)91639-1. [DOI] [PubMed] [Google Scholar]