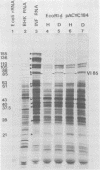
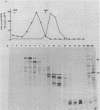
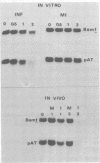
Abstract
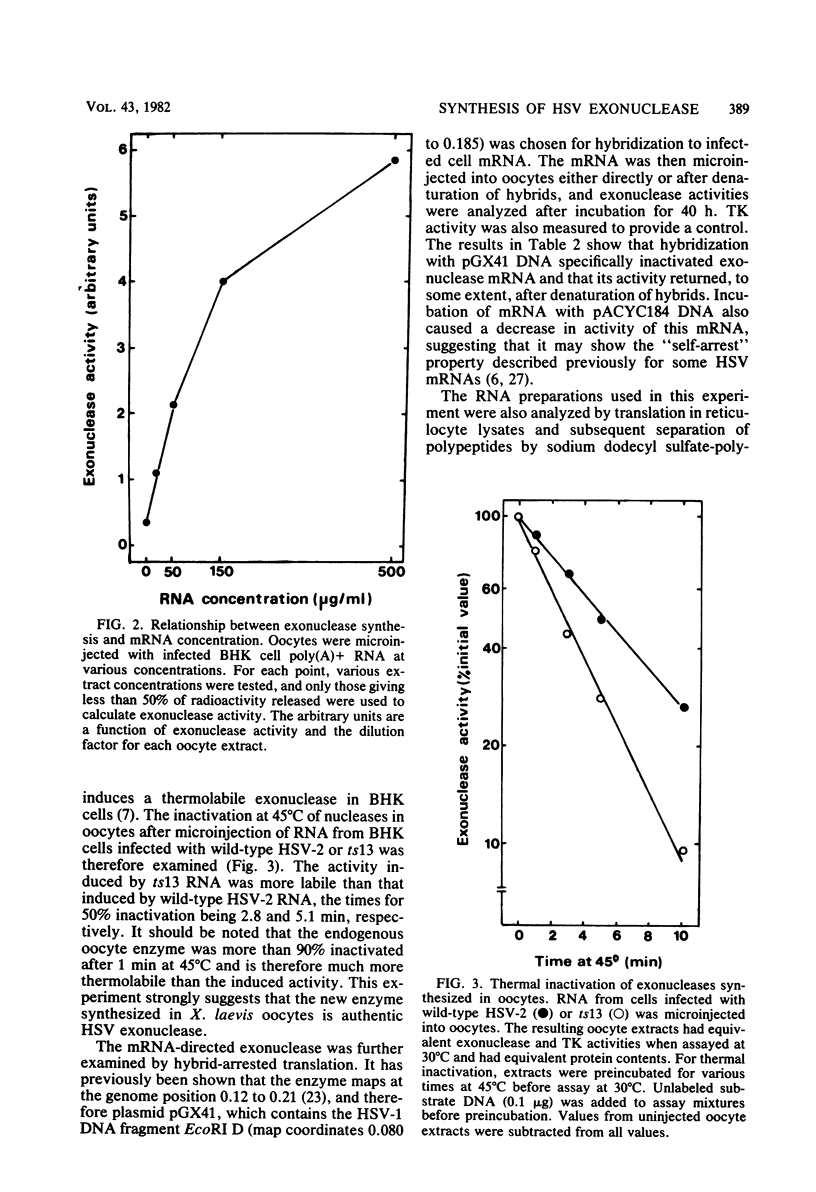
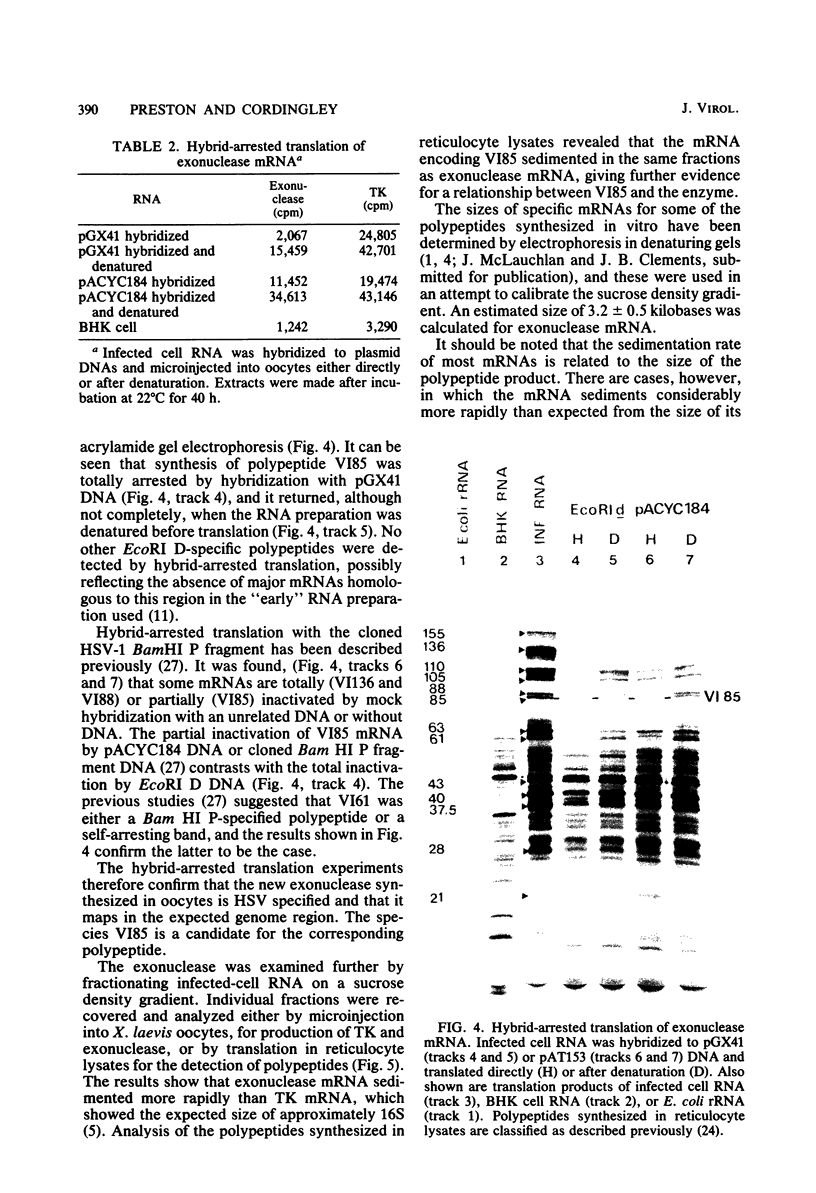
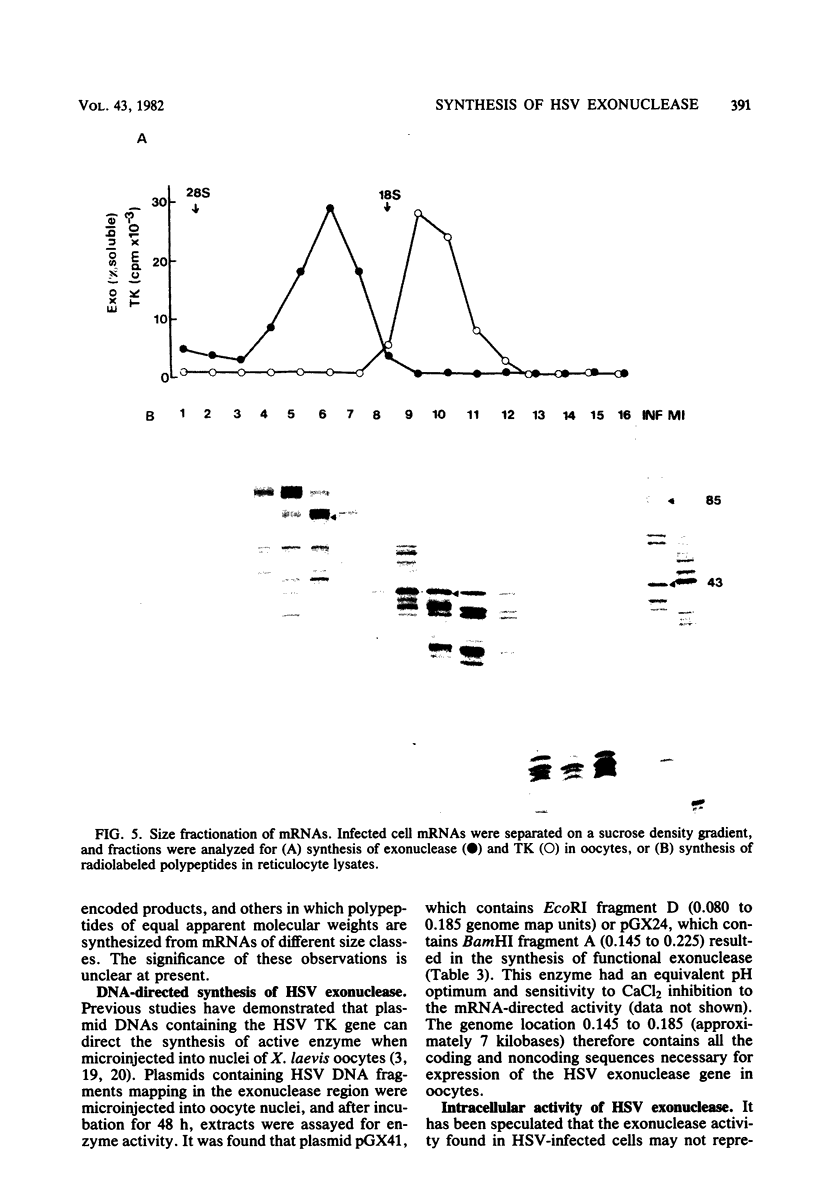
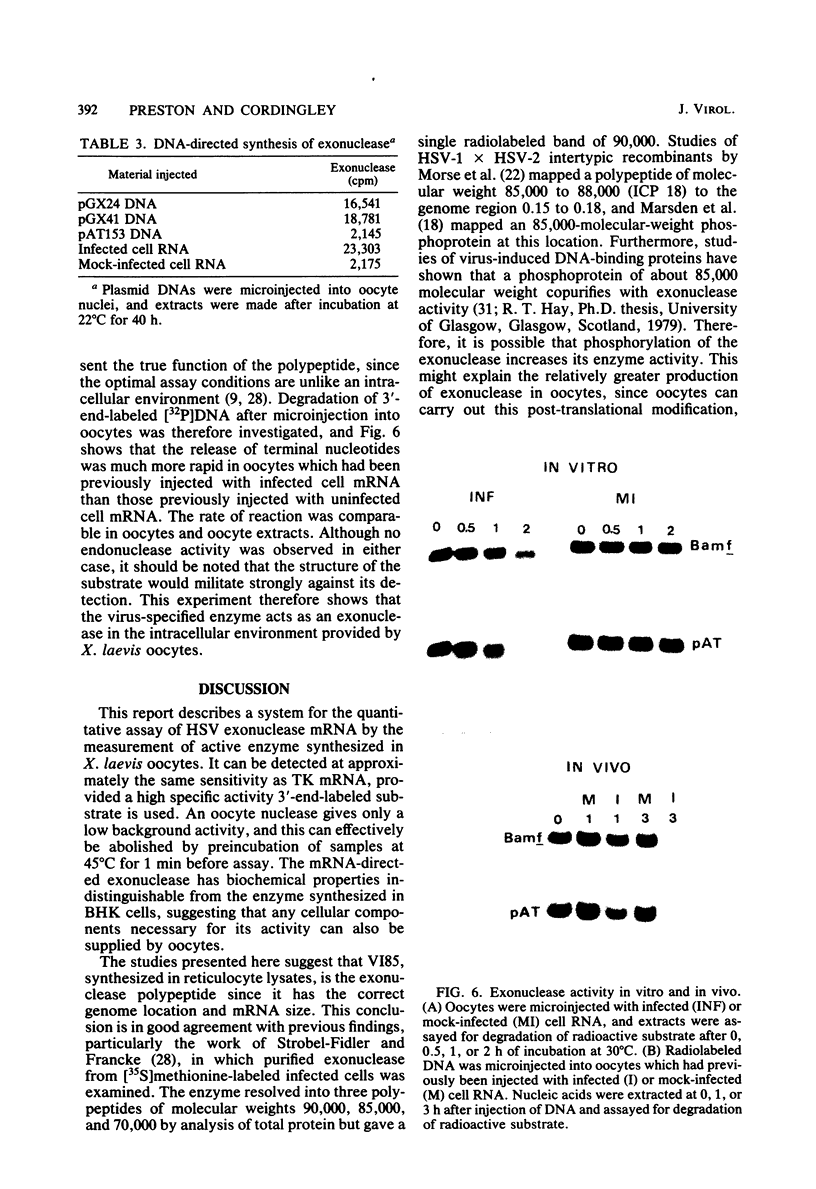
Microinjection of herpes simplex virus (HSV)-infected cell mRNA into Xenopus laevis oocytes resulted in the production of a new exonuclease activity. This enzyme strongly resembled the HSV alkaline exonuclease in many biochemical properties, and hybrid-arrested translation studies showed that it was virus coded, mapping at 0.080 to 0.185 genome map units. Exonuclease mRNA had a size and genome location equivalent to the mRNA encoding V185 in reticulocyte lysates, suggesting that V185 is the exonuclease. The enzyme synthesized in oocytes was found to act as an exonuclease in vivo. Two plasmids containing HSV DNA fragments directed the synthesis of exonuclease when microinjected into oocyte nuclei, and this finding enabled the coding and control sequences for this gene to be localized to 0.155 to 0.185 genome map units.
Full text
PDF

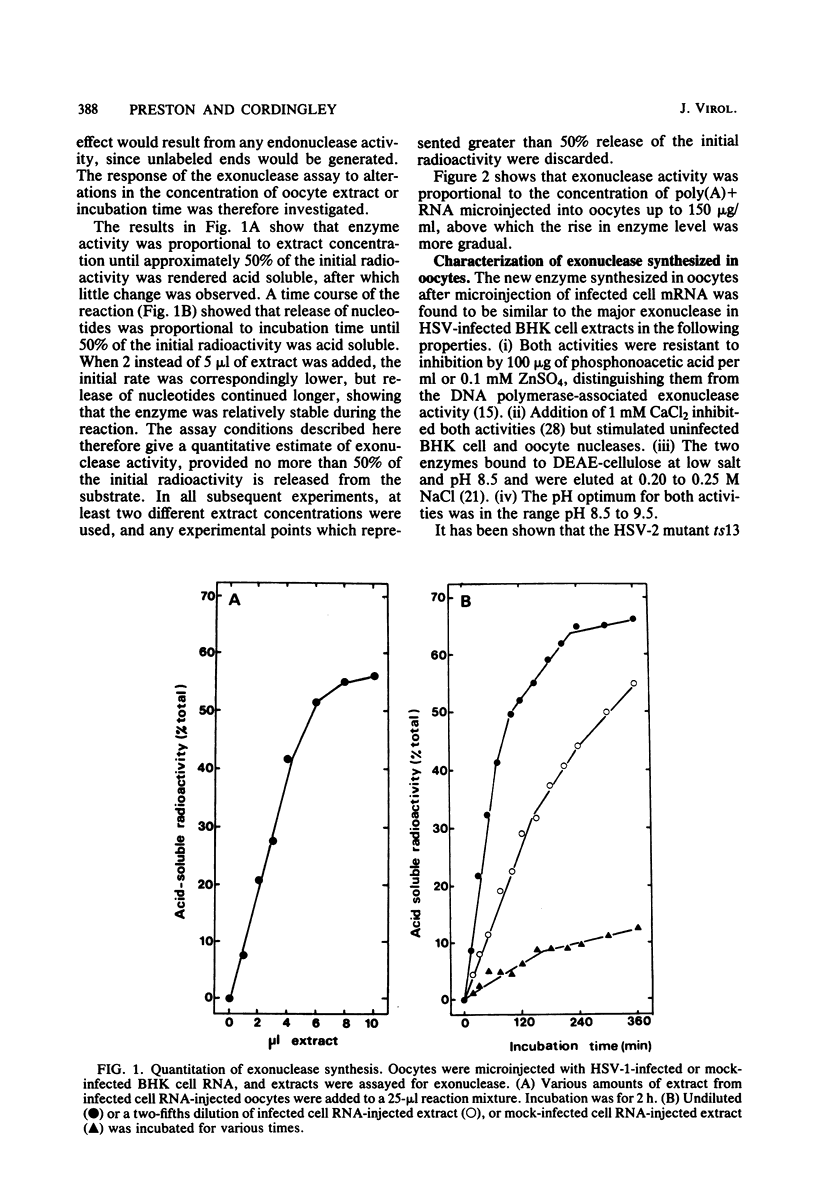


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Holland L. E., Gaylord B. H., Wagner E. K. Isolation and translation of mRNA encoded by a specific region of the herpes simplex virus type 1 genome. J Virol. 1980 Feb;33(2):749–759. doi: 10.1128/jvi.33.2.749-759.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand P., Timbury M. C., Hay J., Moss H. Mutant of herpes simplex virus type 2 with temperature-sensitive lesions affecting virion thermostability and DNase activity: identification of the lethal mutation and physical mapping of the nuc-lesion. J Virol. 1979 Oct;32(1):140–146. doi: 10.1128/jvi.32.1.140-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. B., Cortini R., Wilkie N. M. Analysis of herpesvirus DNA substructure by means of restriction endonucleases. J Gen Virol. 1976 Feb;30(2):243–256. doi: 10.1099/0022-1317-30-2-243. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Preston C. M. Transcription and translation of the herpes simplex virus type 1 thymidine kinase gene after microinjection into Xenopus laevis oocytes. J Gen Virol. 1981 Jun;54(Pt 2):409–414. doi: 10.1099/0022-1317-54-2-409. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Devi B. G., Anderson K. P., Gaylord B. H., Wagner E. K. Characterization of a major late herpes simplex virus type 1 mRNA. J Virol. 1981 May;38(2):483–496. doi: 10.1128/jvi.38.2.483-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K., Bodemer M., Summers W. C. Characterization of the mRNA for herpes simplex virus thymidine kinase by cell-free synthesis of active enzyme. Nucleic Acids Res. 1978 Jul;5(7):2333–2344. doi: 10.1093/nar/5.7.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. J., Subak-Sharpe J. H., Preston C. M. Identification of a virus-specific polypeptide associated with a transforming fragment (BglII-N) of herpes simplex virus type 2 DNA. J Virol. 1981 Oct;40(1):126–132. doi: 10.1128/jvi.40.1.126-132.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Moss H., Timbury M. C., Hay J. Alkaline DNase activity in cells infected with a temperature-sensitive mutant of herpes simplex virus type 2. J Virol. 1978 May;26(2):209–213. doi: 10.1128/jvi.26.2.209-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976 Dec;36(3):523–540. [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. The deoxyribonuclease induced after infection of KB cells by herpes simplex virus type 1 or type 2. I. Purification and characterization of the enzyme. J Biol Chem. 1978 May 25;253(10):3557–3562. [PubMed] [Google Scholar]
- Hoffmann P. J. Mechanism of degradation of duplex DNA by the DNase induced by herpes simplex virus. J Virol. 1981 Jun;38(3):1005–1014. doi: 10.1128/jvi.38.3.1005-1014.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L. E., Anderson K. P., Stringer J. R., Wagner E. K. Isolation and localization of herpes simplex virus type 1 mRNA abundant before viral DNA synthesis. J Virol. 1979 Aug;31(2):447–462. doi: 10.1128/jvi.31.2.447-462.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R. Regulation of herpesvirus thymidine kinase activity in LM(TK) cells transformed by ultraviolet light-irradiated herpes simplex virus. Virology. 1977 Jan;76(1):331–340. doi: 10.1016/0042-6822(77)90306-3. [DOI] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Leiden J. M., Buttyan R., Spear P. G. Herpes simplex virus gene expression in transformed cells. I. Regulation of the viral thymidine kinase gene in transformed L cells by products of superinfecting virus. J Virol. 1976 Nov;20(2):413–424. doi: 10.1128/jvi.20.2.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R. Expression of the herpes thymidine kinase gene in Xenopus laevis oocytes: an assay for the study of deletion mutants constructed in vitro. Nucleic Acids Res. 1980 Dec 20;8(24):5931–5948. doi: 10.1093/nar/8.24.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Morrison J. M., Keir H. M. A new DNA-exonuclease in cells infected with herpes virus: partial purification and properties of the enzyme. J Gen Virol. 1968 Dec;3(3):337–347. doi: 10.1099/0022-1317-3-3-337. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. Cell-free synthesis of herpes simplex virus-coded pyrimidine deoxyribonucleoside kinase enzyme. J Virol. 1977 Sep;23(3):455–460. doi: 10.1128/jvi.23.3.455-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., McGeoch D. J. Identification and mapping of two polypeptides encoded within the herpes simplex virus type 1 thymidine kinase gene sequences. J Virol. 1981 May;38(2):593–605. doi: 10.1128/jvi.38.2.593-605.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. The cell-free synthesis of herpesvirus-induced polypeptides. Virology. 1977 May 1;78(1):349–353. doi: 10.1016/0042-6822(77)90109-x. [DOI] [PubMed] [Google Scholar]
- Strobel-Fidler M., Francke B. Alkaline deoxyribonuclease induced by herpes simplex virus type 1: composition and properties of the purified enzyme. Virology. 1980 Jun;103(2):493–501. doi: 10.1016/0042-6822(80)90206-8. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Summers W. P. [125I]deoxycytidine used in a rapid, sensitive, and specific assay for herpes simplex virus type 1 thymidine kinase. J Virol. 1977 Oct;24(1):314–318. doi: 10.1128/jvi.24.1.314-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Preston C. M., Clements J. B. Separation and characterization of herpes simplex virus type 1 immediate-early mRNA's. J Virol. 1979 Jul;31(1):42–52. doi: 10.1128/jvi.31.1.42-52.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980 Jan;33(1):167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]