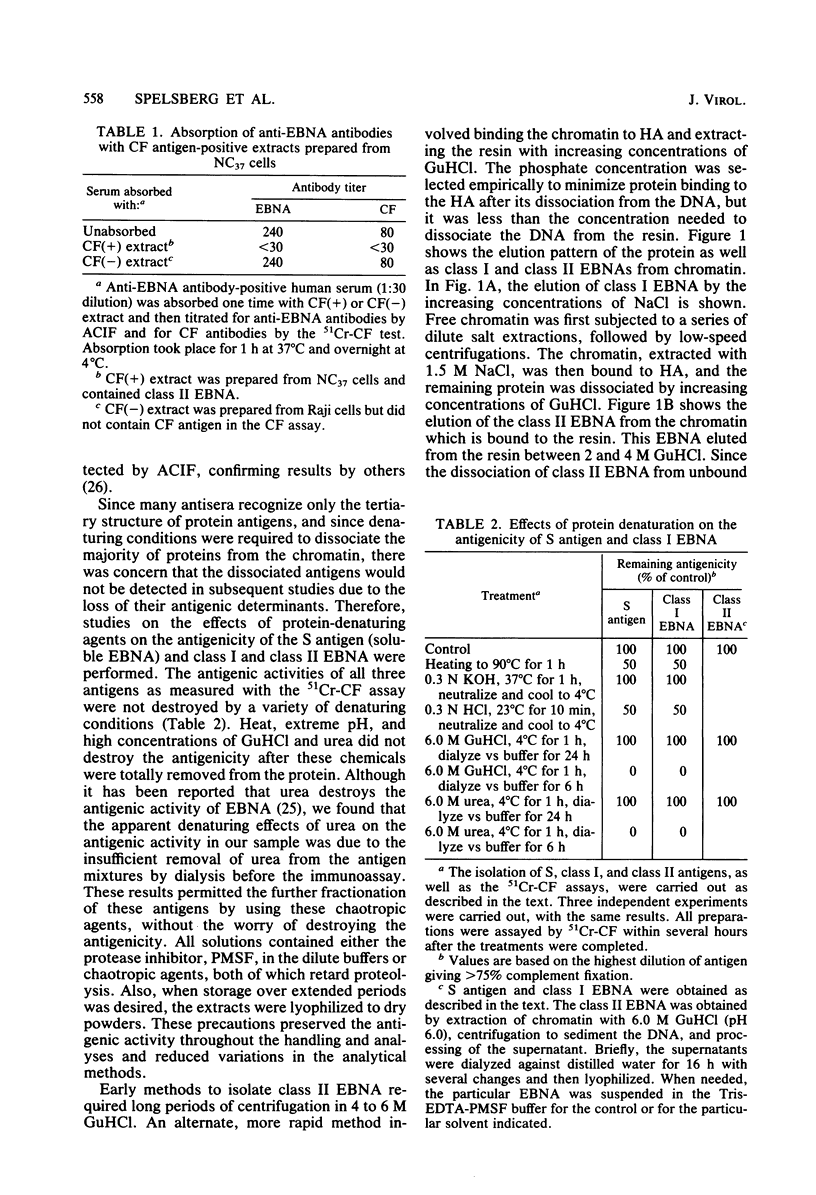
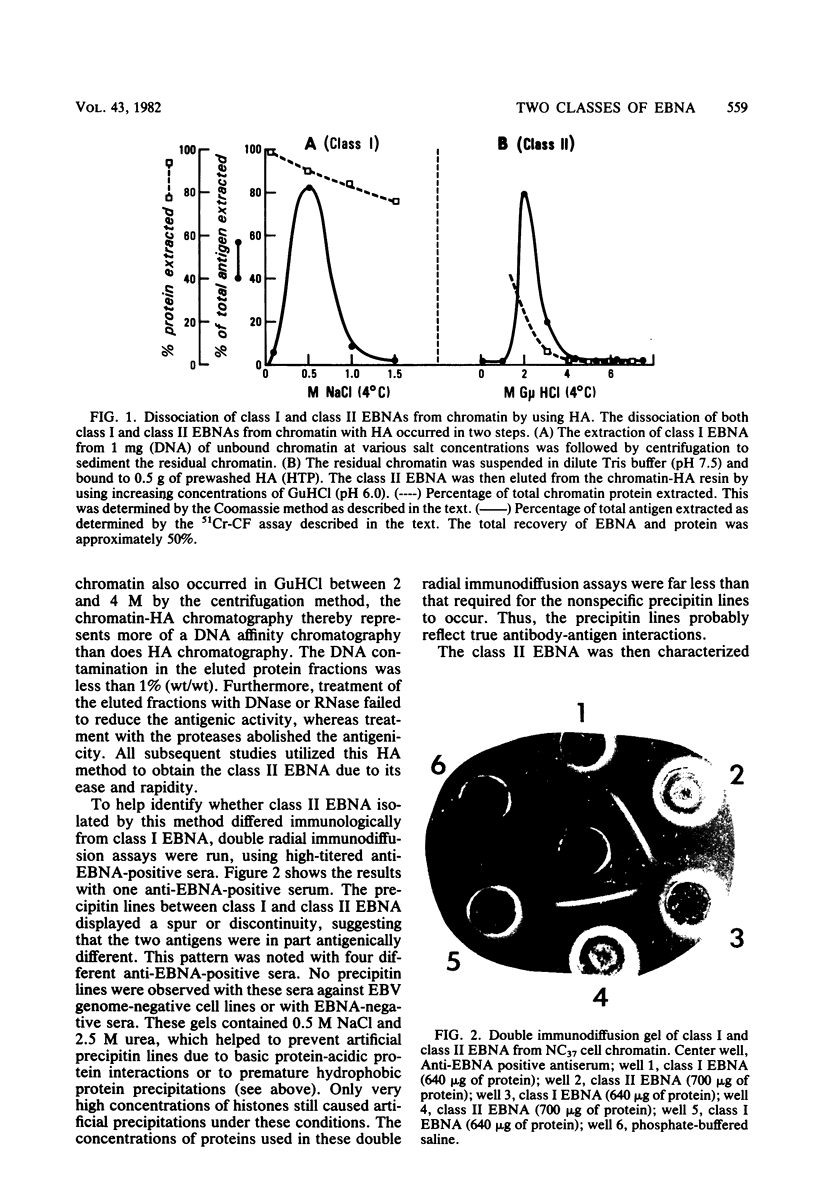
Abstract
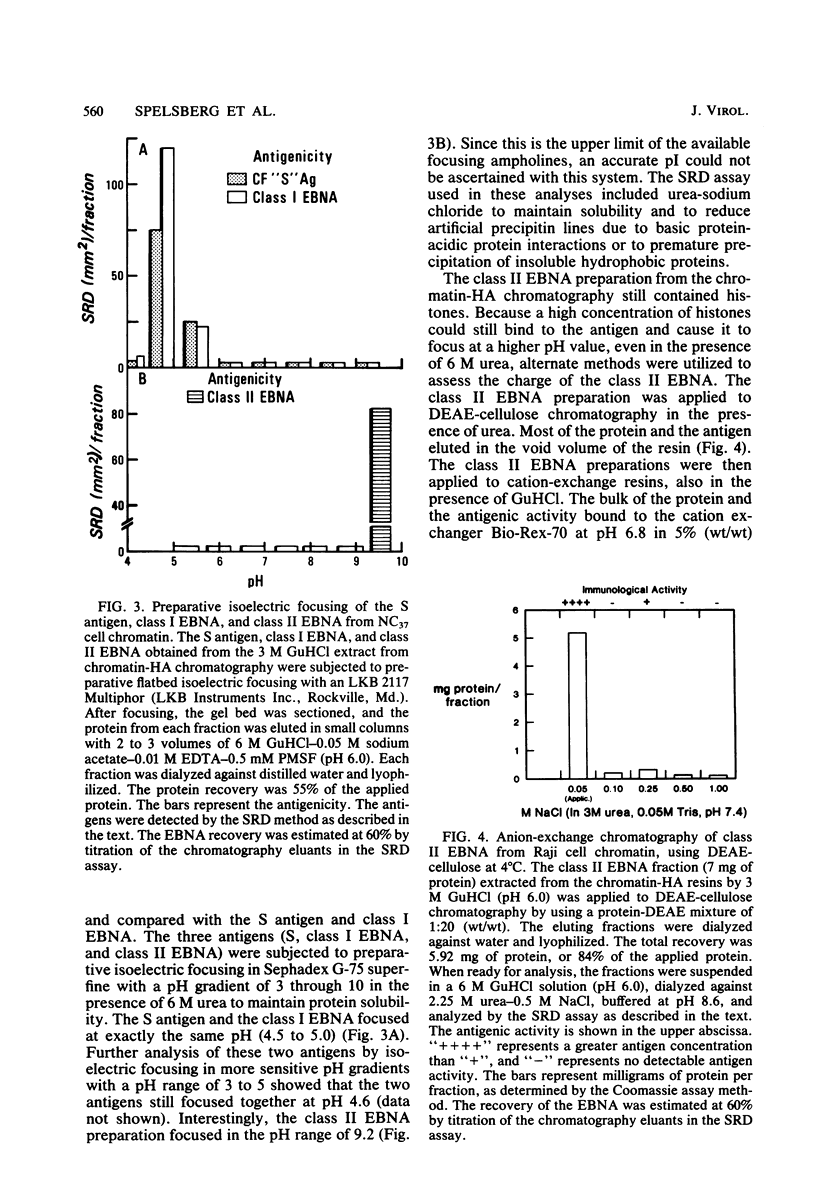
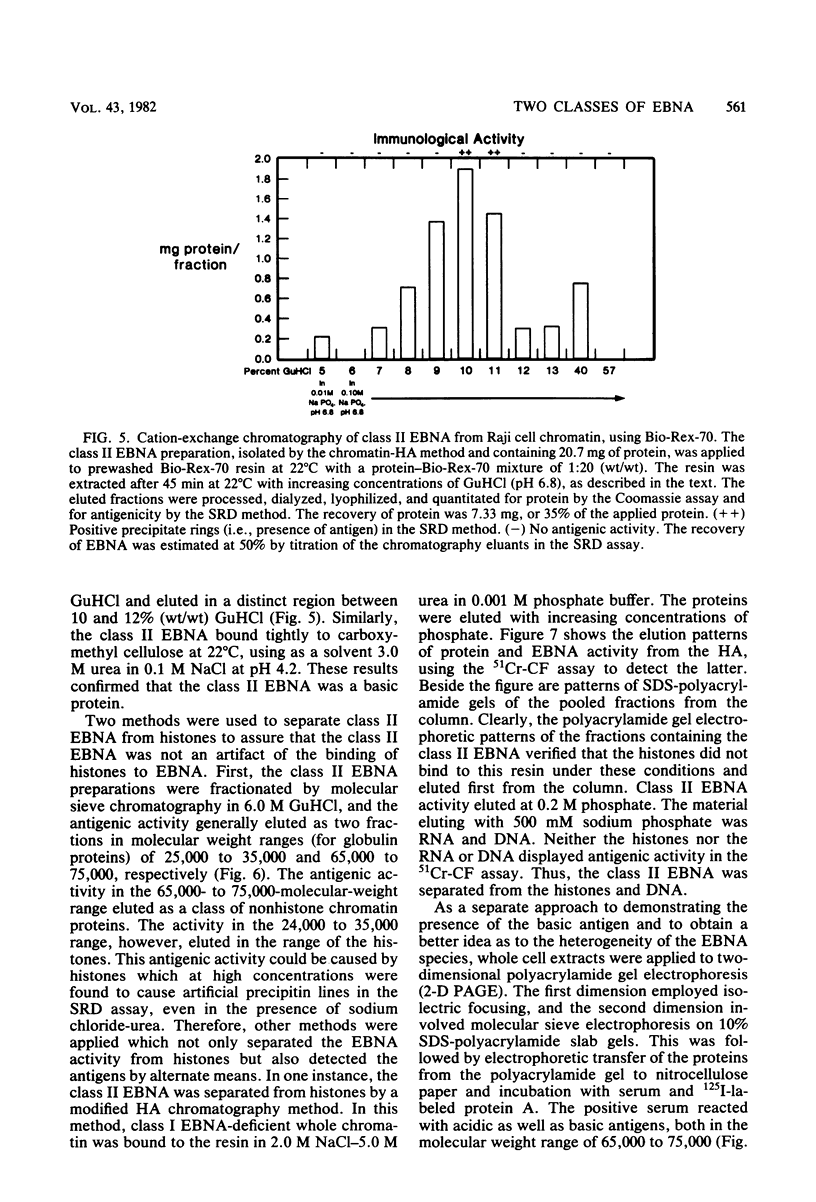
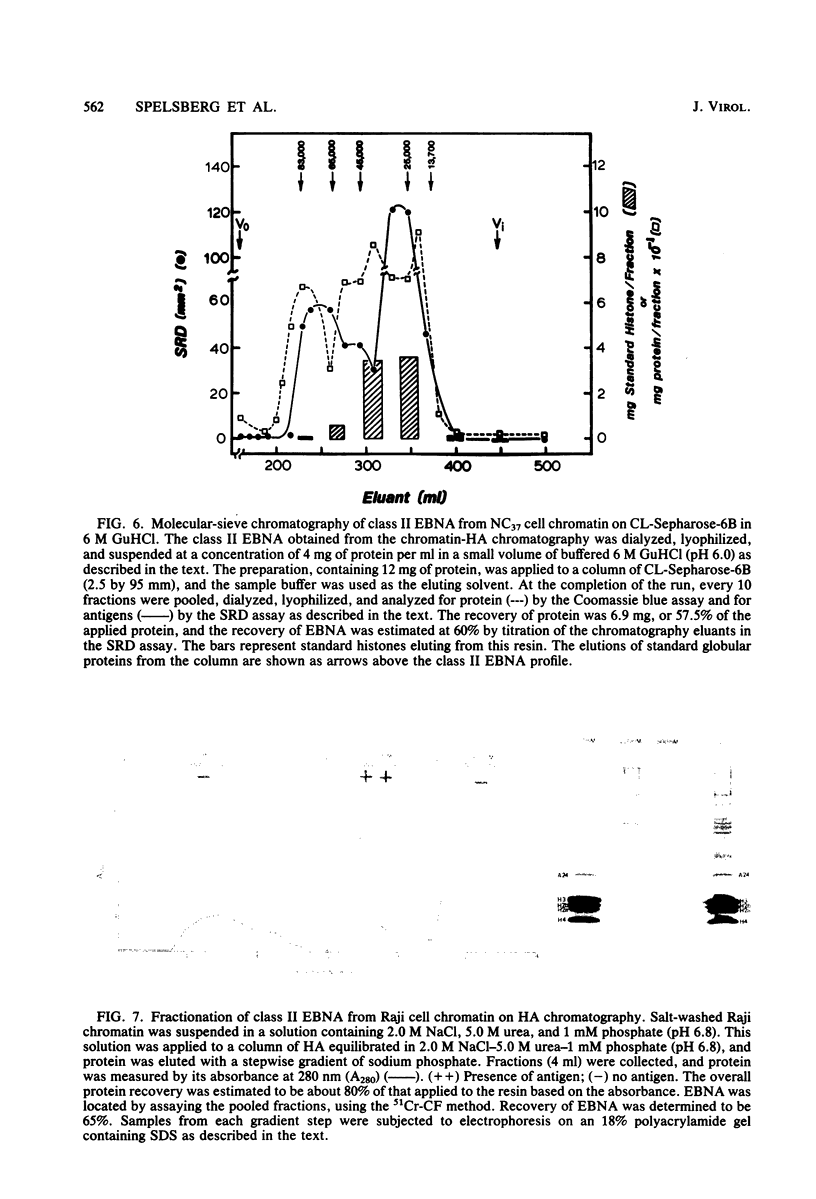
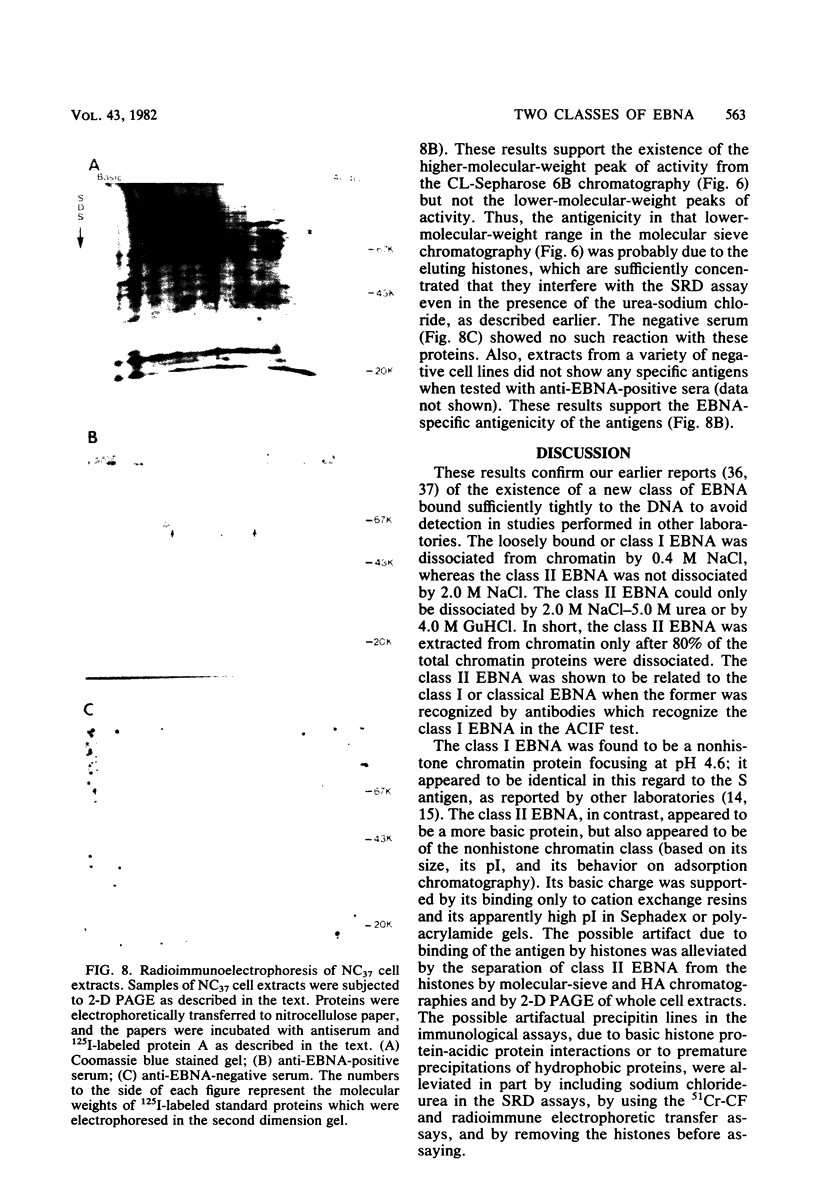
A new class of Epstein-Barr virus nuclear antigen (EBNA) was identified by the complement fixation assay. This new species of EBNA is more tightly bound to chromatin and was termed class II EBNA, as opposed to the more weakly associated species, class I EBNA. Preparations of this new antigen(s) specifically reduced absorption with the titer of anti-EBNA antibodies as determined by the anticomplement immunofluorescence assay. Therefore, the complement fixation antigens (class II EBNA) appear to be related to the classical EBNA (class I EBNA). The class I EBNA was found to focus at the same pH (4.6) as the soluble antigen found in the cytosol. The class II EBNA differed from the class I EBNA with regard to its overall charge, molecular size, antigenicity, and affinity for chromatin. The class II EBNA appeared to be a basic protein, based on its apparent pI of 9.2 and its binding to cation-exchange resins. It differed from histones with regard to its molecular size (molecular weight between 60,000 and 70,000) and its elution from hydroxylapatite chromatography. Steps were taken to prevent proteolysis and artifacts in the immunological assays and in the overall charge estimation of the new antigen by nonspecific basic histone protein-acidic protein interactions. Both class I and class II EBNA were identified by radioimmunoelectrophoresis on two-dimensional polyacrylamide gels with pI values of 5.0 and 8.5, respectively, and a molecular weight range of 60,000 to 70,000 for both. A lower-molecular-weight antigen identified by molecular sieve chromatography appeared to be due to interference by histones in the immunoassays since it was not observed by the two-dimensional gel electrophoresis. Further characterization of this class II EBNA is in progress.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansevin A. T., Hnilica L. S., Spelsberg T. C., Kehm S. L. Structure studies on chromatin and nucleohistones. Thermal denaturation profiles recorded in the presence of urea. Biochemistry. 1971 Dec 7;10(25):4793–4803. doi: 10.1021/bi00801a030. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr G. F., Mikel U., Klein G. Localization and quantitation of EBV-associated nuclear antigen (EBNA) in Raji cells. Beitr Pathol. 1975 May;155(1):72–78. doi: 10.1016/s0005-8165(75)80060-6. [DOI] [PubMed] [Google Scholar]
- Baron D., Strominger J. L. Partial purification and properties of the Epstein-Barr virus-associated nuclear antigen. J Biol Chem. 1978 Apr 25;253(8):2875–2881. [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Brown T. D., Rickwood D., MacGillivray A. J., Klein G. Studies of Epstein-Barr virus (EBV)-associated nuclear antigen: solubilization from Raji cell chromatin with 5 M-urea-2 M-nacl and fractionation on hydroxyapatite. J Gen Virol. 1978 Sep;40(3):511–517. doi: 10.1099/0022-1317-40-3-511. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cikes M. An improved quantitative micro-complement fixation test. J Immunol Methods. 1975;8(1-2):89–100. doi: 10.1016/0022-1759(75)90085-x. [DOI] [PubMed] [Google Scholar]
- Durr F. E., Monroe J. H., Schmitter R., Traul K. A., Hirshaut Y. Studies on the infectivity and cytopathology of Epstein-Barr virus in human lymphoblastoid cells. Int J Cancer. 1970 Nov 15;6(3):436–449. doi: 10.1002/ijc.2910060315. [DOI] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G., Barr Y. M., Zajac B., Henle G., Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst. 1966 Oct;37(4):547–559. [PubMed] [Google Scholar]
- Jackson V. Studies on histone organization in the nucleosome using formaldehyde as a reversible cross-linking agent. Cell. 1978 Nov;15(3):945–954. doi: 10.1016/0092-8674(78)90278-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenoir G., Berthelon M. C., Favre M. C., de-Thé G. Characterization of Epstein-Barr virus antigens. I. Biochemical analysis of the complement-fixing soluble antigen and relationship with Epstein-Barr virus-associated nuclear antigen. J Virol. 1976 Feb;17(2):672–674. doi: 10.1128/jvi.17.2.672-674.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J., Lindahl T., Klein G. Purification of the Epstein-Barr virus-determined nuclear antigen from Epstein-Barr virus-transformed human lymphoid cell lines. J Virol. 1978 Sep;27(3):604–611. doi: 10.1128/jvi.27.3.604-611.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J., Siegert W., Klein G. Solubilization of the Epstein-Barr virus-determined nuclear antigen and its characterization as a DNA-binding protein. J Virol. 1977 Apr;22(1):1–8. doi: 10.1128/jvi.22.1.1-8.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Hibi N., Nishi S., Hirai H., Osato T. Studies on Epstein-Barr virus-related antigens. III. Purification of the virus-determined nuclear antigen (EBNA) from non-producer Raji cells. Int J Cancer. 1978 Dec;22(6):747–752. doi: 10.1002/ijc.2910220618. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Nishi S., Hirai H., Osato T. Studies on Epstein-Barr virus-related antigens. I. Indirect single radial immunodiffusion as a useful method for detection and assay of soluble antigen. Int J Cancer. 1976 Oct 15;18(4):453–457. doi: 10.1002/ijc.2910180409. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Nishi S., Hirai H., Osato T. Studies on Epstein-Barr virus-related antigens. II. Biochemical properties of soluble antigen in Raji Burkitt lymphoma cells. Int J Cancer. 1977 Mar 15;19(3):364–370. doi: 10.1002/ijc.2910190313. [DOI] [PubMed] [Google Scholar]
- Menezes J., Jondal M., Leibold W., Dorval G. Epstein-Barr virus interactions with human lymphocyte subpopulations: virus adsorption, kinetics of expression of Epstein-Barr virus-associated nuclear antigen, and lymphocyte transformation. Infect Immun. 1976 Feb;13(2):303–310. doi: 10.1128/iai.13.2.303-310.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Luka J., Lindahl T., Klein G. Identification of a purified complement-fixing antigen as the Epstein-Barr-virus determined nuclear antigen (EBNA) by its binding to metaphase chromosomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1605–1609. doi: 10.1073/pnas.74.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikler G. M., Pearson G. R., Spelsberg T. C. Isolation of the Epstein-Barr virus nuclear antigen from chromatin preparations. IARC Sci Publ. 1978;(24 Pt 1):243–247. [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S., Ansevin A. T. Proteins of chromatin in template restriction. 3. The macromolecules in specific restriction of the chromatin DNA. Biochim Biophys Acta. 1971 Jan 28;228(2):550–562. doi: 10.1016/0005-2787(71)90061-x. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. II. Specificity of RNA synthesis. Biochim Biophys Acta. 1971 Jan 1;228(1):212–222. doi: 10.1016/0005-2787(71)90561-2. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Knowler J., Boyd P., Thrall C., Martin-Dani G. Support for chromatin acidic proteins as acceptors for progesterone in the chick oviduct. J Steroid Biochem. 1979 Jul;11(1B):373–379. doi: 10.1016/0022-4731(79)90055-4. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Steggles A. W., O'Malley B. W. Progesterone-binding components of chick oviduct. 3. Chromatin acceptor sites. J Biol Chem. 1971 Jul 10;246(13):4188–4197. [PubMed] [Google Scholar]
- Spelsberg T. C., Thrall C., Webster R., Pikler G. Isolation and characterization of the nuclear acceptor that binds the progesterone-receptor complex in hen oviduct. J Toxicol Environ Health. 1977 Sep;3(1-2):309–337. doi: 10.1080/15287397709529567. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Webster R., Pikler G., Thrall C., Wells D. Nuclear binding sites ("acceptors") for progesterone in avian oviduct: characterization of the highest-affinity sites. Ann N Y Acad Sci. 1977 Mar 11;286:43–63. doi: 10.1111/j.1749-6632.1977.tb29404.x. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Schuster T. C., Hopkins R. F., 3rd, Neubauer R. H., Rabin H. Identification of an Epstein-Barr virus nuclear antigen by fluoroimmunoelectrophoresis and radioimmunoelectrophoresis. J Virol. 1981 Jun;38(3):996–1004. doi: 10.1128/jvi.38.3.996-1004.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Fresen K. O. Heterogeneity of Epstein-Barr virus. II. Induction of early antigens (EA) by complementation. Virology. 1977 Aug;81(1):138–143. doi: 10.1016/0042-6822(77)90065-4. [DOI] [PubMed] [Google Scholar]