Abstract
Integrin-mediated adhesion induces several signaling pathways leading to regulation of gene transcription, control of cell cycle entry and survival from apoptosis. Here we investigate the involvement of the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway in integrin-mediated signaling. Plating primary human endothelial cells from umbilical cord and the human endothelial cell line ECV304 on matrix proteins or on antibody to β1- or αv-integrin subunits induces transient tyrosine phosphorylation of JAK2 and STAT5A. Consistent with a role for the JAK/STAT pathway in regulation of gene transcription, adhesion to matrix proteins leads to the formation of STAT5A-containing complexes with the serum-inducible element of c-fos promoter. Stable expression of a dominant negative form of STAT5A in NIH3T3 cells reduces fibronectin-induced c-fos mRNA expression, indicating the involvement of STAT5A in integrin-mediated c-fos transcription. Thus these data present a new integrin-dependent signaling mechanism involving the JAK/STAT pathway in response to cell–matrix interaction.
INTRODUCTION
Endothelial cell adhesion to extracellular matrix is mediated by the integrin family of adhesive receptors, glycoprotein heterodimers that are formed by α and β subunits (Hynes, 1992; Defilippi et al., 1997). Several lines of evidence indicate that integrin-mediated adhesion stimulates signaling pathways leading to actin cytoskeleton organization, cell motility, regulation of cell growth, and control of cell differentiation (for review, see Clark and Brugge, 1995; Schwartz et al., 1995). Integrin-induced signaling has been reported to affect gene expression (Dike and Farmer, 1988; Haskill et al., 1991; Chen et al., 1992; Rana et al., 1994; Schmidhauser et al., 1994; Fan et al., 1995; Tremble et al., 1995; Dike and Ingber, 1996). Among the early growth response genes encoding for transcriptional activators, adhesion-dependent induction of c-fos gene transcription has been described in monocytes (Haskill et al., 1991), in endothelial cells (Dike and Ingber, 1996; Wary et al., 1996), and in fibroblasts (Tremble et al., 1995; Wary et al., 1996) and proposed as a mediator of cell cycle progression controlled by cell adhesion.
The signaling pathways leading to integrin-dependent early growth response gene transcription are not completely defined. A role of activated Erk1/Erk2 MAP kinases in coupling integrins to gene expression has been recently proposed (Chen et al., 1992; Morino et al., 1995; Zhu and Assoian, 1995; Wary et al., 1996). Different signaling pathways, including activation of Shc (Wary et al., 1996), fyn kinase (Wary et al., 1998), phosphinositide 3 kinase (King et al., 1997), Raf (Howe and Juliano, 1998) (for review, see Assoian, 1997; Giancotti, 1997; Howe et al., 1998), and EGF receptor (Moro et al., 1998) have been proposed to trigger adhesion-dependent Erk1/Erk2 MAP kinase activation. Upon activation, Erk1 and Erk2 MAP kinases translocate to the nucleus and phosphorylate their specific Elk1 and SAP1 substrates, that together with the serum response factor regulate transcription of genes containing the serum response element (SRE) in their promoters, such as the c-fos gene. In addition to the SRE sequence, regulated by Erk1/Erk2 MAP kinases, the c-fos promoter contains the serum-inducible element (SIE) sequence, which is a target for the signal transducers and activators of transcription (STAT) (Zhong et al., 1994). STATs are latent cytoplasmic proteins, activated in response to cytokine and/or growth factor receptor stimulation (for review, see Ihle and Kerr, 1995; Leaman et al., 1996; Darnell, 1997; O’Shea, 1997). Among these receptors, those lacking the intrinsic kinase catalytic domain couple ligand binding to tyrosine phosphorylation by using noncovalently associated protein tyrosine kinases belonging to the Janus kinase (JAK) family (Schindler and Darnell, 1995; O’Shea, 1997). In many instances, ligand-activated cytokine receptors lead to a conformational juxtaposition of the JAK thus allowing its transphosphorylation and activation. Activated JAKs then catalyze tyrosine phosphorylation of receptor subunits providing docking sites for STAT proteins. These signaling proteins can then be tyrosine phosphorylated by JAKs and dimerize. As a consequence, they acquire DNA-binding activity, translocate into the nucleus, bind to specific promoter elements, and control the expression of target genes (for review, see Ihle and Kerr, 1995; Schindler and Darnell, 1995; Leaman et al., 1996; Darnell, 1997; O’Shea, 1997). Among the six members of the STAT family, STAT5 was originally described as a transcriptional factor recognizing a specific palindromic sequence, which was originally found in the prolactin-inducible element (PIE) of the β-casein promoter (Wakao et al., 1994). Subsequently two different but highly homologous STAT5 genes were isolated and defined as STAT5A and STAT5B (Mui et al., 1995). These STAT5 proteins undergo activation in response to different stimuli and exert transcriptional activation on a number of genes mainly involved in the control of cell proliferation (Mui et al., 1996). Additional evidence sustaining the role of STAT5 proteins in regulating mitogenic signals comes from the use of dominant negative form (Mui et al., 1996) and activating mutations (Onishi et al., 1998).
In this work we show that JAK2 and STAT5A undergo activation after adhesion of endothelial cells to matrix proteins and that the JAK/STAT pathway has a dominant role in integrin-mediated c-fos gene expression.
MATERIALS AND METHODS
Reagents and Antibodies
FIbronectin (FN) was purified from human plasma as previously described (Defilippi et al., 1994). Laminin (LM) was obtained from Beckton Dickinson (Mountain View, CA). Poly-l-lysine, cytochalasin D, M199 medium (endotoxin tested), bovine serum albumin (BSA) were all from Sigma (St. Louis, MO). Bovine calf serum (endotoxin tested) was obtained from Hyclone (Logan, UT). RPMI medium, G418, and Lipofectin reagents were purchased from Life Technologies (Gaithersburg, MD). Trypsin was purchased from Difco (Detroit, MI). [α-32P]dCTP, nitrocellulose, HRP-conjugated protein A, molecular weight markers, and the ECL reagent were from Amersham (Arlington Heights, IL). Poly(dIdC):poly(dIdC) and protein A-Sepharose were obtained from Pharmacia (Piscataway, NJ). Basic fibroblast growth factor was a gift from Dr. F Bertolero, (Farmitalia, Milan, Italy).
The monoclonal antibody (mAb) BV7 to the human β1 integrin subunit (purchased from Bioline Diagnostici, Torino, Italy) and mAb L230 to the human αv integrin subunit (purchased from American Type Culture Collection, Manassas, VA) were affinity purified on protein A-Sepharose as described (Ey et al., 1978) and the purity of the antibody was higher than 95%. Polyclonal antibody Ab to p125Fak, Fak4, has been previously described (Defilippi et al., 1995). mAb PY20 and RC20 to phosphotyrosine (anti-PY) and mAb to STAT5 were from Transduction Laboratories (Lexington, KY). Polyclonal Ab to STAT5A and STAT5B and polyclonal Ab C-16 to Erk1/MAP kinase, were from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-p44/42 MAP kinase antibody was from New England BioLabs (Beverly, MA). Peroxidase-conjugate goat anti-mouse immunoglobulin G (IgG) and FITC-conjugated goat anti-rabbit IgG were from Sigma.
Cell Culture and Transfection
Endothelial cells were isolated from human umbilical cord vein (HUVECs) within 4 h of delivery by trypsin treatment (0.1%) and cultured in M199 with the addition of 10% bovine calf serum and 10 ng/ml basic fibroblast growth factor. HUVECs were characterized by morphologic criteria and positive immunofluorescence for factor VIII antigen (Brizzi et al., 1993). Contamination with blood leukocytes was assessed by immunofluorescence analysis using an anti-CD45 antibody. They were used at early passage (II–III). The transformed human endothelial ECV304 cell line was provided by European Collection of Animal Cell Cultures (Salisbury, United Kingdom). NIH3T3 cells were stably transfected with the dominant negative STAT5A construct (Mui et al., 1996), by the Lipofectin method and selected with 500 μg/ml G418. Expression of the dominant negative STAT5A protein was analyzed by Western blotting with an anti-STAT5 mAb, and the positive clones were tested by electrophoretic mobility shift assay.
Adhesion Experiments
Tissue culture plates were coated with 20 μg/ml purified FN, LM, or mAb to β1- or αv-integrin subunits by overnight incubation at 4°C and postcoated with BSA for 1 h at 37°C. Poly-l-lysine (PL) was used at 10 μg/ml. ECV304 cells and NIH3T3 fibroblasts were serum deprived at 37°C for 18 h in serum-free medium. HUVECs were deprived for 4 h in medium containing PBS (30% vol/vol), sodium orthovanadate (0.2 mM), and EDTA (1 mM). Cells were then detached by 10 mM EDTA treatment in PBS for 10 min, washed twice in PBS containing 1 mM CaCl2 and 1 mM MgCl2, resuspended in prewarmed Dulbecco’s modified Eagle’s medium, and immediately plated on the tissue culture plates for the indicated times. At the end of the incubation, the cells were washed twice and detergent extracted in lysis buffer [1% Triton X-100, 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid), pH 6.8, 100 mM NaCl, 5 mM MgCl2, 300 mM sucrose, 5 mM EGTA, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonylfluoride, 10 μg/ml leupeptin, 0.15 U/ml trypsin inhibitory unit/ml aprotinin, and 1 μg/ml pepstatin] for 20 min at 4°C and centrifuged at 15,000 × g for 20 min.
Immunoprecipitation, SDS-PAGE, and Immunoblotting
Protein concentration was determined in each cell extract by the Bio-Rad (Munich, Germany) protein assay method based on the Bradford dye-binding procedure. Equal amounts of cell extracts were immunoprecipitated with the indicated antibodies, and immunocomplexes were bound to protein-A-Sepharose beads and recovered by centrifugation. Bound material was eluted by boiling beads in 1% SDS and separated on 8% PAGE in the presence of SDS (SDS-PAGE) in reducing conditions. When cell extracts were analyzed, samples containing equal amounts of proteins were subjected to SDS-PAGE as described above. Proteins were transferred to nitrocellulose using a semidry apparatus (Novablot; Pharmacia, Piscataway, NJ) according to the manufacturer’s instructions. The blots were incubated 1 h at 42°C in 5% BSA in Tris-buffered saline–Tween (TBS-T; 150 mM NaCl, 20 mM Tris-Cl, pH 7.4, and 0.3% Tween), washed with TBS-T, and incubated overnight with the indicated antibodies in TBS and 1% BSA. The blots were washed three times with TBS-T, incubated 2 h with anti-mouse IgG peroxidase conjugate, and washed two times. Phosphotyrosil-containing proteins were visualized by the ECL detection method. Conditions of the development with the chemiluminescent substrate and exposure times were set to obtain a linear response.
Preparation of Nuclear Extracts and Gel Retardation Assay
Nuclear extracts from HUVECs or NIH3T3 cells ectopically transfected with Neo vector or dominant negative STAT5A protein were prepared by Nonidet P40 lysis as described by Sadowski and Gilman (1993). The oligonucleotides used were G GGG GGA CTT CTT GGA ATT AAG GGA and G GGG TCC CTT AAT TCC AAG AAG TCC, corresponding to the PIE of the β-casein promoter (Ruff-Jamison et al., 1995); G GGG CAT TTC CCG TAA ATC and G GGG GAT TTA CGG GAA ATG corresponding to the SIE of c-fos (Zhong et al., 1994). The annealed oligonucleotide was labeled by filling in the overhanging ends with Klenow fragment in the presence of [α-32P]dCTP. Gel retardation reactions were performed in 13 mM HEPES, pH 7.6, 80 mM NaCl, 3 mM NaF, 3 mM NaMoO4, 1 mM DTT, 0.15 mM EDTA, 0.15 mM EGTA, and 8% glycerol (including contribution from the nuclear extract), containing 75 μg/ml poly(dIdC):poly(dIdC), ∼ 0.3 ng of radiolabeled probe, and 5–10 μg of protein. Reactions were carried on at room temperature for 40 min and then resolved on 4% polyacrylamide gels containing 0.25× Tris borate-EDTA (TBE; 1× is 89 mM Tris borate and 1 mM EDTA, pH 8) and 5% glycerol. Gels were run at 4°C in 0.25× TBE at 20 V/cm, dried, and autoradiographed. Oligonucleotide competition was performed by preincubating nuclear extracts with the competitor oligonucleotide (50-fold excess) and poly(dIdC):poly(dIdC) for 30 min at room temperature before the addition of labeled probe. Gel mobility shift assays were done with nuclear extract that had been reacted for 1 h at 4°C with the indicated antibodies.
Northern Blot Analysis
Cytoplasmic RNA was isolated from NIH3T3 cells ectopically transfected with Neo vector or dominant negative STAT5A protein by guanidinium thiocyanate/acid phenol-cloroform extraction (Chomczynski and Sacchi, 1987). Northern blot analysis was performed according to standard methods as previously described (Brizzi et al., 1993). Filters were hybridized to 32P random-priming labeled DNA probes corresponding respectively to mouse c-fos, human c-jun, and β-actin cDNAs, washed for 30 min in 0.1× SSC and 1% SDS at 52°C, and exposed to x-ray film for 2–4 d.
Immunofluorescence
Serum-deprived HUVECs were detached by 10 mM EDTA treatment in PBS for 10 min, washed twice in PBS containing 1 mM CaCl2 and 1 mM MgCl2, resuspended in prewarmed Dulbecco’s modified Eagle’s medium, and immediately plated on a glass coverslip coated with 20 μg/ml FN or 10 μg/ml PL for 1 h. Cells were fixed for 5 min in 3% paraformaldehyde in PBS, pH 7.4, containing 2% sucrose and permeabilized with HEPES-Triton X-100 buffer (20 mM HEPES, pH 7.4, 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, and 0.5% Triton X-100). STAT5A was detected by indirect immunofluorescence with specific anti-STAT5A antiserum and an FITC-conjugated goat anti-rabbit IgG as secondary antibody.
RESULTS
Adhesion to Matrix Proteins Triggers STAT5A and JAK2 Tyrosine Phosphorylation
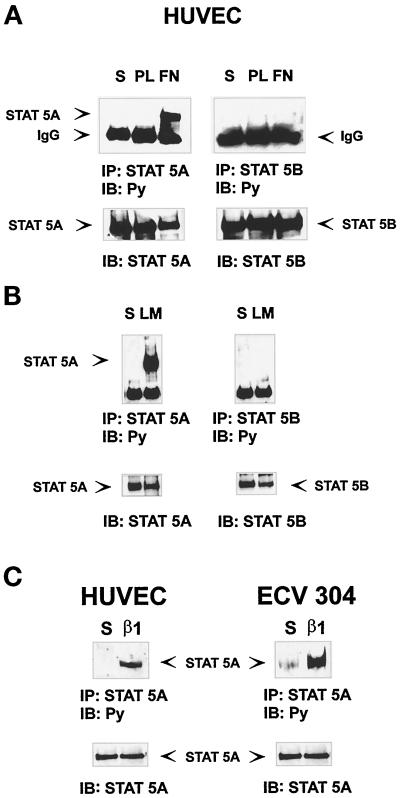
To evaluate the role of the JAK/STAT pathway in integrin-mediated signaling, we first analyzed activation of STAT molecules after adhesion of endothelial cells to extracellular matrix proteins. Among the members of the STAT family, STAT5 proteins are pleiotropic regulators of many genes, including c-fos (Mui et al., 1995; Mui et al., 1996), a well-known target of integrin-mediated adhesion (Haskill et al., 1991; Dike and Ingber, 1996; Wary et al., 1996). We therefore evaluated the ability of integrin binding to extracellular matrix proteins to trigger the activation of the two highly homologous proteins STAT5A and STAT5B. Serum-deprived HUVECs were detached with 10 mM EDTA treatment and plated on PL-, FN-, or LM-coated dishes or kept in suspension. Cell extracts were immunoprecipitated with antibodies to STAT5A or STAT5B proteins and immunoblotted with an anti-phosphotyrosine antibody. As shown in Figure 1, STAT5A but not STAT5B was strongly phosphorylated in cells plated on FN (Figure 1A) and on LM (Figure 1B), whereas no phosphorylation was observed in suspended cells and in cells plated on PL (Figure 1A). Similarly, STAT5A phosphorylation on tyrosine was detected in cells plated on β1-integrin antibodies, thus showing that this event was integrin specific (Figure 1C, left). Similar results were obtained plating cells from the human endothelial cell line ECV304 on β1 antibody-coated dishes (Figure 1C, right). These results show that integrin-mediated adhesion to matrix proteins leads to a specific STAT5A tyrosine phosphorylation.
Figure 1.
Adhesion to matrix proteins induces STAT5A tyrosine phosphorylation. Serum-deprived HUVECs or ECV304 cells were detached with 10 mM EDTA and plated for 15 min on dishes coated with PL, FN (A), LM (B), or β1 integrin antibodies (C) or kept in suspension (S). Cell extracts were immunoprecipitated (IP) with antibodies to STAT5A or STAT5B proteins and subjected to 8% SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose filters and immunoblotted (IB) with an anti-phosphotyrosine antibody (Py) (upper panels). The filters were stripped and reprobed with the indicated antibody (lower panels). The position of the tyrosine-phosphorylated STAT5A is indicated. Five individual experiments were performed with similar results.
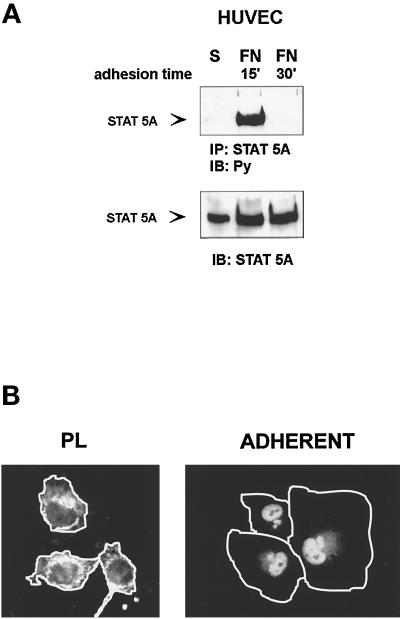
Time course analysis indicated that tyrosine phosphorylation of STAT5A, detected in Triton X-100 cytoplasmic extracts, occurred within 15 min of adhesion to matrix proteins (FN or LM) and completely disappeared within 30 min (Figure 2A), indicating that STAT5A phosphorylation is an early and transient event in integrin signaling. However, when cells adherent to FN were extracted with a modified radioimmunoprecipitation assay buffer, which also solubilizes nuclear proteins, tyrosine phosphorylation of STAT5A was still detectable at 60 min of adhesion (our unpublished results), suggesting that phosphorylated STAT5A rapidly translocates to the nuclear compartment. These results were further confirmed by immunofluorescence experiments with an antiserum to STAT5A. As shown in Figure 2B, although STAT5A was localized in the cytoplasm of HUVECs plated on PL, it acquired a predominant nuclear localization within 1 h of adhesion to FN.
Figure 2.
Time course of adhesion-dependent tyrosine phosphorylation and nuclear translocation of STAT5A. (A) STAT5A activation. Serum-deprived HUVECs were detached with 10 mM EDTA and plated on FN-coated dishes for the indicated times or kept in suspension (S). Cell extracts were immunoprecipitated (IP) with antibodies to STAT5A protein and subjected to 8% SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose filter and immunoblotted (IB) with an anti-phosphotyrosine antibody (Py) (upper panel). The filter was stripped and reprobed with the anti-STAT5A antiserum (lower panel). Migration of the tyrosine-phosphorylated STAT5A is indicated by the arrow. (B) STAT5A nuclear translocation. Serum-deprived HUVECs were detached and plated on FN-coated glass coverslips for 60 min. (ADHERENT) and then fixed in 3% paraformaldehyde in PBS for 10 min. As control, cells were plated for the same time on PL. After permeabilization cells were stained for 1 h at room temperature with STAT5A antibodies, washed, and exposed to a secondary FITC-labeled antibody for 1 h. Coverslips were then mounted in PBS:glycerol, 1:1, viewed on Olympus (Tokyo, Japan) BH2-RFCA fluorescence microscope, and photographed. Photographs were then processed by Adobe (Mountain View, CA) Photo De Luxe 2.0 to magnify cell shape. White lines define the boundaries of the cells.
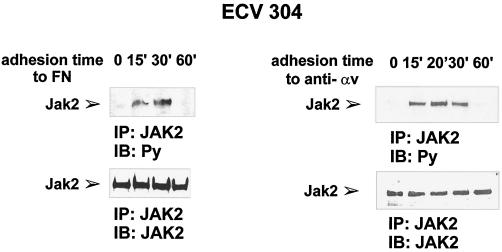
To analyze the mechanisms leading to integrin-mediated STAT5A activation, we first evaluated the involvement of the Janus kinase, JAK2, that is known to be activated by several cytokines that signal to STAT5 (for review, see Ihle and Kerr, 1995; Schindler and Darnell, 1995; Leaman et al., 1996; Darnell, 1997; O’Shea, 1997). Serum-deprived ECV304 cells were plated on dishes coated with FN or antibody to αv-integrin subunit and extracted at different times of adhesion. As shown in Figure 3, tyrosine phosphorylation of JAK2 was detectable within 15 min of adhesion, thus demonstrating that integrin-mediated adhesion is also able to trigger JAK2 tyrosine phosphorylation.
Figure 3.
Time course of adhesion-dependent JAK2 tyrosine phosphorylation. Serum-deprived ECV304 cells were detached with 10 mM EDTA and plated on dishes coated with FN (left panel) or antibodies to αv (anti-αv) (right panel) for the indicated times, kept in suspension (0 on FN) or plated on PL (0 on anti-αv). Cell extracts were prepared and subjected to immunoprecipitation (IP) with an anti-JAK2 antiserum. Proteins were electrophoretically transferred to a nitrocellulose filter that was immunoblotted (IB) with an anti-phosphotyrosine antibody (Py) (upper panels) and reprobed with an anti-JAK2 antiserum (lower panels). The position of the tyrosine-phosphorylated JAK2 is indicated. Similar results were obtained in three individual experiments.
STAT5A Is Translocated to the Nucleus and Binds to the SIE of c-fos Promoter in Response to Adhesion to Matrix Proteins
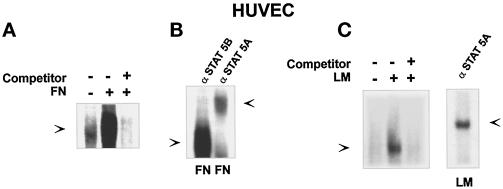
We then evaluated whether the nuclear translocated STAT5A in adherent HUVECs was transcriptionally active. In the c-fos promoter, a region located distally to the SRE, denoted as the c-sis-inducible element (SIE), is known to represent a target region for STAT proteins (Zhong et al., 1994). Formation of the STAT5A–SIE complex in response to integrin-mediated adhesion was evaluated by electrophoretic mobility shift assay. A 32P-labeled SIE sequence incubated with nuclear extracts prepared from HUVECs plated on FN for 40 min led to a SIE-binding complex that was completely competed by addition of unlabeled SIE oligonucleotides (Figure 4A). Similar results were obtained by plating HUVECs on LM (Figure 4C), whereas no DNA-binding activity was detected from nuclear extracts of HUVECs kept in suspension (Figure 4A). The presence of STAT5A in the DNA–protein complex induced by adhesion to matrix proteins was demonstrated by the ability of the antibody to STAT5A to supershift the SIE-binding complex (Figure 4, B and C, right panel). These data indicate that adhesion-mediated STAT5A activation leads to the formation of a STAT5A-containing complex able to interact with the c-fos promoter.
Figure 4.
FN-induced SIE-binding activity. (A and C, left) SIE complex formation. Nuclear extracts were prepared from HUVECs plated for 40 min on FN- or LM-coated dishes (+) or kept in suspension (−). Thirty minutes before the addition of radiolabeled SIE oligonucleotides, the indicated extracts were treated with a 50-fold excess of unlabeled oligonucleotide (Competitor). The DNA–protein complexes were then resolved by nondenaturing PAGE. (B and C, right) FN- or LM-induced SIE-binding complex is antigenically related to STAT5A. Nuclear extracts from HUVECs plated on FN or LM were incubated for 1 h at 4°C with an anti-STAT5A or an anti-STAT5B antiserum as indi-cated before adding a radiolabeled SIE oligonucleotide probe. DNA–protein complexes were resolved on a nondenaturing polyacrylamide gel. The SIE complexes (lower) and the supershifted species (upper) are indicated by the arrows.
Expression of a Dominant Negative STAT5A Protein in NIH3T3 Cells
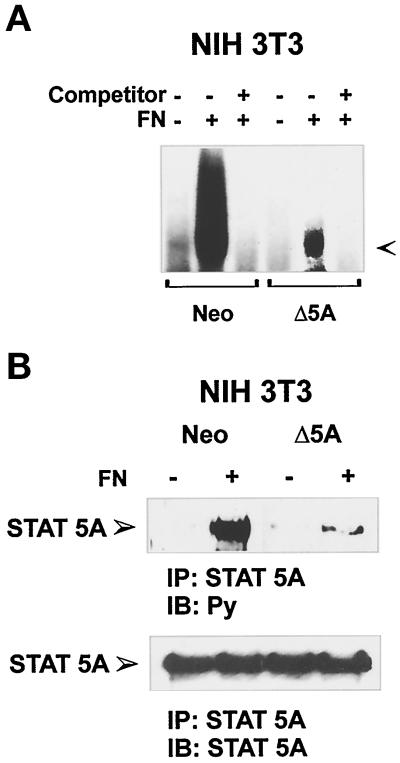
Modified forms of STAT5 proteins, obtained by removing most of the C-terminal tyrosines and acting as dominant negative forms on interleukin 3 (IL-3)-mediated cell proliferation have been recently described as stable transfectants (Mui et al., 1996). These modified proteins retain the ability to bind to activated receptors, whereas they fail to become tyrosine phosphorylated and to translocate to the nucleus to bind DNA target sequence (Mui et al., 1996). To evaluate the biological relevance of STAT5A activation in response to integrin-mediated adhesion, the STAT5A dominant negative construct was stably expressed in NIH3T3 cells. Cells expressing dominant negative STAT5A, selected by G418 treatment, grow more slowly than those transfected with Neo vector alone, as quantified by growth assays (our unpublished results). The analysis of the effects of this dominant-negative STAT5A on endogenous STAT5 proteins was performed on nuclear extracts from cells adherent to FN by electrophoretic mobility shift assay, using the β-casein promoter sequence (PIE) as a probe. As shown in Figure 5A, NIH3T3 cells expressing dominant negative STAT5A construct exhibited a marked decrease in PIE-binding activity, in comparison with NIH3T3 cells expressing the Neo vector. The fact that endogenous STAT5A protein was competed by the expression of STAT5A dominant negative form was also evident by the reduction in the band supershifted by the antibodies to STAT5A (our unpublished results). Indeed, as shown in Figure 5B, adhesion-dependent tyrosine phosphorylation of endogenous STAT5A was strongly decreased in cells expressing the dominant negative STAT5A construct.
Figure 5.
FN-mediated STAT5A activation in NIH3T3 cells ectopically transfected with Neo vector or a dominant negative STAT5A construct. (A) PIE-binding complex. NIH3T3 cells ectopically transfected with a Neo selection marker (Neo) or dominant negative STAT5A cDNA (Δ5A) were plated for 40 min on FN (+) or kept in suspension (−), and nuclear extracts were prepared. Thirty minutes before the addition of radiolabeled PIE oligonucleotides, the indicated extracts were treated with 50-fold excess of unlabeled oligonucleotide (Competitor). The DNA–protein complexes were then resolved by nondenaturing PAGE. The PIE complex is indicated by the arrow. (B) Effect of the dominant negative STAT5A expression on FN-mediated activation of the endogenous STAT5A. Cell extracts from NIH3T3 ectopically transfected with a Neo selection marker (Neo) or dominant negative STAT5A cDNA (Δ5A) plated on FN-coated dishes for 15 min or kept in suspension (S) were prepared and immunoprecipitated (IP) with antibodies to STAT5A protein. Proteins were subjected to 8% SDS-PAGE, electrophoretically transferred to a nitrocellulose filter, and immunoblotted (IB) with an anti-phosphotyrosine antibody (Py) (upper panel). The filter was stripped and reprobed with the anti-STAT5A antiserum (lower panel). Migration of the tyrosine-phosphorylated STAT5A is indicated by the arrow.
Expression of a Dominant Negative STAT5A Protein Drastically Reduces FN-induced c-fos mRNA Induction
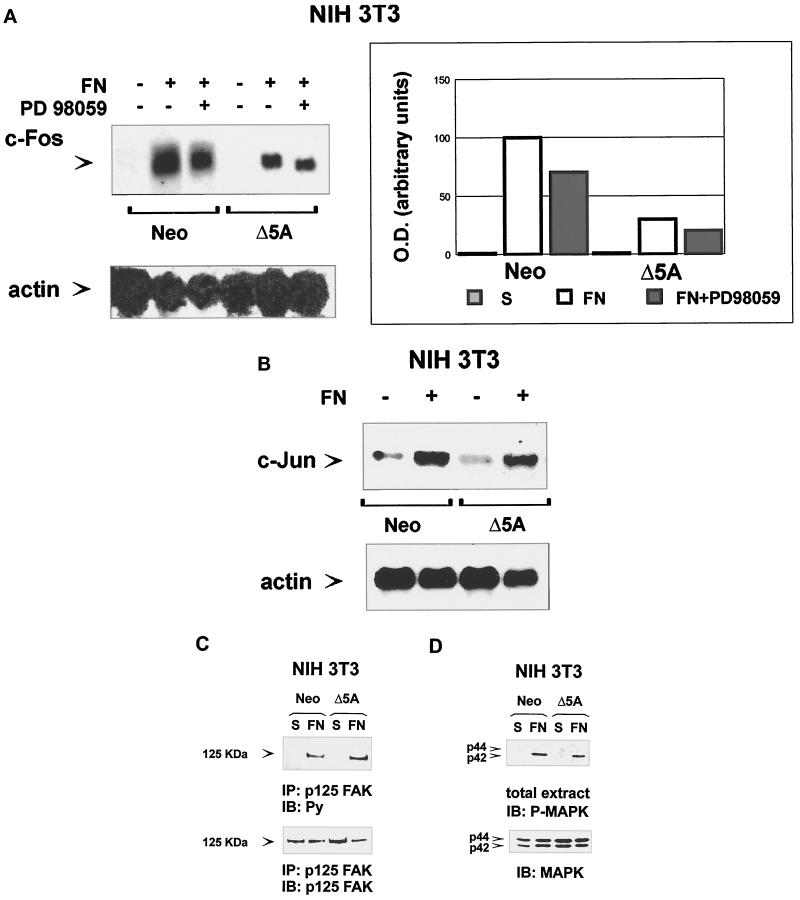
When G0-syncronized cells are plated on FN-coated dishes, early growth response genes, such as c-fos, c-myc, and c-jun, are rapidly induced (Dike and Farmer, 1988; Eierman et al., 1989; Tremble et al., 1995; Varner et al., 1995; Dike and Ingber, 1996). We show that STAT5A activated by integrin-mediated adhesion binds to the SIE oligonucleotide sequence, a known STAT target region in the c-fos promoter (Zhong et al., 1994). To evaluate the in vivo role of STAT5A in integrin-induced c-fos gene expression, NIH3T3 cells expressing Neo selection marker or dominant-negative STAT5A were plated on FN for 1 h or kept in suspension. Northern Blot analysis with a murine c-fos cDNA showed that the expression of dominant-negative STAT5A strongly affects the ability of FN to induce c-fos mRNA (Figure 6A) by reducing c-fos expression of ∼70%. That FN-dependent c-fos gene expression is specifically regulated by STAT5A is indicated by the observation that the level of other adhesion-induced early genes, such as c-jun, was not affected by expression of dominant-negative STAT5A protein (Figure 6B). In addition, tyrosine phosphorylation of p125Fak (Figure 6C) and activation of Erk1/Erk2 MAP kinases (Figure 6D) were unaffected by the expression of the dominant-negative protein, indicating that these adhesion-induced signaling pathways are independent from that involving STAT5A.
Figure 6.
Expression of dominant-negative STAT5A reduces integrin-mediated c-fos gene expression without interfering with integrin-induced p125FAK and Erk1/Erk2 MAP kinase phosphorylation. (A and B) Integrin-mediated c-fos and c-jun gene expression. Serum-deprived NIH3T3 fibroblasts ectopically transfected with Neo vector (Neo) or with a dominant negative STAT5A cDNA (Δ5A) were kept in suspension (−) or plated on FN-coated dishes (+) for 1 h in the presence or in the absence of the MAP–extracellular signal-regulated kinase kinase inhibitor PD98059 (+) as indicated. Total cytoplasmic RNA was isolated by acid phenol-chloroform extraction. Northern blot analysis was performed according to standard methods, and filters were hybridized with a specific mouse c-fos (A, upper panel, left), c-jun (B, upper panel), or β actin (lower panels) cDNA probes. Mouse c-fos hybridization was also quantified by densitometric analysis (A, right) using a Bio-Rad GS 250 molecular imager. (C and D) Integrin-dependent p125FAK and Erk1/Erk2 MAP kinase phosphorylation. Serum-deprived NIH3T3 cells ectopically transfected with a Neo selection marker (Neo) or dominant negative STAT5A cDNA (Δ5A) were processed as above and plated on FN-coated dishes for 15 min or kept in suspension (S). Cells were lysed and either immunoprecipitated (IP) with an anti-p125FAK antiserum or run on 8% SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose filters that were immunoblotted (IB) with an anti-phosphotyrosine antibody (C) or with an anti-phospho-Erk1/Erk2 MAP kinase antibody (D, upper panels) and reprobed with the anti-p125FAK or with an anti-Erk1 MAP kinase antibody (lower panels).
In addition to the SIE region, the c-fos promoter also contains the SRE sequence, regulated by the Erk1/Erk2 MAP kinases. The possible cooperative effect of Erk1/Erk2 MAP kinases and STAT5A pathways on c-fos mRNA induction after adhesion was then evaluated. Treatment of cells with PD98059, a known inhibitor of MAP–extracellular signal-regulated kinase/MAPK kinase pathway, reduced c-fos gene expression in response to FN of ∼30% in NIH3T3 cells expressing Neo selection marker and further decreased the level observed in dominant-negative STAT5A-expressing cells (Figure 6A). In the same experiment, treatment with PD98059 completely inhibited integrin-induced MAPK activation (our unpublished results). Therefore, our data show that c-fos gene expression is almost completely abolished when STAT5A and MAP kinase pathways are both down-regulated, indicating that STAT5A and MAP kinases are the main components regulating c-fos gene expression in response to FN.
DISCUSSION
JAKs and STAT proteins participate in signaling for DNA synthesis mediated by different stimuli such as cytokines and growth factors (for review, see Ihle and Kerr, 1995; Schindler and Darnell, 1995; Leaman et al., 1996; Darnell, 1997; O’Shea, 1997). In this report we show that, in addition to soluble mediators, the JAK/STAT pathway can be also activated by cell matrix interaction mediated by integrins. In mammary gland epithelial cells, prolactin-dependent transcription of milk protein genes through the DNA-binding activity of STAT5 has been shown to occur only in cells cultured on basement membrane, indicating that, in this model, cell–basement membrane interaction is required to propagate a cytokine signal (Streuli et al., 1995). Herein we demonstrate that, in primary endothelial cells and murine fibroblasts, cell–matrix interaction is sufficient to trigger activation of the JAK/STAT pathway. Indeed, the presence of soluble factors can be excluded because endothelial cells were serum deprived before plating on matrix proteins in serum-free medium. A further indication that autocrine production of soluble regulators is not involved in JAK/STAT activation by adhesion rises from the observation that, in confluent monolayers of serum-deprived cells, activation of the JAK/STAT pathway was never detected in the absence of exogenous stimuli (Brizzi et al., 1999). Indeed, STAT5A phosphorylation has been observed by plating endothelial cells on dishes coated with antibodies to β1- or αv-integrin subunits and not on the nonspecific substrate PL, indicating that this activation is an integrin-specific event. Moreover, because the level of STAT5A tyrosine phosphorylation obtained by integrin-mediated adhesion can be further increased twofold by addition of a known activator of STAT5A, such as IL-3 (our unpublished results), we may assume that only a partial activation of STAT5A is triggered by adhesion to matrix proteins.
The molecular mechanisms underlying the activation of the JAK/STAT pathway by integrin-mediated adhesion remain to be defined. In cytokine-mediated JAK activation, a ligand-dependent transphosphorylation of JAK has been reported (Schindler and Darnell, 1995; O’Shea, 1997). As a consequence, JAK triggers phosphorylation on tyrosine residues of the cytokine receptor’s tail, creating a docking site for the Src homology 2 domains of STAT proteins, thus recruiting STATs into the receptor complex (for review, see Ihle and Kerr, 1995; Schindler and Darnell, 1995; Leaman et al., 1996; Darnell, 1997; O’Shea, 1997). Similarly, the activation of JAK2/STAT5A by integrin-mediated adhesion could occur through the formation of an intermolecular complex containing JAK2, which, either directly or through an adaptor molecule, interacts with integrins. However, the failure to detect an immunoprecipitable complex between β1-integrin subunit and JAK2 (our unpublished results) suggests that the association of these two molecules may occur either indirectly or through low-affinity interactions.
After activation, STAT proteins migrate to the nucleus to regulate the expression of a wide range of genes (for review, see Ihle and Kerr, 1995; Schindler and Darnell, 1995; Leaman et al., 1996; Darnell, 1997; O’Shea, 1997). Besides the β-casein promoter (Wakao et al., 1994), STAT5 proteins act as transcriptional activators for a number of genes, including c-fos (Mui et al., 1995, 1996). In the murine IL-3-dependent hemopoietic cell line, Ba/F3, regulation of c-fos transcription by IL-3 treatment has been shown to be dependent on STAT5 activation (Mui et al., 1996). In nuclear extracts prepared from endothelial cells plated on FN (Figure 4) or on LM (our unpublished results), the formation of a SIE-binding complex containing STAT5A indicates that also integrin-mediated adhesion can promote transcription of c-fos gene through STAT5A activation. Erk1/Erk2 MAP kinases have been previously shown to regulate c-fos expression in response to adhesion (Wary et al., 1996). Herein we report that together with the Erk1/Erk2 MAP kinases, STAT5A is required to induce transcription of the c-fos gene in response to adhesion. These data are supported by the following observations: 1) in NIH3T3 fibroblasts ectopically transfected with a dominant-negative STAT5A construct, the level of c-fos mRNA induced by adhesion to FN is strongly decreased in comparison with that obtained from NIH3T3 cells expressing the selection marker; and 2) addition of PD98059, a known inhibitor of MAP kinase activation, further decreases c-fos mRNA level in response to adhesion in cells expressing the dominant-negative form of STAT5A. In addition, adhesion-dependent transcription of the c-jun gene was not affected by the ectopic expression of the dominant negative STAT5A protein, indicating that among the early growth response genes, activated in response to adhesion, c-fos represents a specific STAT5A target.
Activation of STAT5A signaling represents a novel pathway triggered by integrins, independently from those previously identified, such as activation of Erk1/Erk2 MAP kinases and tyrosine phosphorylation of p125FAK kinase (for review, see Clark and Brugge, 1995; Schwartz et al., 1995). Indeed, in NIH3T3 cells expressing dominant-negative STAT5A protein, both tyrosine phosphorylation of p125Fak and activation of Erk1/Erk2 MAP kinases in response to adhesion were comparable with those observed in control NIH3T3 cells. Moreover we found that cytochalasin D, which affects actin cytoskeleton organization and inhibits integrin-dependent p125Fak tyrosine phosphorylation (Defilippi et al., 1995), does not interfere with STAT5A transcriptional activity (our unpublished results), indicating that organization of actin cytoskeleton is not required for integrin-dependent activation of the STAT5 pathway.
In conclusion, the results presented here demonstrate that activation of the JAK/STAT pathway is a downstream event in integrin-mediated adhesion and that this pathway is involved in the transcriptional regulation of the adhesion-dependent early growth response gene c-fos.
ACKNOWLEDGMENTS
We are very grateful for the excellent technical assistance of L. Dolce, P. Dentelli, and M. Pavan. This work was supported by grants from the Italian Association for Cancer Research to G.T. and L.P., Ministero dell’Universitá e della Ricerca Scientifica e Tecnologica to P.D., L.S., G.T., and L.P., and Istituto Superiore di Sanitá, Progetto “Sostituzioni funzionali, organi artificiali e trapianti di organi,” to P.D.
REFERENCES
- Assoian RK. Anchorage-dependent cell cycle progression. J Cell Biol, 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzi MF, Battaglia E, Montrucchio G, Dentelli P, Del Sorbo L, Garbarino G, Pegoraro L, Camussi G. Thrombopoietin stimulates endothelial cell motility and neoangiogenesis by platelet-activating factor-dependent mechanism. Circ Res. 1999;84:785–796. doi: 10.1161/01.res.84.7.785. [DOI] [PubMed] [Google Scholar]
- Brizzi MF, Garbarino G, Rossi PR, Pagliardi GL, Arduino C, Avanzi GC, Pegoraro L. Interleukin-3 stimulates proliferation and triggers endothelial-leukocyte adhesion molecule 1 gene activation of human endothelial cells. J Clin Invest. 1993;91:2887–2892. doi: 10.1172/JCI116534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Magnuson V, Hill S, Arnaud C, Steffensen B, Klebe RJ. Regulation of integrin gene expression by substrate adherence. J Biol Chem. 1992;267:23502–23506. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single stem step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–161. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Bozzo C, Volpe G, Romano G, Venturino M, Silengo M, Tarone G. Integrin-mediated signal transduction in human endothelial cells: analysis of tyrosine phosphorylation events. Cell Adhes Commun. 1994;2:75–86. doi: 10.3109/15419069409014203. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Retta FS, Olivo C, Palmieri M, Venturino M, Silengo L, Tarone G. p125FAK tyrosine phosphorylation and focal adhesion assembly: studies with phosphotyrosine phosphatase inhibitors. Exp Cell Res. 1995;221:141–152. doi: 10.1006/excr.1995.1361. [DOI] [PubMed] [Google Scholar]
- Defilippi P, van Hinsbergh V, Bertolotto A, Rossino P, Silengo L, Tarone G. Differential distribution and modulation of expression of alpha1/beta1 integrin on human endothelial cells. J Cell Biol. 1997;114:855–863. doi: 10.1083/jcb.114.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dike LE, Farmer SR. Cell adhesion induces expression of growth-associated genes in suspension-arrested fibroblasts. Proc Natl Acad Sci USA. 1988;85:6792–6796. doi: 10.1073/pnas.85.18.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dike LE, Ingber DE. Integrin-dependent induction of early growth response genes in capillary endothelial cells. J Cell Sci. 1996;109:2855–2863. doi: 10.1242/jcs.109.12.2855. [DOI] [PubMed] [Google Scholar]
- Eierman DF, Johnson CE, Haskill JS. Human monocyte inflammatory mediator gene expression is selectively regulated by adherence substrates. J Immunol. 1989;142:1970–1976. [PubMed] [Google Scholar]
- Ey PL, Prowse SJ, Jenkin CR. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978;15:429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fan ST, Mackman N, Cui MZ, Edgington TS. Integrin regulation of an inflammatory effector gene. Direct induction of the tissue factor promoter by engagement of beta 1 or alpha 4 integrin chains. J Immunol. 1995;154:3266–3274. [PubMed] [Google Scholar]
- Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Haskill S, Beg AA, Tompkins SM, Morris JS, Yurochko AD, Sampson-Johannes A, Mondal K, Ralph P, Baldwin AS., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991;65:1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol, 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Howe AK, Juliano RL. Distinct mechanisms mediate the initial and sustained phases of integrin-mediated activation of the Raf/MEK/mitogen-activated protein kinase cascade. J Biol Chem. 1998;273:27268–27274. doi: 10.1074/jbc.273.42.27268. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaman DW, Leung S, Li X, Stark GR. Regulation of STAT-dependent pathways by growth factors and cytokines. FASEB J. 1996;10:1578–1588. [PubMed] [Google Scholar]
- Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Takehara K, Kadowaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induced activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui AL-F, Wakao H, Kinoshita T, Kitamura T, Miyajima A. Suppression of interleukin-3-induced gene expression by a C-terminal truncated STAT5: role of STAT5 in proliferation. EMBO J. 1996;15:2425–2433. [PMC free article] [PubMed] [Google Scholar]
- Mui AL-F, Wakao H, O’Farrell AM, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995;14:1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi M, Nosaka K, Mui AL, Gorman D, McMahon M, Miyajima A, Kitamura T. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- Rana B, Mischoulon D, Xie Y, Bucher NL, Farmer SR. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBP alpha and immediate-early growth response transcription factors. Mol Cell Biol. 1994;14:5858–5869. doi: 10.1128/mcb.14.9.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff-Jamison S, Chen C, Cohen S. Epidermal growth factor induces the tyrosine phosphorylation and nuclear translocation of Stat 5 in mouse liver. Proc Natl Acad Sci USA. 1995;92:4215–4218. doi: 10.1073/pnas.92.10.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski HB, Gilman MZ. Cell-free activation of a DNA-binding protein by epidermal growth factor. Nature. 1993;362:79–83. doi: 10.1038/362079a0. [DOI] [PubMed] [Google Scholar]
- Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- Schmidhauser C, Casperson GF, Bissell MJ. Transcriptional activation by viral enhancers: critical dependence on extracellular matrix-cell interactions in mammary epithelial cells. Mol Carcinog. 1994;10:66–71. doi: 10.1002/mc.2940100203. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Edwards GM, Delcommenne M, Whitelaw CB, Burdon TG, Schindler C, Watson CJ. Stat5 as a target for regulation by extracellular matrix. J Biol Chem. 1995;270:21639–21644. doi: 10.1074/jbc.270.37.21639. [DOI] [PubMed] [Google Scholar]
- Tremble P, Damsky CH, Werb Z. Components of the nuclear signaling cascade that regulate collagenase gene expression in response to integrin-derived signals. J Cell Biol. 1995;129:1707–1720. doi: 10.1083/jcb.129.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner JA, Emerson DA, Juliano RL. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol Biol Cell. 1995;6:725–740. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H, Gouilleux F, Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994;13:2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]