Abstract
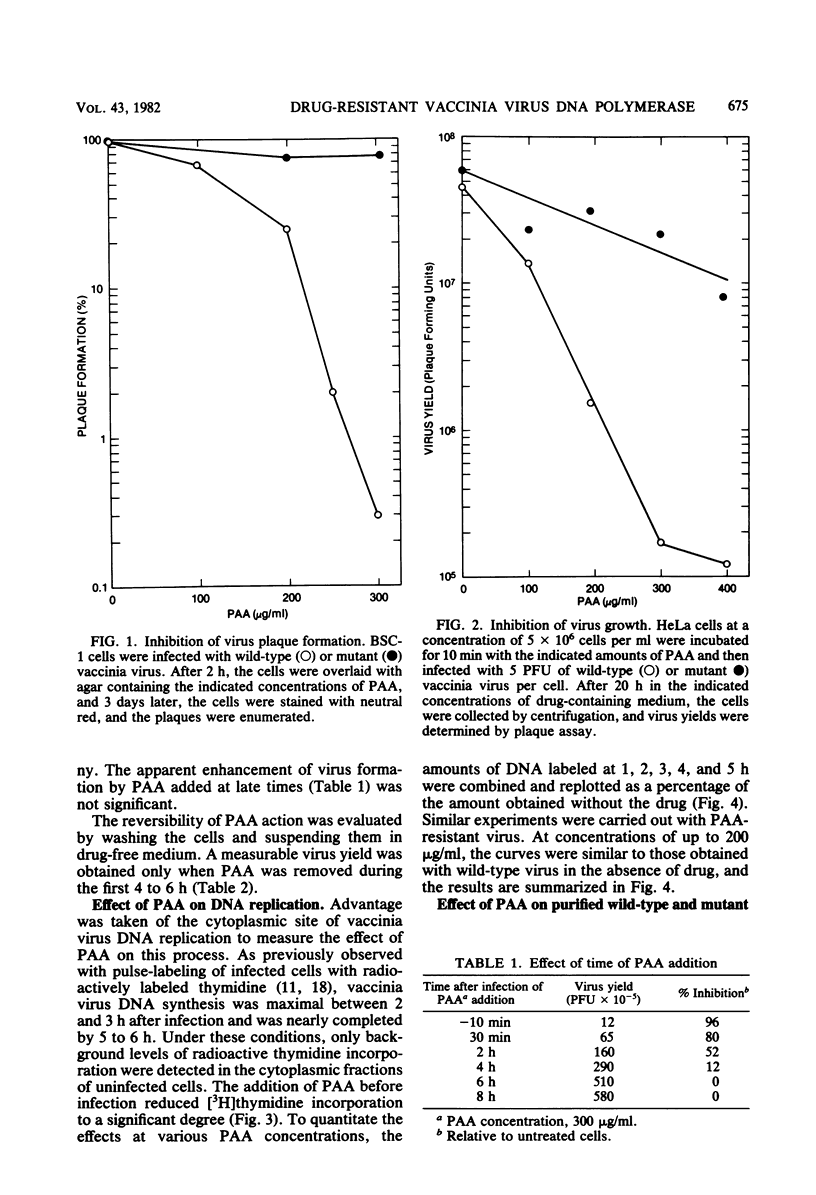
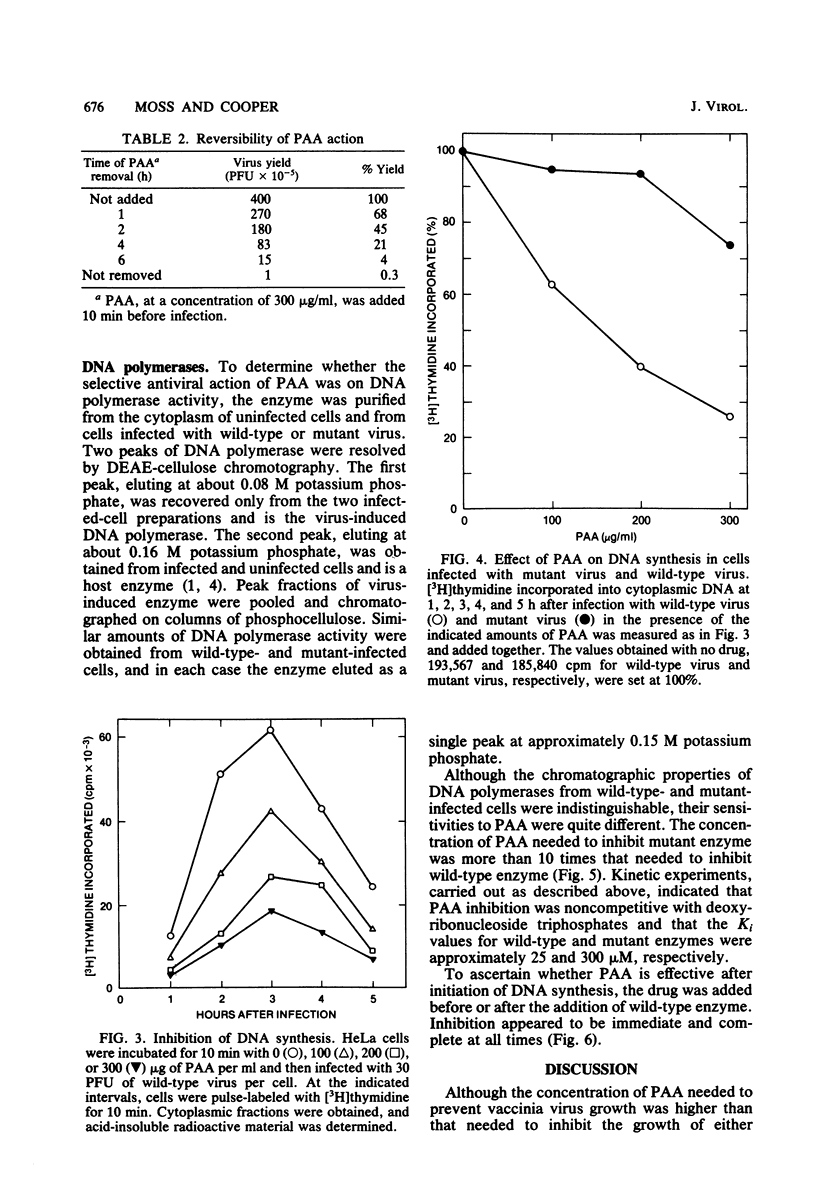
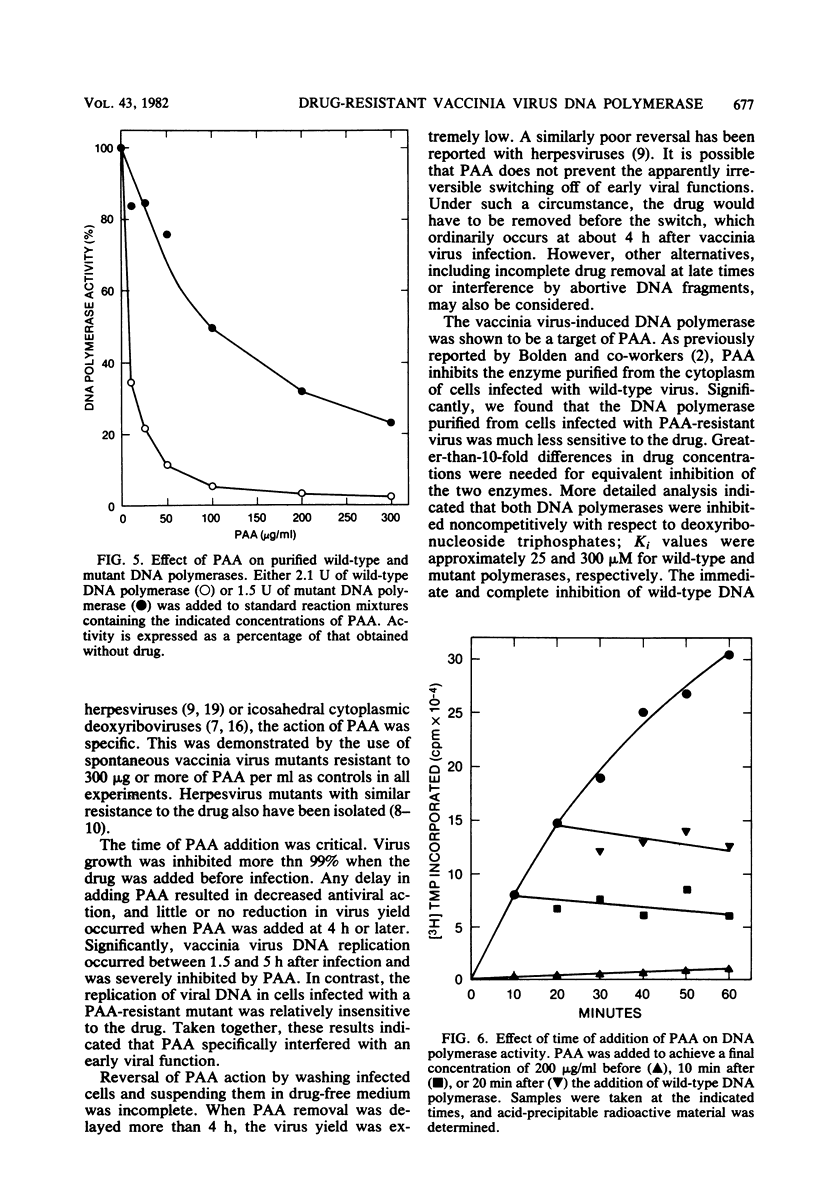
Phosphonoacetate (PAA), at concentrations of 200 micrograms/ml or more, prevented growth of vaccinia virus in HeLa and BSC-1 cells. Spontaneous vaccinia virus mutants, selected at high PAA levels, were resistant to the antiviral effects of the drug. The action of PAA was directed toward an early viral function, since the drug was inhibitory only during the first 4 h of the approximately 15-h growth cycle. Conversely, significant reversal of the antiviral effects was obtained only when the drug was removed at or before the fourth hour of infection. Incorporation of [3H]thymidine into cytoplasmic viral DNA was severely inhibited in cells infected with wild-type virus but not in cells infected with mutant virus. Virus-induced DNA polymerase isolated from the cytoplasm of cells infected with wild-type or mutant virus had indistinguishable chromatographic properties on DEAE-cellulose and phosphocellulose columns. However, the wild-type enzyme was inhibited by relatively low concentrations of PAA, whereas 10-fold higher concentrations were needed for equivalent inhibition of the mutant enzyme. Kinetic analysis indicated that PAA inhibition was noncompetitive with deoxyribonucleoside triphosphates; Ki values for wild-type and mutant DNA polymerases were approximately 25 and 300 microM, respectively. Inhibition of wild-type DNA polymerase was immediate and complete even when PAA was added after initiation of DNA synthesis in vitro, suggesting that chain elongation was affected. These results established that the DNA polymerase is a target of the antiviral action of PAA and provided genetic evidence that this enzyme is virus encoded.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns K. I., Silverman C., Weissbach A. Separation of a new deoxyribonucleic acid polymerase from vaccinia-infected HeLa cells. J Virol. 1969 Jul;4(1):15–23. doi: 10.1128/jvi.4.1.15-23.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden A., Aucker J., Weissbach A. Synthesis of herpes simplex virus, vaccinia virus, and adenovirus DNA in isolated HeLa cell nuclei. I. Effect of viral-specific antisera and phosphonoacetic acid. J Virol. 1975 Dec;16(6):1584–1592. doi: 10.1128/jvi.16.6.1584-1592.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. Purification and properties of the deoxyribonucleic acid polymerase induced by vaccinia virus. J Biol Chem. 1979 Aug 25;254(16):7812–7819. [PubMed] [Google Scholar]
- Citarella R. V., Muller R., Schlabach A., Weissbach A. Studies on vaccinia virus-directed deoxyribonucleic acid polymerase. J Virol. 1972 Oct;10(4):721–729. doi: 10.1128/jvi.10.4.721-729.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. ISOLATION AND PROPERTIES OF VACCINIA MUTANTS DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Feb;22:214–225. doi: 10.1016/0042-6822(64)90006-6. [DOI] [PubMed] [Google Scholar]
- Elliott R. M., Bateson A., Kelly D. C. Phosphonoacetic Acid inhibition of frog virus 3 replication. J Virol. 1980 Jan;33(1):539–542. doi: 10.1128/jvi.33.1.539-542.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J Virol. 1977 Feb;21(2):584–600. doi: 10.1128/jvi.21.2.584-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- Jofre J. T., Schaffer P. A., Parris D. S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977 Sep;23(3):833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Hinkle D. C., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Purification and properties. J Biol Chem. 1975 Jan 25;250(2):461–469. [PubMed] [Google Scholar]
- Magee W. E., Miller O. V. Immunological evidence for the appearance of a new DNA-polymerase in cells infected with vaccinia virus. Virology. 1967 Jan;31(1):64–69. doi: 10.1016/0042-6822(67)90008-6. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Moreno M. A., Carrascosa A. L., Ortín J., Viñuela E. Inhibition of African swine fever (ASF virus replication by phosphonoacetic acid. J Gen Virol. 1978 May;39(2):253–258. doi: 10.1099/0022-1317-39-2-253. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Katz E., Grimley P. M. Rifampicin: a specific inhibitor of vaccinia virus assembly. Nature. 1969 Dec 27;224(5226):1280–1284. doi: 10.1038/2241280a0. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Duff R. G., Mao J. C. Antiviral potential of phosphonoacetic acid. Ann N Y Acad Sci. 1977 Mar 4;284:310–320. doi: 10.1111/j.1749-6632.1977.tb21966.x. [DOI] [PubMed] [Google Scholar]
- Sabourin C. L., Reno J. M., Boezi J. A. Inhibition of eucaryotic DNA polymerases by phosphonoacetate and phosphonoformate. Arch Biochem Biophys. 1978 Apr 15;187(1):96–101. doi: 10.1016/0003-9861(78)90010-3. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]



