Abstract
The rab11 GTPase has been localized to both the Golgi and recycling endosomes; however, its Golgi-associated function has remained obscure. In this study, rab11 function in exocytic transport was analyzed by using two independent means to perturb its activity. First, expression of the dominant interfering rab11S25N mutant protein led to a significant inhibition of the cell surface transport of vesicular stomatitis virus (VSV) G protein and caused VSV G protein to accumulate in the Golgi. On the other hand, the expression of wild-type rab11 or the activating rab11Q70L mutant had no adverse effect on VSV G transport. Next, the membrane association of rab11, which is crucial for its function, was perturbed by modest increases in GDP dissociation inhibitor (GDI) levels. This led to selective inhibition of the trans-Golgi network to cell surface delivery, whereas endoplasmic reticulum–to–Golgi and intra-Golgi transport were largely unaffected. The transport inhibition was reversed specifically by coexpression of wild-type rab11 with GDI. Under the same conditions two other exocytic rab proteins, rab2 and rab8, remained membrane bound, and the transport steps regulated by these rab proteins were unaffected. Neither mutant rab11S25N nor GDI overexpression had any impact on the cell surface delivery of influenza hemagglutinin. These data show that functional rab11 is critical for the export of a basolateral marker but not an apical marker from the trans-Golgi network and pinpoint rab11 as a sensitive target for inhibition by excess GDI.
INTRODUCTION
Members of the rab GTPase family have emerged as important regulators of vesicular membrane traffic. Individual rab proteins govern discrete endocytic and exocytic transport steps (reviewed in Novick and Zerial, 1997). Along the exocytic pathway, endoplasmic reticulum (ER)–to–Golgi transport is regulated by two rab proteins, rab1 and rab2, and intra-Golgi transport depends on the action of rab6 (Plutner et al., 1991; Tisdale et al., 1992; Martinez et al., 1994). Transport from the trans-Golgi network (TGN) to the cell surface requires rab8 and possibly rab11 (Huber et al., 1993; Urbéet al., 1993). Rab8 was shown to be important in the transport of vesicular stomatitis virus (VSV) G protein and Semliki Forest virus spike glycoproteins to the cell surface of polarized and nonpolarized cells, respectively (Ikonen et al., 1995; Peränen et al., 1996).
Rab11 has been detected on a variety of subcellular membranes. In nonpolarized cells rab11 was found associated with both the Golgi and the recycling endosome (Ullrich et al., 1996; Ren et al., 1998). In polarized and regulated secretory cells rab11 has been localized to the Golgi as well as a variety of specialized membrane compartments (Urbéet al., 1993; Deretic et al., 1996; Goldenring et al., 1996, 1997; Sheehan et al., 1996; Calhoun and Goldenring, 1997). The complex localization profiles of rab11 in diverse cell types have complicated the assessment of its precise function in intracellular membrane transport.
Dominant negative mutant forms of numerous rab proteins have been generated over the last decade and have proven extremely useful for dissecting rab protein function in mammalian cell systems. Ullrich et al. (1996) generated such mutant forms of rab11 and used these to analyze the role of rab11 in endocytic membrane transport. In a carefully controlled study the dominant negative rab11S25N mutant was shown to reduce the rate of transferrin recycling in Chinese hamster ovary cells. Transferrin internalization on the other hand was not affected. On the basis of the data it was suggested that rab11 functioned to control the flux of internalized molecules through recycling endosomes (Ullrich et al., 1996). In polarized cells rab11 is also frequently disposed on membranes and vesicles typically derived from plasma membrane recycling pathways (e.g., synaptic vesicles, apical recycling endosomes, and the Na+/K+-ATPase tubulovesicular compartment) (Goldenring et al., 1996, 1997; Sheehan et al., 1996; Calhoun and Goldenring, 1997). Therefore, rab11 may carry out some parallel functions in membrane recycling in both polarized and nonpolarized cells.
The evidence that mammalian rab11 might also play a role in exocytic membrane traffic is largely circumstantial and based solely on localization and cofractionation studies (Urbéet al., 1993; Deretic et al., 1996). The recent finding that the closest homologues of rab11 in yeast, Ypt31/32, are required for exit from the trans-Golgi suggested that this issue needed to be examined more directly (Jedd et al., 1997). Here we analyzed the intracellular distribution of wild-type and mutant forms of rab11 and tested its functional involvement in exocytic transport in baby hamster kidney (BHK) cells.
MATERIALS AND METHODS
Cells and Cell Culture
The BHK21 cell line was obtained from American Type Culture Collection (Rockville, MD) and grown in complete G-MEM (5% FCS, 2 mM glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2.6 mg/ml tryptose phosphate broth) as described (Feng et al., 1995).
Antibodies
Mouse mAb I1 (8G5F11), recognizing an ectoplasmic epitope of VSV G protein (Lefrancois and Lyles, 1982), was kindly provided by Douglas Lyles (The Bowman Gray School of Medicine, Wake Forest University, Winston-Salem, NC). Mouse mAb P5D4, recognizing a cytoplasmic epitope of VSV G protein (Kreis and Lodish, 1986), was a gift from Thomas Kreis (Université de Genève, Geneva, Switzerland). Rabbit antiserum to the influenza virus A/chicken/Germany/34 (fowl plaque virus [FPV] Rostock) hemagglutinin (HA) (Takeuchi and Lamb, 1994) were generously provided by Robert Lamb (Northwestern University). Mouse anti-myc antibody was a kind gift from Karen J. Colley (University of Illinois College of Medicine, Chicago, IL). Mouse anti-actin mAb was purchased from Chemicon International (Temecula, CA). Rabbit antiserum directed against rab2 (Chavrier et al., 1990) was kindly supplied by Marino Zerial (European Molecular Biology Laboratory [EMBL], Heidelberg, Germany). A rabbit polyclonal antiserum to rab8 (Peränen et al., 1996) was generously supplied by Kai Simons (EMBL) and affinity purified as described (Peränen et al., 1996). A rabbit polyclonal antiserum cross-reactive with GDI-1 and GDI-2 was raised using His6-tagged bovine GDI as an antigen. Similarly, a rabbit polyclonal antiserum reactive against rab11 was raised using purified, recombinant canine rab11 as an antigen. A goat polyclonal antiserum against human transferrin receptor was a gift from Suzanne Pfeffer (Stanford University, Stanford, CA). Texas Red–conjugated horse anti-mouse antibodies, biotinylated horse anti-goat antibodies, and Texas Red–conjugated avidin D were purchased from Vector Laboratories (Burlingame, CA).
Plasmids and Subcloning
Plasmids pGEM1-rab11, -rab11Q70L and -rab11S25N (Ullrich et al., 1996) were kindly provided by Marino Zerial. Sequencing of all rab11 inserts revealed that both wild-type rab11 and rab11S25N had a silent nucleotide change, substituting G for A in the third position of the threonine 67 codon. Rab11Q70L bears a different silent mutation in this region, which was purposefully introduced to create a second XbaI site (Ullrich et al., 1996). Plasmids pEGFP-rab11, -rab11S25N, and -rab11Q70L were made by subcloning the cDNA fragments in frame to pEGFP-C3 vector (CLONTECH Laboratories, Palo Alto, CA).
The full-length cDNAs of canine GDI-1 and GDI-2 isolated from a Madin–Darby canine kidney (MDCK) cDNA library (see below) in pJG4-5 were cloned into pGEM3 (Promega, Madison, WI) and pcDNA3 vectors (Invitrogen, Carlsbad, CA) under the T7 promoter as EcoRI–XhoI fragments. A BamHI–EcoRI DNA fragment encoding a VSV G epitope tag (MGTDIEMNRLGKGS, recognized by mouse mAb P5D4 antibody [Kreis and Lodish, 1986]) was inserted 5′ in frame to the GDI-1 and GDI-2 genes of plasmids pcDNA3-GDI-1 and pcDNA3-GDI-2 to create the pcDNA3-G-GDI-1 and -GDI-2 plasmids.
Plasmids encoding wild-type (pAR-G) (Whitt et al., 1989) and temperature-sensitive forms (pGtsO45-2/T7) (Gallione and Rose, 1985) of VSV G protein under the control of T7 promoter were kindly provided by John Rose (Yale University, New Haven, CT) and Marino Zerial, respectively. The pcDNA3-G plasmid encoding wild-type VSV G protein under the control of a human cytomegalovirus (CMV) promoter was made by subcloning the BamHI insert from pAR-G into the pcDNA3 vector. The pcDNA3-GtsO45 plasmid was made by subcloning the cDNA from pGtsO45-2/T7 into the pcDNA3 vector. Plasmid pcDNA3-myc-ST encoding myc epitope-tagged rat α2,6-sialyltransferase (ST) tyr form (Ma et al., 1997) under the control of a human CMV promoter was kindly provided by Karen J. Colley. Plasmid pTM3-FPV-HA encoding the FPV HA protein (Takeuchi and Lamb, 1994) was a kind gift from Robert Lamb. Plasmid pRAB8-wt encoding wild-type rab8 protein (Peränen et al., 1996) was kindly provided by Johan Peränen (University of Helsinki, Helsinki, Finland). Plasmid pGEM1-hTfR was obtained from Marino Zerial, and an EcoRI fragment containing the human transferrin receptor cDNA was subcloned into pcDNA3 to generate pcDNA3-hTfR.
Confocal Immunofluorescence Microscopy
BHK cells were grown on 15-mm square coverslips in 35-mm dishes for 18 h before transfection. After washing once with serum-free medium, cells were transfected with equal amounts of pcDNA3-myc-ST, pcDNA3-G, or pcDNA3-hTfR in combination with pEGFP-rab11, -rab11Q70L, or -rab11S25N plasmids. LipofectAMINE was used for transfections according to the manufacturer’s instructions (Life Technologies, Grand Island, NY): 1 μg DNA/9 μl LipofectAMINE. The DNA/LipofectAMINE transfection mixture was removed 5 h after transfection, and complete G-MEM was added. The transfected cells were cultured for an additional 12 h at 37°C in a 5% CO2 incubator and processed for immunofluorescence as described below. When pcDNA3-GtsO45 (encoding a temperature-sensitive variant of VSV G protein) was used in cotransfections with rab11, the cells were transfected at 39°C (restrictive temperature) for 15 h. Under these conditions the VSV G tsO45 protein accumulated in the endoplasmic reticulum. Cells were then incubated at 20°C for 2 h and either processed for immunofluorescence immediately or transferred to 37°C (31°C for VSV G tsO45) for 1 additional hour where indicated before permeabilization and fixation.
In preparation for immunofluorescence staining, cells were washed once with PBS and permeabilized with 0.5% saponin (Sigma, St. Louis, MO) in 80 mM piperazine-N,N′-bis(2-ethanesulfonic acid)-KOH (pH 6.8), 5 mM EGTA, and 1 mM MgCl2 for 5 min. Cells were then fixed with 3% paraformaldehyde in PBS+ (PBS containing 1 mM CaCl2 and 1 mM MgCl2) for 15 min at room temperature. After fixation cells were washed with 0.5% saponin in PBS for 5 min, and free aldehyde groups were quenched with 50 mM NH4Cl in PBS for 10 min. Cells were washed with 0.5% saponin in PBS for 5 min and then incubated with mouse anti-myc antibody (for detection of myc epitope-tagged ST), goat anti-human transferrin receptor, or monoclonal P5D4 antibody (for detection of VSV G) in PBS containing 0.5% saponin for 20 min. After rinsing cells three times with 0.5% saponin in PBS (5 min each), they were incubated with the appropriate fluorophore-conjugated secondary antibody in PBS containing 0.5% saponin for 20 min. Cells were then washed once with PBS containing 0.5% saponin and three times with PBS (5 min each). The coverslips were then mounted on glass slides in Mowiol 4-88 (Calbiochem, La Jolla, CA). Confocal imaging was performed using a Zeiss (Thornwood, NY) LSM410 confocal microscope (fitted with an Ar laser with a band at 488 nm for FITC and an He-Ne laser with a band at 543 nm for rhodamine). GFP was detected using an FITC filter set. Imaging was performed with a 63× (1.4 numerical aperture) or 100× (1.4 numerical aperture) oil immersion lens (Zeiss). Optical sections were 0.4 μm in the z plane.
GFP was fused in frame to the N-terminus of wild-type and mutant rab11. To demonstrate that the GFP moiety did not alter rab protein localization, GFP-rab protein chimeras were coexpressed in BHK cells with the same rab protein bearing only a small N-terminal VSV G epitope tag (see above). In all cases examined the GFP-rab protein chimeras exhibited a localization that was identical to that of the VSV G-tagged rab proteins (our unpublished results).
MDCK cDNA Library
Poly(A)+ mRNA was isolated from MDCK cells grown to 70% confluence by two rounds of chromatography on oligo(dT) cellulose. A ZAP-cDNA synthesis kit (Stratagene, Menasha, WI) was used to prepare cDNA according to the manufacturer’s instructions. After digestion with EcoRI–XhoI, cDNAs were cloned into the EcoRI–XhoI window of plasmid pJG4-5 (2μ TRP1+, Ampr) (Gyuris et al., 1993; Estojak et al., 1995). The plasmids were then introduced into Epicurian coli sure cells (Stratagene) by electroporation. Transformants were selected on LB ampicillin plates. The library plasmids were prepared from a pool of unamplified 3.0 × 106 primary transformants. More than 95% of the library plasmids contained MDCK cDNA inserts with an average size of 1.3 kb.
Yeast Two-Hybrid Assays and Identification of GDI Isoforms
The yeast strains, expression vectors, and reporter plasmids were kindly provided by Roger Brent (Massachusetts General Hospital, Boston, MA) (Golemis and Brent, 1992; Gyuris et al., 1993; Zervos et al., 1994; Estojak et al., 1995). The yeast reporter strain EGY48 (MATa trp1 ura3 his3 LEU2::pLexAop6-LEU2) was used as a host for all two-hybrid assays. Rab7Q67L was generated for use as a two-hybrid bait by PCR-mediated, site-directed mutagenesis using the following primer: AGCAGGCCTGGAACGGTTCCA (changed base is underlined). The nucleotide sequence was confirmed by sequencing. The plasmid pLexA-rab7Q67L (for two-hybrid screening) was constructed by transferring rab7Q67L into pEG202 (2μ, HIS3+, Ampr) such that the resulting bait protein was fused to amino acids 1–202 of LexA and was expressed under the control of the constitutive ADH1 promoter (Estojak et al., 1995). EGY48 was sequentially transformed with the pLexA-rab7Q67L plasmid and with the MDCK cDNA library in pJG4-5. Transformants were plated on synthetic medium lacking tryptophan, histidine, and uracil and incubated at 30°C for 2 d. The transformants were isolated, and Leu+ and LacZ+ yeast were selected as described (Brent and Ptashne, 1985; Estojak et al., 1995). Library plasmids from positive clones were rescued using Escherichia coli XL1-Blue cells and subsequently analyzed by transformation tests and DNA sequencing. Approximately 20% of the positive clones were found to encode the canine GDI-1 and GDI-2 isoforms. The GDI clones also showed reactivity in a two hybrid assay, albeit of varying intensity, with plasmids encoding other rab proteins.
GDI Sequence Comparisons
The canine form of GDI-1 was 98.7% identical to the bovine GDI-1 protein. This protein was originally identified as smg p25A (rab3A) GDI and is also called GDI-α (Sasaki et al., 1990; Nishimura et al., 1994; Shisheva et al., 1994b). The second isoform corresponds most closely to the mouse GDI-2 protein, sharing 96.9% amino acid identity with this protein (Shisheva et al., 1994b). Comparison with another closely related mouse isoform known as GDI-β yielded a slightly lower amino acid sequence identity (95%). Our data, in concordance with that of others, supports the idea that GDI-1 (or GDI-α isoforms), GDI-2, and GDI-β represent three distinct isoforms (Shisheva et al., 1994b; Janoueix-Lerosey et al., 1995; Pfeffer et al., 1995). The nucleotide sequence data for the canine GDI isoforms are available from GenBank/EMBL/DDBJ under accession numbers AF027360 (canine GDI-1) and AF027361 (canine GDI-2).
A recent report on the structure of bovine GDI-1 identified the critical amino acid residues involved in the formation of a rab binding domain (Schalk et al., 1996). These amino acids map within three sequence-conserved regions. All three sequence-conserved regions were readily identified in both canine isoforms. Furthermore, the consensus residues constituting the three sequence-conserved regions were invariant among GDI-1, GDI-2, and GDI-β isoforms. Therefore, it appears that the GDI-2 and GDI-β isoforms have a rab binding domain similar to that reported for GDI-1 (Schalk et al., 1996).
Exocytosis of VSV G and HA Proteins
Transient overexpression studies were performed using the T7 RNA polymerase recombinant vaccinia virus expression system as described (Fuerst et al., 1986; Feng et al., 1995). Equal amounts of the plasmids encoding GDI (pGEM3-GDI or pcDNA3-G-GDI as indicated) or rab11 and VSV G protein (pAR-G) were used for cotransfection experiments. Mock transfections were performed using vectors lacking an insert. Under the experimental conditions used, transfection efficiencies are very high (>90%). After 4.5 h, transfected BHK cells were incubated for 30 min in medium without methionine and cysteine and metabolically labeled with Tran35S-label (ICN Biomedicals, Irvine, CA) for 10 min (100 μCi/35-mm dish) at 37°C. Subsequently, the cells were transferred to complete G-MEM and incubated for various lengths of time at 37°C. In some cases 40 μg/ml cycloheximide was added as indicated to increase the synchrony of the molecules in transit to the cell surface. The cells were then transferred to ice and subjected to surface biotinylation using sulfo-NHS-SS-biotin (Pierce Chemical, Rockford, IL) as described (Feng et al., 1995). For analysis, cells were lysed in 500 μl radioimmunoprecipitation assay (RIPA) buffer (1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 7.4, and 150 mM NaCl) with PB/CLAP protease inhibitor mixture (1 mM PMSF, 1 mM benzamidine, and 1 μg/ml chymostatin, leupeptin, antipain, and pepstatin A) and 10 mM iodoacetamide (Sigma) added fresh.
RIPA lysates were precleared by centrifugation at 15,000 rpm in an Eppendorf (Madison, WI) microfuge for 15 min at 4°C. VSV G protein was immunoprecipitated on ice using mouse mAb P5D4, a polyclonal rabbit anti-mouse linker antibody, and protein A-Sepharose. The immune complexes were collected by centrifugation and washed twice with RIPA buffer, twice with high-salt RIPA buffer (500 mM NaCl), and twice more with 50 mM Tris-HCl (pH 7.4). The immunoprecipitates were boiled in 80 μl 10% SDS to release bound VSV G protein and clarified by a brief centrifugation at 15,000 rpm in an Eppendorf microfuge.
Biotinylated VSV G protein was precipitated from the released immunoprecipitates as follows. An aliquot (40 μl) of each sample was diluted 10-fold with RIPA buffer containing fresh PB/CLAP, 10 mM iodoacetamide, and 0.2% BSA and incubated for 60 min at 4°C with 40 μl 50% slurry of streptavidin-Sepharose (Pierce). The biotinylated VSV G protein was released from the streptavidin-Sepharose by boiling in SDS-PAGE sample buffer and resolved by SDS-PAGE on 10% gels. These samples were used as a measure of the fraction of VSV G protein delivered to the cell surface. The remaining 40 μl of each immunoprecipitate was resolved by SDS-PAGE directly and was used as a measure of the total VSV G protein.
In cases in which pGtsO45-2/T7 (encoding a temperature-sensitive variant of VSV G protein) was used in cotransfections with GDI, the cells were radiolabeled at 39°C (restrictive temperature). Under these conditions the VSV G tsO45 protein accumulated in the endoplasmic reticulum. Subsequently, the cells were transferred to 31°C for various lengths of time to allow cell surface transport of VSV G protein. All other procedures were the same as those outlined for the wild-type VSV G protein.
The exocytosis of FPV HA was monitored as outlined for wild-type VSV G protein. For these experiments the pTM3-FPV-HA plasmid was used for cotransfections with rab11 or GDI, and rabbit anti-FPV-HA antiserum was used for immunoprecipitation.
Radiolabeled proteins were quantified by exposing gels to phosphoimager plates and analyzing the photo-stimulated luminescence on a Fuji Medical Systems (Stamford, CT) Bioimager equipped with MacBas software (Fuji). The amount of cell surface VSV G protein was calculated as [biotinylated VSV G protein]/[total VSV G protein] × 100. The amount of cell surface HA was calculated as [biotinylated HA1 + HA2]/[total HA0 + HA1 + HA2] × 100. The amount of cleaved HA was calculated as [total HA1 + HA2]/[ total HA0 + HA1 + HA2] × 100.
Subcellular Fractionation and Immunoblotting
VSV G epitope-tagged GDI proteins were transiently overexpressed for 6 h using the T7 RNA polymerase recombinant vaccinia virus expression system as described (Feng et al., 1995). After two washes with PBS+, the cells were scraped in PBS+ at 4°C. The cells were pelleted by centrifugation at 4000 rpm in a Sorvall SA600 rotor (DuPont, Newtown, CT) for 10 min at 4°C. The cell pellets were resuspended in the homogenization buffer (10 mM HEPES, pH 7.4, 250 mM sucrose, and PB/CLAP, 400 μl for two 10-cm dishes of cells) and homogenized at 4°C using a 1-ml Dounce homogenizer. Cell debris and nuclei were removed by two centrifugation steps at 2500 rpm for 5 min in a microfuge at 4°C. The resulting postnuclear supernatant was subjected to centrifugation at 4°C for 1 h in a Beckman TLA 100.2 rotor at 100,000 × g to separate the cytosol from a total membrane fraction. The membrane pellet was resuspended in 500 μl SDS-PAGE sample buffer, and the supernatant (cytosol, ∼350 μl) was also adjusted to 500 μl with concentrated SDS-PAGE sample buffer. The samples were boiled for 5 min, and equal volumes of the cytosol and the total membrane fractions were used for immunoblot analysis.
For immunoblot detection of rab2, rab8, rab11, and VSV G epitope-tagged GDI proteins, samples were resolved on 12.5% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were blocked, washed, and probed as described previously (Feng et al., 1995).
Analysis of GDP and GTP Bound to Rab Proteins
BHK cells were labeled for 2 h with 0.6 mCi/ml [32P]orthophosphate (ICN Biomedicals) in phosphate-free DMEM and lysed as described (Satoh et al., 1988, 1990). The ratio of GDP to GTP bound to rab8 or rab11 was analyzed in the same way as described for p21 (Satoh et al., 1988, 1990). Radiolabeled GDP and GTP were quantified with a Fuji Bioimager equipped with MacBas software. The molar ratio of rab-bound GTP was calculated as [GTP]/([GDP] × 1.5 + [GTP]) × 100.
RESULTS
Wild-Type and Mutant Rab11 Proteins Are Differentially Localized
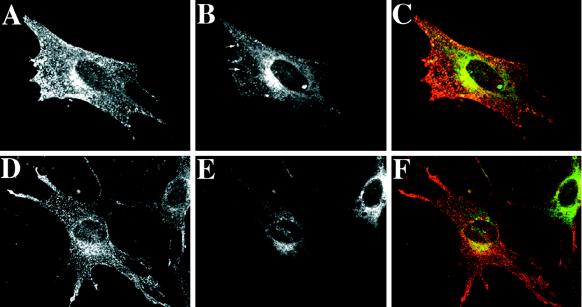
The distribution of wild-type and mutant forms of rab11 between the TGN and recycling endosomes was examined in an effort to obtain clues regarding the site of rab11 recruitment. GFP-tagged variants of wild-type and two mutant forms of canine rab11 were generated. These chimeras were transiently overexpressed in BHK cells together with myc epitope-tagged α2,6-ST, a TGN marker, or human transferrin receptor, a marker of early and recycling endosomes. Consistent with previous observations (Ullrich et al., 1996), the wild-type rab11 protein was found partially colocalized with ST (Figure 1, A–C) and extensively colocalized with the transferrin receptor both in the perinuclear recycling endosomes as well as in disperse, peripheral early endosomes (Figure 2, A–C). The “constitutively active” rab11Q70L mutant protein exhibited a localization pattern similar to that of the wild-type protein (our unpublished results). In contrast, the dominant negative rab11S25N mutant protein was markedly enriched on ST-positive Golgi elements in the perinuclear region (Figure 1, D–F) and exhibited only limited overlap with the transferrin receptor (Figure 2, D–F). In particular, peripheral transferrin receptor positive early endosomes appeared to be devoid of the rab11S25N mutant protein, and there was reduced colocalization of the two proteins in recycling endosomes. Based on these results it appears that the wild-type rab11 protein transits between the Golgi and endosomal compartments. Because the mutant protein accumulated on the Golgi when GTP loading was diminished by the S25N mutation, this serves as an indication that the Golgi serves as a site of rab11 recruitment and nucleotide exchange.
Figure 1.
Localization of rab11 and the Golgi marker α2,6-ST in BHK cells. Myc epitope-tagged ST was transiently overexpressed in BHK cells under the control of a constitutive CMV promoter together with GFP-tagged wild-type rab11 (A–C) or rab11S25N (D–F). At 17 h after transfection, cells were incubated at 20°C for 2 h to increase the ST signal in the Golgi (Ma et al., 1997). Cells were then fixed and processed for confocal microscopy. GFP-tagged rab11 proteins were visualized directly (A and D), whereas ST was detected using a mouse anti-myc antibody as the primary antibody and a horse anti-mouse, Texas Red–conjugated secondary antibody (B and E). Each image represents a single 0.4-μm section with ST and GFP-tagged rab11 proteins viewed in the same focal plane. (C and F) Merged images.
Figure 2.
Localization of rab11 and transferrin receptor in BHK cells. BHK cells were cotransfected with human transferrin receptor and GFP-tagged wild-type rab11 (A–C) or rab11S25N (D–F). At 17 h after transfection, cells were fixed and processed for confocal microscopy. Transferrin receptor was visualized using a goat anti-transferrin receptor antibody as the primary antibody and a biotinylated horse anti-goat antibody and Texas Red–conjugated avidin for detection purposes (A and D). GFP-tagged rab11 proteins were visualized directly (B and E). Each image represents a single 0.4-μm section with transferrin receptor and GFP-tagged rab11 proteins viewed in the same focal plane. (C and F) Merged images. The arrows in B designate some of the peripheral endosomes positive for wild-type rab11 and transferrin receptor.
Overexpression of Mutant Rab11S25N Causes VSV G Protein to Accumulate in the Golgi
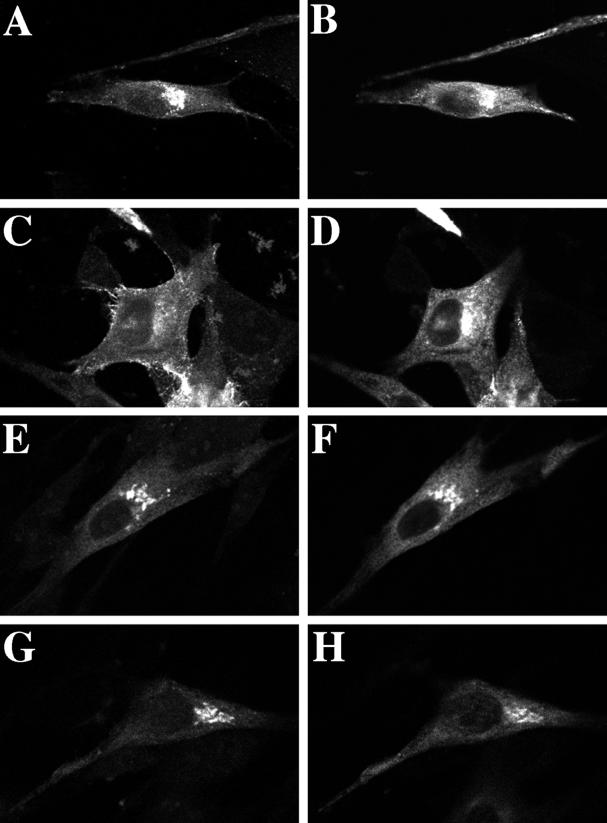
The involvement of rab11 in TGN–to–cell surface transport was tested directly by overexpressing VSV G protein together with wild-type rab11 or the dominant negative rab11S25N mutant. VSV G is a membrane protein encoded by the vesicular stomatitis virus, which is transported to the cell surface along the exocytic pathway. The availability of the cloned gene and numerous excellent antibody reagents makes VSV G protein an ideal marker for use in such cotransfection studies. Export of the VSV G protein from the Golgi was then scored using a morphological assay. VSV G protein was reversibly accumulated in the TGN by incubation at 20°C (Hughson et al., 1988). At 20°C VSV G protein was found colocalized with the subset of the wild-type rab11 present in the Golgi (Figure 3, A and B). A more significant overlap was seen between VSV G and rab11S25N at 20°C because of the preferential association of the mutant rab11 with the TGN (Figure 3, E and F). Coexpression of wild-type rab11 with VSV G protein did not influence the capacity of VSV G protein to reach the cell surface (Figure 3, C and D). Transfer to 37°C caused the VSV G protein to become concentrated at the plasma membrane (Figure 3C), whereas wild-type rab11 remained intracellular and relatively unchanged in its distribution (Figure 3D). However, coexpression of the mutant rab11S25N protein caused a substantial fraction of VSV G protein to remain in the Golgi, where both proteins colocalized, for up to 1 h after rewarming to 37°C (Figure 3, G and H). Analogous results were obtained using the temperature-sensitive VSV G tsO45 protein as the transport marker to follow a single, synchronous wave of exocytosis (our unpublished results; for details see MATERIALS AND METHODS).
Figure 3.
Overexpression of the dominant negative rab11S25N mutant causes VSV G protein to accumulate in the Golgi. VSV G protein was transiently overexpressed in BHK cells under the control of a constitutive CMV promoter in conjunction with GFP-tagged wild-type rab11 (A–D) or rab11S25N (E–H). At 17 h after transfection, cells were incubated at 20°C for 2 h (A, B, E, and F). Parallel samples were transferred to 37°C for 1 h immediately after the 20°C incubation (C, D, G, and H). Cells were then fixed and processed for confocal microscopy. VSV G protein was detected using mouse mAb P5D4 as the primary antibody and a horse anti-mouse, Texas Red–conjugated secondary antibody (A, C, E, and G). GFP-tagged rab11 proteins were visualized directly (B, D, F, and H). Each image represents a single 0.4-μm section with VSV G protein (left panels) and GFP-tagged rab11 proteins (right panels) viewed in the same focal plane.
Functional Rab11 Is Required for Efficient Export of VSV G Protein from the Golgi
The morphological studies shown above provided the first indication that functional rab11 was essential for constitutive exocytosis to proceed. The role of rab11 in exocytic transport was explored further using a biochemical assay. For this purpose a recombinant vaccinia virus system (details given in MATERIALS AND METHODS) was used on account of the ease with which the rab11 proteins could be overexpressed in combination with various transport markers. Furthermore, the contribution of secondary effects is minimized in this system because of the narrow time frame during which overexpression occurs.
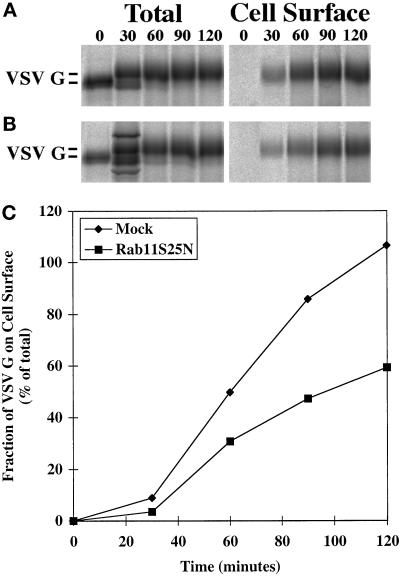
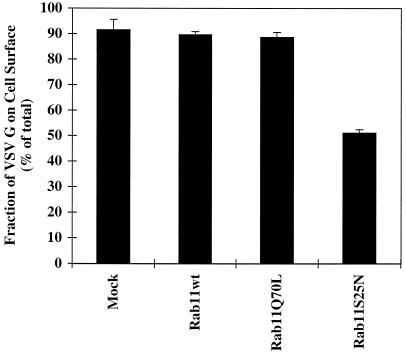
BHK cells were infected and transfected as detailed in MATERIALS AND METHODS to induce the expression of VSV G protein alone or in combination with the rab11S25N mutant protein. The kinetics of radiolabeled VSV G protein transport to the cell surface was scored by cell surface biotinylation. VSV G protein was quantitatively immunoprecipitated after various chase periods, and the fraction of VSV G protein disposed at the cell surface was quantified by retrieval using streptavidin agarose. In the absence of mutant rab11S25N expression, VSV G protein was detected at the cell surface within a 30-min chase period and increased steadily for up to 120 min (Figure 4A). After 120 min all of the labeled VSV G protein had reached the cell surface (Figure 4C, diamonds). However, when rab11S25N mutant was expressed in combination with the VSV G protein marker, there was a noticeable decrease in the cell surface delivery at all time points (Figure 4B). After a 120-min chase period only about half of the total VSV G protein had reached the cell surface (Figure 4C, squares). Triplicate samples revealed that expression of the dominant negative rab11S25N mutant reproducibly decreased cell surface transport of VSV G protein by approximately twofold at the 120-min time point (Figure 5). As expected, overexpression of either wild-type rab11 or activating mutant rab11Q70L had no such inhibitory effect (Figure 5). The observed reduction in transport is consistent with other studies in mammalian systems, in which dominant negative mutant rab proteins generally decreased the efficiency of affected membrane transport steps between 50 and 66% (Bucci et al., 1992; Feng et al., 1995).
Figure 4.
The kinetics of VSV G protein cell surface delivery is markedly altered by overexpression of the dominant negative rab11S25N mutant. Rab11S25N was coexpressed with VSV G protein in BHK cells. At 5 h after transfection, cells were metabolically labeled for 10 min at 37°C and subsequently incubated for various chase times (0–120 min). The kinetics of cell surface delivery was monitored by biotinylation at 4°C. Biotinylated (cell surface) and total cellular VSV G fractions were prepared, analyzed by SDS-PAGE, and quantified with a Fuji Bioimager. Quantitation of VSV G protein cell surface appearance in mock-treated control cells (A) and cells overexpressing rab11S25N mutant (B) is shown in C. (A and B) Left panels, quantitative immunoprecipitation of total VSV G protein from cell lysates after different chase periods using mouse mAb P5D4 (Note that there are two unidentified background bands in the bottom 30-min lane); right panels, cell surface, biotinylated VSV G protein recovered using streptavidin-Sepharose after different chase periods. Representative data from one of three independent trials are shown.
Figure 5.
Cell surface delivery of VSV G protein is reduced in cells expressing the dominant negative rab11S25N mutant. BHK cells were transfected with VSV G together with wild-type (wt) rab11, rab11Q70L, or rab11S25N. At 5 h after transfection, cells were metabolically labeled for 10 min. After a chase period of 120 min, cell surface delivery of the VSV G protein was monitored by surface biotinylation as described in MATERIALS AND METHODS. Each column represents the mean ± SEM of triplicate samples from one of three independent experiments.
Overexpressed Mutant Rab11S25N Selectively Interferes with Exocytic Transport along the Basolateral Cognate Pathway
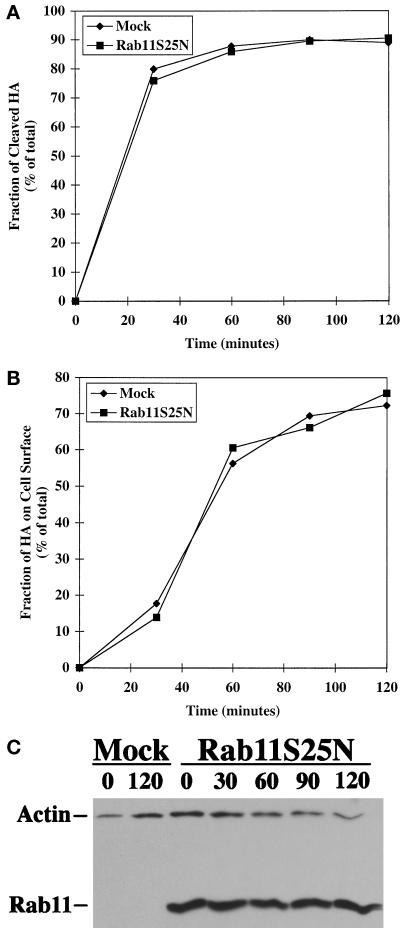
Influenza HA is a second model plasma membrane protein that has been used to follow cell surface transport in both polarized and nonpolarized cells (Rodriguez-Boulan and Pendergast, 1980; Yoshimori et al., 1996). A key distinction between the two proteins is engendered by the fact that VSV G protein is basolaterally targeted, whereas influenza HA is apically targeted in polarized cells. Recently, it has been shown that the requirements for VSV G protein and influenza HA cell surface transport are distinct in both polarized and nonpolarized cells (Ikonen et al., 1995; Yoshimori et al., 1996). In particular, the delivery of influenza HA was suggested to occur independent of the targeting and fusion machinery involving rab proteins, NEM-sensitive factor, and SNAREs. On the other hand, VSV G protein transport to the cell surface was critically dependent on these accessory factors. Therefore, the cell surface delivery of influenza HA was analyzed to test whether the inhibitory effect of mutant rab11S25N protein overexpression on cell surface transport was limited to specific pathways or whether the inhibition reflected a more general and perhaps less specific effect of the overexpressed protein.
The infection–transfection scheme was used to express influenza HA (derived from FPV Rostock) together with or without mutant rab11S25N protein. The newly synthesized influenza HA was metabolically labeled for a short period, and the progress of its cell surface transport was monitored by cell surface biotinylation. FPV HA is initially synthesized as a single precursor (HA0), which is cleaved in the TGN to a disulfide-linked product composed of the membrane-spanning HA2 and the ectoplasmic HA1 (Stieneke-Gröber et al., 1992). Within a 30- to 60-min chase period cleavage of influenza HA was maximal (Figure 6A), and within a 60- to 90-min chase period this cleaved HA were found at the cell surface (Figure 6B).
Figure 6.
Exocytosis of influenza HA is unaffected by overexpression of the dominant negative rab11S25N mutant. Rab11S25N was coexpressed with influenza HA in BHK cells. At 5 h after transfection, cells were metabolically labeled for 10 min at 37°C and subsequently incubated for various chase times (0–120 min). Cell surface biotinylation and immunoprecipitation were performed as described in MATERIALS AND METHODS. (A) Quantitation of the kinetics of FPV HA cleavage as a measure of transport through the TGN. (B) Quantitation of influenza HA cell surface appearance in cells overexpressing rab11S25N compared with mock-treated control cells. (C) Detection of rab11 in transfected cell lysates by immunoblotting with actin serving as a control for protein loading.
The overall kinetics of influenza HA delivery to the cell surface was identical in mock-transfected cells and those transfected with mutant rab11S25N (Figure 6, A and B). Overexpression of mutant rab11S25N protein in the transfected cell lysates was confirmed by immunoblot analysis using actin levels as a control for equal protein loading (Figure 6C). The anti-rab11 antibody was diluted for optimal detection of the overexpressed protein, which precluded the detection of endogenous rab11 protein in the mock-transfected samples. These data showed that the mutant rab11S25N protein had no deleterious effect on influenza HA exocytosis.
Rab11 Is Selectively Released from Membranes by Overexpressed GDI
To further exclude the possibility that the dominant rab11S25N mutant might exert pleiotropic effects on membrane transport, it was of interest to modulate rab11 function by a different means and to reexamine the consequences for VSV G export from the TGN. Overexpression of GDI can be used to alter the membrane association of rab proteins and thereby affect their function. Excess GDI added in vitro has been demonstrated to release GDP-bound rab proteins from membranes by forming a soluble complex (Dirac-Svejstrup et al., 1994; Peter et al., 1994; Ullrich et al., 1994). Excess GDI causes a marked inhibition in membrane transport most likely because rebinding of rab proteins to membranes is extremely inefficient under these conditions. Soluble GDI-rab protein complexes exist in the cytosol of cells normally (Yang et al., 1994), but in the absence of excess GDI most rab proteins exist predominantly in the membrane-bound state (Ullrich et al., 1993).
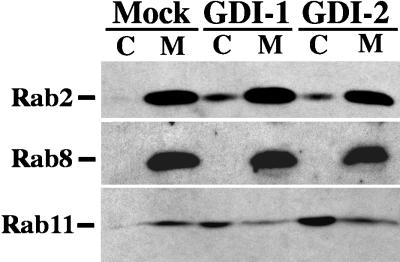
The canine isoforms of GDI-1 and GDI-2 (for details on how these were cloned, see MATERIALS AND METHODS) were modestly overexpressed, and the distribution of individual exocytic rab proteins in membrane and cytosolic fractions was analyzed by immunoblot analysis (Figure 7). In mock-transfected cells all of the rab proteins examined were exclusively membrane bound. Overexpression of either GDI-1 or GDI-2 resulted in a modest increase in cytosolic levels of rab2, a protein involved in ER-to-Golgi transport. Under the same conditions the membrane association of rab8, a rab protein previously shown to be required for TGN cell surface transport of VSV G (Peränen et al., 1996), was completely unaltered by either GDI isoform. In contrast, overexpression of both GDI isoforms was found to have a very significant impact on the membrane association of rab11, causing it to become largely cytosolic.
Figure 7.
Distribution of rab2, rab8, and rab11 between cytosol and total membrane fractions after GDI overexpression. VSV G epitope-tagged GDI-1 or GDI-2 proteins were overexpressed in BHK cells; 6 h after transfection, the cells were homogenized, and cytosol and total membrane fractions were prepared. Cytosol (C) and total membrane (M) fractions were resolved by SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membranes, and blots were probed individually with antibodies against rab2, rab8, or rab11. An HRP-conjugated secondary antibody was used to detect the immune complexes by chemiluminescence.
The observation that individual rab proteins are differentially sensitive to extraction by GDI in vivo is a novel one. Sensitivity to extraction by GDI hinges on the rab protein being in its GDP-bound form. Our results are consistent with the possibility that individual rab proteins vary in their GDP- versus GTP-bound ratios. Support for this suggestion was drawn from comparisons of the fraction of rab11 or rab8 in the two nucleotide-bound states in vivo. Cells were radiolabeled with [32P]orthophosphate, and subsequently rab8 and rab11 were immunoprecipitated from cell lysates. The bound nucleotides were resolved by thin-layer chromatography and identified by comparisons with standards. Quantitative analysis using a Fuji Bioimager revealed that a significant fraction (68%) of rab11 was present in the GDP-bound form, whereas rab8 was significantly more enriched in the GTP-bound state (91%).
TGN–to–Cell Surface Transport Is Particularly Sensitive to Overexpressed GDI
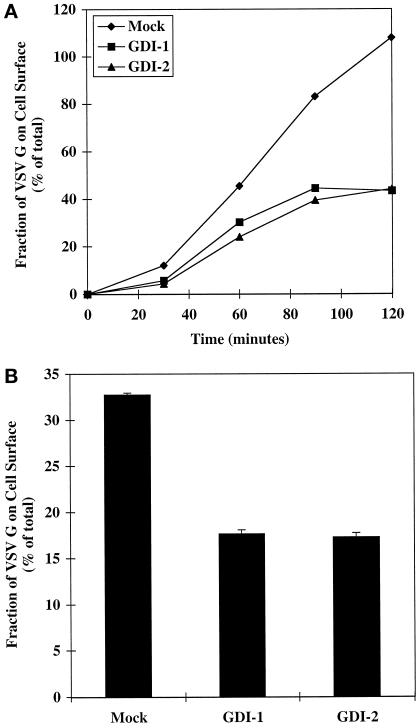
Knowing that GDI overexpression selectively perturbed the membrane association of wild-type rab11, we next examined its consequences on the exocytosis of VSV G protein. For this series of experiments we used the temperature sensitive variant VSV G tsO45 protein as the transport marker, because it permits the analysis of a synchronous wave of molecules in transit. At the nonpermissive temperature of 39°C the VSV G tsO45 protein is synthesized but fails to exit the endoplasmic reticulum. Subsequent transfer of cells to permissive temperature (31°C) initiates the synchronous transport of the viral proteins through the Golgi and on to the cell surface. Cell surface delivery of VSV G tsO45 protein was again monitored by biotinylation. Overall cell surface transport of VSV G tsO45 upon overexpression of either GDI isoform was markedly inhibited relative to control cells, such that after a 120-min chase period only 40% of the total VSV G protein was detected at the cell surface (Figure 8A, squares and triangles).
Figure 8.
TGN–to–cell surface transport of VSV G tsO45 protein is inhibited by overexpression of either GDI isoform. GDI-1 and GDI-2 tagged with a VSV G epitope were coexpressed with the temperature-sensitive VSV G tsO45 protein in BHK cells. This enabled the simultaneous monitoring of both overexpressed proteins. Transfected cells were maintained and metabolically labeled (10 min, 5 h after transfection) at nonpermissive temperature (39°C). Cell surface transport of VSV G tsO45 protein was induced by transfer to permissive temperature (31°C) for various chase times (0–120 min). (A) The cell surface delivery of the VSV G protein as a function of time was monitored by surface biotinylation as described in MATERIALS AND METHODS. Representative data from one of three independent experiments is shown. (B) TGN–to–cell surface transport of VSV G tsO45 proteins was monitored by transferring transfected cells from 39 to 20°C for 1 h to accumulate VSV G in the TGN and subsequently transferring them to 31°C for 1 h. Cell surface delivery was quantified by biotinylation as described in MATERIALS AND METHODS. Each column represents the mean ± SEM of triplicate samples from one of two independent experiments.
Diagnostic assays for individual transport events were used in an effort to pinpoint the membrane transport step(s) affected by the overexpression of GDI. The impact of the GDI isoforms on export from the TGN was assessed using a similar experimental outline as above, except that transfer to permissive temperature was preceded by a 1-h incubation at 20°C (Matlin and Simons, 1983). Under these conditions the VSV G tsO45 protein can exit the ER and traverse the Golgi stacks but accumulates in the TGN (Hughson et al., 1988). Immediately after incubation at 20°C the cells were shifted to permissive temperature to allow cell surface delivery of the accumulated VSV G protein. There was approximately a twofold decrease in the cell surface delivery of VSV G protein when either GDI isoform was overexpressed (Figure 8B). The effect was the same even when the accumulation of any new VSV G protein or GDI was inhibited by the addition of cycloheximide during the chase period (our unpublished results). The reduced total surface delivery of VSV G protein after release of the 20°C transport block can be explained by the fact that only half of the protein reached the Golgi during the 20°C incubation as determined by the acquisition of endo-β-N-acetylglucosaminidase H resistance (our unpublished results). It was notable that the magnitude of the inhibitory effect of GDI overexpression on TGN–to–plasma membrane transport was in the same range as the overall inhibitory effect of GDI overexpression on exocytosis (Figure 8A) and the inhibitory effect by overexpression of rab11S25N mutant (Figures 4 and 5). Overexpressed GDI did not impair the cell surface delivery or the cleavage kinetics of influenza HA, in correspondence with the results obtained when mutant rab11S25N was overexpressed (our unpublished results).
The acquisition of endo-β-N-acetylglucosaminidase H resistance was used to monitor the transport kinetics from the ER to the medial Golgi. Several independent trials revealed no inhibitory effect of either GDI isoform on export of VSV G or influenza HA from the ER (our unpublished results). This is actually not surprising, because neither GDI isoform could extract a significant amount of rab2 from membranes (Figure 6). In each trial the amount of GDI overexpressed was carefully monitored and found to be in the range of 2–5 μM (our unpublished results), which is two- to fivefold above endogenous levels (Ullrich et al., 1993). These data suggest that overexpression of GDI caused a specific block in transport between the TGN and the plasma membrane affecting selected transport markers, this inhibitory effect being most likely caused by extracting rab11 from membranes.
Active Forms of Rab11 Can Reverse the Inhibitory Effect of Excess GDI on Exocytic Transport
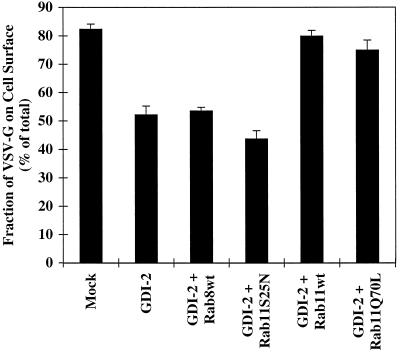
The data presented in the previous sections are consistent with the idea that limited overexpression of GDI results in the sequestration of a single critical rab protein, namely rab11, which in turn is sufficient to significantly inhibit TGN–to–cell surface transport. To address this directly we tested whether the simultaneous overexpression of rab11 would reverse the transport inhibition. Cell surface delivery of VSV G protein was examined while simultaneously overexpressing GDI-2 and various forms of rab11 or rab8 (Figure 9). Overexpression of GDI-2 alone inhibited surface delivery of VSV G protein, as before (Figure 8). Coexpression of wild-type rab8 failed to relieve the inhibitory effect of GDI-2, as did the dominant negative rab11S25N mutant. In marked contrast, coexpression of GDI-2 with either wild-type rab11 or the activating rab11Q70L mutant fully restored surface delivery of VSV G protein to normal levels. Overexpression of the rab proteins was confirmed in each case by immunoblot analysis (our unpublished results). This dramatic rescue offered strong support for the experimental premise that overexpressed GDI inhibited exocytosis by sequestering rab11. Therefore, by two independent means rab11 was shown to be required for efficient exocytic transport of selected markers from the TGN.
Figure 9.
The inhibition of cell surface delivery of VSV G tsO45 protein by GDI-2 overexpression can be relieved by coexpression of wild-type rab11 but not wild-type rab8. BHK cells were transfected with the VSV G tsO45 plasmid (0.3 μg/35-mm dish) and GDI-2 epitope tagged with VSV G plasmid (0.6 μg/35-mm dish) together with wild-type (wt) rab8, rab11S25N, wild-type (wt) rab11, or rab11Q70L (0.3 μg/35-mm dish). At 5 h after transfection, cells were metabolically labeled for 10 min and then subjected to a 90-min chase period. The cell surface delivery of the VSV G tsO45 protein was monitored by surface biotinylation as described in MATERIALS AND METHODS. Each column represents the mean ± SEM of triplicate samples from one of three independent experiments.
DISCUSSION
We have shown that a dominant negative mutant form of rab11 (rab11S25N) is preferentially associated with the TGN. In contrast, wild-type rab11 is much more prevalent on transferrin receptor–positive endosomes. Cells expressing rab11S25N also manifested a twofold reduction in the cell surface transport of VSV G protein, resulting in Golgi accumulation of the VSV G protein. Modest overexpression of GDI caused a similar reduction in TGN–to–cell surface transport of VSV G protein and accumulation of VSV G in the Golgi (our unpublished results). We could show that this was correlated with the specific depletion of rab11 from the membrane fraction. Other exocytic rab proteins and transport steps such as ER-to-Golgi and intra-Golgi transport were relatively unaffected by modest GDI overexpression. The inhibitory effect of excess GDI could be compensated by coexpression of wild-type rab11 but not by the dominant negative rab11S25N or wild-type rab8. Influenza HA, which follows an alternative route, served as a control and was unaffected by either mutant rab11S25N or GDI overexpression (our unpublished results). Two different GDI isoforms were used and found to be functionally indistinguishable in all aspects tested. On the basis of these results a number of conclusions can be drawn regarding the potential role of rab11 in exocytosis and GDI function.
Rab11 Function in Constitutive Exocytosis
Mutations affecting the nucleotide binding or hydrolysis of small GTPases can influence both their membrane localization and transport functions. Careful analysis of the phenotypic changes brought about by individual mutant GTPases has offered clues regarding GTPase function. For example, large, swollen early endosomes were shown to result from the expression of rab5Q79L, a mutant deficient in GTP hydrolysis (Stenmark et al., 1994). This provided the first evidence that GTP hydrolysis was not a prerequisite for rab protein function in membrane fusion events. Wild-type ARF6 has been found uniformly associated with both the plasma membrane and endosomes, whereas two mutant forms were found preferentially associated with one or the other of the two membranes (D’Souza-Schorey et al., 1998). The activated mutant form, ARF6(Q67L), was primarily detected on the plasma membrane, whereas the dominant negative mutant form, ARF6(T27N), was principally present on perinuclear, endocytic vesicles. Extrapolating from these data and observed alterations in transferrin recycling, ARF6 was proposed to function in endocytic recycling to the plasma membrane (D’Souza-Schorey et al., 1998). In the case of rab11, localization studies have detected it on a variety of subcellular membranes, including the Golgi, endosomes, and vesicles of both endocytic and exocytic origins. The striking restriction of the dominant negative rab11S25N mutant protein to the TGN shown in this study hints that the TGN might serve as a site of rab11 recruitment and activation. The lack of activated rab11 on the TGN was associated with an inhibition in export of VSV G protein but not influenza HA from the TGN. Taken together these data suggest a possible role for rab11 in the budding of a subset of TGN vesicles. Although rab proteins have been primarily associated with vesicle-targeting functions, there are a number of examples in which the rab proteins have been suggested to influence vesicle budding (Plutner et al., 1991; Riederer et al., 1994; Jedd et al., 1995, 1997). Perhaps the most compelling evidence derives from in vitro assays showing a direct role for rab5 in coated vesicle formation at the plasma membrane (McLauchlan et al., 1998). Rab5 has also been demonstrated to control the fusion of clathrin-coated vesicles with endosomes as well as the homotypic fusion of early endosomes (Gorvel et al., 1991). Using rab5 as a paradigm, it is interesting to consider that vesicle budding and targeting are coordinately regulated.
Our studies made use of the same rab11 mutants used in the study of transferrin recycling (Ullrich et al., 1996; Ren et al., 1998). The inhibitory effect exerted by the rab11S25N mutant on exocytosis was at least as dramatic as its inhibitory effect on transferrin receptor recycling. To reconcile these findings, at least two scenarios can be envisioned. In one case rab11 is seen to control exit from the Golgi directly, perhaps by regulating Golgi vesicle budding as discussed above. A proposed function for rab11 in regulating exit from the Golgi would place it upstream of rab8, which has been observed on post-Golgi exocytic vesicles (Huber et al., 1993). Rab8 has also been shown to mediate post-Golgi vesicle interaction with the cytoskeleton and subsequent delivery to the plasma membrane (Peränen et al., 1996). Again this would be consistent with our finding that excess rab8 could not bypass the requirement for rab11 under circumstances in which rab11 was sequestered by excess GDI. In this sense rab8 would fulfill distal targeting functions very similar to those ascribed to its yeast counterpart Sec4p (Walch-Solimena et al., 1997). The functional requirement for rab11 in transferrin receptor recycling would be an expected outcome if the targeting of components essential for the membrane recycling pathway were also mediated by rab11.
It is interesting to consider the alternative possibility that rab11 regulates flux through the recycling endosome originating from both the endocytic and exocytic pathways. There is growing precedence for the transport of a fraction of newly synthesized molecules through endosomes en route to the cell surface. For example, transferrin receptor, asialoglycoprotein receptor H1, and the Semliki Forest virus p62 precursor are all examples of basolaterally directed proteins with clathrin-coated pit localization signals, which have been shown to reach the cell surface after passage through endosomes (Futter et al., 1995; Leitinger et al., 1995; Sariola et al., 1995). Variable fractions of these molecules were reported to contact endosomes en route to the surface. In the case of transferrin receptor only 15–20% of the newly synthesized molecules cofractionated with endosomes (Futter et al., 1995). Using coimmunoisolation with resident endosomal markers or contact with internalized antibody, 60–80% of newly synthesized asialoglycoprotein H1 or Semliki forest virus p62 were scored as passing through endosomes, respectively (Leitinger et al., 1995; Sariola et al., 1995). In epithelia, VSV G protein is a basolaterally targeted protein with a classical tyrosine-coated pit internalization motif; by analogy this would make it an excellent candidate for an endosomal-based sorting pathway to the cell surface. Rab11S25N inhibited cell surface transport of VSV G protein by 40–50%. This might reflect the fraction of VSV G protein traversing endosomes en route to the cell surface, whereas the remainder follows a direct, rab11-independent, and perhaps rab8-dependent route to the surface. This would also be consistent with our observation that wild-type rab8 failed to rescue the transport inhibition induced when rab11 was sequestered by GDI and the observation by Peränen et al. (1996) that a dominant mutant form of rab8 impaired VSV G transport to the cell surface by only 30%.
The fact that the mutant rab11S25N protein had no effect on the exocytosis of a second plasma membrane marker (influenza HA) is also in accordance with such a model. In epithelial cells influenza HA is targeted to the apical cell surface (Rodriguez-Boulan and Pendergast, 1980). Transport to this domain is mediated by a distinct class of vesicles with different targeting and fusion requirements compared with those destined for the basolateral surface (Wandinger-Ness et al., 1990; Ikonen et al., 1995). Rab8, for example, was not found to be involved in influenza HA transport (Huber et al., 1993). Two independent routes for cell surface transport have also been suggested to exist in nonpolarized cells (Yoshimori et al., 1996). Influenza HA sorting has been linked to the formation of glycolipid rafts, which often include glycosylphosphatidylinositol-anchored proteins. Interestingly, the glycosylphosphatidylinositol-anchored protein alkaline phosphatase did not traffic through endosomes en route to the plasma membrane (Futter et al., 1995). The cumulative data suggest that constitutive transport to the cell surface may occur via several routes. Apical markers and basolateral markers may be delivered directly from the TGN to the cell surface via distinct carrier vesicles. In addition, basolateral molecules may traffic through endosomes and, thereby, depend partially on rab11 function.
Two yeast homologues of rab11 were originally isolated in a suppresser screen to identify further regulators of ER-to-Golgi transport (Benli et al., 1996; Jedd et al., 1997). Deletion of both Ypt31 and Ypt32 caused newly synthesized proteins to accumulate in the trans-Golgi, lending support to the suggested role for these proteins in Golgi vesicle budding (Jedd et al., 1997). This is analogous to what we observed for VSV G protein when mutant rab11S25N protein was expressed. At present it is unknown whether Ypt31p and Ypt32p have any role in endocytosis. Furthermore, expression of the human wild-type rab11 gene under the control of the inducible Gal10 promoter did not affect the growth of a ypt31null/ypt32A141D mutant in yeast (Segev, personal communication). Therefore, although there are parallels between the yeast and mammalian homologues, functional specialization of these proteins in the two systems remains a distinct possibility. This suggestion is further supported by the fact that Ypt31p and Ypt32p are functionally redundant in yeast. In mammalian systems a second isoform, termed rab11B, is enriched in brain (Lai et al., 1994). The stingray homologue of rab11B (Ora3) was found on axonal synaptic vesicles, whereas the original isolate (now also designated rab11A) was found restricted to dendrites (Ngsee et al., 1991; Volknandt et al., 1993; Sheehan et al., 1996). This would suggest that at least in neurons the two isoforms have distinct distributions and likely different functions. Because rab11A is ubiquitously expressed in all mammalian cell types, functional analyses of rab11 (including our study) have centered on this isoform, but clearly the functional specialization of rab11 in polarized cells will be of significant interest.
Rab11 as a Select Target of GDI In Vivo
It is of interest to consider what causes rab11 to be a select target of GDI among the rab proteins analyzed. It is possible that the specific sequestration of rab11 by GDI in vivo is reflective of a higher affinity for rab11 compared with other rab proteins. Because GDI-binding constants have been determined for only a limited number of rab proteins, this cannot be conclusively evaluated. However, available data on the GDI-binding constants for rab3A and rab9 show these to be on the same order of magnitude (Shapiro and Pfeffer, 1995; Schalk et al., 1996), and no difference was observed between the binding constants for rab5 and rab6 (Possmayer and Goud, personal communication). Furthermore, GDP dissociation from rab11 was affected in a dose-dependent manner by GDI over a similar concentration range as rab3A, suggesting that the binding constants of these two rab proteins are also on the same order of magnitude (Sakurada et al., 1991; Nishimura et al., 1994). If differences in affinity prove to be minimal, as suggested by these examples, then differences in the GDP-bound concentrations of individual rab proteins could offer an alternative explanation for the observed selectivity of GDI in vivo.
The relative nucleotide-bound state (GTP or GDP bound) of a given membrane-bound rab protein is governed by its nucleotide exchange and hydrolysis rates. The rab guanine nucleotide exchange factors and GTPase-activating proteins, which contribute to the regulation of these rates, appear to be specific for individual rab family members (Novick and Zerial, 1997). In addition, the intrinsic nucleotide exchange and hydrolysis rates for individual rab proteins varies quite dramatically (Zerial and Huber, 1995). Therefore, it is plausible that rab11 remains a sensitive target, because it has a longer residency in the GDP-bound state relative to other rab proteins. To test this possibility we compared the in vivo nucleotide-bound states of two rab proteins, which we observed to have significantly different sensitivities to increased GDI levels. Rab8, which was insensitive to GDI, was preferentially recovered from labeled cell extracts in the GTP-bound state. Under the same circumstances rab11 was preferentially in the GDP-bound state. A longer residency in the GDP-bound state could be brought about by a slower rate of guanine nucleotide exchange, lack of stabilization in the GTP-bound form by a suitable effector, or a rapid GTPase-activating protein–stimulated GTP hydrolysis rate. In this respect it is interesting to note that rab5, which has a high intrinsic GTPase activity and rapidly cycles between the GTP and GDP-bound form on membranes (Rybin et al., 1996), is relatively insensitive to release by GDI under our assay conditions (our unpublished results). In contrast, both rab7 and rab11 have significantly slower intrinsic GTPase activities (Urbé and Parton, 1995; Simon et al., 1996), yet both are sensitive to release from membranes by excess GDI (our unpublished results). Taken together, the data hint that the factors involved in stable membrane recruitment, including GDI displacement factor and guanine nucleotide exchange factor, may be the most critical in dictating the GDP-/GTP-bound ratio of an individual rab protein.
The observation that the inhibition of VSV G exocytosis caused by excess GDI could be overcome by overexpression of active forms of rab11 (wild-type rab11 and rab11Q70L) and not by the dominant negative form (rab11S25N) argues for specificity. It suggests that the delivery of sufficient functional rab11 to membranes is a critical parameter in relieving the transport inhibition. The dominant negative rab11S25N protein binds GDP preferentially, and even though it was clearly detected on membranes (see Figures 1–3), it failed to restore efficient transport. The fact that functional rab11 was all that was required to restore transport argued strongly that the observed transport inhibition was not caused by a pleiotropic effect of GDI. Excess wild-type rab8 was ineffective in restoring transport to normal levels, presumably because it functions in a different transport step (possibilities described above) and, therefore, could not substitute for a rab11 deficiency on membranes.
Excess GDI has been shown to block both ER-to-Golgi and intra-Golgi transport events in vitro (Elazar et al., 1994; Peter et al., 1994). Our finding that excess GDI had relatively little effect on these transport steps in vivo is not necessarily contradictory. In both assay systems, excess GDI is present, which shifts the equilibrium to favor GDP-bound rab protein release from membranes. In vitro, limiting amounts of GTP may result in the accumulation of rab proteins in their GDP-bound conformations, thereby making them sensitive to GDI-mediated release. Once released, the large volume of most in vitro reactions, relative to the cytosolic volume of an intact cell, may also come into play. The large fold dilution of released rab–GDI complexes makes the likelihood of membrane rebinding and rab protein recruitment into the active state very low. In contrast, the abundance of GTP in vivo and the relatively constant cytosolic volume allow for the continuous recruitment of rab proteins into their GTP-bound forms on membranes. Consequently, differences in the nucleotide-bound status of ER- and Golgi-specific rab proteins are likely to exist under the two circumstances and may account for the observed differences between the in vitro and in vivo assay systems.
Function of Multiple GDI Isoforms
Several GDI isoforms have been identified in mammalian systems; however, the function of individual isoforms remains unclear (Nishimura et al., 1994; Shisheva et al., 1994b; Janoueix-Lerosey et al., 1995). Our data support the view that there are at least three distinct GDI isoforms (Janoueix-Lerosey et al., 1995; Pfeffer et al., 1995). Yeast (Saccharomyces cerevisiae) encodes only one GDI (Gdi1p), and it has been shown to be an essential gene product (Garrett et al., 1994). A variety of explanations have been put forward for the existence of multiple mammalian isoforms. The observation that the GDI-1 and GDI-2 isoforms were differentially expressed in some cells and bound rab proteins in accordance with their cell type abundance led to the postulate that multiple isoforms might function in a tissue-specific manner (Bachner et al., 1995). However, because these two isoforms were clearly not mutually exclusive in their expression profiles, this appeared not to be the complete explanation (Shisheva et al., 1994b; Yang et al., 1994). It was observed that the mouse GDI-1 and GDI-2 isoforms exhibited overlapping but distinct subcellular distributions, and a suggestion was put forward that this difference might contribute to differences in function (Shisheva et al., 1994a, 1995).
The results reported here provide no evidence for functional distinctions between GDI-1 and GDI-2 in the regulation of exocytic rab proteins on the basis of their localization. The canine GDI-1 and GDI-2 isoforms exhibited the same differences in membrane localization (our unpublished results) described previously for the mouse homologues (Shisheva et al., 1995), yet both isoforms inhibited late exocytic membrane transport to a similar extent. In addition, both isoforms exhibited the same capacity for dissociating various rab proteins from membranes in vivo. Rab8 was not dissociated by either isoform, whereas rab11 was dissociated to a significant extent. This is perhaps not surprising, because the two GDI proteins displayed an absolute conservation of their rab protein binding domains (Schalk et al., 1996).
Summary
Our data point to an essential function for rab11 in the export of selected molecules from the Golgi, which has not been characterized previously. Rab11 was also revealed as a sensitive target for membrane dissociation in the presence of excess GDI. This is most likely attributed to the longevity of the protein in its GDP-bound form in vivo. Consequently, we would predict that the transport step regulated by rab11 is an important rate-limiting step in intracellular membrane traffic, because active transport depends on the GTP-bound status of the protein at equilibrium.
ACKNOWLEDGMENTS
We extend our sincere thanks to those individuals who kindly provided us with reagents and made this study possible. Hybridomas producing mouse mAbs to VSV G protein were the kind gifts of Drs. Douglas Lyles and Thomas Kreis; Drs. Robert Lamb, Johan Peränen, Suzanne Pfeffer, Kai Simons, and Marino Zerial generously provided antisera and plasmids. We gratefully acknowledge Mary Slater Venkata for expert laboratory management throughout this project. We also thank Drs. Bruno Goud and Nava Segev for communicating their results in advance of publication. Drs. Robert Lamb, Marino Zerial, Bruno Goud, and Nava Segev are thanked for their critical reading of the early drafts of the manuscript. This work was supported by grants from The Council for Tobacco Research, USA (3980) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK50141) and a National Science Foundation Career award (MCB-9507206) to A.W.N. W.C. was supported in part by a Gramm travel fellowship award from the Lurie Cancer Center of Northwestern University. D.C. was supported by the Howard Hughes Medical Institute (HHMI) while a postdoctoral fellow in the laboratory of Dr. Robert Lamb (Investigator, HHMI and Department of Biochemistry, Molecular Biology, and Cell Biology, Northwestern University).
Footnotes
This article is dedicated to the memory of Dr. Thomas Kreis, an innovative, dedicated, and generous colleague, whose productive career was tragically cut short by the September 2, 1998, crash of SwissAir Flight 111.
REFERENCES
- Bachner D, Sedlacek Z, Korn B, Hameister H, Poustka A. Expression patterns of two human genes coding for different rab GDP-dissociation inhibitors (GDIs), extremely conserved proteins involved in cellular transport. Hum Mol Genet. 1995;4:701–708. doi: 10.1093/hmg/4.4.701. [DOI] [PubMed] [Google Scholar]
- Benli M, Döring F, Robinson DG, Yang X, Gallwitz D. Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Brent R, Ptashne M. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell. 1985;43:729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Calhoun BC, Goldenring JR. Rab proteins in gastric parietal cells: evidence for the membrane recycling hypothesis. Yale J Biol Med. 1997;69:1–8. [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Deretic D, Puleo-Scheppke B, Trippe C. Cytoplasmic domain of rhodopsin is essential for post-Golgi vesicle formation in a retinal cell-free system. J Biol Chem. 1996;271:2279–2286. doi: 10.1074/jbc.271.4.2279. [DOI] [PubMed] [Google Scholar]
- Dirac-Svejstrup AB, Soldati T, Shapiro AD, Pfeffer SR. Rab-GDI presents functional Rab9 to the intracellular transport machinery and contributes selectivity to Rab9 membrane recruitment. J Biol Chem. 1994;269:15427–15430. [PubMed] [Google Scholar]
- D’Souza-Schorey C, van Donselaar E, Hsu VW, Yang C, Stahl PD, Peters PJ. ARF6 targets recycling vesicles to the plasma membrane: insights from an ultrastructural investigation. J Cell Biol. 1998;140:603–616. doi: 10.1083/jcb.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazar Z, Mayer T, Rothman JE. Removal of Rab GTP-binding proteins from Golgi membranes by GDP dissociation inhibitor inhibits inter-cisternal transport in the Golgi stacks. J Biol Chem. 1994;269:794–797. [PubMed] [Google Scholar]
- Estojak J, Brent R, Golemis EA. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- Gallione CJ, Rose JK. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J Virol. 1985;54:374–382. doi: 10.1128/jvi.54.2.374-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett MD, Zahner JE, Cheney CM, Novick PJ. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994;13:1718–1728. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring JR, Bhartur S, Navarre J, Wang X, Casanova J. Rabb11 and Rab25 localize to the apical recycling system in MDCK Cells. Mol Biol Cell. 1997;8:408a. doi: 10.1091/mbc.10.1.47. (abstract). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol. 1996;270:G515–G525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- Golemis EA, Brent R. Fused protein domains inhibit DNA binding by LexA. Mol Cell Biol. 1992;12:3006–3014. doi: 10.1128/mcb.12.7.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson E, Wandinger-Ness A, Gausepohl H, Griffiths G, Simons K. The cell biology of enveloped virus infection of epithelial tissues. In: Schwartz M, editor. Centenary Symposium of the Pastuer Institute. Paris: Elsevier; 1988. pp. 75–89. [Google Scholar]
- Ikonen E, Tagaya M, Ullrich O, Montecucco C, Simons K. Different requirements for NSF, SNAP, and Rab proteins in apical and basolateral transport in MDCK cells. Cell. 1995;81:571–580. doi: 10.1016/0092-8674(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Janoueix-Lerosey I, Jollivet F, Camonis J, Marche PN, Goud B. Two-hybrid system screen with the small GTP-binding protein Rab6. Identification of a novel mouse GDP dissociation inhibitor isoform and two other potential partners of Rab6. J Biol Chem. 1995;270:14801–14808. doi: 10.1074/jbc.270.24.14801. [DOI] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Richardson C, Litt R, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J Cell Biol. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE, Lodish HF. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986;46:929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Stubbs L, Artzt K. Molecular analysis of mouse Rab11b: a new type of mammalian YPT/Rab protein. Genomics. 1994;22:610–616. doi: 10.1006/geno.1994.1434. [DOI] [PubMed] [Google Scholar]
- Lefrancois L, Lyles DS. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982;121:157–167. [PubMed] [Google Scholar]
- Leitinger B, Hille-Rehfeld A, Spiess M. Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc Natl Acad Sci USA. 1995;92:10109–10113. doi: 10.1073/pnas.92.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Qian R, Rausa FMR, Colley KJ. Two naturally occurring alpha2,6-sialyltransferase forms with a single amino acid change in the catalytic domain differ in their catalytic activity and proteolytic processing. J Biol Chem. 1997;272:672–679. doi: 10.1074/jbc.272.1.672. [DOI] [PubMed] [Google Scholar]
- Martinez O, Schmidt A, Salamero J, Hoflack B, Roa M, Goud B. The small GTP-binding protein rab6 functions in intra-Golgi transport. J Cell Biol. 1994;127:1575–1588. doi: 10.1083/jcb.127.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin KS, Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983;34:233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Ngsee JK, Elferink LA, Scheller RH. A family of ras-like GTP-binding proteins expressed in electromotor neurons. J Biol Chem. 1991;266:2675–2680. [PubMed] [Google Scholar]
- Nishimura N, Nakamura H, Takai Y, Sano K. Molecular cloning and characterization of two rab GDI species from rat brain: brain-specific and ubiquitous types. J Biol Chem. 1994;269:14191–14198. [PubMed] [Google Scholar]
- Novick P, Zerial M. The divertsity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Peränen J, Auvinen P, Virta H, Wepf R, Simons K. Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J Cell Biol. 1996;135:153–167. doi: 10.1083/jcb.135.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter F, Nuoffer C, Pind SN, Balch WE. Guanine nucleotide dissociation inhibitor is essential for Rab1 function in budding from the endoplasmic reticulum and transport through the Golgi stack. J Cell Biol. 1994;126:1393–1406. doi: 10.1083/jcb.126.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR, Dirac-Svejstrup AB, Soldati T. Rab GDP dissociation inhibitor: putting rab GTPases in the right place. J Biol Chem. 1995;270:17057–17059. doi: 10.1074/jbc.270.29.17057. [DOI] [PubMed] [Google Scholar]
- Plutner H, Cox AD, Pind S, Khosravi-Far R, Bourne JR, Schwaninger R, Der CJ, Balch WE. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115:31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes [in process citation] Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer MA, Soldati T, Shapiro AD, Lin J, Pfeffer SR. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J Cell Biol. 1994;125:573–582. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980;20:45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rybin V, Ullrich O, Rubino M, Alexandrov K, Simon I, Seabra C, Goody R, Zerial M. GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature. 1996;383:266–269. doi: 10.1038/383266a0. [DOI] [PubMed] [Google Scholar]
- Sakurada K, et al. Molecular cloning and characterization of a ras p21-like GTP-binding protein (24KG) from rat liver. Biochem Biophys Res Commun. 1991;177:1224–1232. doi: 10.1016/0006-291x(91)90672-t. [DOI] [PubMed] [Google Scholar]
- Sariola M, Saraste J, Kuismanen E. Communication of post-Golgi elements with early endocytic pathway: regulation of endoproteolytic cleavage of Semliki Forest virus p62 precursor. J Cell Sci. 1995;108:2465–2475. doi: 10.1242/jcs.108.6.2465. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kikuchi A, Araki S, Hata Y, Isomura M, Kuroda S, Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265:2333–2337. [PubMed] [Google Scholar]
- Satoh T, Endo M, Nakafuku M, Nakamura S, Kaziro Y. Platelet-derived growth factor stimulates formation of active p21ras. GTP complex in Swiss mouse 3T3 cells. Proc Natl Acad Sci USA. 1990;87:5993–5997. doi: 10.1073/pnas.87.15.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Endo M, Nakamura S, Kaziro Y. Analysis of guanine nucleotide bound to ras protein in PC12 cells. FEBS Lett. 1988;236:185–189. doi: 10.1016/0014-5793(88)80311-9. [DOI] [PubMed] [Google Scholar]
- Schalk I, Zeng K, Wu SK, Stura EA, Matteson J, Huang M, Tandon A, Wilson IA, Balch WE. Structure and mutational analysis of Rab GDP-dissociation inhibitor. Nature. 1996;381:42–48. doi: 10.1038/381042a0. [DOI] [PubMed] [Google Scholar]
- Shapiro AD, Pfeffer SR. Quantitative analysis of the interactions between prenyl Rab9, GDP dissociation inhibitor-alpha, and guanine nucleotides. J Biol Chem. 1995;270:11085–11090. doi: 10.1074/jbc.270.19.11085. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Ray GS, Calhoun BC, Goldenring JR. A somatodendritic distribution of Rab11 in rabbit brain neurons. NeuroReport. 1996;7:1297–1300. doi: 10.1097/00001756-199605170-00016. [DOI] [PubMed] [Google Scholar]
- Shisheva A, Buxton J, Czech MP. Differential intracellular localizations of GDP dissociation inhibitor isoforms. Insulin-dependent redistribution of GDP dissociation inhibitor-2 in 3T3–L1 adipocytes. J Biol Chem. 1994a;269:23865–23868. [PubMed] [Google Scholar]
- Shisheva A, Doxsey SJ, Buxton JM, Czech MP. Pericentriolar targeting of GDP-dissociation inhibitor isoform 2. Eur J Cell Biol. 1995;68:143–158. [PubMed] [Google Scholar]
- Shisheva A, Südhof TC, Czech MP. Cloning, characterization, and expression of a novel GDP dissociation inhibitor isoform from skeletal muscle. Mol Cell Biol. 1994b;14:3459–3468. doi: 10.1128/mcb.14.5.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon I, Zerial M, Goody RS. Kinetics of interaction of Rab5 and Rab7 with nucleotides and magnesium ions. J Biol Chem. 1996;271:20470–20478. doi: 10.1074/jbc.271.34.20470. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieneke-Gröber A, Vey M, Angliker H, Shaw E, Thomas G, Roberts C, Klenk HD, Garten W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 1992;11:2407–2414. doi: 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Lamb RA. Influenza virus M2 protein ion channel activity stabilizes the native form of fowl plague virus hemagglutinin during intracellular transport. J Virol. 1994;68:911–919. doi: 10.1128/jvi.68.2.911-919.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbé S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Stenmark H, Alexandrov K, Huber LA, Kaibuchi K, Sasaki T, Takai Y, Zerial M. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J Biol Chem. 1993;268:18143–18150. [PubMed] [Google Scholar]
- Urbé S, Huber LA, Zerial M, Tooze SA, Parton RG. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- Urbé S, Parton RG. Rab11. In: Zerial M, Huber LA, editors. Guidebook to the Small GTPases. Oxford: Oxford University Press; 1995. pp. 345–347. [Google Scholar]
- Volknandt W, Pevsner J, Elferink LA, Scheller RH. Association of three small GTP-binding proteins with cholinergic synaptic vesicles. FEBS Lett. 1993;317:53–56. doi: 10.1016/0014-5793(93)81490-q. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RC, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A, Bennett MK, Antony C, Simons K. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990;111:987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt MA, Chong L, Rose JK. Glycoprotein cytoplasmic domain sequences required for rescue of a vesicular stomatitis virus glycoprotein mutant. J Virol. 1989;63:3569–3578. doi: 10.1128/jvi.63.9.3569-3578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Slepnev VI, Goud B. Rab proteins form in vivo complexes with two isoforms of the GDP-dissociation inhibitor protein (GDI) J Biol Chem. 1994;269:31891–31899. [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, Huber LA. The rab subfamily. In: Zerial M, Huber LA, editors. Guidebook to the Small GTPases. Oxford: Oxford University Press; 1995. pp. 295–373. [Google Scholar]
- Zervos AS, Gyuris J, Brent R. Mxi1, a protein that specifically interacts with Max to bind Myc-Max recognition sites. Cell. 1994;72:223–232. doi: 10.1016/0092-8674(93)90662-a. [DOI] [PubMed] [Google Scholar]