Abstract
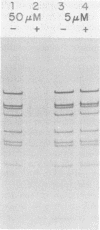
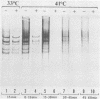
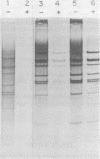
Aphidicolin is a highly specific inhibitor of DNA polymerase α and has been most useful for assessing the role of this enzyme in various replication processes (J. A. Huberman, Cell 23:647-648, 1981). Both nuclear DNA replication and simian virus 40 DNA replication are highly sensitive to this drug (Krokan et al., Biochemistry 18:4431-4443, 1979), whereas mitochondrial DNA synthesis is completely insensitive (Zimmerman et al., J. Biol. Chem. 255:11847-11852, 1980). Adenovirus DNA replication is sensitive to aphidicolin, but only at much higher concentrations. These patterns of sensitivity are seen both in vivo and in vitro (Krokan et al., Biochemistry 18:4431-4443, 1979). A temperature-sensitive mutant of adenovirus type 5 known as H5ts125 is able to complete but not initiate new rounds of replication at nonpermissive temperatures (P. C. van der Vliet and J. S. Sussenbach, Virology 67:415-426, 1975). When cells infected with H5ts125 were shifted from permissive (33°C) to nonpermissive (41°C) conditions, the residual DNA synthesis (elongation) showed a striking increase in sensitivity to aphidicolin. The temperature-sensitive mutation of H5ts125 is in the gene for the 72-kilodalton single-stranded DNA-binding protein. This demonstrated that the increased resistance to aphidicolin shown by adenovirus DNA replication was dependent on that protein. It also supports an elongation role for both DNA polymerase α and the 72-kilodalton single-stranded DNA-binding protein in adenovirus DNA replication. Further support for an elongation role of DNA polymerase α came from experiments with permissive temperature conditions and inhibiting levels of aphidicolin in which it was shown that newly initiated strands failed to elongate to completion.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arens M., Yamashita T., Padmanabhan R., Tsuruo T., Green M. Adenovirus deoxyribonucleic acid replication. Characterization of the enzyme activities of a soluble replication system. J Biol Chem. 1977 Nov 25;252(22):7947–7954. [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Ostrove J. M., Kelly T. J., Jr Initiation of adenovirus DNA replication: detection of covalent complexes between nucleotide and the 80-kilodalton terminal protein. J Virol. 1982 Jan;41(1):265–270. doi: 10.1128/jvi.41.1.265-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zubay G., Ginsberg H. S. The replication pattern of adenovirus DNA in vivo reproduced in vitro. Eur J Biochem. 1980 Mar;104(2):587–594. doi: 10.1111/j.1432-1033.1980.tb04462.x. [DOI] [PubMed] [Google Scholar]
- Eichelman B., Solomon J. K., Qureshi A. A. L-histidine-induced suppression of lipogenic enzymes. Biochem Biophys Res Commun. 1978 Jun 14;82(3):1034–1039. doi: 10.1016/0006-291x(78)90887-2. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Lichy J. H., Ikeda J. E., Hurwitz J. Adenovirus DNA replication in vitro: purification of the terminal protein in a functional form. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6779–6783. doi: 10.1073/pnas.78.11.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Berget S. M., Sharp P. A. Characterization of single-stranded viral DNA sequences present during replication of adenovirus types 2 and 5. Cell. 1976 Dec;9(4 Pt 1):559–571. doi: 10.1016/0092-8674(76)90038-6. [DOI] [PubMed] [Google Scholar]
- Foster D. A., Spangler R., Zubay G. Inhibition of host DNA synthesis by adenovirus infection is reversed at elevated temperatures. J Virol. 1981 Jan;37(1):493–495. doi: 10.1128/jvi.37.1.493-495.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S. Temperature-sensitive replication of H5ts125 adenovirus DNA in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4291–4295. doi: 10.1073/pnas.75.9.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Ikeda J. E., Enomoto T., Hurwitz J. Replication of adenovirus DNA-protein complex with purified proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):884–888. doi: 10.1073/pnas.78.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- Krokan H., Wist E., Krokan R. H. Aphidicolin inhibits DNA synthesis by DNA polymerase alpha and isolated nuclei by a similar mechanism. Nucleic Acids Res. 1981 Sep 25;9(18):4709–4719. doi: 10.1093/nar/9.18.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwant M. M., van der Vliet P. C. Differential effect of aphidicolin on adenovirus DNA synthesis and cellular DNA synthesis. Nucleic Acids Res. 1980 Sep 11;8(17):3993–4007. doi: 10.1093/nar/8.17.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro M., Suzuki-Hori C., Nagano H., Mano Y., Ikegami S. The mode of inhibitory action by aphidicolin on eukaryotic DNA polymerase alpha. Eur J Biochem. 1979 Jul;97(2):603–607. doi: 10.1111/j.1432-1033.1979.tb13149.x. [DOI] [PubMed] [Google Scholar]
- Pincus S., Robertson W., Rekosh D. Characterization of the effect of aphidicolin on adenovirus DNA replication: evidence in support of a protein primer model of initiation. Nucleic Acids Res. 1981 Oct 10;9(19):4919–4938. doi: 10.1093/nar/9.19.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. Studies on the mechanism of replication of adenovirus DNA. V. The location of termini of replication. Virology. 1977 Mar;77(1):149–157. doi: 10.1016/0042-6822(77)90414-7. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. The mechanism of replication of adenovirus DNA. VI. Localization of the origins of the displacement synthesis. Virology. 1978 Feb;84(2):509–517. doi: 10.1016/0042-6822(78)90266-0. [DOI] [PubMed] [Google Scholar]
- Van der Vliet P. C., Zandberg J., Jansz H. S. Evidence for a function of the adenovirus DNA-binding protein in initiation in DNA synthesis as well as in elongation of nascent DNA chains. Virology. 1977 Jul 1;80(1):98–110. doi: 10.1016/0042-6822(77)90383-x. [DOI] [PubMed] [Google Scholar]
- Vliet P. C., Sussenbach J. S. An adenovirus type 5 gene function required for initiation of viral DNA replication. Virology. 1975 Oct;67(2):415–426. doi: 10.1016/0042-6822(75)90443-2. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Chen S. M., Bolden A., Weissbach A. Mitochondrial DNA replication does not involve DNA polymerase alpha. J Biol Chem. 1980 Dec 25;255(24):11847–11852. [PubMed] [Google Scholar]
- van der Vliet P. C., Kwant M. M. Role of DNA polymerase gamma in adenovirus DNA replication. Nature. 1978 Nov 30;276(5687):532–534. doi: 10.1038/276532a0. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Bouché J. P., Méchali M., Girard M. Involvement of both DNA polymerases alpha and gamma in the replication of adenovirus deoxyribonucleic acid in vitro. Virology. 1980 Jul 15;104(1):56–72. doi: 10.1016/0042-6822(80)90365-7. [DOI] [PubMed] [Google Scholar]