Abstract
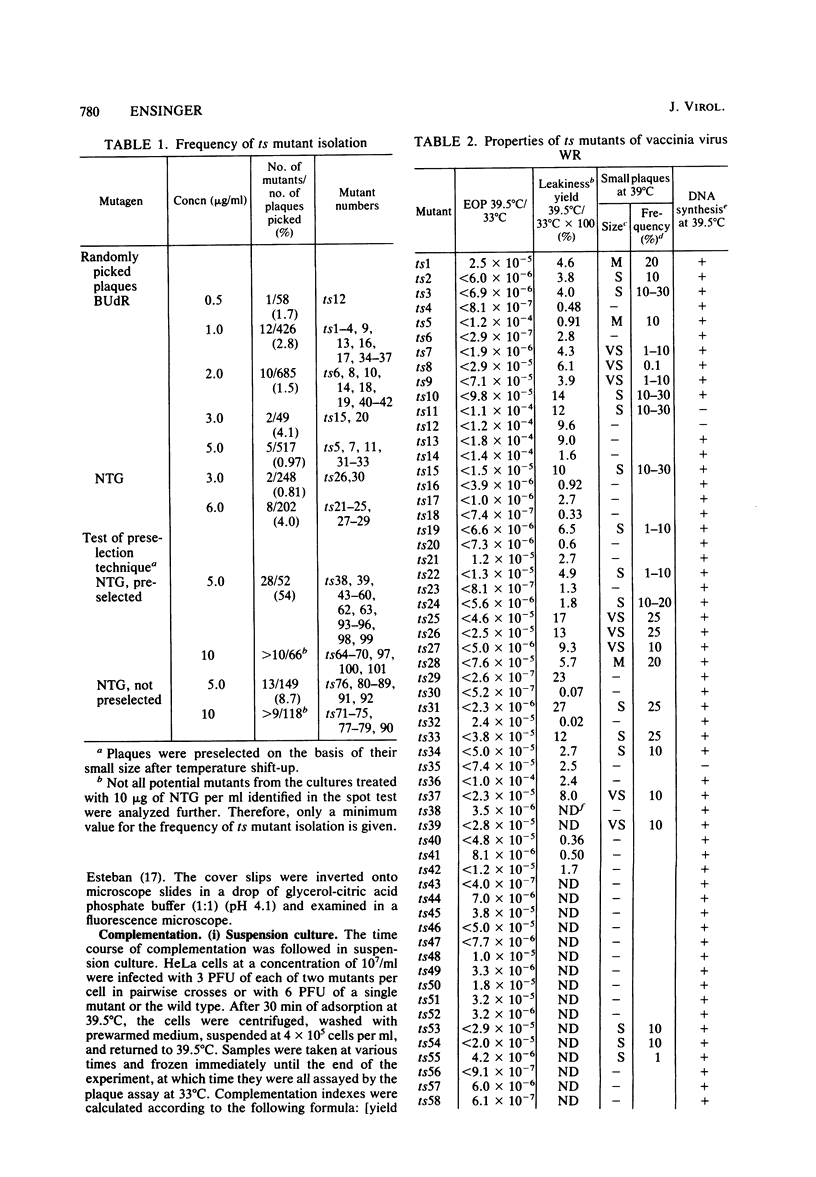
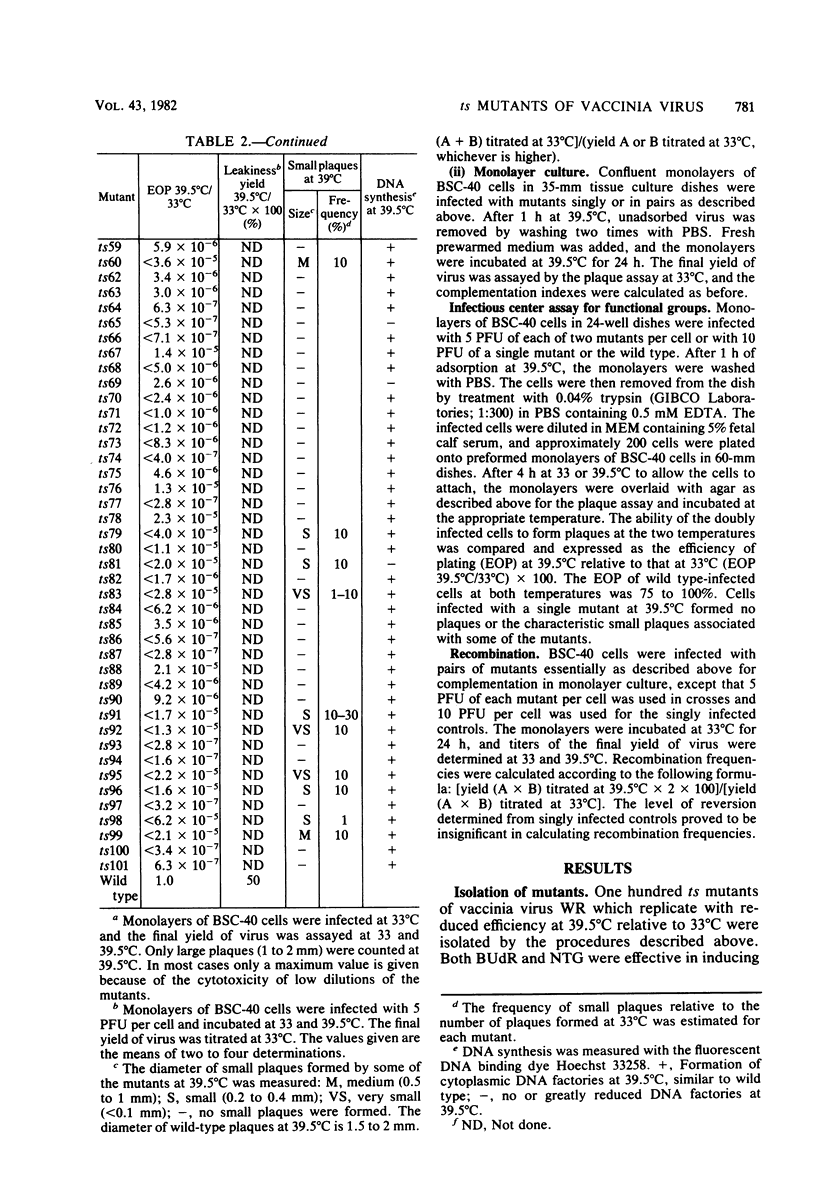
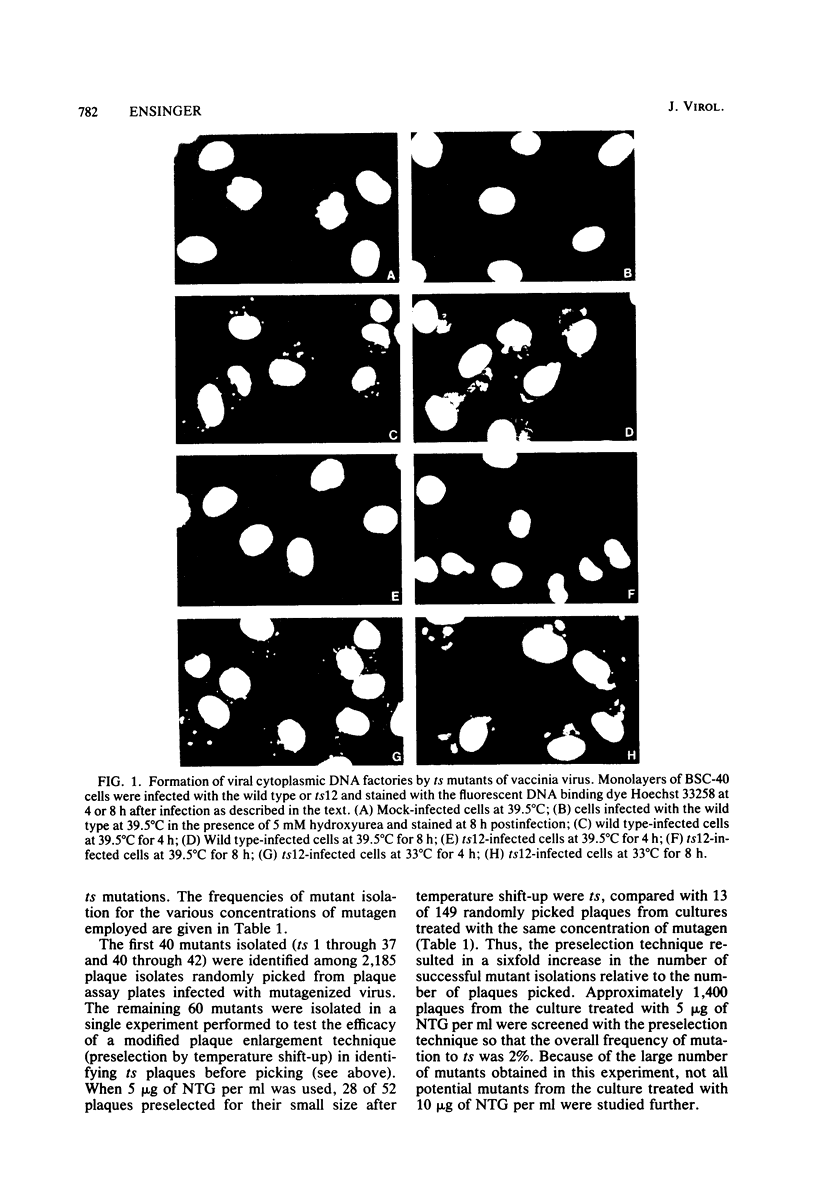
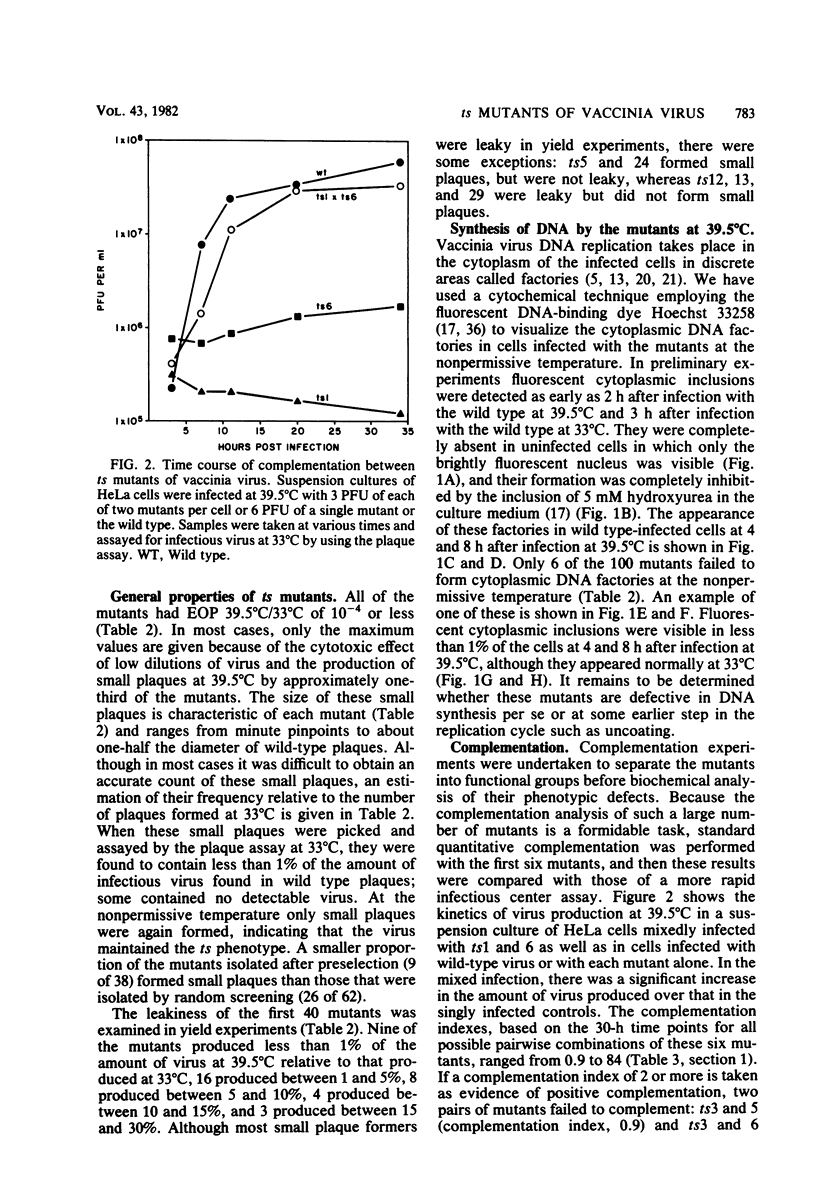
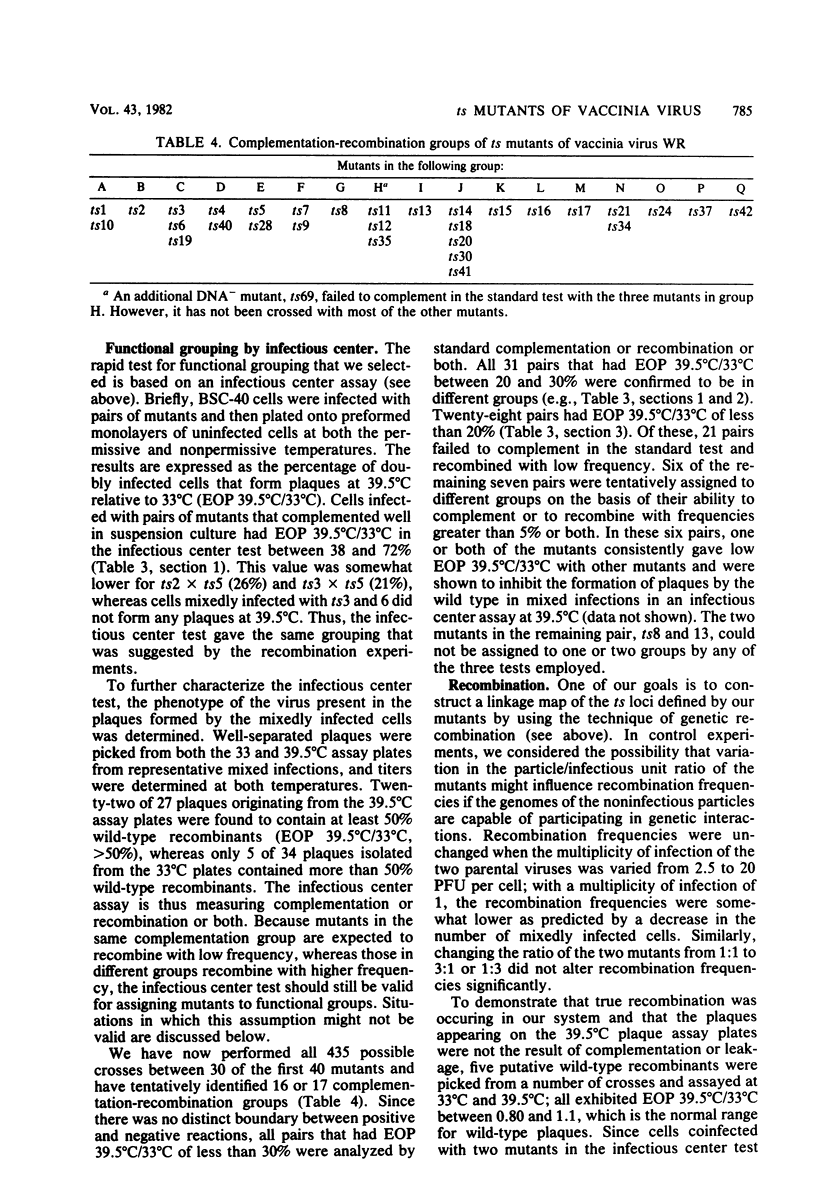
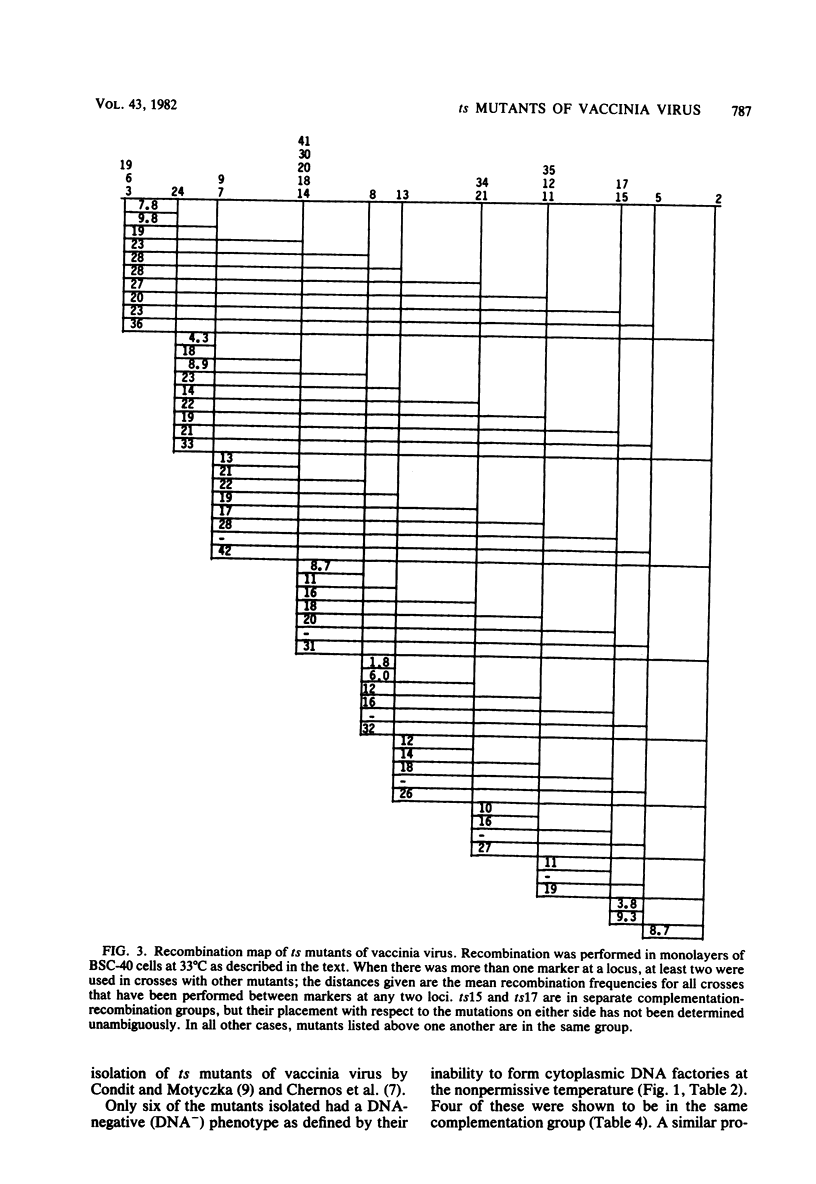
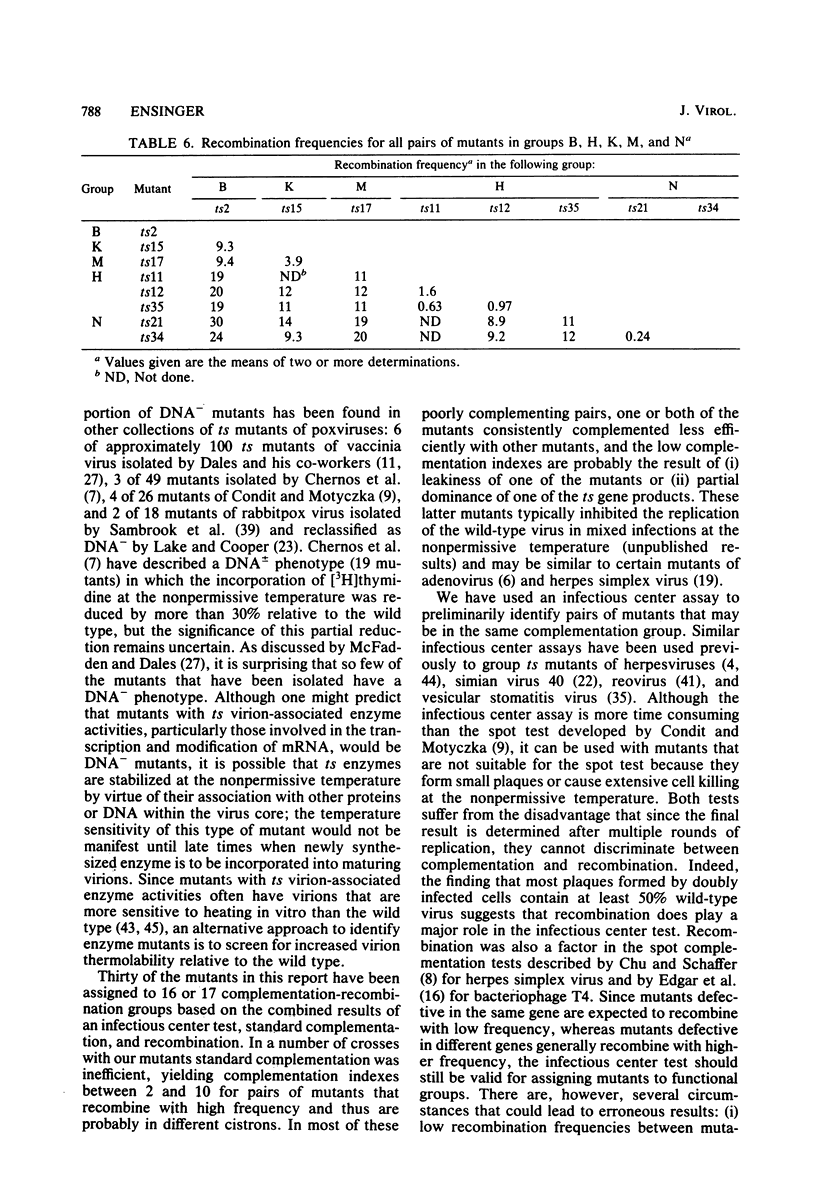
One hundred temperature-sensitive mutants of vaccinia virus WR were isolated from virus that had been mutagenized with 5-bromodeoxyuridine or N-methyl-N'-nitro-N-nitrosoguanidine. A rapid screening procedure based on the ability of vaccinia virus to form plaques under liquid overlay medium was used to identify potential mutants among randomly picked plaque isolates or plaques preselected for their small size after temperature shift-up. The preselection technique resulted in a sixfold increase in the number of successful mutant isolations relative to the number of plaques picked. All of the mutants had efficiencies of plating at 39.5 degrees C relative to that at 33 degrees C of 10(-4) or less, and 33 of 40 produced 10% or less of the amount of virus at the nonpermissive temperature (39.5 degrees C) relative to that at the permissive temperature (33 degrees C). Experiments with the fluorescent DNA binding dye Hoechst 33258 demonstrated that 6 of the 100 mutants failed to form characteristic cytoplasmic DNA factories at 39.5 degrees C. To facilitate the functional grouping of such a large number of mutants, a rapid infectious center assay was developed. Thirty of the mutants were assigned to 16 or 17 complementation-recombination groups by using this assay. Recombination experiments have allowed the construction of a genetic map representing 22 mutants in 12 of these groups.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroudy B. M., Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. J Biol Chem. 1980 May 10;255(9):4372–4380. [PubMed] [Google Scholar]
- Boone R. F., Moss B. Sequence complexity and relative abundance of vaccinia virus mRNA's synthesized in vivo and in vitro. J Virol. 1978 Jun;26(3):554–569. doi: 10.1128/jvi.26.3.554-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W., Nathans D. The isolation of simian virus 40 variants with specifically altered genomes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):942–946. doi: 10.1073/pnas.71.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- Chernos V. I., Belanov E. F., Vasilieva N. N. Temperature-sensitive mutants of vaccinia virus. I. Isolation and preliminary characterization. Acta Virol. 1978 Mar;22(2):81–90. [PubMed] [Google Scholar]
- Chu C. T., Schaffer P. A. Qualitative complementation test for temperature-sensitive mutants of herpes simplex virus. J Virol. 1975 Nov;16(5):1131–1136. doi: 10.1128/jvi.16.5.1131-1136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. Transcription of vaccinia virus mRNA coupled to translation in vitro. Virology. 1978 Jul 1;88(1):149–165. doi: 10.1016/0042-6822(78)90118-6. [DOI] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Drillien R., Koehren F., Kirn A. Host range deletion mutant of vaccinia virus defective in human cells. Virology. 1981 Jun;111(2):488–499. doi: 10.1016/0042-6822(81)90351-2. [DOI] [PubMed] [Google Scholar]
- Drillien R., Tripier F., Koehren F., Kirn A. A temperature-sensitive mutant of vaccinia virus defective in an early stage of morphogenesis. Virology. 1977 Jun 15;79(2):369–380. doi: 10.1016/0042-6822(77)90364-6. [DOI] [PubMed] [Google Scholar]
- EDGAR R. S., DENHARDT G. H., EPSTEIN R. H. A COMPARATIVE GENETIC STUDY OF CONDITIONAL LETHAL MUTATIONS OF BACTERIOPHAGE T4D. Genetics. 1964 Apr;49:635–648. doi: 10.1093/genetics/49.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban M. Rifampin and vaccinia DNA. J Virol. 1977 Feb;21(2):796–801. doi: 10.1128/jvi.21.2.796-801.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Control of expression of the vaccinia virus thymidine kinase gene. J Virol. 1981 Nov;40(2):456–464. doi: 10.1128/jvi.40.2.456-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre J. T., Courtney R. J., Schaffer P. A. A dominant lethal temperature-sensitive mutant of herpes simplex virus type 1. Virology. 1981 May;111(1):173–190. doi: 10.1016/0042-6822(81)90663-2. [DOI] [PubMed] [Google Scholar]
- KATO S., OGAWA M., MIYAMOTO H. NUCLEOCYTOPLASMIC INTERACTION IN POXVIRUS-INFECTED CELLS. I. RELATIONSHIP BETWEEN INCLUSION FORMATION AND DNA METABOLISM OF THE CELLS. Biken J. 1964 Jul;7:45–56. [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lake J. R., Cooper P. D. Synthesis of virus DNA and polypeptides by temperature-sensitive mutants of rabbitpox virus. J Gen Virol. 1980 Apr;47(2):243–259. doi: 10.1099/0022-1317-47-2-243. [DOI] [PubMed] [Google Scholar]
- Lake J. R., Silver M., Dales S. Biogenesis of vaccinia: complementation and recombination analysis of one group of conditional-lethal mutants defective in envelope self-assembly. Virology. 1979 Jul 15;96(1):9–20. doi: 10.1016/0042-6822(79)90167-3. [DOI] [PubMed] [Google Scholar]
- Mackett M., Archard L. C. Conservation and variation in Orthopoxvirus genome structure. J Gen Virol. 1979 Dec;45(3):683–701. doi: 10.1099/0022-1317-45-3-683. [DOI] [PubMed] [Google Scholar]
- McFadden G., Dales S. Biogenesis of poxviruses: preliminary characterization of conditional lethal mutants of vaccinia virus defective in DNA synthesis. Virology. 1980 May;103(1):68–79. doi: 10.1016/0042-6822(80)90126-9. [DOI] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Nakano E., Panicali D., Paoletti E. Molecular genetics of vaccinia virus: demonstration of marker rescue. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1593–1596. doi: 10.1073/pnas.79.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Grady L. J. Transcriptional complexity of vaccinia virus in vivo and in vitro. J Virol. 1977 Sep;23(3):608–615. doi: 10.1128/jvi.23.3.608-615.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Dumont R., Baltimore D. Screening procedure for complementation-dependent mutants of vesicular stomatitis virus. J Virol. 1975 Jan;15(1):41–49. doi: 10.1128/jvi.15.1.41-49.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. Viral protein and DNA synthesis in vaccinia virus-infected HeLacell cultures. Virology. 1963 Apr;19:542–550. doi: 10.1016/0042-6822(63)90049-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F., Padgett B. L., Tomkins J. K. Conditional lethal mutants of rabbitpox virus. I. Isolation of host cell-dependent and temperature-dependent mutants. Virology. 1966 Apr;28(4):592–599. doi: 10.1016/0042-6822(66)90244-3. [DOI] [PubMed] [Google Scholar]
- Schümperli D., Menna A., Schwendimann F., Wittek R., Wyler R. Symmetrical arrangement of the heterologous regions of rabbit poxvirus and vaccinia virus DNA. J Gen Virol. 1980 Apr;47(2):385–398. doi: 10.1099/0022-1317-47-2-385. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Infectious center assay for complementation and recombination between mutant of reovirus. J Virol. 1976 Jun;18(3):1151–1154. doi: 10.1128/jvi.18.3.1151-1154.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E., Shuman S., Hurwitz J. Purification and properties of vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1980 Jun 10;255(11):5388–5395. [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutations on the virion-associated RNA transcriptase of vesicular stomatitis virus. J Mol Biol. 1972 Nov 14;71(2):281–291. doi: 10.1016/0022-2836(72)90351-8. [DOI] [PubMed] [Google Scholar]
- Timbury M. C. Temperature-sensitive mutants of herpes simplex virus type 2. J Gen Virol. 1971 Nov;13(2):373–376. doi: 10.1099/0022-1317-13-2-373. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Varmus H. E., Hunter E. Characterization of "early" temperature-sensitive mutants of avian sarcoma viruses: biological properties, thermolability of reverse transcriptase in vitro, and synthesis of viral DNA in infected cells. Virology. 1976 Oct 1;74(1):16–29. doi: 10.1016/0042-6822(76)90124-0. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Menna A., Müller H. K., Schümperli D., Boseley P. G., Wyler R. Inverted terminal repeats in rabbit poxvirus and vaccinia virus DNA. J Virol. 1978 Oct;28(1):171–181. doi: 10.1128/jvi.28.1.171-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Menna A., Schümperli D., Stoffel S., Müller H. K., Wyler R. HindIII and Sst I restriction sites mapped on rabbit poxvirus and vaccinia virus DNA. J Virol. 1977 Sep;23(3):669–678. doi: 10.1128/jvi.23.3.669-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]