Abstract
Lentiviral encephalitis has been hypothesized to be associated with altered monocyte migration into the brain. CD14hi/CD16lo and CD14lo/CD16hi monocytes were expanded during acute infection; however, this expansion was not unique or greater in macaques that developed encephalitis. The proportion of monocytes that expressed CD62L, HLA-DR, CD16, CD64, and CD40 varied during the course of infection in macaques that eventually developed encephalitis. Taken together, these results suggest that changes in the proportion of circulating activated monocytes are not predictive of development of encephalitis, but this does not rule out the importance of activated monocytes in the development of encephalitis.
Keywords: Simian immunodeficiency virus, Encephalitis, Monocytes, Activation markers, Cluster of differentiation, Flow cytometry
1. Introduction
Twenty-five percent of HIV-infected patients develop a clinical syndrome known as HIV-associated dementia (HAD) (Adamson et al., 1999; Brew, 1999; Cinque et al., 1997; Dalpan et al., 1998; Dore et al., 1999; McArthur et al., 1997). In the absence of opportunistic infections, HIV encephalitis (HIVE) is the pathological correlate of HAD. Within the central nervous system (CNS), the predominant infected cell in HIVE is the macrophage (Fauci, 1988; Griffin et al., 1994; Merrill and Chen, 1991; Pulliam et al., 1991; Wiley et al., 1986). Although virus can be detected in the CNS soon after infection (Davis et al., 1992), dementia and encephalitis develop late in disease when the patient is severely immunosuppressed (Brew, 1999). Development of encephalitis is thought to be the result of activated infected blood monocytes trafficking into the CNS.
By migrating from blood into tissue, monocytes differentiate into macrophages (van Furth, 1992). While trafficking of activated T cells into the CNS has been studied in a variety of diseases, less is known about monocyte trafficking. Migration of activated T cells is directed by tissue specific chemokines and integrin receptors (Ebert et al., 2005). Tcells found in CSF express CXCR3, CCR5, and CCR6 (Kivisakk et al., 2002), and inflammatory chemokine CXCR3 is thought to be involved in T cell accumulation in the CNS (Trebst et al., 2003). Little is known about the normal turnover of CNS macrophages (microglia) (Williams and Hickey, 1995) or how this might be augmented during disease (Hume et al., 2002).
It is reasonable to speculate that migration of activated monocytes is regulated by expression of integrins such as LFA-1 and VLA-4 and chemokine receptors. Monocyte integrins bind to endothelial cell adhesion molecules ICAM-1 and VCAM-1 initiating adherence followed by migration into tissue (Issekutz and Issekutz, 1995; Shang and Issekutz, 1998). It is largely unknown how monocytes cross the brain endothelium, but it is believed that that VLA-4/VCAM and PECAM/PECAM interactions are important in facilitating transmigration (Chuluyan and Issekutz, 1993; Meerschaert and Furie, 1994; Nottet et al., 1996; Sasseville et al., 1994; Verdegaal et al., 1993). Monocyte α4β1 integrin and endothelial VCAM-1 interactions are thought to be necessary for transmigration into the CNS during simian immunodeficiency virus (SIV) encephalitis (Sasseville et al., 1994). Recent reports indicate that monocytes express high amounts of monocyte chemotactic protein-1 receptor CCR2 (Kaufmann et al., 2001). Upon differentiation into macrophages, CCR2 expression progressively decreases and CCR1 and CCR5 expression progressively increases (Kaufmann et al., 2001). In multiple sclerosis, reports suggest CCR1+/CCR5+/CD14+ cells are the sub-population of monocytes able to enter the inflamed CNS (Trebst et al., 2001). CCR1 is thought to play an integral role in the migration of monocytes into the CNS (Trebst et al., 2003).
Blood monocytes can be categorized on the basis of CD14 and CD16 expression with CD14hiCD16− monocytes as the major population and CD14+CD16+ monocytes as the minor population (Ziegler-Heitbrock, 2000). Cross-linking of immunoglobulin receptors expressed on monocytes can either inhibit or activate monocytes. Binding of FcgammaIIb inhibits monocytes while activation of FcgammaRIIa, FcgmmaRI (CD64), and FcgammaRIII (CD16) activates monocytes (Clynes et al., 1999; Gerber and Mosser, 2001; Tridandapani et al., 2002). Activation of monocytes by CD16 binding IgG immune complexes initiates cellular responses such as phagocytosis, antibody-dependent cellular cytotoxicity, and release of inflammatory molecules such as cytokines (Gerber and Mosser, 2001).
It has been shown that CD14+CD16+ cells are expanded during pro-inflammatory conditions such as infectious diseases (Ziegler-Heitbrock, 2000), including bacterial infections (Katayama et al., 2000), and other disorders such as patients with coronary artery disease (Schlitt et al., 2004), rheumatoid arthritis (Kawanaka et al., 2002), acute Kawasaki disease (Katayama et al., 2000), and asthma (Rivier et al., 1995). This minor monocyte population is also increased within minutes after exercising (Gabriel et al., 1994). HIV-infected patients also have increased percentages of CD14lo/CD16+ cells (Dunne et al., 1996; Thieblemont et al., 1995), and patients with HAD have been reported to show increased proportions of CD14+CD16+ and CD14+CD69+ blood monocytes (Kusdra et al., 2002; Pulliam et al., 1997).
Since lentiviral infection of the brain has been hypothesized to be associated with augmented monocyte migration into the CNS and an increased proportion of blood monocytes that express CD16 and CD69 has been reported in patients with HAD, we prospectively analyzed the percent expression and mean fluorescent intensity (MFI) of a panel of phenotypic markers on CD14+ monocytes during disease progression using a primate model. Blood monocytes from SIV-infected macaques that did or did not develop encephalitis (determined retrospectively) were analyzed for CD16, CCR5, CD69, HLA-DR, CD62L, CD40, CD64, and CD163 expression. We hypothesized that macaques that would develop encephalitis would have an increased proportion of monocytes expressing these activation markers compared to macaques that would not develop encephalitis during the course of infection.
2. Materials and methods
2.1. Monkeys and blood
Six male pigtailed macaques (Macaca nemestrina) were inoculated intravenously with the viral swarm SIVDeltaB670. Whole blood was collected from each monkey in heparinized tubes once weekly through the first month of infection and every 2 weeks thereafter. Macaque activity, appetite, stool consistency, and general condition were monitored daily. Physical examinations were performed at each blood draw. Examinations consisted of body temperature and weight measurements, palpation and size grading of lymph nodes and spleen, abdominal palpation, and assessment of general condition. Macaques were provided full supportive care and humanely euthanized when they developed clinical AIDS. A macaque is considered to have terminal clinical AIDS when either systemic or neuropathologic SIV infection has progressed to the end stage and is non-responsive to treatment as determined by clinical examination (e.g. increased body temperature, 20% or greater sustained weight loss, anorexia, lymphadenopathy, splenomegaly, lethargy and other changes in activity, diarrhea, opportunistic infections, and changes in overall condition), peripheral blood analysis (e.g. complete blood counts/differentials), and T cell subset changes. Treatments were conservative, minimally invasive and mainly limited to sedation during procedures and, if necessary, antibiotics to control diarrhea or skin infections at the end stages of disease.
2.2. Pathological assessment of SIV encephalitis
Formalin-fixed coronal sections of macaque brains were examined for the presence of SIV encephalitis. Paraffin sections containing mid-frontal, parietal and occipital cortical gray and white matter, corpus callosum, caudate, putamen, globus pallidus, thalamus, hippocampal formation, brain stem, cerebellum, leptomeninges, and choroid plexus were stained with hematoxylin and eosina, macrophages (CD68; clone KP1; DakoCytomation) and SIV Envelope protein gp110 (generously provided by Dr. Kelly Stefano Cole and Dr. Ron Montelaro, University of Pittsburgh, Pittsburgh, PA) (Bissel et al., 2002). Sections were examined microscopically and the presence of encephalitis was scored on a scale of 0, 1, 2, 3 in three different brain areas (deep gray matter, deep white matter, central gray matter) according to the abundance of microglial nodules, multinucleated giant cells, perivascular chronic inflammation, and SIV-infected macrophages. Nonencephalitic animals have a score of 0 for all three areas; while encephalitic animals score 2 or greater for at least one area. All brain regions were examined for the presence of opportunistic agents.
2.3. Preparation of peripheral blood monocytes for flow cytometry
100 μL whole heparinized blood was incubated with fluorescein isothiocyanate (FITC)-conjugated anti-CD14, clone RM052 (Beckman Coulter, Hialeah, FL), phycoerythrin (PE)-Cy5-conjugated anti-CD16, clone 3G8 (Beckman Coulter), and one of the following: PE-conjugated anti-human CCR5, clone 3A9 (BD PharMingen, San Diego, CA), CD69, clone TP1.55.3 (Beckman Coulter), HLA-DR, clone G46-6 (BD PharMingen), CD62L, clone SK11 (BD PharMingen), CD64, clone 22 (Beckman Coulter), CD40, clone MAB89 (Beckman Coulter), CD163, clone GHI/61 (BD PharMingen) for 30 min, 4 °C. Red blood cells were lysed using 2 mL Vitalyse (BioE, Inc., St. Paul, MN), 30 min, room temperature. Cell suspensions were centrifuged and washed with phosphate-buffered saline (PBS) containing 4% fetal bovine serum. Cell suspensions were centrifuged again and resuspended in PBS containing 1% paraformaldehyde. A small percentage of the hundreds of stains could not be analyzed due to sample unavailability or technical problems at a few time points; therefore, these time points are not plotted in the data graphs.
2.4. Flow cytometric and statistical analyses
Cells were analyzed with an EPICS XL-2 flow cytometer (Beckman Coulter) within 24 h of staining. At least 100,000 total events per sample were collected. Monocytes were gated by CD14 fluorescence and side-scatter log (SS Log). Proper compensation was set by singly stained PBMC from each animal. The flow cytometer was calibrated daily for laser fluctuation and photomultiplier tube (PMT) voltage setting by using FlowCheck beads (Beckman Coulter). Daily fluctuation is logged and checked every week to monitor range in variation. There was not a significant fluctuation in the PMT voltage settings for any PMT. Data analysis and graphic representations were performed using FlowJo (Tree Star, Inc., Ashland, OR). The percent marker expression of CD14+ cells was derived by the number of events in the upper right (CD14+ double positives) quadrant.
Statistical analyses of the difference in the percent expression and mean fluorescent intensity (MFI) of activation markers on CD14hi or CD14lo cells between macaques that did or did not develop SIVE was performed in terms of time post-infection and separately in terms of weeks prior to death (6 weeks prior to death). The data illustrated in the figures represents the time post-infection. Differences in percent expression and MFI observed in the weeks prior to death are stated in the results section.
Statistical analyses of the time course curves for each group (macaques that did or did not develop SIVE) were performed using two-way repeated-measures analysis of variance (Prism, version 4.0b, GraphPad Software, Inc., San Diego, CA). Bonferroni post-tests were performed to identify differences in each group over time. However, statistical analyses between groups were not performed because having a group with two members violates assumptions needed for statistical comparison. Changes in the mean percentage of CD14/CD16 subsets as a function of duration of infection were estimated using linear regression analyses (Prism). Mean percentage expression and MFI is displayed as mean±S.E.M. P<0.05 was considered to be statistically significant.
3. Results
3.1. CD14 expression on pigtailed macaque monocytes can be divided into two subsets
Based on side-scatter log and CD14 expression, monocytes can be classified as CD14hi or CD14lo (Fig. 1). Both of these monocyte subsets were followed every 2 weeks throughout the course of SIV infection in six pigtailed macaques and analyzed for percentage expression and mean fluorescent intensity of a panel of activation markers. A description of each activation marker is listed in Table 1. Phenotypic changes in monocyte subsets were evaluated retrospectively in macaques that developed SIV encephalitis (SIVE) and compared to macaques that did not develop encephalitis (Table 2). Upon development of end-stage AIDS illness, four of the six macaques were euthanized at 83–300 days post-infection; two macaques died unexpectedly at 83 and 185 days post-infection. At the time of death, one macaque showed neurological signs, while the remaining three that developed SIVE exhibited breathing difficulties (Table 2). One macaque had acute terminal bacterial meningitis, but this presumably did not affect the longitudinal measurements taken weeks before necropsy. Opportunistic infections of the CNS were not observed in any macaque in this study.
Fig. 1.
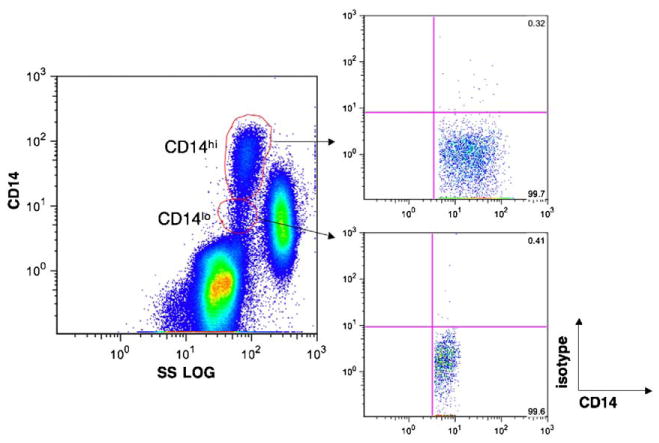
CD14 expression on monocytes can be divided into two subsets. Whole blood was stained with anti-CD14 and one of the antibodies listed in Table 1. Based on CD14 expression and side-scatter log characteristics, monocytes can be divided into CD14hi and CD14lo subsets. Each of these gated populations was monitored for changes in expression of phenotypic markers (Table 1) throughout the course of the disease in 6 SIV-infected pigtailed macaques. Isotype controls from CD14hi and CD14lo subsets are shown.
Table 1.
Description of activation markers analyzed on monocyte subsets during the course of SIV infection
| Phenotypic marker | Monocyte expression changes |
|---|---|
| CCR5 | Increased in multiple sclerosis
(Martinez-Caceres et al., 2002). |
| CD69 | Increased in patients with HAD (Kusdra et al., 2002; Pulliam et al., 1997) and Alzheimer's disease (Kusdra et al., 2000). |
| HLA-DR | Decreased in systemic lupus erythematosus (Nagai et al., 1984), Hodgkin's disease (Nagai et al., 1986), acute pancreatitis (Yu et al., 2004), and Trypanosoma cruzi infection (Sathler-Avelar et al., 2003). |
| CD62L | Increased in diabetes type II (van Oostrom et al., 2004). |
| CD40 | Increased expression after in vitro treatment with interferon-β (Marckmann et al., 2004). |
| CD64 | Decreased in acute hemolytic uremic syndrome (Fernandez et al., 2005), Bordetella pertussis infection (Hodge et al., 2003), and hemoglobin H disease. |
| CD163 | Increased after coronary artery bypass graft surgery. (Goldstein et al., 2003). |
| CD16 | Increased in HIV (Dunne et al., 1996; Thieblemont et al., 1995), SIV (Otani et al., 1998), HAD (Kusdra et al., 2002; Pulliam et al., 1997), hemolytic uremic syndrome (Brauner et al., 1998), bacterial infections (Katayama et al., 2000), asthma (Rivier et al., 1995), rheumatoid arthritis (Kawanaka et al., 2002), acute Kawasaki disease (Katayama et al., 2000), sarcoidosis (Okamoto et al., 2003), and liver cirrhosis (Panasiuk et al., 2005). |
Table 2.
Pigtailed macaque age, sex, infection parameters, and neuropathological and clinical diagnosis
| Monkey number | Age at death
(month) |
Sex | Disease at time of sacrifice | Length of Infection (days) | Neuropath Dx | Clinical Dx |
|---|---|---|---|---|---|---|
| M156 | 52 | m | AIDS | 83 | Normal | Cough, epistaxis, interstitial pneumonia, scrotum and enlarged and necrotic prepuce |
| M160 | 52 | m | AIDS | 300 | Normal, acute hypoxic changes | Hemorrhagic lung, pneumonitis |
| M157 | 50 | m | AIDS | 137 | Subacute SIV encephalitis with diffuse granulomas, low-grade meningitis, poliomyelitis | Screaming, orchitis, muscle tics |
| M158 | 55 | m | AIDS | 185 | SIV encephalitis, SIV myelitis | Died unexpectedly, SIV hepatitis, SIV enteritis |
| M159 | 51 | m | AIDS | 83 | SIV encephalitis, severe meningitis (Gram-positive bacteria), severe edema with ischemic changes | Died unexpectedly, CMV pnemonitis, glomerulosclerosis |
| M161 | 59 | m | AIDS | 119 | SIV encephalitis, SIV myelitis | Ataxia, splenomegaly, severe pneumonia (Pneumocystis carinii) |
3.2. Macaques that would or would not develop encephalitis were not distinguished on the basis of monocyte CCR5 expression during the course of infection
Prior to infection, 10% or less of CD14hi and CD14lo cells expressed CCR5 in all but one macaque (Figs. 2a and 3a). Three macaques exhibited increased proportions of CD14hi/CCR5+ cells (range = 19.2%–31.1%, median = 15.2%, mean = 25.2 ± 3.4%) and CD14lo/CCR5+ cells (range = 11.7%–16.9%, median = 25.3%, mean = 13.9 ± 1.5%) at 1 week post-infection (wpi) regardless of whether the macaque eventually developed encephalitis (SIVE, CD14hi, P<0.05) (Figs. 2a and 3a). In the weeks prior to death, there was a general increase in proportion of CD14hi/CCR5+ cells in most macaques. CCR5 MFI varied during the course of infection on both CD14hi (Fig. 4a) and CD14lo cells, but this was independent of development of encephalitis.
Fig. 2.
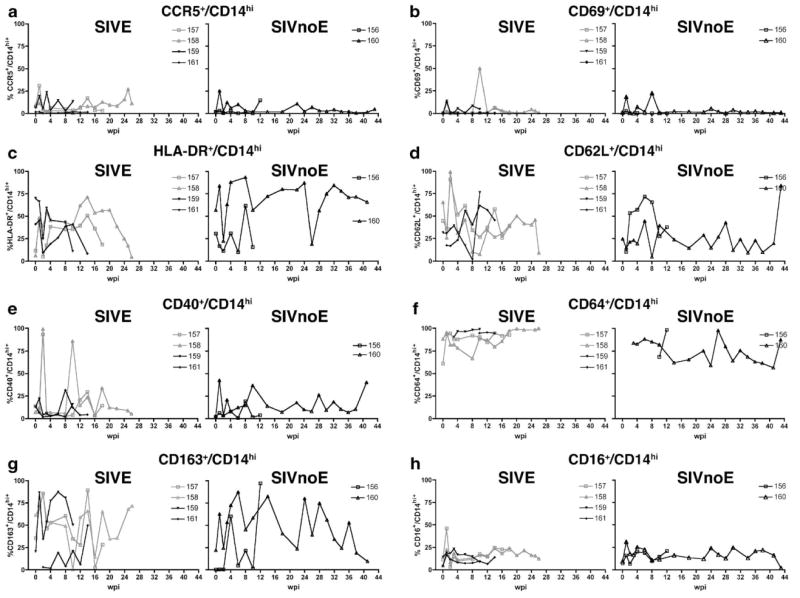
Proportion of CD14hi cells that expressed phenotypic markers of activation during the course of infection in 6 pigtailed macaques infected with SIV/DeltaB670. Based on histological findings, macaques were retrospectively classified at post-mortem for the presence of SIV encephalitis (SIVE). For each phenotypic marker, macaques that developed SIVE (M157, M158, M159, and M161) are shown in the left graph, while macaques that did not develop SIVE (SIVnoE) (M156 and M160) are shown in the right graph. At indicated weeks post-infection (wpi), whole blood was stained with anti-CD14 and the panel of antibodies listed in Table 1. CD14hi cells were gated as shown in Fig. 1. Shown here are longitudinal changes in percent expression of each phenotypic marker: (a) CCR5+/CD14hi, (b) CD69+/CD14hi, (c) HLA-DR+/CD14hi, (d) CD62L+/CD14hi, (e) CD40+/CD14hi, (f) CD64+/CD14hi, (g) CD163+/CD14hi, and (h) CD16+/CD14hi.
Fig. 3.
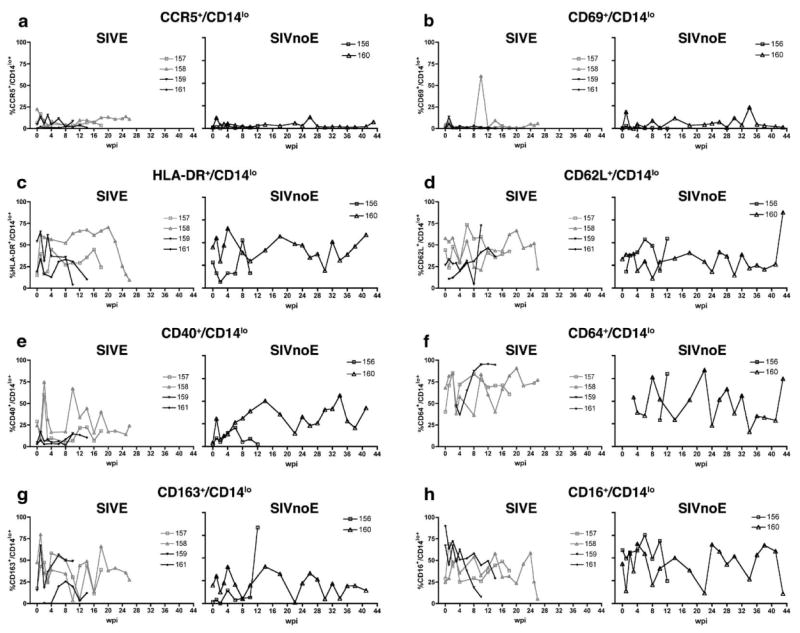
Proportion of CD14lo cells that expressed phenotypic markers of activation during the course of infection in 6 pigtailed macaques infected with SIV/DeltaB670. Based on histological findings, macaques were retrospectively classified at post-mortem for the presence of SIV encephalitis. For each phenotypic marker, macaques that developed SIVE (M157, M158, M159, and M161) are shown in the left graph, while macaques that did not develop SIVE (SIVnoE) (M156 and M160) are shown in the right graph. At indicated weeks post-infection (wpi), whole blood was stained with anti-CD14 and the panel of antibodies listed in Table 1. CD14lo cells were gated as shown in Fig. 1. Shown here are longitudinal changes in percent expression of each phenotypic marker: (a) CCR5+/CD14lo, (b) CD69+/CD14lo, (c) HLA-DR+/CD14lo, (d) CD62L+/CD14lo, (e) CD40+/CD14lo, (f) CD64+/CD14lo, (g) CD163+/CD14lo, and (h) CD16+/CD14lo.
Fig. 4.
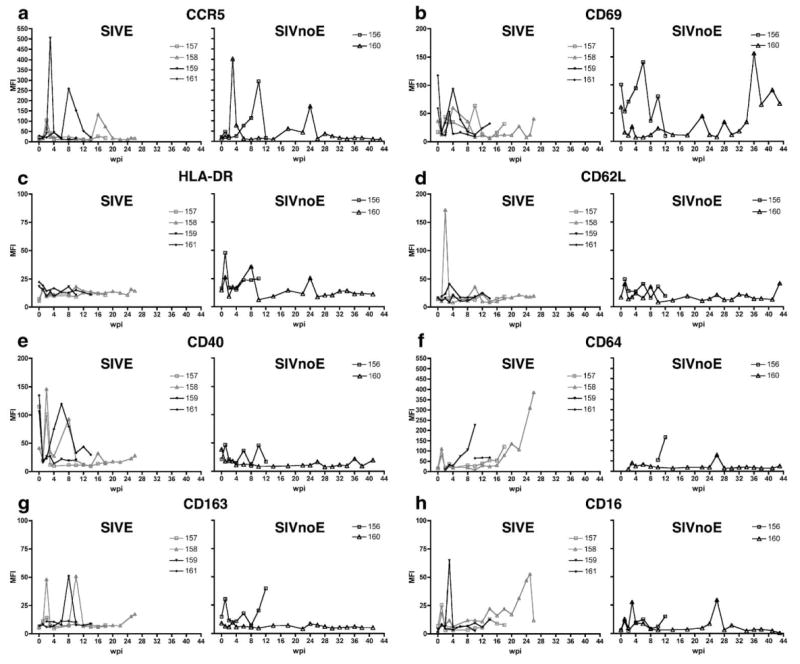
Mean fluorescent intensity (MFI) of activation markers on CD14hi cells during the course of infection in 6 pigtailed macaques infected with SIV/DeltaB670. Based on histological findings, macaques were retrospectively classified at post-mortem for presence of SIV encephalitis. For each phenotypic marker, macaques that developed SIVE (M157, M158, M159, and M161) are shown in the left graph, while macaques that did not develop SIVE (SIVnoE) (M156 and M160) are shown in the right graph. At indicated weeks post-infection (wpi), whole blood was stained with anti-CD14 and the panel of antibodies listed in Table 1. MFI of each phenotypic marker on CD14hi cells was followed every 2 wpi: (a) CCR5, (b) CD69, (c) HLA-DR, (d) CD62L, (e) CD40, (f) CD64, (g) CD163, and (h) CD16. Note the difference in y-axes scales.
3.3. CD69+ monocyte percentage and intensity varied during the course of infection but was independent of the development of encephalitis
The proportion of CD14hi cells that expressed CD69 at baseline was 1.12% or lower (median = 0.41, mean = 0.49 ± 0.13) for all macaques (Fig. 2b), while 0.28%–4.58% (median = 0.67, mean = 1.38 ± 0.67) of CD14lo cells expressed CD69 (Fig. 3b). During the first week of infection, there was a 3.3–57.3-fold increase in the proportion of CD14hi cells that expressed CD69 and a 1.3–27.7-fold increase in the proportion of CD14lo cells that expressed CD69 (Figs. 2b and 3b). At 1 wpi, CD69 MFI on CD14hi cells decreased from baseline for all macaques (SIVE, P<0.05) (Fig. 4b). At 2 weeks before death, CD69 MFI on CD14hi cells was significantly lower in macaques that developed SIVE compared to macaques that did not develop SIVE.
3.4. Monocyte HLA-DR MFI was lower in macaques that would develop encephalitis during the first weeks of infection
The proportions of CD14hi and CD14lo cells that expressed HLA-DR were variable prior to SIV infection (median = 36%, mean = 36.1 ± 10.2%, range = 6.01%–70% and median = 24%, mean = 30.3 ± 4.8%, range = 15%–54%, respectively) (Figs. 2c and 3c). At 2 wpi, all but one macaque had decreased proportions of CD14hi (SIVE, P<0.05) and CD14lo cells that expressed HLA-DR (Figs. 2c and 3c). After acute infection, the percentage of CD14hi and CD14lo cells that expressed HLA-DR remained variable with all macaques showing decreased proportions of CD14hi/HLA-DR+ and CD14lo/HLA-DR+ cells in the weeks before death. Macaques that developed SIVE had a lower proportion of CD14hi/HLA-DR+ cells 4 weeks before death. HLA-DR MFI was higher for macaques that did not develop encephalitis on the CD14hi population at 1 and 8 wpi (P<0.05) (Fig. 4c) and on the CD14lo subset at 1 and 4 wpi (data not shown).
3.5. Early in infection, macaques that would develop encephalitis showed increased proportion of CD62L+ monocytes
Prior to infection, the proportion of CD14hi cells that expressed CD62L was higher in macaques that developed SIVE (mean = 47.2%) than macaques that did not develop encephalitis (24.6%) (Fig. 2d). Baseline CD62L expression was not available for one macaque that developed SIVE and one macaque that did not develop SIVE. At 1 wpi, the proportion of CD14hi cells that expressed CD62L decreased in all macaques (Fig. 2d). The proportion of CD14hi/CD62L+ cells was variable for the remainder of infection. Macaques that developed SIVE had decreased CD62L MFI on CD14hi cells at 1 wpi (Fig. 4d).
3.6. Monocytes of macaques that would develop SIVE showed higher expression of CD40 prior to infection that decreased below that of macaques that would not develop SIVE at 4 weeks
Prior to infection, the proportion of CD14hi/ CD40+ cells was significantly higher for macaques that developed SIVE (Fig. 2e). After infection, the proportions of CD14hi/CD40+ and CD14lo/CD40+ cells were variable for most macaques (Figs. 2e and 3e). Compared to baseline, CD40 MFI for the CD14hi subset decreased in macaques that developed SIVE at 1, 4, and 10 wpi (P<0.05) (Fig. 4e). Four weeks prior to death, macaques that developed SIVE had higher CD40 MFI on CD14hi cells than macaques that did not develop SIVE.
3.7. Four weeks prior to death, macaques that would develop SIVE had a greater proportion of CD64+/CD14+ cells
The percentage of CD14hi cells that expressed CD64 either increased or remained high for most macaques during the course of infection (Fig. 2f). Four weeks prior to death, macaques that developed SIVE had higher proportions of CD64+/CD14hi and CD64+/CD14lo cells than macaques that did not develop SIVE (Figs. 2f and 3f).
3.8. Two weeks prior to death, CD14lo monocytes of macaques that would develop SIVE had greater CD163 MFI than macaques that would not develop encephalitis
During the first 2 wpi, the percentage of CD14lo cells that expressed CD163 was higher in macaques that developed SIVE (Fig. 3g). Both CD14hi and CD14lo subsets showed large increases and decreases in the proportions of cells that expressed CD163 throughout the length of infection (Figs. 2g and 3g). Two weeks prior to death, macaques that developed SIVE had higher CD163 MFI on CD14lo cells than macaques that did not develop SIVE (data not shown).
3.9. Increased proportions of CD14+/CD16+ cells occurred in all SIV-infected macaques independent of development of encephalitis
Prior to infection, the mean proportion of CD14lo cells that expressed CD16 was 52.19 ± 10.1% (median = 51.2%) (Fig. 3h) and 8.65 ± 1.57% (median = 8.4%) for CD14hi cells compared to baseline (SIVE, P<0.05) (Fig. 2h). All macaques had an increased percentage of CD14hi/CD16+ cells at 1 wpi (Fig. 2h), while the proportion of CD14lo/CD16+ cells decreased in four macaques at 1 wpi (Fig. 3h). Regardless of development of encephalitis, the proportion of CD14hi/CD16+ cells remained high at most time periods for the duration of infection. Compared to baseline, CD16 MFI increased in most animals at 1 (SIVE, P<0.05, CD14hi subset) and 3 wpi for both CD14hi and CD14lo subsets (Fig. 4h and data not shown).
Fig. 5a is a representative example of the changes in the proportions of CD14+/CD16+ cell populations during the course of infection. Three CD16+ cell populations can be observed as shown in Fig. 5a, week 0; A = CD14hi/CD16lo; B = CD14lo/CD16hi; C = CD14−/CD16hi. The proportion of cells in C decreased during the course of infection (m = −0.18 ± 0.05, P = 0.001) (Fig. 5a and d). The A and B subsets were present at smaller proportions than the C subset (means ± S.D.: A = 0.53 ± 0.15%; B = 0.89 ± 0.32%; C = 7.77 ± 1.95%) (Fig. 5a–d), but the proportions of the CD14hi/CD16lo subset increased at least 2–3-fold or higher during the course of infection (m = 0.05 ± 0.01, P = 0.006) for all monkeys especially during 2 and 6 wpi (Fig. 5b). The proportion of CD14lo/CD16hi cells mirrored the trend of the CD14hi/CD16lo population for most macaques with an increase at 1 wpi. However, due to variability, the mean percentage as a function of duration of infection showed no estimated change (m = 0.004 ± 0.01, P = 0.774) (Fig. 5c).
Fig. 5.
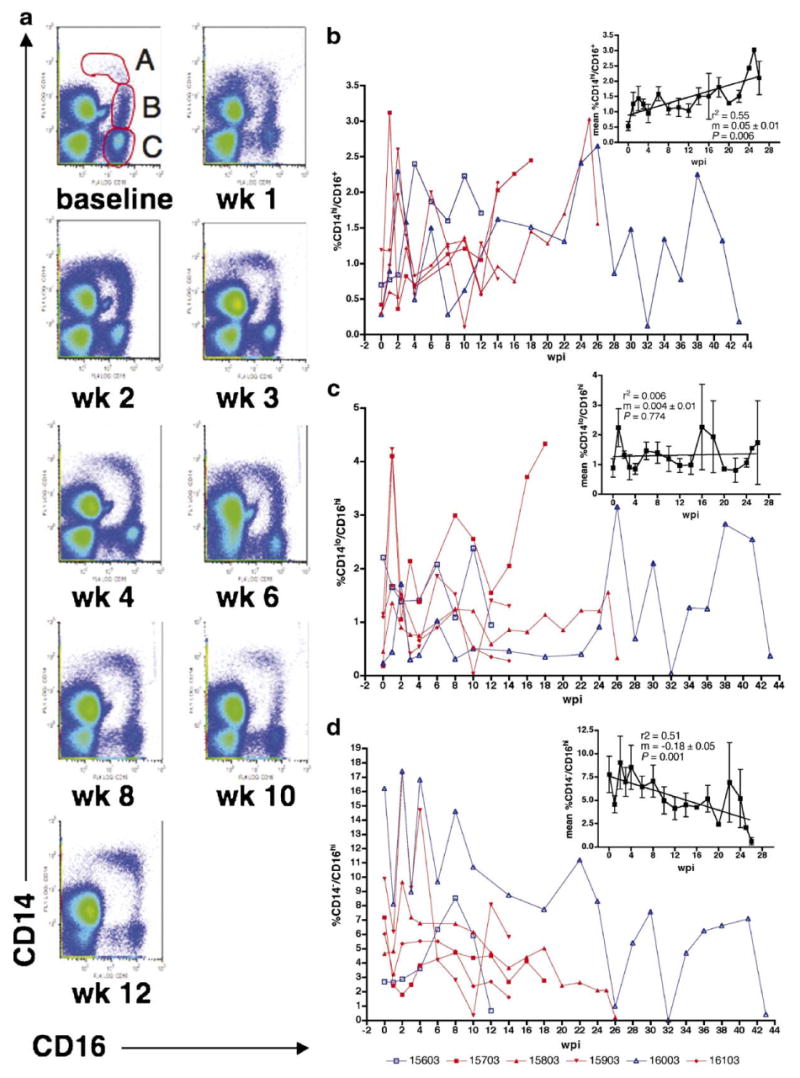
The proportion of CD14hi/CD16lo cells increased while the percentage of CD14−/CD16hi cells decreased during the course of infection in 6 pigtailed macaques infected with SIV/DeltaB670 regardless of development of encephalitis. At each week post-infection (wpi), whole blood was stained with anti-CD14 and anti-CD16 antibodies. (a) These representative pseudo-color plots (ungated) from M156 show the proportions of CD14hi/CD16lo and CD14lo/CD16hi cells increased during the course of infection. The baseline plot shows three CD16+ cell populations: A = CD14hi/CD16lo; B = CD14lo/CD16hi; and C = CD14−/CD16hi. (b–d) Macaques that developed SIVE (M157, M158, M159, and M161) are shown in red, while macaques that did not develop SIVE (M156 and M160) are shown in blue. The A, B, and C subsets cells were gated as shown in (a). Insets show changes in the mean percentage of each subset as a function of duration of infection (wpi). (b) A subset. The proportion of CD14hi/CD16lo cells increased during the course of infection, r2 = 0.55, m = 0.05 ± 0.01 and P = 0.006. (c) B subset. The proportion of CD14lo/CD16hi cells was variable during the course of infection. Most macaques showed increased proportions at 1 wpi. However, there was no estimated change in mean percentage during the course of infection, r2 = 0.006, m = 0.004 ± 0.01 and P = 0.774. (d) C subset. The proportion of CD14−/CD16hi cells decreased during the course of infection, r2 = 0.51, m = −0.18 ± 0.05 and P = 0.001. r2 = measure of goodness of fit. m = slope of line. Since only one animal survived after 26 wpi, the insets show data from 0 to 26 wpi to avoid inaccuracies in statistical analyses presented. Note the difference in y-axes scales for (b–d).
4. Discussion
In the past decade, it has been suggested that patients with HAD demonstrated increased proportions of CD14+/CD16+ monocytes (Pulliam et al., 1997). Several reports have described increases in the proportion or absolute number of CD14+/CD16+ monocytes during HIV or SIV infection (Dunne et al., 1996; Pulliam et al., 1997; Thieblemont et al., 1995; Williams et al., 2005). During acute SIV infection of pigtailed and cynomolgus macaques, increased numbers of monocytes (Israel et al., 1993) and specifically CD14loCD16+ monocytes were found in the blood (Otani et al., 1998). We have performed longitudinal analyses of CD14+/CD16+ monocyte subsets along with other phenotypic markers of activation on monocytes in a group of six SIV-infected pigtailed macaques. The SIV-infected macaque provides an excellent model to determine whether expansion of the CD14+/CD16+ monocyte subset is predictive of the development of lentiviral encephalitis.
4.1. Activation markers that distinguished macaques that would develop encephalitis from macaques that would not develop encephalitis
Prior to SIV infection, macaques that would develop SIVE had a higher proportion of CD14hi monocytes that expressed CD62L and CD40 than macaques that would not develop SIVE. CD40 MFI on CD14hi monocytes was also increased in macaques that would develop SIVE compared to macaques that would not develop SIVE. Although the proportion of monocytes that expressed CD69 was similar, the MFI varied from animal to animal. Even before infection, the proportion and MFI varied greatly in all macaques.
After infection, macaques that would develop SIVE had a higher proportion of CD62L+CD14hi monocytes than macaques that would not develop SIVE, but this was accompanied by decreased CD62L MFI. During the course of infection, the proportions of CD40+CD14hi, CD40+CD14lo, and CD16+CD14hi cells were decreased in macaques that developed SIVE. Interestingly, CD16 MFI was also decreased on CD16+CD14hi cells in macaques that would develop SIVE, while CD40+CD14hi cells showed increased CD40 MFI. Although these markers distinguished macaques that would develop SIVE from those that would not, differences were observed at single time points during the course of infection. All other activation markers fluctuated but showed no correlation with the development of encephalitis.
Prior to death, the proportion of CD64+/CD14+ monocytes was higher in encephalitic macaques, while the proportions of HLA-DR+/CD14hi and HLA-DR+/CD14lo monocytes were decreased compared to nonencephalitic macaques. MFI for CD40 and CD163 on CD14hi and CD14lo cells, respectively, was also increased prior to death. Decreased proportions of HLA-DR+/CD14hi cells in the weeks before death suggest circulating cells might have trafficked into the CNS and other tissues.
4.2. Activation markers that did not distinguish macaques that would develop encephalitis from macaques that would not develop encephalitis
In HIV-infected humans, emphasis has been placed on the potential expansion of CD14+/CD16+ monocytes being associated with presence of HAD. This was not replicated in our macaque model comparing those that would versus those that would not develop SIVE. It remains a possibility that macaques that developed SIVE have increased numbers of SIV-infected CD14+/CD16+ cells.
Since neither the proportion of CD14+/CCR5+ cells nor the MFI of CCR5 on CD14+ cells distinguished macaques that would develop SIVE from those that would not, increased presence of SIV-infected CNS macrophages was not associated with increased proportion or intensity of CCR5 (a co-receptor for SIV-associated with macrophage infection) on blood monocytes. It has also been suggested that CCR1+/CCR5+/CD14+ cells are the population of monocytes that enter the inflamed CNS (Trebst et al., 2001), but we were unable to detect such trafficking by assessing the peripheral blood. Our results in a small number of SIV-infected macaques do not support the observation that the proportion of circulating CD14+CD69+ monocytes predicts development of encephalitis (Kusdra et al., 2002; Pulliam et al., 1997).
4.3. Activation markers that changed during the course of infection in all macaques regardless of development of encephalitis
Interestingly, CD16 expression on ungated cells changed during the course of SIV infection in both macaques that would and would not develop encephalitis. We analyzed changes in the proportion of three CD16+ cell populations. The CD14−CD16hi subset likely contained NK cells. We observed a decrease in the proportion of NK cells during the course of SIV infection. This corroborates reports that the frequency of the cytolytic subset of NK cells (CD16+) decreases during HIV infection (Lucia et al., 1995; Meier et al., 2005; Tarazona et al., 2002).
The other two CD16+ cell populations were CD14hi/CD16lo and CD14lo/CD16hi. These subsets contained the monocyte populations described in previous reports, CD14++CD16+ and CD14+CD16+ (Ziegler-Heitbrock, 2000). As others have described, our study showed that the proportion of CD14hiCD16hi/lo cells changed significantly as a function of duration of infection, but this was independent of whether the macaque developed encephalitis. An increased percentage was also observed in the CD14lo/CD16hi subset during the course of infection, but this was not statistically significant. This may be due to the presence of CD14+/CD16+ granulocytes. We have expanded previous observations by following the macaques throughout the course of infection. The strength of our longitudinal study suggests that increases in the proportion of CD14+/CD16+ cells are not predictive of the development of encephalitis but rather an indication of chronic disease.
There are at least two possible explanations for changes in CD16+ populations: either these populations expanded over time (increased number of cells) or the proportions of these subsets increased because other populations were decreasing. Assuming the number of events in each subset is a rough indication of the actual number of cells in each subset, it appears that during acute infection, the increased proportion of monocytes seen was due to an expansion in the number of monocytes in both subsets. After acute infection, the increased proportion of monocytes was due to expansion of the number of monocytes alternating with times where changes in other cell populations increased the proportion of monocytes. At the last time point prior to death, approximately half of the macaques had expanded monocyte populations. Expansion of the monocyte subsets and loss of the NK cell subset did not correlate with viral load or CD4+ T cell counts (data not shown).
We have reported our data as the proportion of cells expressing activation markers, rather than actual cell numbers. Determining absolute cell numbers by multiplying the proportion of each subset by the number of monocytes present in whole blood at each time point confirmed that both percentage and actual number of cells that expressed activation markers had similar trends (data not shown).
Since SIV-infection does not produce a uniform disease, the macaques in this study have different survival times. Because we did not observe any differences between activation markers on monocytes from macaques that would or would not develop SIVE in terms of length of time post-infection, we also compared the results in terms of time prior to death. These results are arguably more meaningful; however, we did not observe any significant differences using this strategy.
It is possible that we did not observe differences between activation markers on monocytes from macaques that would and would not develop SIVE because we did not look at the correct activation marker(s). We have also analyzed these activation markers along with others on monocytes from a group of eight rhesus macaques. Longitudinal analysis of the proportion of monocytes that expressed CD49 (α4 integrin), CD80, CD86, CD166, and any of the markers listed in Table 1 did not reveal any differences between macaques that would and would not develop encephalitis (unpublished observations). Inclusion of CCR1 on monocytes would be germane since the chemokine ligand of this receptor is reportedly involved in monocyte CNS trafficking, but there are no commercial antibodies available to this macaque marker. Alternatively, it is possible that the pneumonitis observed in the two macaques that would not develop SIVE affected expression of activation markers on circulating monocytes. If true, it would suggest that monitoring differences in activation markers during the course of lentiviral infection would not be specific to development of encephalitis but would be a nonspecific harbinger of disease.
The present study followed activation markers on blood monocytes from six SIV infected pigtailed macaques during the course of infection. Analysis of blood monocytes showed there was an expansion of CD14hi/CD16lo and CD14lo/CD16hi monocytes during acute infection and at various time points during the course of infection; however, this expansion was not unique or greater in macaques that developed SIVE. The proportion of monocytes that expressed other activation markers was variable for all macaques during the course of infection and none of the tested phenotypic markers predicted development of encephalitis. Taken together, these results suggest that assessment of the proportion of circulating activated monocytes will not predict development of SIVE, but this does not rule out the importance of activated monocytes in the development of encephalitis.
Acknowledgments
We thank Aki Hoji for assistance with flow cytometry analysis; Dawn L. McClemens-McBride for assistance in scheduling blood draws; and LuAnn Borowski and Charles Rinaldo, Jr., for the use and assistance with the flow cytometry facility. This work was supported in part by NIH grants R01 MH64921 (Wiley), R01 MH071151 (Wiley), and K24 MH01717 (Wiley).
References
- Adamson DC, Kopnisky KL, Dawson TM, Dawson VL. Mechanisms and structural determinants of HIV-1 coat protein, gp41-induced neurotoxicity. J Neurosci. 1999;19:64–71. doi: 10.1523/JNEUROSCI.19-01-00064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissel SJ, Wang G, Ghosh M, Reinhart TA, Capuano S, III, Cole K Stefano, Murphey-Corb M, Piatak MJ, Lifson JD, Wiley CA. Macrophages relate presynaptic and postsynaptic damage in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;160:927–941. doi: 10.1016/S0002-9440(10)64915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner A, Lu Y, Hallden G, Hylander B, Lundahl J. Difference in the blood monocyte phenotype between uremic patients and healthy controls: its relation to monocyte differentiation into macrophages in the peritoneal cavity. Inflammation. 1998;22:55–66. doi: 10.1023/a:1022395723972. [DOI] [PubMed] [Google Scholar]
- Brew BJ. AIDS dementia complex. Neurol Clin. 1999;17:861–881. doi: 10.1016/s0733-8619(05)70170-5. [DOI] [PubMed] [Google Scholar]
- Chuluyan HE, Issekutz AC. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Invest. 1993;92:2768–2777. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque P, Scarpellini P, Vago L, Linde A, Lazzarin A. Diagnosis of central nervous system complications in HIV-infected patients—cerebrospinal fluid analysis by the polymerase chain reaction. AIDS. 1997;11:1–17. doi: 10.1097/00002030-199701000-00003. [DOI] [PubMed] [Google Scholar]
- Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med. 1999;189:179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpan GJ, Farzadegan H, Selnes O, Hoover DR, Miller EN, Skolasky RL, Nancesproson TE, McArthur JC. Sustained cognitive decline in HIV infection—relationship to CD4+ cell count, plasma viremia and P24 antigenemia. J Neurovirology. 1998;4:95–99. doi: 10.3109/13550289809113486. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 1999;13:1249–1253. doi: 10.1097/00002030-199907090-00015. [DOI] [PubMed] [Google Scholar]
- Dunne J, Feighery C, Whelan A. Beta-2-microglobulin, neopterin and monocyte Fc gamma receptors in opportunistic infections of HIV-positive patients. Br J Biomed Sci. 1996;53:263–269. [PubMed] [Google Scholar]
- Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fernandez GC, Ramos MV, Gomez SA, Dran GI, Exeni R, Alduncin M, Grimoldi I, Vallejo G, Elias-Costa C, Isturiz MA, Palermo MS. Differential expression of function-related antigens on blood monocytes in children with hemolytic uremic syndrome. J Leukoc Biol. 2005;78:853–861. doi: 10.1189/jlb.0505251. [DOI] [PubMed] [Google Scholar]
- Gabriel H, Urhausen A, Brechtel L, Muller HJ, Kindermann W. Alterations of regular and mature monocytes are distinct, and dependent of intensity and duration of exercise. Eur J Appl Physiol Occup Physiol. 1994;69:179–181. doi: 10.1007/BF00609414. [DOI] [PubMed] [Google Scholar]
- Gerber JS, Mosser DM. Stimulatory and inhibitory signals originating from the macrophage Fcgamma receptors. Microbes Infect. 2001;3:131–139. doi: 10.1016/s1286-4579(00)01360-5. [DOI] [PubMed] [Google Scholar]
- Goldstein JI, Goldstein KA, Wardwell K, Fahrner SL, Goonan KE, Cheney MD, Yeager MP, Guyre PM. Increase in plasma and surface CD163 levels in patients undergoing coronary artery bypass graft surgery. Atherosclerosis. 2003;170:325–332. doi: 10.1016/s0021-9150(03)00297-1. [DOI] [PubMed] [Google Scholar]
- Griffin DE, Wesselingh SL, McArthur JC. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Annals Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- Hodge G, Hodge S, Markus C, Lawrence A, Han P. A marked decrease in L-selectin expression by leucocytes in infants with Bordetella pertussis infection: leucocytosis explained? Respirology. 2003;8:157–162. doi: 10.1046/j.1440-1843.2003.00459.x. [DOI] [PubMed] [Google Scholar]
- Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–627. [PubMed] [Google Scholar]
- Israel ZR, Dean GA, Maul DH, O'Neil SP, Dreitz MJ, Mullins JI, Fultz PN, Hoover EA. Early pathogenesis of disease caused by SIVsmmPBj14 molecular clone 1.9 in macaques. AIDS Res Hum Retrovir. 1993;9:277–286. doi: 10.1089/aid.1993.9.277. [DOI] [PubMed] [Google Scholar]
- Issekutz AC, Issekutz TB. Monocyte migration to arthritis in the rat utilizes both CD11/CD18 and very late activation antigen 4 integrin mechanisms. J Exp Med. 1995;181:1197–1203. doi: 10.1084/jem.181.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Matsubara T, Fujiwara M, Koga M, Furukawa S. CD14+ CD16+ monocyte subpopulation in Kawasaki disease. Clin Exp Immunol. 2000;121:566–570. doi: 10.1046/j.1365-2249.2000.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Salentin R, Gemsa D, Sprenger H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J Leukoc Biol. 2001;69:248–252. [PubMed] [Google Scholar]
- Kawanaka N, Yamamura M, Aita T, Morita Y, Okamoto A, Kawashima M, Iwahashi M, Ueno A, Ohmoto Y, Makino H. CD14+, CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002;46:2578–2586. doi: 10.1002/art.10545. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Trebst C, Liu Z, Tucky BH, Sorensen TL, Rudick RA, Mack M, Ransohoff RM. T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin Exp Immunol. 2002;129:510–518. doi: 10.1046/j.1365-2249.2002.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusdra L, Rempel H, Yaffe K, Pulliam L. Elevation of CD69+ monocyte/macrophages in patients with Alzheimer's disease. Immunobiology. 2000;202:26–33. doi: 10.1016/S0171-2985(00)80049-2. [DOI] [PubMed] [Google Scholar]
- Kusdra L, McGuire D, Pulliam L. Changes in monocyte/macrophage neurotoxicity in the era of HAART: implications for HIV-associated dementia. Aids. 2002;16:31–38. doi: 10.1097/00002030-200201040-00005. [DOI] [PubMed] [Google Scholar]
- Lucia B, Jennings C, Cauda R, Ortona L, Landay AL. Evidence of a selective depletion of a CD16+ CD56+ CD8+ natural killer cell subset during HIV infection. Cytometry. 1995;22:10–15. doi: 10.1002/cyto.990220103. [DOI] [PubMed] [Google Scholar]
- Marckmann S, Wiesemann E, Hilse R, Trebst C, Stangel M, Windhagen A. Interferon-beta up-regulates the expression of co-stimulatory molecules CD80, CD86 and CD40 on monocytes: significance for treatment of multiple sclerosis. Clin Exp Immunol. 2004;138:499–506. doi: 10.1111/j.1365-2249.2004.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Caceres EM, Espejo C, Brieva L, Pericot I, Tintore M, Saez-Torres I, Montalban X. Expression of chemokine receptors in the different clinical forms of multiple sclerosis. Mult Scler. 2002;8:390–395. doi: 10.1191/1352458502ms841oa. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McClernon DR, Cronin MF, Nance-Sproson TE, Saah AJ, Clair M, Lanier ER. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain [see comments] Annals Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- Meerschaert J, Furie MB. Monocytes use either CD11/CD18 or VLA-4 to migrate across human endothelium in vitro. J Immunol. 1994;152:1915–1926. [PubMed] [Google Scholar]
- Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, Tang K, Newton P, Pellegrino P, Williams I, Klenerman P, Borrow P. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–12374. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Chen IS. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991;5:2391–2397. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- Nagai H, Sztein MB, Steeg PS, Hooks JJ, Oppenheim JJ, Steinberg AD. Diminished peripheral blood monocyte DR antigen expression in systemic lupus erythematosus. Clin Exp Rheumatol. 1984;2:131–137. [PubMed] [Google Scholar]
- Nagai H, Fisher RI, Cossman J, Oppenheim JJ. Decreased expression of class II major histocompatibility antigens on monocytes from patients with Hodgkin's disease. J Leukoc Biol. 1986;39:313–321. doi: 10.1002/jlb.39.3.313. [DOI] [PubMed] [Google Scholar]
- Nottet H, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- Okamoto H, Mizuno K, Horio T. Circulating CD14+ CD16+ monocytes are expanded in sarcoidosis patients. J Dermatol. 2003;30:503–509. doi: 10.1111/j.1346-8138.2003.tb00424.x. [DOI] [PubMed] [Google Scholar]
- Otani I, Akari H, Nam KH, Mori K, Suzuki E, Shibata H, Doi K, Terao K, Yosikawa Y. Phenotypic changes in peripheral blood monocytes of Cynomolgus monkeys acutely infected with simian immunodeficiency virus. AIDS Res Hum Retrovir. 1998;14:1181–1186. doi: 10.1089/aid.1998.14.1181. [DOI] [PubMed] [Google Scholar]
- Panasiuk A, Zak J, Kasprzycka E, Janicka K, Prokopowicz D. Blood platelet and monocyte activations and relation to stages of liver cirrhosis. World J Gastroenterol. 2005;11:2754–2758. doi: 10.3748/wjg.v11.i18.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Herndier BG, Tang NM, McGrath MS. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991;87:503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Rivier A, Pene J, Rabesandratana H, Chanez P, Bousquet J, Campbell AM. Blood monocytes of untreated asthmatics exhibit some features of tissue macrophages. Clin Exp Immunol. 1995;100:314–318. doi: 10.1111/j.1365-2249.1995.tb03670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasseville VG, Newman W, Brodie SJ, Hesterberg P, Pauley D, Ringler DJ. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced aids encephalitis is mediated by vascular cell-adhesion molecule-1/alpha-4-beta-1 integrin interactions. Am J Pathol. 1994;144:27–40. [PMC free article] [PubMed] [Google Scholar]
- Sathler-Avelar R, Lemos EM, Reis DD, Medrano-Mercado N, Araujo-Jorge TC, Antas PR, Correa-Oliveira R, Teixeira-Carvalho A, Eloi-Santos SM, Favato D, Martins-Filho OA. Phenotypic features of peripheral blood leucocytes during early stages of human infection with Trypanosoma cruzi. Scand J Immunol. 2003;58:655–663. doi: 10.1111/j.1365-3083.2003.01340.x. [DOI] [PubMed] [Google Scholar]
- Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ. CD14+ CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- Shang XZ, Issekutz AC. Contribution of CD11a/CD18, CD11b/CD18, ICAM-1 (CD54) and -2 (CD102) to human monocyte migration through endothelium and connective tissue fibroblast barriers. Eur J Immunol. 1998;28:1970–1979. doi: 10.1002/(SICI)1521-4141(199806)28:06<1970::AID-IMMU1970>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tarazona R, Casado JG, Delarosa O, Torre-Cisneros J, Villanueva JL, Sanchez B, Galiani MD, Gonzalez R, Solana R, Pena J. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. 2002;22:176–183. doi: 10.1023/a:1015476114409. [DOI] [PubMed] [Google Scholar]
- Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- Trebst C, Sorensen TL, Kivisakk P, Cathcart MK, Hesselgesser J, Horuk R, Sellebjerg F, Lassmann H, Ransohoff RM. CCR1+/CCR5+ mononuclear phagocytes accumulate in the central nervous system of patients with multiple sclerosis. Am J Pathol. 2001;159:1701–1710. doi: 10.1016/s0002-9440(10)63017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst C, Staugaitis SM, Tucky B, Wei T, Suzuki K, Aldape KD, Pardo CA, Troncoso J, Lassmann H, Ransohoff RM. Chemokine receptors on infiltrating leucocytes in inflammatory pathologies of the central nervous system (CNS) Neuropathol Appl Neurobiol. 2003;29:584–595. doi: 10.1046/j.0305-1846.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J Biol Chem. 2002;277:5082–5089. doi: 10.1074/jbc.M110277200. [DOI] [PubMed] [Google Scholar]
- van Furth R. Production and Migration of Monocytes and Kinetics of Macrophages. Kluwer Academic Publishers; Dordrecht: 1992. pp. 3–12. [Google Scholar]
- van Oostrom AJ, van Wijk JP, Sijmonsma TP, Rabelink TJ, Castro Cabezas M. Increased expression of activation markers on monocytes and neutrophils in type 2 diabetes. Neth J Med. 2004;62:320–325. [PubMed] [Google Scholar]
- Verdegaal EM, Beekhuizen H, Blokland I, van Furth R. Increased adhesion of human monocytes to IL-4-stimulated human venous endothelial cells via CD11/CD18, and very late antigen-4 (VLA-4)/vascular cell adhesion molecule-1 (VCAM-1)-dependent mechanisms. Clin Exp Immunol. 1993;93:292–298. doi: 10.1111/j.1365-2249.1993.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Traffic of hematogenous cells through the central nervous system. Curr Top Microbiol Immunol. 1995;202:221–245. doi: 10.1007/978-3-642-79657-9_15. [DOI] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WK, Li WQ, Li N, Li JS. Mononuclear histocompatibility leukocyte antigen-DR expression in the early phase of acute pancreatitis. Pancreatology. 2004;4:233–243. doi: 10.1159/000078748. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW. Definition of human blood monocytes. J Leukoc Biol. 2000;67:603–606. doi: 10.1002/jlb.67.5.603. [DOI] [PubMed] [Google Scholar]


