Abstract
The tumor suppressor retinoblastoma protein, Rb, plays a pivotal role in the regulation of cell proliferation and sensitivity to apoptosis through binding to E2F transcription factors. Loss of Rb in response to genotoxic stress or inflammatory cytokines can enhance cell death, in part, by eliminating Rb-mediated repression of proapoptotic gene transcription. Here we show that calpain cleavage of Rb facilitates Rb loss by proteasome degradation and that this may occur during TNFα induced apoptosis. The cytoprotective, Rb-binding protein, SerpinB2 (Plasminogen activator inhibitor type 2; PAI-2) protects Rb from calpain cleavage, increasing Rb levels and enhancing cell survival. Chromatin immunoprecipitation assays show that the increased Rb levels selectively enhance Rb- repression of proapoptotic gene transcription. This cytoprotective role of SerpinB2 is illustrated by reduced susceptibility of SerpinB2-deficient mice to multistage skin carcinogenesis, where Rb dependent cell proliferation competes with apoptosis during initiation of papilloma development. These data identify SerpinB2 as cell survival factor that modulates Rb-repression of proapoptotic signal transduction, and define a new post-translational mechanism for selective regulation of the intracellular levels of Rb.
Keywords: Retinoblastoma protein, Plasminogen Activator Inhibitor type 2, PAI-2, SerpinB2, apoptotic signal transduction
Introduction
The retinoblastoma tumor suppressor protein (Rb), the product of the Rb1 susceptibility gene, was the first tumor suppressor gene to be identified and has emerged to play a central role in limiting cell cycle progression through regulation of the E2F family of transcription factors. In addition to its anti-proliferative function, Rb possesses prosurvival activity which is mediated by Rb's ability to suppress apoptosis directly, independent of growth suppression (1,2). In mice, genetic deletion of Rb1 results in excessive apoptosis associated with abnormal degeneration of neurons and lens fiber cells (3). Rb1 inactivation in epidermis causes increased apoptosis at an early stage of epidermal oncogenic progression, rendering mice less susceptible to skin carcinogenesis (4). Mechanisms implicated in mediating Rb's anti-apoptotic activities include its ability to repress E2F gene transcription (5) and its direct inhibition of proapoptotic signal transduction (1,6). Rb ablation leads to up-regulation of E2F, which can sensitize cells to apoptosis (7). Gene expression profiling studies and direct transcription experiments show that up-regulation of E2F1 induces transcription of cell cycle genes, such as the G1/S cyclins, and also genes encoding proapoptotic cell-death machinery, including Apaf-1, p73, procaspases-3 and -7 (8,9). Since Rb is capable of regulating the expression of cell cycle and apoptotic gene targets, additional factors in association with Rb likely contribute to ultimate cell fate decisions.
The activities of Rb are determined by the cellular proteins that interact with Rb and the functional consequences of these interactions. We recently identified the intracellular serine protease inhibitor SerpinB2 [also called Plasminogen Activator Inhibitor type 2 (PAI-2)] as a Rb-binding protein that co-localizes with Rb in the nucleus and protects Rb from proteolytic degradation, resulting in enhanced Rb protein levels (10). SerpinB2 is a multifunctional protein of the serine protease inhibitor (serpin) family, that is synthesized by a variety of cells (11), and promotes cell survival (12-14). Transgenic overexpression of SerpinB2 in proliferating basal keratinocytes of mice inhibits apoptosis and promotes keratinocyte survival during skin carcinogenesis (15). SerpinB2 protects cells from the cytolytic effects of cytopathic viruses (16,17), mycobacterial infection (18,19) and confers resistance to death induced by the inflammatory cytokine TNFα. SerpinB2 biosynthesis is an acute phase response to TNFα (20) and SerpinB2 inhibits the characteristic morphological changes and DNA fragmentation patterns associated with TNFα-induced apoptosis (18). Although the cell survival activity of SerpinB2 is now well established, the molecular mechanism of SerpinB2 cytoprotection is not known. SerpinB2 protection from TNFα apoptosis cannot be explained by loss of TNFα receptors, impaired ability of TNFα to bind to receptors, impaired TNFα receptor signal transduction or direct inhibition of caspases (18).
Ablation of Rb either through inactivation, or protease-induced degradation, is associated with apoptosis initiated by genotoxic cell stress or TNFα (6). To date, only a few proteolytic mechanisms targeting Rb degradation have been identified. Rb may be targeted by viral oncoproteins, including the human papillomavirus (HPV) E7, which accelerate Rb degradation by proteasomes (21,22). Alternatively, in response to death receptor signals, Rb may be cleaved and inactivated through the action of caspases (23). Cleavage of Rb at the major caspase consensus DEAD886-G site within the C-terminus of Rb, and subsequent degradation, occurs during TNF receptor-I induced apoptosis (24). Mice engineered to express the caspase-resistant Rb mutant (Rb-MI) show resistance to TNFα-induced apoptosis in several tissues (25). Notably, caspase-resistant Rb could be eliminated under certain experimental conditions, suggesting the existence of additional intracellular proteolytic mechanisms for eliminating Rb.
Calpains are Ca2+ dependent, nucleocytoplasmic cysteine proteases that have emerged to play important roles in cell death signaling via the cleavage and/or degradation of a number of regulatory proteins and transcription factors (26,27). The calpain family includes the ubiquitously expressed calpain-1 (μ-calpain) and calpain-2 (m-calpain), as well as a number of tissue specific calpains (26). Both calpain-1 and calpain-2 are heterodimers containing a large ∼80 kDa subunit, encoded by the genes, capn1 and capn2 respectively, and a common 28 kDa regulatory small subunit, encoded by capn4. Here we report that Rb is a calpain substrate. Calpain cleavage of Rb precedes proteasome degradation, and participates in the regulation of Rb turnover. We identify the calpain cleavage site within the C-terminal domain of Rb, which overlaps the SerpinB2 binding site contained within this domain (10). SerpinB2 blocks calpain cleavage of Rb, enhancing Rb levels and Rb anti-apoptotic activities, including repression of pro-apoptotic gene transcription and promotion of keratinocyte survival. We conclude that SerpinB2 functions as a cytoprotective factor that influences cell death signaling pathways through Rb protection.
Materials and Methods
Cell culture
HeLa cells and the HeLa cell line stably expressing SerpinB2 (S1a) have been described previously (18). SV40 transformed CAPN4-/- and CAPN4+/+ MEFs were provided by John Elce, Queen's University, Kingston, Canada (28). Jurkat cells and RAW264.7 cells were obtained from the ATCC. Cell viability and cell cycle analyses were performed by flow cytometry following propidium iodide staining.
Rb cleavage assays
For cell-free assays, Jurkat lysates were incubated at 30°C for 1 hr with the indicated enzymes and inhibitors. For in vitro Rb cleavage, purified recombinant Rb (QED Biosciences, Cat. No. 3108) was incubated with active calpain-1 (>90% purity) or recombinant calpain 2 (>98% purity) (Calbiochem) in the presence of 25 mM Hepes pH 7.4, 5 mM CaCl2, 1mM DTT for 15min at 30°C. Where indicated, Rb was incubated with caspase-3 (R&D Systems), calpain inhibitors II or III (Calbiochem), PD145305 or PD150606 (Calbiochem), or preincubated with recombinant human SerpinB2 (Biotech Australia, Pty. Ltd.).
Immunoblot analyses
Lysates were prepared in ice-cold lysis buffer (50 mM Tris at pH 7.4, 2 mM EDTA, 150 mM NaCl, 1% Nonidet P-40) with protease inhibitor cocktail (Roche). To dephosphorylate Rb proteins, all lysates were incubated with 10U calf intestine alkaline phosphatase (CIP, NEB) at 37°C for 30min. These conditions were sufficient to completely dephosphorylate all Rb species, verified using anti-phosphoRb antibodies (not shown). Immunoblotting was performed as described (18) using antibodies specific for calpain-1 (2H2D7C2), calpain-2 (107-82) and the calpain small subunit (8E9) (Calbiochem); GAPDH and Rb(C-15; detecting the C-terminus of Rb-aa 913-928) (Santa Cruz); caspase-3, -7, -8,-9 and PARP (Cell Signaling); SerpinB2 mAb (American Diagnostica); Rb(G3-245; detecting Rb-aa 332-344) and G99-2005 (detecting the N-terminus of Rb-aa 1-240)(BD Bioscience).
N-terminal Amino Acid Sequence Analysis
Purified recombinant Rb was incubated with calpain and Rb protein fragments were separated by SDS-PAGE, transferred to a PVDF membrane, and the transferred proteins stained with Coomassie blue. The ∼10kDa fragment was excised from the membrane, and the N-terminal amino acid residues were identified by 8-12 Edman cycles on an Applied Biosystems Procise 494 sequencer.
Calpain Activity Assays
Calpain zymography was performed on cell lysates using calpain zymograms incorporating 0.2% casein in the gel as described (29). Assay of calpain activity using the fluorogenic peptide substrate (Suc-Leu-Tyr-AMC) is described in the Online Supplementary Data.
Transfection
Human SerpinB2 in pRC/CMV has been described previously (12,18). The mammalian expression vector encoding full length CAPN4 was purchased from the ATCC (IMAGE Clone #8578411 cloned into pCMV-SPORT6). The Rb-810W mutant was generated from pcDNA3.1-Rb by site directed mutagenesis. Primer sequences are given in the Online Supplementary Data. Transient transfections in CAPN4-/- cells were performed using the AMAXA Nucleofector Kit R (Program U-30). HeLa cells were transfected using Lipofectamine 2000 (Invitrogen). Stable clonal cell lines were selected with Hygromycin B (Invitrogen).
TNFα induced apoptosis
Cells were treated with 10 ng/ml TNFα (R&D Systems) in the presence of 10 μg/ml cycloheximide (CHX), or with CHX alone. Cell death was assessed after the indicated time by counting live cells using trypan blue exclusion, by flow cytometry after propidium iodide staining, or immunoblotting for procaspase 3 activation and PARP cleavage.
ChIP Assays
Chromatin immunoprecipitation was performed using the ChIP Assay Kit (Upstate). After stimulation cells were fixed in formaldehyde at a final concentration of 1%. Chromatin was sheared by sonication (4 × 10 sec at 30% maximum potency). Immunoprecipitations were performed at 4°C overnight with anti-Rb mAb (G3-245). Immune complexes were collected with protein A and protein-DNA crosslinks reverted by heating at 65°C for 6 hrs. Immunoprecipitated DNA was isolated and used for PCR amplification. The sequences of the promoter specific primers used are given in the Online Supplementary Data.
Quantitative PCR
RNA was extracted using the RNeasy mini kit (Qiagen). For quantitative real-time PCR, specific primers and fluorescence-labeled probes for Cyclin A2 (Hs 00153138_m1), p73 (Hs 00232088_m1), Thymidine Kinase (Hs 00177406_m1), Caspase-7 (Hs 00169152_m1), Caspase-8 (Hs 00154256_m1), Caspase-10 (Hs 00154268_m1) or β-actin (Hs 99999903_m1) were obtained from Applied Biosystems Assay-on-Demand Gene Expression products and assayed using the ABI PRISM 7900HT sequence detector system.
Chemical carcinogenesis
The SerpinB2-/- mice on the C57BL/6 background (backcrossed 7 generations) were provided by D. Ginsburg (30). Both gender matched wild type littermates from heterozygote crosses and C57BL/6 mice (Jackson Laboratories) were used as controls. For the skin carcinogenesis experiments, the dorsal area of mice 8-14 weeks old were shaved and treated with a single application of 7,12-dimethyl benz(a)anthracene (DMBA) [25μg] followed by promotion with phorbol-12-myristate-13-acetate (PMA) [12.5μg], twice weekly (experiment 1) or three times weekly for 3 weeks and then twice weekly (experiment 2). Mice were monitored weekly for formation of papillomas (≥1mm). No papillomas formed as a result of treatment with vehicle alone. Animals were maintained and all experiments performed under approved University of Maryland IACUC protocols.
Immunohistochemistry on formalin-fixed tissues was performed using standard protocols with antibodies specific for Keratin 5 (AF-138, Covance) and mSerpinB2 (protein G affinity purified rabbit polyclonal generated as described (30)). Apoptosis was monitored using DeadEnd Colorimetric TUNEL System (Promega).
Statistical analyses
Student's t test was used to compare averages of normal distributed data with equal variance. Chi-square analysis was used for analysis of frequency distributions. A threshold of P<0.05 was considered significant.
Results
Proteolytic cleavage of Rb precedes Rb degradation by proteasomes
We reported previously that SerpinB2 increases Rb protein levels, resulting from inhibition of Rb degradation (10). While Rb is ultimately degraded via the proteasome pathway (22), a number of considerations indicate that it is unlikely that SerpinB2 is a direct inhibitor of the protease activity of the proteasome. Therefore, we postulated that proteasome-mediated Rb degradation may be preceded by a separate, SerpinB2-inhibitable, proteolytic cleavage event. If this was the case, then inhibition of proteasome activity could enable a normally transient Rb cleavage intermediate to be stabilized. Indeed, when Jurkat cells were treated with the proteasome inhibitors, lactacystin or MG-132, the accumulation of a faster migrating Rb intermediate of ∼95kDa was detected, which was accompanied by the loss of full length 110kD-Rb (Fig. 1A). Rb proteolytic cleavage was assessed specifically by treatment of lysates with alkaline phosphatase to dephosphorylate Rb, thus eliminating bands due to multi-phosphorylated Rb species (10). Detection of the ∼95kDa species required the presence of the caspase inhibitor, Boc-D-FMK, to inhibit caspases activated by proteasome inhibitors (31) which may also cleave and degrade Rb (23). This ‘trapped’ 95kDa-Rb intermediate showed strong immunoreactivity with an N-terminal specific Rb mAb (Rb amino acids 1-240); however, immunoreactivity was lost with a C-terminal specific Rb mAb (C-15), indicating loss of a ∼10-15kDa C-terminal Rb peptide. These data suggested the occurrence of a C-terminal, caspase-independent proteolytic cleavage of the Rb protein, producing a 95kDa-Rb intermediate targeted for proteasomal degradation.
Figure 1. Rb is a calpain substrate.

A: Trapping of a 95kDa-Rb proteolytic cleavage intermediate by proteasome inhibition. Jurkat cells were incubated for 16 hrs with proteasome inhibitors lactacystin (10 μM) or MG-132 (3 μM), in the presence of 25 μM of the caspase inhibitor, Boc-D-FMK. Harvested cell lysates were heated to 90°C for 10 min to inactivate enzymes, and then proteins were dephosphorylated by CIP treatment. Cells lysates were immunoblotted for Rb (1-240 and C-15 antibodies) or cyclin A loading control.
B: Rb cleavage by calpain generates 95kDa-Rb and is distinct from caspase cleavage. Rb containing Jurkat cell extracts in lanes 1-4 were treated with 0.2U of calpain-1 in the presence of 2 mM CaCl2 and 25 μM Boc-D-FMK with combinations of 300 mM EGTA or 1.5 μg recombinant SerpinB2. Lanes 5 and 6 were treated with 100ng of Caspase-3 in the absence or presence of 1.5 μg recombinant SerpinB2. Reactions were heated to 90°C for 10 min to inactivate enzymes, and then proteins were dephosphorylated by CIP treatment, and analysed by immunoblotting using Rb antibodies (C-15, G3-245) and GAPDH. In 3 separate experiments the magnitude of the protection of Rb by SerpinB2 in the presence of calpain averaged 56±3%, as determined by densitometric analyses.
C: Calpain cleaves Rb to directly generate 95kDa-Rb. Recombinant Rb (1 μg) was incubated with 0.02U, 0.04U or without recombinant calpain-1 for 15min at 30°C. Cleavage peptides were separated by 4-12% SDS-PAGE and visualized by (left panel) silver staining, (middle panel) by immunoblotting for Rb with the C-terminal specific antibody (C-15), or (right panel) with an anti-Rb antibody that detects the Rb N-terminus (G99-2005). Arrow indicates the 10kDa polypeptide fragment that was gel purified and subjected to N-terminal sequence analysis. The N-terminal sequence was determined to be S-P-Y-K-I-S-E-G-L, identifying cleavage at Lys810-Ser811.
D: SerpinB2 inhibits Rb cleavage by calpain. Recombinant Rb (1 μg) was incubated with a 10-fold molar excess of recombinant SerpinB2 for 15min and then 0.02U calpain-1 for 5min. The reactions were visualized by silver staining (left panel) or by immunoblot using the C-terminal Rb antibody C-15 (right panel). SerpinB2 blocked the cleavage associated with the appearance of the 95kD-Rb peptide. Full length uncleaved Rb is indicated by immunoreactivity with the C-15 antibody. The bottom diagram illustrates the C-terminal calpain and caspase cleavage sites. The calpain cleavage site is situated at the Serpin-B2-Rb interaction site (10)
Rb is C-terminally cleaved by calpain, distinct from caspase cleavage
To investigate whether intracellular calpains cleave Rb, Jurkat cell extracts containing Rb were treated with calpain and immunoblotted for detection of Rb protein. Mild calpain treatment resulted in a decrease in full length 110kDa-Rb and the appearance of a 95kDa-Rb cleavage product (Fig. 1B, Rb(G3-245)), which was identical to the Rb intermediate trapped by proteasome inhibition. An additional 100kDa-Rb band was also observed, but subsequent experiments showed that this cleavage product was generated only under cell-free conditions (data not shown), indicating it was not relevant in vivo. The 95kDa-Rb was not immunoreactive with the Rb(C-15) antibody (Fig. 1B, Rb(C-15), lane 2), showing that proteolytic cleavage occurred near the Rb C-terminus. Cleavage was calpain and Ca2+ dependent, since cleavage did not occur in the presence of Ca2+ alone (data not shown) or when Ca2+ was chelated by EGTA (Fig. 1B, lane 4). Cell extracts treated with caspase-3 did not produce the same Rb cleavage products, but instead generated 105kD-Rb (Fig. 1B, lane 5), expected from the C-terminal release of a 5kD peptide (24). These data demonstrate that calpain can mediate the cleavage of Rb near the C-terminus to generate a 95kDa-Rb cleavage product.
When recombinant SerpinB2 was added to the Jurkat cell extract prior to calpain addition, SerpinB2 inhibited the appearance of 95kDa-Rb (Fig. 1B, Rb(G3-245), lane 3 vs lane 2), and stabilized full length 110kD-Rb (Fig. 1, Rb(C-15), lane 3 vs lane 2). The cleavage of Rb by caspase was unaffected by SerpinB2 (Fig. 1B, lane 6). These data suggested that SerpinB2 could protect Rb from C-terminal cleavage by calpain.
C-terminal Rb cleavage by calpain is inhibited by SerpinB2
To determine whether calpain cleaves Rb directly, we used a purified system in which purified recombinant Rb protein was treated with catalytic amounts of calpain-1. Multiple cleavage fragments were detected (Fig. 1C), demonstrating that Rb is a direct calpain substrate. Generation of the 95kDa-Rb, recognized by the N-terminal Rb mAb (N-240) (Fig. 1C, left and right panels), occurred very rapidly, and was accompanied by the release of a ∼10kDa Rb fragment (Fig. 1C, left panel, arrow) demonstrated to be C-terminal by immunoreactivity with the C-terminal specific Rb(C-15) antibody (Fig. 1C middle panel, arrow). Experiments using calpain-2 generated similar Rb cleavage products (data not shown). When SerpinB2 was added to Rb prior to addition of calpain, the generation of the 95kDa-Rb was specifically abolished, showing that this calpain-mediated proteolytic cleavage was directly inhibitable by SerpinB2 (Fig. 1D, lane 5 vs 4). While calpain was capable of cleaving Rb at multiple sites under these cell-free conditions, the 95kDa-Rb fragment was the only cleavage product observed in cultured cells in vivo (Fig.1A and data not shown).
Identification of the SerpinB2 protected calpain cleavage site at Rb-Lys810
The identity of the calpain cleavage site within the C-terminal domain of Rb was determined by N-terminal sequence analysis of the purified 10kDa calpain cleavage product (Fig 1C, arrow). Ser811 was identified at the N-terminus of this peptide fragment, revealing the calpain cleavage site at P1 Lys810. This cleavage is consistent with calpain substrate preference for a basic amino acid at the P1 position (27,32). Using fluorogenic peptide assays of calpain protease activity, SerpinB2 did not inhibit calpain protease activity directly (data not shown). Considering that Lys810 is centered within the SerpinB2-Rb binding domain previously mapped to Rb amino acids 768-785 and 825-840 (10)(Fig. 1D), these data may suggest that SerpinB2-Rb binding interaction may interfere with accessibility of calpain to its cleavage site at Rb-Lys810.
Calpain inhibitors stabilize Rb, decreasing Rb turnover
Our data provided evidence for calpain cleavage of Rb in cell-free systems, which could control Rb levels by regulating Rb turnover. To determine whether inhibition of calpain affects Rb turnover in cells, we exposed several different cell types to a series of cell-permeable calpain inhibitors (Fig. 2A). Calpain inhibition specifically enhanced the accumulation of full length Rb protein (Fig. 2A (left)), which was not accompanied by changes in Rb mRNA levels (measured by Q-PCR, data not shown), consistent with post-transcriptional stabilization of Rb protein and inhibition of Rb turnover. The increased Rb was not a general stress response to calpain inhibitors since cell viability did not change throughout the time period of the experiments (data not shown). Time course studies in Jurkat cells revealed that Rb stabilization was time dependent, being maximally effective during the first 4-8 hrs after exposure to calpin inhibitors (Fig. 2A (right)).
Figure 2. Calpain induces Rb degradation in calpain-deficient MEFs.
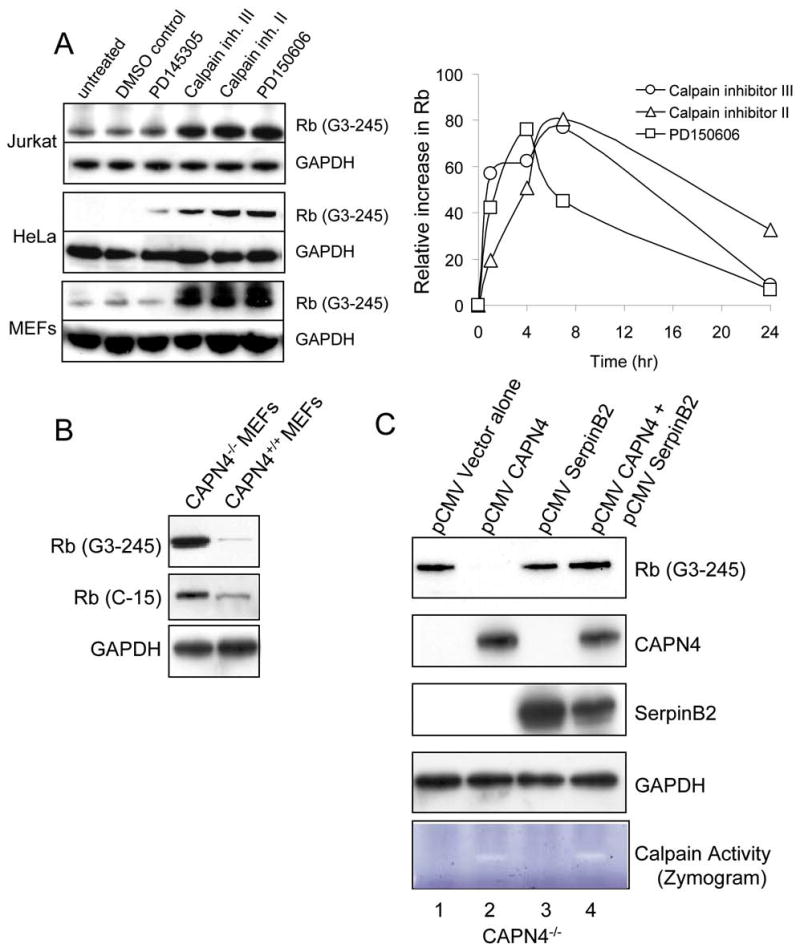
A: Inhibition of calpain activity stabilizes Rb and inhibits Rb turnover. (left) Jurkat cells were incubated in the presence of the cell permeable competitive inhibitors, calpain inhibitor II (N-acetyl-leu-leu-methional (aLLM), 230nM), calpain inhibitor III (carbobenzoxy-valyl-phenylalanal, 8 nM), the noncompetitive selective calpain inhibitor PD150606 (3-(4-iodophenyl)-2-mercapto-(Z)-2-propenoic acid, 400 nM) which targets the Ca2+ binding sites of calpains and its noninhibitory PD145305 analogue, or DMSO vehicle control. HeLa, RAW 264.7 and MEFs were cultured in the presence of 10 μM calpain inhibitors. After 7hrs, cell lysates were immunoblotted for Rb(G3-245) or GAPDH control. (right) Time course of Rb stabilization in Jurkat cells. Jurkat cells were incubated in the presence of the different cell permeable calpain inhibitors for up to 24 hrs. Rb protein was detected by immunoblot and signal intensity normalized to the untreated control at each time point.
B: Rb protein levels are enhanced in calpain deficient MEFs. Lysates from CAPN4+/+ and CAPN4-/- cells were immunoblotted for Rb(G3-245 or C-15) or GAPDH control.
C: Calpain mediates cleavage and degradation of Rb. CAPN4-/- cells were transfected with expression plasmids encoding the calpain regulatory small subunit CAPN4, SerpinB2, both of these plasmids or vector alone. Transfected cells were cultured in complete media containing 25μM Boc-D-FMK (ICN), added fresh every 12 hrs. Forty eight hrs after transfection, cells were treated with 1μM Ca2+ ionophore A23187 for 1 hr to activate calpain, prior to immunoblot analysis. Restored calpain-1 proteolytic activity was detected by calpain zymography after incubation of the cells with the Ca2+ ionophore A23187 for 1 hr.
Calpain regulates Rb turnover directly, through a mechanism inhibitable by SerpinB2
To further investigate the participation of calpain in the regulation of Rb turnover, we utilized calpain-deficient mouse embryonic fibroblasts (CAPN4-/- MEFs) (28). Genetic deletion of the Capn4 gene abolishes both calpain-1 and calpain-2 activities (28). The CAPN4-/- MEFs had substantially increased Rb protein levels compared with control CAPN4+/+ MEFs (Fig. 2B), that could not be accounted for by increased Rb gene transcription (data not shown), implicating calpain deficiency with stabilization of Rb. We attempted to rescue the CAPN4-/- MEF phenotype by transfecting an expression plasmid encoding CAPN4 (pCMV-CAPN4) into the CAPN4-/- MEFs (Fig. 2C). Restoration of calpain activity in the CAPN4-/- MEFs was demonstrated by the presence of CAPN4 protein expression (Fig. 2C, lanes 2&4, CAPN4) and detection of restored calpain-1 proteolytic activity by calpain zymography (Fig. 2C, lanes 2&4, Zymogram). Restoration of calpain activity resulted in a dramatic decrease in Rb protein levels (Fig. 2C, lane 2 vs lane 1, Rb(G3-245)), showing that calpain specifically mediates Rb turnover in these cells. Strikingly, when an expression plasmid encoding SerpinB2 (pCMV-SerpinB2) was co-transfected along with the pCMV-CAPN4 into the CAPN4-/- MEFs, Rb was stabilized and detected at a level indistinguishable from control transfected cells (Fig. 2C, Rb(G3-245), lane 4 vs lane 1), even though calpain activity was rescued (Fig. 2C, lane 4, CAPN4 and Zymogram). SerpinB2 expression alone in the absence of calpain did not affect Rb levels (Fig. 2C; lane 3 vs. 1, Rb(G3-245)). Together, these data provide compelling evidence for regulation of Rb turnover though calpain cleavage, by a mechanism inhibitable by SerpinB2.
Rb cleavage by calpain occurs during TNFα-induced cell death
We previously identified SerpinB2 as a cytoprotective factor that confers resistance to TNFα-induced apoptosis in HeLa cells (12,18). Calpains have been implicated in orchestrating the induction of apoptosis (34) and Rb loss is associated with enhanced susceptibility to apoptosis when cells are exposed to TNFα (6,12-14). To determine whether SerpinB2 may confer resistance to apoptosis though the calpain-Rb pathway, we investigated the effect of SerpinB2 on Rb levels during apoptosis induced by TNFα in HeLa cells. Exposure of HeLa cells to TNFα and CHX initiates cell death (18) evidenced by activated caspase-3 and appearance of the marker of caspase activation, poly(ADP-ribose) polymerase (PARP) cleavage within 3hrs (Fig. 3A). The HeLa cell line expressing SerpinB2 (S1a) shows resistance to TNFα induced death (18) and further demonstrated delayed caspase activation (Fig. 3A). These TNFα-resistant S1a cells showed increased levels of C-terminally intact Rb (Fig. 3A, Rb(C-15)), suggesting that resistance to TNFα-induced death could be mediated by SerpinB2 inhibition of Rb loss, via protection of Rb from calpain cleavage. In contrast, full length Rb begins to disappear in HeLa cells after exposure to TNF for 3 hrs (Supplementary Figure 1). To specifically investigate the role of calpain and SerpinB2 in protecting Rb during TNFα-induced apoptosis, we investigated calpain deficient MEFs. CAPN4-/- MEFs, which have elevated Rb (Fig. 2B), show resistance to TNFα-induced cell death compared with CAPN4+/+ MEFs (Fig. 3B). When calpain activity was restored in CAPN4-/- MEFs by transfection of CAPN4, the sensitivity of the CAPN4-/- MEFs to TNFα-induced death was rescued (Fig. 3B, CAPN4). Co-expression of SerpinB2 together with CAPN4, could block calpain-dependent cell death, resulting in resistance to TNFα (Fig. 3B, CAPN4+SerpinB2). These data show that calpain cleavage of Rb contributes to initiation of TNFα-induced death and that this activity can be blocked by SerpinB2.
Figure 3. Rb cleavage by calpain occurs during TNFα induced apoptosis and is protected by SerpinB2.
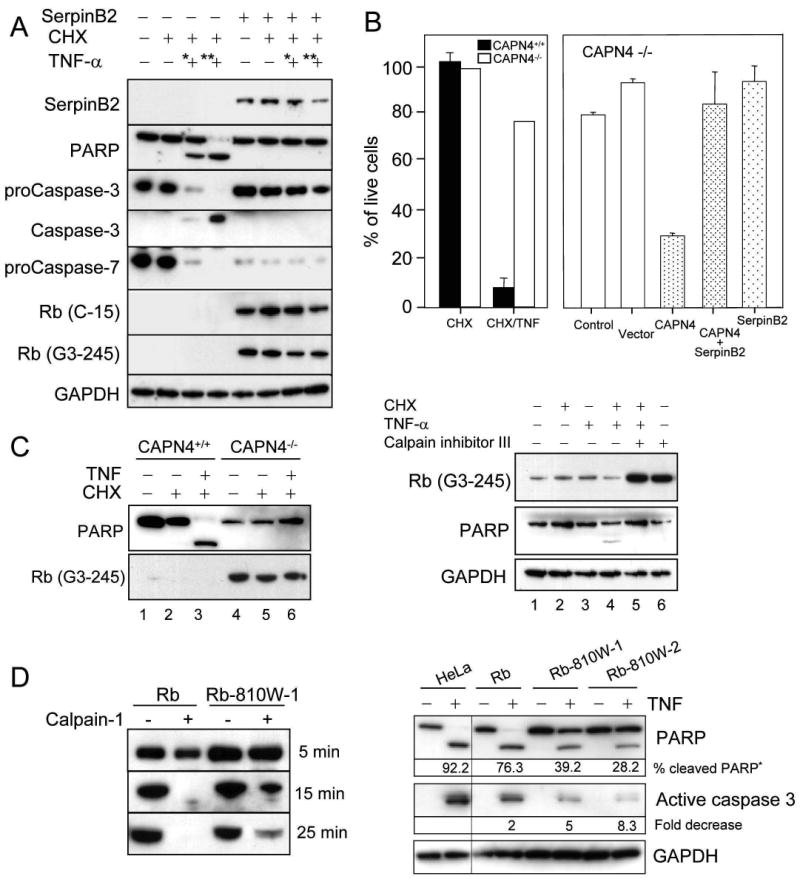
A: SerpinB2 delays caspase activation in HeLa cells by stabilizing Rb. HeLa and HeLa expressing SerpinB2 (S1a)(18) cells were treated with CHX alone or in combination with * 1 ng/ml or ** 10 ng/ml of human TNFα for 3 hrs. Cell lysates were immunoblotted with the indicated antibodies.
B: CAPN4-/- MEFs are more resistant to TNFα-induced death, and can become sensitive by restoration of calpain. CAPN4-/- and CAPN4+/+ cells were treated with mouse TNFα and CHX for 4hrs and live cells quantitated by trypan blue exclusion. Each treatment was assessed in duplicate, error bars represent standard deviation. In addition, CAPN4-/- cells were transfected with plasmids encoding CAPN4 or SerpinB2 or co-transfected with both plasmids. Twenty-four hrs after transfection, cells were exposed to CHX alone, or TNFα and CHX for 3 hrs and live cells counted by Trypan Blue exclusion. Graph shows % live cells relative to treatment with CHX alone in each sample, and represents the mean and SD from triplicate experiments.
C: Calpain deficiency in CAPN4-/- MEFs or inhibition of calpain in TNFα sensitive HeLa cells decreases caspase activation. (left) CAPN4+/+ or CAPN4-/- cells were exposed to mouse TNFα and CHX for 3hrs and cell death monitored by immunoblotting for Rb (G3-245) and PARP cleavage. (right) HeLa cells were incubated for 1hr with 10 μM calpain inhibitor III and then subjected to TNFα and CHX for 2 hrs. Cells lysates were immunoblotted for Rb (G3-245) or GAPDH for loading control. Induction of cell death was monitored by immunoblotting for PARP cleavage.
D: The dominant-negative Rb-810W mutant shows resistance to calpain cleavage and delays caspase activation in response to TNFα-induced cell death. (left) Lysates from HeLa cells stably producing Rb or Rb(K810W) mutant were subjected to 0.2U calpain-1 digestion for the indicated times. Proteins were separated and immunoblotted for Rb(G3-245). (right) HeLa cells and HeLa cell lines stably expressing wild type Rb or 2 independent clonal cell lines expressing the Rb(K810W) mutant were exposed to human TNFα and CHX for 2 hrs. Immunoblots for PARP and active caspase-3 were quantified by densitometry using Scion Image analysis software and the latter normalized for protein levels using GAPDH. * relative to TNFα treated samples.
Resistance to TNFα-induced cell death in the CAPN4-/- MEFs was associated with delayed caspase activation, evidenced by delayed PARP cleavage in CAPN4-/- MEFs compared with CAPN4+/+ MEFs upon exposure to TNFα (Fig. 3C, left, lane 6 vs lane 3). In a similar way, pretreatment of TNFα–sensitive HeLa cells (18) with calpain inhibitor III to inhibit calpain activity, stabilized Rb and delayed caspase activation in response to TNFα (Fig. 3C, right, lane 5 vs lane 4).
These data implicate regulation of Rb by calpain during induction of the TNFα-induced cell death response. To explore this directly, a mutant Rb (Rb-810W) was generated and when expressed in HeLa cells (Supplementary Figure 2), showed resistance to cleavage by exogenous calpain at Rb-Lys810 compared with wild type Rb (Fig. 3D, left panel). HeLa cells transfected with Rb-810W were more resistant to caspase activation after exposure to TNFα, compared with HeLa cells transfected with wild type Rb or parental HeLa cells, evidenced by significantly less PARP cleavage and several-fold less active caspase 3 (Fig. 3D, right panel). These studies suggest that regulation of Rb by calpain contributes to induction of TNFα–induced apoptotic responses and that inhibition or blocking of calpain cleavage of Rb can delay caspase activation and confer resistance to TNFα induced death.
SerpinB2 sustains Rb mediated repression of proapoptotic genes
How does SerpinB2 stabilization of Rb cause anti-apoptotic activity? Increased stabilization of Rb might be expected to mediate anti-apoptotic activity though repression of the transcription of proapoptotic E2F target genes. A Rb-containing signal transduction module present on selective E2F-regulated proapoptotic gene promoters, (eg. APAF1, p21, p73, and several caspases) has been associated with repression of apoptotic cell death (9,36). To determine whether SerpinB2 stabilization of Rb contributes to Rb-mediated repression of proapoptotic genes, HeLa and S1a cells were compared for differential Rb binding to promoters of E2F-regulated genes during induction of TNFα-induced death using chromatin immunoprecipitation assays. Rb binding to the promoters of the proapoptotic genes, p73, caspase-7 and p21 was substantially decreased after exposure of HeLa cells to TNFα, whereas, Rb binding to these same proapoptotic promoters in SerpinB2 expressing S1a cells was unaffected (Fig. 4A). We observed no significant changes in Rb promoter occupancy upon treatment with TNFα for the cell cycle-regulated CDC24B, CDC2 or thymidine kinase gene promoters. Interestingly, SerpinB2 also sustained repression of the cell cycle gene, cyclin A2, which has been reported previously to be subject to regulatory mechanisms similar to the proapoptotic genes (9). PCR amplification of the β-actin promoter (a control to monitor specificity of the immunoprecipitation), did not amplify any DNA (data not shown). Measurement of transcription of several of these E2F regulated genes and additional caspases by quantitation of mRNA (Fig. 4B) confirmed their repression by Rb in SerpinB2 expressing S1a cells.
Figure 4. SerpinB2 stabilization of Rb sustains transcriptional repression of proapoptotic genes.

A: Rb repression of proapoptotic genes is enhanced in the presence of SerpinB2. In vivo detection of promoter occupancy by Rb by chromatin immunoprecipitation assay. HeLa and HeLa expressing SerpinB2 cells were treated with 2.5 μg/ml CHX with or without 10ng/ml human TNFα for 3hrs. Lysates were immunoprecipitated with Rb(G3-245) antibody and precipitated chromatin samples were amplified with primers specific for E2F regulated gene promoters. Input corresponds to PCR reactions performed on total DNA prior to immunoprecipitation to ensure equal amounts of DNA were present in samples.
B: SerpinB2 enhances Rb repression of transcription of E2F regulated genes and caspase genes after apoptotic stimuli. Quantitative PCR analysis of mRNA in Hela and HeLa expressing SerpinB2 cells following treatment with TNFα and CHX for 30 min. mRNA levels are normalized to β-actin. Error bars represent the mean and standard deviation from triplicate experiments.
SerpinB2-deficiency reduces susceptibility to Rb-dependent skin carcinogenesis
Constitutive Rb-mediated repression of proapoptotic gene promoters is postulated to be a host-surveillance mechanism whereby cells that have lost Rb function activate a cell death response, thereby eliminating would-be tumor cells early in the transformation process (3). Accordingly, Rb inactivation in epidermal keratinocytes renders mice less susceptible to DMBA/PMA induced skin carcinogenesis (4). Since SerpinB2 is abundantly expressed by proliferating basal keratinocytes (Fig. 5A) we postulated a role for SerpinB2 as a protector of Rb during skin tumor surveillance. SerpinB2+/+ mice (n=11) and SerpinB2-/- mice (n=12) were treated with DMBA followed by twice weekly applications of PMA. After 9 weeks, hyperplasia and then the formation of papillomas were observed in SerpinB2+/+ control mice, whereas the SerpinB2-/- mice showed a delay in onset of these features. After 21 weeks, when all of the SerpinB2+/+ control group had developed papillomas (example illustrated in Fig. 5A, last panel), only 42% (5/12) of the SerpinB2-/- mice developed papillomas (P<0.01; Chi Squared). Tumor multiplicity (mean number of tumors per mice) was markedly reduced in SerpinB2-/- mice [mean 0.8±0.3 tumors; range 0-3] compared with control SerpinB2+/+ mice [mean 5.8±0.8 tumors; range 3-10].
Figure 5. SerpinB2-deficiency reduces susceptibility to Rb-dependent skin carcinogenesis.
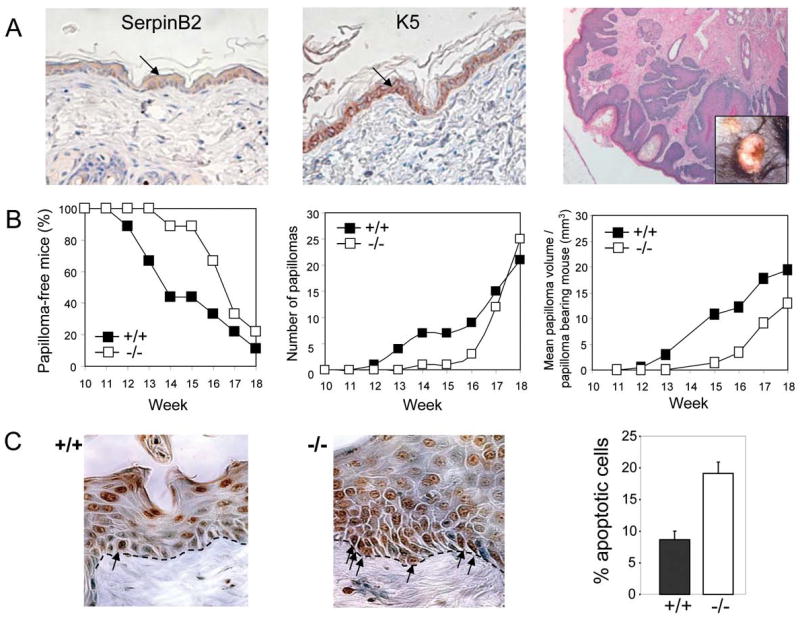
A: Histopathology of representative papillomas. In normal adult mouse epidermis, SerpinB2 is present in suprabasal and basal keratinocytes (left), where it co-localizes with basal K5 (middle), a predominant keratin of proliferating basal keratinocytes. (right) Hemotoxylin & eosin stained section of a representative SerpinB2+/+ papilloma. Inset shows gross appearance.
B: SerpinB2 deficiency reduces susceptibility to tumor formation during skin carcinogenesis. SerpinB2+/+ (n=9) and SerpinB2-/- mice (n=9) were treated with DMBA/PMA as per experiment 2 and the onset of papilloma formation followed with time. There was a significant delay in the onset of papillima incidence in the SerpinB2-/- animals (left panel). Tumor multiplicity (middle panel) represents the total number of papillomas per genotype (n=9). The mean papilloma volume per papilloma bearing mouse is represented in the right panel. Once formed, papillomas did not produce SerpinB2, and SerpinB2 deficiency had no effect on the rate of papilloma progression (data not shown).
C: Increased apoptosis in SerpinB2 deficient hyperplastic skin lesions. TUNEL staining of apoptotic keratinocytes in the basal layer of SerpinB2+/+ (left panel) and SerpinB2-/- (middle panel) epidermis. Arrows indicate TUNEL positive basal keratinocytes localized along the basement membrane (dotted line). Percentage of apoptotic cells in the basal layer (right panel). The number of TUNEL positive cells relative to the total number of cells along the basement membrane were counted. Data represents specimens from 4 mice of each genotype (2 sections per mouse) (bars, ± SD; P ≤0.001, Students t test).
In a second experiment, where a more aggressive regimen of PMA application was followed, there was again a significant delay in the onset of tumor incidence in the SerpinB2-/- animals (Fig. 5B, left panel). By week 15, 56% of the SerpinB2+/+ mice had formed papillomas compared with 11% of the SerpinB2-/- mice (P<0.03; Chi Squared). Consistent with the delay in tumor onset, the numbers of papillomas present on SerpinB2-/- mice were initially reduced, although with repeated weekly PMA applications, the numbers of tumors eventually reached the levels present on the wild type counterparts (Fig. 5B, middle panel). Tumor volumes were consistently reduced in SerpinB2-/- mice (Fig. 5B, right panel), most likely due to a delay in the initiation of tumor formation and not due to an altered tumor growth rate. Once initiated, tumors progressed at comparable rates in both genotypes (data not shown). The response of the SerpinB2-/- mice is very similar to the keratinocyte-targeted Rb-/- phenotype (37), with genetic loss of Rb or SerpinB2 leading to fewer and smaller papillomas.
SerpinB2-deficiency is associated with increased apoptosis
In the initiation stages of tumor development during DMBA/PMA skin carcinogenesis, cell proliferation competes with apoptosis (38), and Rb plays a critical role (37). Quantitation of apoptotic cells in hyperplastic lesions of SerpinB2-/- mice revealed a marked increase in apoptotic index (Fig. 5C), analogous to enhanced apoptosis detected in hyperplastic lesions of keratinocyte-targeted Rb-/- mice (4). These data suggest that SerpinB2 deficiency decreases keratinocyte survival early during the transformation process, resulting in delayed onset of papilloma development. We can speculate that TNFα induced by PMA during skin carcinogenesis (39) could initiate a calpain-dependent pathway to eliminate Rb, that would be blocked in cells expressing SerpinB2. These findings suggest that sustained SerpinB2 expression is important for the continued survival of basal keratinocytes and acts by reducing their sensitivity to apoptosis during stress, thereby enabling better survival and paradoxically, promoting tumor development.
Discussion
The elimination of Rb is a cellular response to stress induced by genotoxic agents and TNFα (16). Depending on the biological context, Rb loss can precipitate induction of a cell death responses and apoptotic cell death. Here we show that the cytoprotective protein SerpinB2 protects cells from Rb loss and delays cell death. The mechanism of SerpinB2 protection involves inhibition of calpain cleavage of Rb and subsequent proteasome degradation, thus promoting elevated Rb levels and enhancing Rb-mediated repression of E2F-regulated proapoptotic genes. Loss of this cytoprotective pathway, such as occurs with SerpinB2 deficiency, increases susceptibility to cell death. These data define a novel pathway for rapid and selective modulation of Rb that impacts cell survival pathways.
Rb degradation is required for TNFα-receptor I to signal apoptosis (25). Our findings that Rb is a calpain substrate and that calpain regulates Rb levels, provide an additional mechanism in addition to caspase cleavage of Rb (25), for eliminating Rb. The role of calpains in cell death, however, is clearly complex. Calpains are reported to play both proapoptotic and anti-apoptotic roles in several different cell systems and in response to a wide range of stimuli (40). Some of the complexity in calpain functions may be due to cell-specific regulatory factors such as the presence of SerpinB2. Our data shows that calpain, likely activated as a consequence of stress-induced signaling, contributes to elimination of Rb and subsequent proapoptotic signaling events through loss of Rb mediated transcriptional repression.
Calpain cleaves Rb within the C-terminal domain (Rb-Lys810) and the calpain-cleaved Rb1-810 intermediate is targeted for proteasomal degradation. The Rb C-terminal domain is recognized to contribute to Rb-mediated growth suppression through binding to transcriptional regulators such as c-Abl (41) and E2F1 (42). It may be that differential protein-protein interactions associated with this domain potentially dictate the diversity of Rb functions in transcription, chromatin remodeling, differentiation, and cell survival. Further studies are required to determine whether calpain cleavage of Rb affects binding of transcriptional regulators that associate with Rb, and subsequent downstream signaling events.
SerpinB2 protects Rb from calpain cleavage. SerpinB2 is a member of the intracellular clade B or ov-serpin sub-family (43). Many cells involved in the innate immune response produce clade B serpins. SerpinB2 is expressed in a limited number of cells, placental trophoblasts, monocytes/macrophages, and keratinocytes, however it may be strongly up-regulated in multiple cell types following exposure to inflammatory and cellular stress mediators, including cytokines, growth factors, viruses and bacterial endotoxin (11). Several other clade B members protect cells from exogenous and endogenous proteinase-mediated injury triggered by various death-inducing stimuli utilizing a wide range of mechanisms (44-46,46-49). In addition, clade B serpins are implicated in direct inhibition of the activity of specific proteolytic enzymes, mostly serine and/or papain-like cysteine proteases. We did not find that SerpinB2 inhibited calpain protease activity directly, suggesting a unique mechanism of inhibition that likely involves Rb binding via the PENF motif within the C-D interhelical loop region of SerpinB2 (10).
The induction of cytoprotective SerpinB2 could impact cell survival for multiple physiological stress induced pathways, but, in addition, would likely be restricted to cells or situations where Rb is a determinant of the death response. DMBA/PMA induced skin carcinogenesis is an Rb dependent process (4,37,50). Transgenic over-expression of SerpinB2 in mouse skin increases survival of transformed keratinocytes during DMBA/PMA skin carcinogenesis (15), and we show here that SerpinB2 deficiency decreases their survival by reducing their susceptibility to transformation. PMA tumor promotion during skin carcinogenesis induces TNFα (39), which may initiate a calpain-dependent pathway to eliminate Rb, and thus initiate the activation of a cell death response unless protective factors such as SerpinB2 are present. These data provide new insight into cell-specific and serpin-dependent mechanisms by which cell death versus prosurvival cell fates may be determined.
Supplementary Material
Online supplementary methods include the oligonucleotide primer sequences for construction of the Rb mutant and for the ChIP Assay.
Acknowledgments
The work was supported by grants from the NCI (R01 CA098369) to TMA and the NH&MRC (Australia) to TMA and AS. MB is supported by a CJ Martin Training Fellowship from the NH&MRC (Australia). We thank Dr John Elce from the Queen's University, Canada for CAPN4-/- MEFs and Mr. Brian Hampton for amino-terminal sequence analyses.
Abbreviations used
- PAI
plasminogen activator inhibitor
- DMBA
7,12-dimethyl benz(a)anthracene
- PARP
poly(ADP-ribose) polymerase
- PMA
phorbol-12-myristate-13-acetate
- Rb
retinoblastoma
- TNFα
tumor necrosis factor alpha
References
- 1.Du W, Pogoriler J. Retinoblastoma family genes. Oncogene. 2006;25:5190–200. doi: 10.1038/sj.onc.1209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chau BN, Pan CW, Wang JY. Separation of Anti-Proliferation and Anti-Apoptotic Functions of Retinoblastoma Protein through Targeted Mutations of Its A/B Domain. PLoS ONE. 2006;1:e82. doi: 10.1371/journal.pone.0000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chau BN, Wang JY. Coordinated regulation of life and death by RB. Nat Rev Cancer. 2003;3:130–8. doi: 10.1038/nrc993. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz S, Santos M, Lara MF, Segrelles C, Ballestin C, Paramio JM. Unexpected roles for pRb in mouse skin carcinogenesis. Cancer Res. 2005;65:9678–86. doi: 10.1158/0008-5472.CAN-05-1853. [DOI] [PubMed] [Google Scholar]
- 5.Young AP, Longmore GD. Differential regulation of apoptotic genes by Rb in human versus mouse cells. Oncogene. 2004;23:2587–99. doi: 10.1038/sj.onc.1207330. [DOI] [PubMed] [Google Scholar]
- 6.Wang JY. Nucleo-cytoplasmic communication in apoptotic response to genotoxic and inflammatory stress. Cell Res. 2005;15:43–8. doi: 10.1038/sj.cr.7290263. [DOI] [PubMed] [Google Scholar]
- 7.DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci U S A. 1997;94:7245–50. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller H, Bracken AP, Vernell R, et al. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001;15:267–85. doi: 10.1101/gad.864201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahle Z, Polakoff J, Davuluri RV, et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nat Cell Biol. 2002;4:859–64. doi: 10.1038/ncb868. [DOI] [PubMed] [Google Scholar]
- 10.Darnell GA, Antalis TM, Johnstone RW, et al. Inhibition of retinoblastoma protein degradation by interaction with the serpin plasminogen activator inhibitor 2 via a novel consensus motif. Mol Cell Biol. 2003;23:6520–32. doi: 10.1128/MCB.23.18.6520-6532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruithof EK, Baker MS, Bunn CL. Biological and clinical aspects of plasminogen activator inhibitor type 2. Blood. 1995;86:4007–24. [PubMed] [Google Scholar]
- 12.Kumar S, Baglioni C. Protection from tumor necrosis factor-mediated cytolysis by overexpression of plasminogen activator inhibitor type-2. J Biol Chem. 1991;266:20960–4. [PubMed] [Google Scholar]
- 13.Kasyapa CS, Kunapuli P, Hawthorn L, Cowell JK. Induction of the plasminogen activator inhibitor-2 in cells expressing the ZNF198/FGFR1 fusion kinase that is involved in atypical myeloproliferative disease. Blood. 2006;107:3693–9. doi: 10.1182/blood-2005-04-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varro A, Noble PJ, Pritchard DM, et al. Helicobacter pylori induces plasminogen activator inhibitor 2 in gastric epithelial cells through nuclear factor-kappaB and RhoA. Implications for invasion and apoptosis. Cancer Res. 2004;64:1695–702. doi: 10.1158/0008-5472.can-03-2399. [DOI] [PubMed] [Google Scholar]
- 15.Zhou HM, Bolon I, Nichols A, Wohlwend A, Vassalli JD. Overexpression of plasminogen activator inhibitor type 2 in basal keratinocytes enhances papilloma formation in transgenic mice. Cancer Res. 2001;61:970–6. [PubMed] [Google Scholar]
- 16.Antalis TM, La Linn M, Donnan K, et al. The serine proteinase inhibitor (serpin) plasminogen activation inhibitor type 2 protects against viral cytopathic effects by constitutive interferon alpha/beta priming. J Exp Med. 1998;187:1799–811. doi: 10.1084/jem.187.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Losick VP, Isberg RR. NF-kappaB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J Exp Med. 2006;203:2177–89. doi: 10.1084/jem.20060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson JL, Bates EJ, Ferrante A, Antalis TM. Plasminogen activator inhibitor type 2 inhibits tumor necrosis factor alpha-induced apoptosis. Evidence for an alternate biological function. J Biol Chem. 1995;270:27894–904. doi: 10.1074/jbc.270.46.27894. [DOI] [PubMed] [Google Scholar]
- 19.Gan H, Newman GW, Remold HG. Plasminogen activator inhibitor type 2 prevents programmed cell death of human macrophages infected with Mycobacterium avium, serovar 4. J Immunol. 1995;155:1304–15. [PubMed] [Google Scholar]
- 20.Pytel BA, Peppel K, Baglioni C. Plasminogen activator inhibitor type-2 is a major protein induced in human fibroblasts and SK-MEL-109 melanoma cells by tumor necrosis factor. J Cell Physiol. 1990;144:416–22. doi: 10.1002/jcp.1041440308. [DOI] [PubMed] [Google Scholar]
- 21.Helt AM, Galloway DA. Mechanisms by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis. 2003;24:159–69. doi: 10.1093/carcin/24.2.159. [DOI] [PubMed] [Google Scholar]
- 22.Ying H, Xiao ZX. Targeting retinoblastoma protein for degradation by proteasomes. Cell Cycle. 2006;5:506–8. doi: 10.4161/cc.5.5.2515. [DOI] [PubMed] [Google Scholar]
- 23.Tan X, Wang JY. The caspase-RB connection in cell death. Trends Cell Biol. 1998;8:116–20. doi: 10.1016/s0962-8924(97)01208-7. [DOI] [PubMed] [Google Scholar]
- 24.Tan X, Martin SJ, Green DR, Wang JY. Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J Biol Chem. 1997;272:9613–16. doi: 10.1074/jbc.272.15.9613. [DOI] [PubMed] [Google Scholar]
- 25.Chau BN, Borges HL, Chen TT, Masselli A, Hunton IC, Wang JY. Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat Cell Biol. 2002;4:757–65. doi: 10.1038/ncb853. [DOI] [PubMed] [Google Scholar]
- 26.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 27.Sato K, Kawashima S. Calpain function in the modulation of signal transduction molecules. Biol Chem. 2001;382:743–51. doi: 10.1515/BC.2001.090. [DOI] [PubMed] [Google Scholar]
- 28.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4. calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–81. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raser KJ, Posner A, Wang KK. Casein zymography. a method to study mu-calpain, m-calpain, and their inhibitory agents. Arch Biochem Biophys. 1995;319:211–16. doi: 10.1006/abbi.1995.1284. [DOI] [PubMed] [Google Scholar]
- 30.Dougherty KM, Pearson JM, Yang AY, Westrick RJ, Baker MS, Ginsburg D. The plasminogen activator inhibitor-2 gene is not required for normal murine development or survival. Proc Natl Acad Sci U S A. 1999;96:686–91. doi: 10.1073/pnas.96.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandra J, Niemer I, Gilbreath J, et al. Proteasome inhibitors induce apoptosis in glucocorticoid-resistant chronic lymphocytic leukemic lymphocytes. Blood. 1998;92:4220–9. [PubMed] [Google Scholar]
- 32.Tompa P, Buzder-Lantos P, Tantos A, et al. On the sequential determinants of calpain cleavage. J Biol Chem. 2004;279:20775–85. doi: 10.1074/jbc.M313873200. [DOI] [PubMed] [Google Scholar]
- 33.Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–7. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- 34.Johnson DE. Noncaspase proteases in apoptosis. Leukemia. 2000;14:1695–703. doi: 10.1038/sj.leu.2401879. [DOI] [PubMed] [Google Scholar]
- 35.McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun. 1997;239:357–66. doi: 10.1006/bbrc.1997.7409. [DOI] [PubMed] [Google Scholar]
- 36.Young AP, Longmore GD. Differences in stability of repressor complexes at promoters underlie distinct roles for Rb family members. Oncogene. 2004;23:814–23. doi: 10.1038/sj.onc.1207187. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz S, Santos M, Paramio JM. Is the loss of pRb essential for the mouse skin carcinogenesis? Cell Cycle. 2006;5:625–9. doi: 10.4161/cc.5.6.2580. [DOI] [PubMed] [Google Scholar]
- 38.Hennings H, Glick AB, Lowry DT, Krsmanovic LS, Sly LM, Yuspa SH. FVB/N mice. an inbred strain sensitive to the chemical induction of squamous cell carcinomas in the skin. Carcinogenesis. 1993;14:2353–8. doi: 10.1093/carcin/14.11.2353. [DOI] [PubMed] [Google Scholar]
- 39.Scott KA, Moore RJ, Arnott CH, et al. An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol Cancer Ther. 2003;2:445–51. [PubMed] [Google Scholar]
- 40.Tan Y, Wu C, De Veyra T, Greer PA. Ubiquitous calpains promote both apoptosis and survival signals in response to different cell death stimuli. J Biol Chem. 2006;281:17689–98. doi: 10.1074/jbc.M601978200. [DOI] [PubMed] [Google Scholar]
- 41.Whitaker LL, Su H, Baskaran R, Knudsen ES, Wang JY. Growth suppression by an E2F-binding-defective retinoblastoma protein (RB). contribution from the RB C pocket. Mol Cell Biol. 1998;18:4032–42. doi: 10.1128/mcb.18.7.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dick FA, Dyson N. pRB contains an E2F1-specific binding domain that allows E2F1-induced apoptosis to be regulated separately from other E2F activities. Mol Cell. 2003;12:639–49. doi: 10.1016/s1097-2765(03)00344-7. [DOI] [PubMed] [Google Scholar]
- 43.Remold-O'Donnell E. The ovalbumin family of serpin proteins. FEBS Lett. 1993;315:105–8. doi: 10.1016/0014-5793(93)81143-n. [DOI] [PubMed] [Google Scholar]
- 44.Bird PI. Serpins and regulation of cell death. Results Probl Cell Differ. 1998;24:63–89. doi: 10.1007/978-3-540-69185-3_4. [DOI] [PubMed] [Google Scholar]
- 45.Suminami Y, Nagashima S, Vujanovic NL, Hirabayashi K, Kato H, Whiteside TL. Inhibition of apoptosis in human tumour cells by the tumour-associated serpin, SCC antigen-1. Br J Cancer. 2000;82:981–9. doi: 10.1054/bjoc.1999.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeda A, Kajiya A, Iwasawa A, Nakamura Y, Hibino T. Aberrant expression of serpin squamous cell carcinoma antigen 2 in human tumor tissues and cell lines. evidence of protection from tumor necrosis factor-mediated apoptosis. Biol Chem. 2002;383:1231–6. doi: 10.1515/BC.2002.136. [DOI] [PubMed] [Google Scholar]
- 47.Schleef RR, Chuang TL. Protease inhibitor 10 inhibits tumor necrosis factor alpha -induced cell death. Evidence for the formation of intracellular high M(r) protease inhibitor 10-containing complexes. J Biol Chem. 2000;275:26385–9. doi: 10.1074/jbc.C000389200. [DOI] [PubMed] [Google Scholar]
- 48.Welss T, Sun J, Irving JA, et al. Hurpin is a selective inhibitor of lysosomal cathepsin L and protects keratinocytes from ultraviolet-induced apoptosis. Biochemistry. 2003;42:7381–9. doi: 10.1021/bi027307q. [DOI] [PubMed] [Google Scholar]
- 49.Bailey CM, Khalkhali-Ellis Z, Seftor EA, Hendrix MJ. Biological functions of maspin. J Cell Physiol. 2006;209:617–24. doi: 10.1002/jcp.20782. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz S, Santos M, Segrelles C, et al. Unique and overlapping functions of pRb and p107 in the control of proliferation and differentiation in epidermis. Development. 2004;131:2737–48. doi: 10.1242/dev.01148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online supplementary methods include the oligonucleotide primer sequences for construction of the Rb mutant and for the ChIP Assay.


