Abstract
Serotonin (5-HT) is an indirect modulator of the electric organ discharge (EOD) in the weakly electric gymnotiform fish, Brachyhypopomus pinnicaudatus. Injections of 5-HT enhance EOD waveform “masculinity”, increasing both waveform amplitude and the duration of the second phase. This study investigated the pharmacological identity of 5-HT receptors that regulate the electric waveform and their effects on EOD amplitude and duration. We present evidence that two sets of serotonin receptors modulate the EOD in opposite directions. We found that the 5HT1AR agonist 8-OH-DPAT diminishes EOD duration and amplitude while the 5HT1AR antagonist WAY100635 increases these parameters. In contrast, the 5HT2R agonist α-Me-5-HT increases EOD amplitude but not duration, yet 5-HT-induced increases in EOD duration can be inhibited by blocking 5HT2A/2C-like receptors with ketanserin. These results show that 5-HT exerts bi-directional control of EOD modulations in B. pinnicaudatus via action at receptors similar to mammalian 5HT1A and 5HT2 receptors. The discordant amplitude and duration response suggests separate mechanisms for modulating these waveform parameters.
Keywords: Serotonin, Electric organ discharge, Communication signal, 5HT1A receptor, 5HT2 receptor, Social interaction, Dominance hierarchy
Serotonin (5-HT) is a neuromodulator so ubiquitous that hardly any physiological function or behavior is free from its direct or indirect effects. As a result, a full account of neuroendocrine control of behavior requires a thorough assessment of 5-HT’s function across a wide range of circumstances, even as the pervasive presence of 5-HT in the nervous system complicates unambiguous assessment of its functions. Particularly, serotonin is involved in the regulation of diametrically opposed behaviors, aggression and subordinance (Summers and Winberg, 2006). Serotonin activity rises in both dominant and subordinate males but rapidly returns to baseline in dominants while it stays chronically high in subordinates (Overli et al., 1999; Summers and Winberg, 2006). Prior social defeat or success during aggressive interactions affects future aggressive behaviors and the activity of the serotonergic system (Winberg et al., 1992; Winberg et al., 1997b). Furthermore, social experience affects the regulatory effect of serotonin on dominant behaviors via serotonin receptors 1A and 2A (Yeh et al., 1996).
Pharmaceutical 5HT1A agonists inhibit aggression or induce submissive behaviors in a wide range of non-mammalian vertebrates including green anoles (Deckel and Fuqua, 1998), Arctic charr (Hoglund et al., 2002; Winberg and Nilsson, 1993), rainbow trout (Winberg et al., 1997a), and sticklebacks (Bell et al., 2007). In addition, 5HT1A receptors operate as both post-synaptic receptors and as pre-synaptic autoreceptors to either suppress or stimulate stress responses in teleosts as they do in mammals (Hoglund et al., 2002). Despite the functionally and anatomically conserved nature of the serotonin system in vertebrates (Parent et al., 1984) and the abundance of data indicating a role for 5HT1A receptors in regulating social behaviors in fish, nothing is known about the function of 5HT2-like receptors in teleosts (Bagdy, 1996; Eison and Mullins, 1996). The complexity of the 5-HT receptor system and the multiple levels upon which serotonin influences physiology and behavior hints at a system capable of producing contextually appropriate responses to a wide range of stimuli using the same structures, circuits, and ligands.
Gymnotiform fish are excellent models to evaluate the processes underlying the connection between environmental stimuli, motivational state and behavioral output. These fish emit an easily-quantifiable electric signal generated by a well-mapped neural motor network. Male–male interactions alter these electric signals. Furthermore, the electrocommunication network of these fish is dynamic, and is modulated by hormones, modulators, and neurotransmitters. Thus, changes in the electric signal of males during aggressive interactions give us a real-time broadcast of the neuromodulatory regulation of the electro-communication signals of competing males.
The electric organ discharge (EOD) of weakly electric gymnotiform fish is a dual-purpose signal used to navigate and communicate in total darkness. The EOD pulse of Brachyhypopomus pinnicaudatus is a biphasic sinusoidal wave that increases or decreases in amplitude and in the duration of the second phase (Fig. 1). The EOD is produced by electrocytes —specialized excitable cells in the peripheral electric organ. Social encounters and environmental stimuli modulate the EOD waveform by altering the membrane biophysics and discharge waveforms of individual electrocytes (Ferrari et al., 1995; McAnelly and Zakon, 2000; Mills and Zakon, 1991), resulting in either a larger or smaller waveform. Social encounters modulate the EOD within minutes and large waveforms and/or rapid enhancements are associated with dominance status (Franchina et al., 2001; Hagedorn and Zelick, 1989; Stoddard et al., 2003).
Fig. 1.
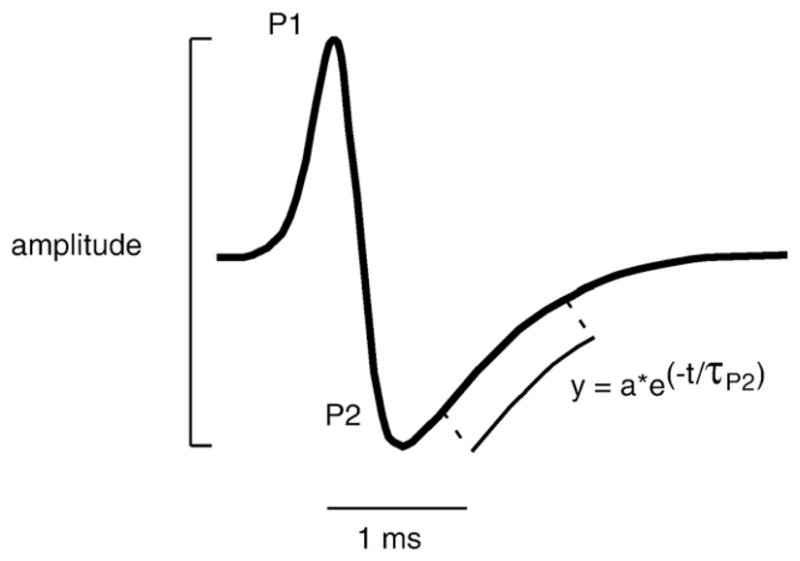
Measure of Brachyhypopomus pinnicaudatus EOD waveform parameters. Amplitude was measured peak-to-peak for the whole waveform. The time constant of repolarization of the 2nd phase, τP2, was estimated by fitting an inverse exponential function fit to the decay segment.
We have shown that intramuscular injections of 5-HT cause male B. pinnicaudatus to increase their EOD amplitude and duration akin to the waveform changes observed during male–male social interactions (Stoddard et al., 2003). These effects of 5-HT do not result from its direct action on the electrocytes, whereas melanocortin peptides do modulate the discharge waveforms of single electrocytes directly (Markham and Stoddard, 2005). Thus, it is possible that 5-HT modulates EOD waveform by acting centrally to elicit release of melanocortins into circulation. Therefore we sought to clarify the proximate mechanisms of serotonin’s action on EOD modulations in B. pinnicaudatus.
The 5-HT system consists of extensive projections of serotonergic neurons throughout the brain and a labyrinthine system of serotonin receptors. Seven distinct 5-HT receptor families (5HT1R–5HT7R) have been identified and some families possess multiple receptor subtypes (e.g., 5HT2A, 2B, 2C) (Glennon et al., 2000). Stimulating (or blocking) different serotonin receptor types often results in opposite actions on target tissues (Welch et al., 1993). The seemingly incongruous role of the same agonist as activator and inhibitor can be explained by evidence that different serotonin receptor types activate different serotonergic signaling pathways, each of which produces different results downstream (Jorgensen et al., 1998; Saphier et al., 1995; Welch et al., 1993). Studies have confirmed that 5-HT receptors with pharmacological profiles similar to mammalian 5HT1 and 5HT2-like receptors are present in teleosts, and at least three different 5-HT receptor types have been localized in whole teleost brain homogenates (Dietl and Palacios, 1988; McDonald and Walsh, 2004; Winberg and Nilsson, 1996; Yamaguchi and Brenner, 1997).
Our objective in this study was to clarify our understanding of serotonergic regulation of weakly electric social signals and to identify which 5-HT receptor types were pharmacologically linked to changes in EOD. We used various serotonergic compounds to assess activity and directionality of EOD modulations in response to activating or inhibiting specific 5-HT receptor types. We found that the opposing actions of two serotonin receptors showing pharmacology characteristic of the mammalian 5HT1AR and 5HT2R regulate the EOD waveform.
Methods
Animals
Sexually mature male B. pinnicaudatus (Hopkins, 1991), bred and maintained on Florida International University campus, were randomly collected from outdoor breeding pools throughout 2002–2006 and brought indoors for pharmacological challenge tests (n=4–12 males per trial). Fish were weighed and measured prior to placement in separate recording tanks to calculate appropriate injection doses and then left undisturbed for a minimum of 24-h before pharmacological challenges were administered to allow individuals to acclimate to their tanks and to measure baseline EODs.
All methods used in these experiments were approved in advance by the FIU IACUC and complied with the “Principles of Animal Care” publication No. 86-23, revised 1985, of the National Institutes of Health.
Electric signal recording
We recorded EODs with an automated, calibrated recording system previously described in detail (Stoddard et al., 2003). The system automatically records EODs when the fish passes through or rests in the geometric center of the tank. EODs are collected approximately once a minute around the clock throughout the duration of each experiment. We measured amplitude of the EOD waveform peak-to-peak, and duration of the second phase as τP2, the time constant of an inverse exponential function fit to the decay segment of the second phase (P2) of the EOD waveform (Fig. 1).
Chemicals and reagents
Table 1 shows reported mammalian 5-HT receptor affinities of the serotonergic drugs that we used to characterize the mechanisms of 5-HT activity. Alpha-methyl-5-hydroxytryptamine (α-Me-5-HT, 5HT2 agonist), 8-hydroxy-din-propylamino tetralin (8-OH-DPAT, 5HT1A and 5HT7 agonist), and 6-chloro-2-(1-piperazinyl) pyrazine (MK212, 5HT2 agonist) were purchased from Tocris Cookson Inc. (Ballwin, MO, US). N-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]-N-(2-pyridinyl) cyclohexanecarboxamide trihydrochloride (WAY100635, 5HT1A antagonist), 2,5-dimethoxy-4-iodoamphetamine (DOI, 5HT2 agonist), and ketanserin tartrate salt (ketanserin, 5HT2A/2C antagonist) were purchased from Sigma-Aldrich (St. Louis, MO, US). Ketanserin was dissolved at pH 6.7, titrated to pH 7.2 with NaOH, and diluted to a final concentration of 2.5 mM. Other pharmacological compounds were dissolved in physiological saline as described previously (Stoddard et al., 2003).
Table 1.
Receptor affinities (pKis) for serotonergic agents used in this study, based on mammalian studies
| 5HT1AR | 5HT2AR | 5HT2BR | 5HT2CR | 5HT7R | |
|---|---|---|---|---|---|
| 5-HT | 8.8 (Peroutka and Howell, 1994) | 8.2 (Peroutka and Howell, 1994) | 7.6 (Peroutka and Howell, 1994) | 8.0 (Peroutka and Howell, 1994) | 8.7–9.2 (Ruat et al., 1993; Shen et al., 1993) |
| 8-OH-DPAT (5HT1A agonist) | 9.2 (Millan et al., 1992) | 5.2 (Millan et al., 1992) | <5 (Millan et al., 1992) | 7.7 (Sleight et al., 1995) | |
| DOI (5HT2 agonist) | 5.2 (van Wijngaarden et al., 1990) | 7.6 (Schechter and Simansky, 1988) | 7.0 (Schechter and Simansky, 1988) | ||
| MK212 (5HT2 agonist) | 5.3 (Smythe et al., 1988) | 4.8 (Smythe et al., 1988) | 6.2 (Hoyer et al., 1994) | 6.2 (Smythe et al., 1988) | |
| WAY100635 (5HT1A antagonist) | 8.9 (Fletcher et al., 1996) | <7 (Fletcher et al., 1996) | <7 (Fletcher et al., 1996) | ||
| Ketanserin (5HT2A/2C antagonist) | 5.9 (Hoyer, 1989) | 8.7 (Schreiber et al., 1995) | 5.4 (Hoyer et al., 1994) | 7.2 (Schreiber et al., 1995) | |
| α-Me-5-HT (5HT2 agonist) | 6.1 (Baxter et al., 1995) | 8.4 (Baxter et al., 1995) | 6.2 (Baxter et al., 1995) |
Pharmacological challenges
We found previously that EOD response to intramuscular injections of 5-HT saturates at 2.5 nM g−1 body weight (bw). We used this concentration as the starting point to select doses for serotonergic drugs, but if we obtained either no effect or an equivocal effect we performed additional injections at higher and lower doses (Fig. 2). We had little choice but to inject the drugs peripherally. Central injection can only be done on cannulated or anesthetized fish, and the fish are too small for cannulation. Every anesthetic tested increases the EOD waveform parameters (M. Markham unpubl. data). Thus we chose intramuscular injection, which had proven to be successful with serotonin (Stoddard et al., 2003). We prepared the injection solutions to produce the desired dose when injected intramuscularly at 1 μl g−1 body weight. Saline injections (1 μl g−1 bw) served as a control condition for effects of handling and injection. All injections were given midday (10:00–15:00) and EOD recording resumed immediately after fish were returned to the tank. Handling time from capture, through injection, to replacement in the tank was less than one minute, and produced little change in EOD waveform (Stoddard et al., 2003).
Fig. 2.
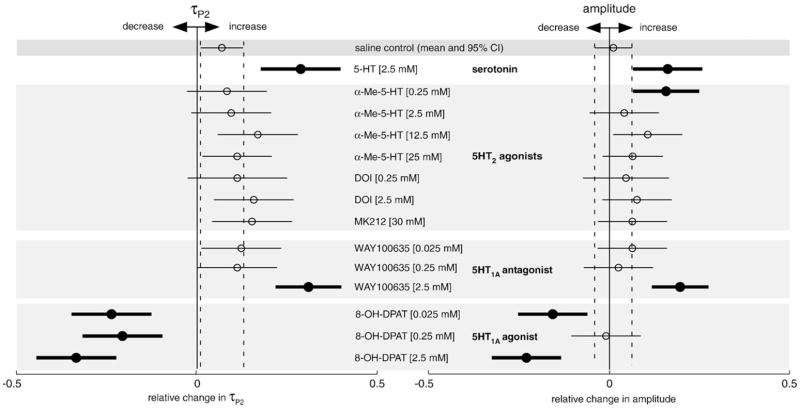
EOD waveform responses to intramuscular injections of 5-HT receptor agonists and antagonists vary depending on which receptor is targeted. Data are shown as mean ± 95% confidence intervals of combined data measured as responses to pharmacological challenge relative to individual baseline [(peak − baseline)/baseline]. Responses presented as thick black lines and solid black circles represent challenge responses that were significantly different (p<0.05) than responses to saline control determined by Dunnett’s t-test. Injections of 5HT2 receptor-specific agents differentially modulate the EOD waveform. Injections of MK212 or DOI had no effect on either amplitude or τP2, while injections of α-Me-5-HT enhanced EOD amplitude, but not τP2. 5HT1A receptor-specific agents modulated EOD waveform in opposite directions. The low and high dose of the 5HT1A agonist 8-OH-DPAT inhibited amplitude, while all three doses inhibited τP2 response to challenge. Conversely, the highest dose of the 5HT1AR antagonist, WAY100635, enhanced both amplitude and τP2.
To probe for central regulation of the EOD by the 5HT2R family, we used three partially selective agonists: MK212 [30 mM], DOI [0.25 and 2.5 mM], and α-Me-5-HT [0.25, 2.5, 12.5, and 25 mM]. While each of these agonists has high affinity for 5HT2 receptors and differentiates well against other 5-HT receptor families, they do not exhibit reliable selectivity between the three receptor subtypes in this family: 5HT2A, 2B, and 2C (Baxter et al., 1995; Hoyer et al., 2002; Jerman et al., 2001; Ramage, 2005; Van de Kar et al., 2001). Modulations of the EOD in response to one or more of these ligands would therefore support involvement of at least one 5HT2 receptor type; however such a response would not exclude involvement of the other subtypes in regulating the EOD.
The silent antagonist (i.e., ligand with no intrinsic effect) ketanserin is more useful for distinguishing which of the 5HT2 receptor subtypes may be exerting an effect. Ketanserin, unlike 5HT2R agonists, is highly selective for the 5HT2A/2CR over the 5HT2BR (Table 1). We found that ketanserin alone neither increased nor decreased the EOD waveform, suggesting that given alone it does not influence or interfere with normal EOD modulations. We therefore conducted serial challenges to determine whether ketanserin could block the effects of a successive 5-HT injection. We pre-treated a control group of fish with saline and the test group with ketanserin [2.5 mM]. Fish in both groups were then given a second injection of 5-HT [2.5 mM] 15–45 min later and any changes in their EOD waveforms were recorded.
To probe for waveform regulation by the 5HT1AR, we capitalized on ligands highly selective for that receptor in a broad array of taxa: the agonist 8-OH-DPAT [0.025, 0.25, and 2.5 mM] and the antagonist WAY100635 [0.025, 0.25, and 2.5 mM]. We also sought to explore whether there were any interactions between the 5HT1A and the 5HT2 receptors in the regulation of EOD waveform. To test the hypothesis that the 5HT1AR and the 5HT2R are consecutively aligned within the same neural pathway, we sought to enhance the EOD through the 5HT1AR after blocking the 5HT2R. Thus we pre-treated males with ketanserin [2.5 mM] followed 15–45 min later by WAY100635 [2.5 mM]. Control treatment consisted of saline pre-treatment followed by WAY100635.
Data analysis
Changes in the EOD waveform resulting from our pharmacological challenge trials are superimposed upon circadian cycles in waveform parameters (Franchina and Stoddard, 1998; Stoddard et al., 2003). We therefore mathematically isolated challenge-induced changes in these measures by subtracting the circadian oscillation as reported previously (Stoddard et al., 2003). This analysis allowed us to measure changes in EOD amplitude and τP2 caused by the pharmacological challenges, apart from normal circadian modulations in the EOD. Additionally, our earlier work showed that responses to 5-HT challenge are closely related to an individual’s baseline values of EOD amplitude and τP2 (Stoddard et al., 2003). These parameters vary considerably between individuals; therefore, we quantified individual challenge responses as the proportion change relative to that individual’s EOD baseline on the day of that challenge: [(peak value after injection − baseline value)/baseline value].
We analyzed amplitude and τP2 responses to single-injection serotonergic challenges using separate omnibus one-way ANOVA with significance level set at p ≤0.05. We then conducted post-hoc analysis using Dunnett’s pairwise multiple comparison t-test to compare responses to each challenge against responses to a single control (saline injection) (Dunnett, 1955, 1964). We compared test statistics to two-tailed critical values because we had no a priori expectation for directionality of effect of each drug on EOD.
Responses to ketanserin+5-HT and ketanserin+WAY100635 challenges were analyzed using two-sample Student’s t-tests assuming unequal variances. We compared the calculated test statistic to values from a one-tailed critical region because we anticipated that ketanserin would either have no effect on or block EOD response. Data analysis was generated using MATLAB v7.1 and SPSS 15.0. Data are reported in figures as mean ± 95% confidence intervals and significance values from Dunnett’s t-test are reported in Table 2.
Table 2.
Results of Dunnett’s pairwise multiple comparison t-test (p-values) comparing responses to individual challenges against responses to saline control
| Receptor targeted | Action | Amplitude | tP2 | |
|---|---|---|---|---|
| 5-HT [2.5 mM] | Agonist | 0.008 | <0.001 | |
| α-Me-5-HT [0.25 mM] | 5HT2 | Agonist | 0.016 | 1.000 |
| α-Me-5-HT [2.5 mM] | 5HT2 | Agonist | 1.000 | 1.000 |
| α-Me-5-HT [12.5 mM] | 5HT2 | Agonist | 0.3 | 0.438 |
| α-Me-5-HT [25 mM] | 5HT2 | Agonist | 0.885 | 0.994 |
| DOI [0.25 mM] | 5HT2 | Agonist | 1.000 | 1.000 |
| DOI [2.5 mM] | 5HT2 | Agonist | 0.816 | 0.620 |
| MK212 [30 mM] | 5HT2 | Agonist | 0.955 | 0.699 |
| WAY100635 [0.025 mM] | 5HT1A | Antagonist | 0.956 | 0.983 |
| WAY100635 [0.25 mM] | 5HT1A | Antagonist | 1.000 | 0.998 |
| WAY100635 [2.5 mM] | 5HT1A | Antagonist | <0.001 | <0.001 |
| 8-OH-DPAT [0.025 mM] | 5HT1A | Agonist | 0.002 | <0.001 |
| 8-OH-DPAT [0.25 mM] | 5HT1A | Agonist | 1.000 | <0.001 |
| 8-OH-DPAT [2.5 mM] | 5HT1A | Agonist | <0.001 | <0.001 |
Results
The one-way ANOVAs for both amplitude and τP2 responses to single-injection serotonergic challenges showed that some challenge responses were significantly different (p<0.001 for both amplitude and τP2). We report the results from Dunnett’s multiple comparison t-tests below.
Effect of 5HT2R selective ligands on EOD waveform
Of the three 5HT2R agonists tested, only the lowest concentration of α-Me-5-HT [0.25 mM] increased the EOD amplitude response (Dunnett’s t-test; p=0.016). No 5HT2R agonist had an effect on τP2 at any dose (Fig. 2). The EOD response to one 5HT2R agonist implicates the involvement of a receptor in the 5HT2R family in EOD waveform modulation.
The 5HT2A/2CR silent antagonist ketanserin had no effect on its own (data not shown), as expected for a silent antagonist, but blocked the typical 5-HT enhancement of EOD τP2 (Fig. 3a). Fish pre-treated with ketanserin showed little or no increase in τP2 in response to the 5-HT injection, compared to fish pre-treated with saline which increase τP2 following injection with 5-HT (two-sample t-test; p=0.02). Fish pre-treated with ketanserin exhibited amplitude responses similar to fish receiving saline pre-treatment (two-sample t-test; p=0.26) (Fig. 3b).
Fig. 3.
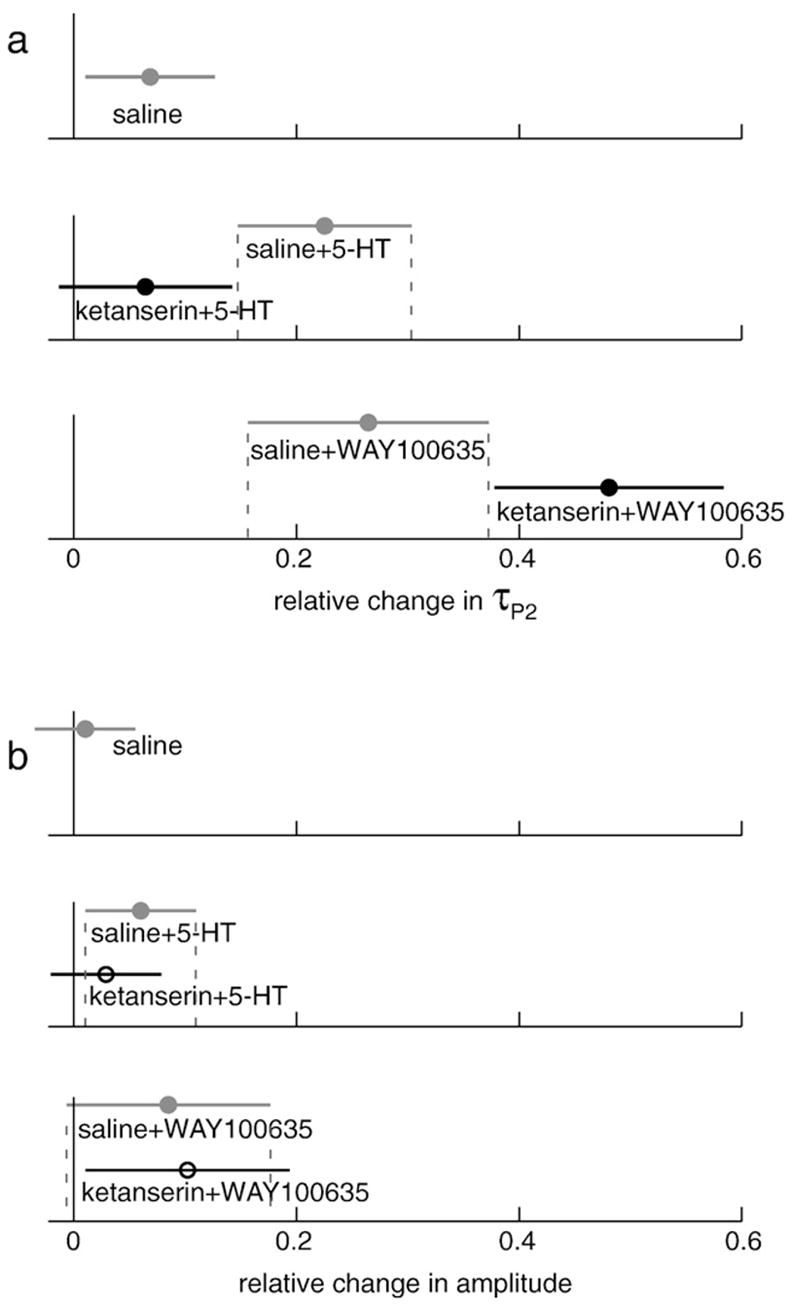
Injecting fish with the 5HT2A receptor silent antagonist ketanserin blocked the 5-HT-typical EOD enhancement in τP2 (a) but had no effect on amplitude response to 5-HT injection (b). Pre-treating fish with ketanserin followed by 5HT1A antagonist, WAY100635, does not block enhancement of τP2, rather the ketanserin+WAY100635 response is greater than response to WAY100635 alone (a). Amplitude responses to challenge with ketanserin+ WAY100635 were no different than responses to WAY100635 given alone (b). Responses were measured relative to individual baseline [(peak − baseline)/baseline] and error bars represent mean ± 95% confidence intervals of combined data. A solid black circle denotes statistically significant differences ( p<0.05) between responses to ketanserin pre-treatment and saline pre-treatment challenge responses as determined by one-tailed Student’s t-test.
Effect of 5HT1AR selective ligands on EOD waveform
Each of the three doses of the highly selective 5HT1AR agonist 8-OH-DPAT reduced both amplitude and τP2 relative to controls (Dunnett’s t-test; p<0.01), with the exception of amplitude response at the middle dose [0.25 mM] (Dunnett’s t-test; p=1.0) (Fig. 2). Treatment with the ostensibly silent 5HT1AR antagonist WAY100635 resulted in enhancement of both amplitude and τP2 at the highest dose tested [2.5 mM] (Dunnett’s t-test; p<0.001). In the serial challenges designed to explore possible interactions between 5HT1A and 5HT2 receptors, we found that the saline+WAY100635 group showed the expected increases in the amplitude response to WAY100635 and fish that received ketanserin+WAY100635 exhibited similar increases (two-sample t-test, p=0.42), showing that blocking the 5HT2A/2C receptors has no effect on the EOD amplitude response to 5HT1A antagonism (Fig. 3b). Quite the opposite, and not expected, τP2 response was significantly higher following ketanserin+WAY100635 than following saline+WAY100635 challenge (two-sample t-test; p=0.02) (Fig. 3a).
Discussion
Our results suggest that two families of serotonin receptors are involved in regulating changes in EOD waveform. We found that serotonin receptors pharmacologically similar to mammalian 5HT2 and 5HT1A receptors are capable of producing EOD waveform modulations and that these receptors modulate the EOD waveform in different ways. We also found that EOD τP2 was more responsive to some serotonergic agents than EOD amplitude in general, which was unexpected given that both parameters can be enhanced by 5-HT (Stoddard et al., 2003). Perhaps, EOD amplitude and τP2 are differentially regulated by parallel neuroendocrine pathways. 5HT1A-like and possibly 5HT2-like receptors have been localized in Arctic charr brain homogenate (Winberg and Nilsson, 1996); at the same time distributions of 5-HT receptors in the teleost brain are still unknown. We propose this model as a guide for future research investigating the placement, distribution, and molecular identity of these different 5-HT receptors in weakly electric fish brains. Currently, the specific location(s) and distribution of 5-HT receptors in the brains of electric fish are not known; however, our results provide a blueprint for which 5-HT receptors to target first in future receptor localization experiments.
The 5HT1A receptor agonist 8-OH-DPAT reduced EOD amplitude and τP2 while the 5HT1A antagonist WAY100635 lead to increased waveforms. The silent 5HT1AR antagonist WAY100635 should have no intrinsic effect on its own, yet both waveform parameters increased in response to this challenge. These results signify that: (1) a 5HT1AR-like receptor is present and pharmacologically relevant in this species and, (2) endogenous release of 5-HT tonically suppresses EOD enhancement via 5HT1A receptors in this circuit and blocking these receptors with WAY100635 releases this inhibition.
Recent evidence has shown that WAY100635 is a “potent” dopamine receptor agonist as well as a 5HT1A antagonist, possibly obfuscating interpretation of our results (Chemel et al., 2006). To dispel this ambiguity, we injected fish with dopamine [25 mM] and saw a depression of EOD waveform parameters (data not shown). If the increased EOD responses to WAY100635 challenge were the result of actions at dopamine receptors, we would have expected responses to WAY100635 and dopamine to be in the same direction. Consequently, we doubt that the results of our WAY100635 injections reflect any known activity at a dopamine receptor. While our 5HT1A agonist/antagonist challenges clearly support our assertion that this receptor type is present and relevant to regulation of the EOD, differences in responses to different doses of these ligands suggest the role of the 5HT1AR may be more complex in our fish than tonic inhibition of melanocortin release.
The 5HT1AR antagonist WAY100635 enhanced both EOD parameters only at the highest dose tested, whereas 8-OH-DPAT inhibited both parameters (except at the middle dose [0.25 mM]). The lack of response at the two lower doses of WAY100635 might be explained by 5HT1A receptors possessing greater affinity for agonists than antagonists (Gozlan et al., 1983) or the possibility that this ligand is competing with tonic levels of 5-HT present at the 5HT1AR. An explanation for the irregular response pattern we observed due to 8-OH-DPAT challenge, however, is not as straightforward.
In vertebrates, including teleosts, the 5HT1AR operates both as a somatodendritic autoreceptor on serotonergic neurons and as a post-synaptic receptor on cells receiving projections from 5-HT neurons (Gozlan et al., 1983; Hoglund et al., 2002). Autoreceptor activation leads to reduced neuronal firing and thus reduced 5-HT activity in the brain (Invernizzi et al., 1991; Sharp et al., 1989; VanderMaelen et al., 1986) whereas post-synaptic 5HT1A activation mediates behavioral effects of brain 5-HT (Carey et al., 2005; Rabiner et al., 2004). Half of the fish that were given 8-OH-DPAT at [0.25 mM] enhanced their amplitudes and the other half in this group reduced theirs after injection, raising the possibility that both types of 5HT1A receptor are operational within this system. If this was so, higher density or activity of post-synaptic 5HT1A receptors might have been present in those fish for which 8-OH-DPAT increased EOD amplitude, subsequently overriding the inhibitory effect of 8-OH-DPAT on 5HT1A autoreceptors. Another hypothesis, not incompatible with this one, is that multiple serotonergic pathways bearing different combinations of receptors drive EOD parameters in different directions.
Not all drugs we used cross the blood–brain barrier to the same extent. For instance 5HT2R agonists readily penetrate the blood–brain barrier while 5HT2R antagonists like ketanserin do not (Michiels et al., 1988). Indeed, ketanserin acts on 5HT2 receptors to block the passage of 5-HT through the blood–brain barrier (Sharma et al., 1995). Thus ketanserin could block 5-HT action on the EOD by preventing its passage into the brain. Alternately our ketanserin results are consistent with a 5HT2AR expressed in the pituitary or circumventricular organs of hypothalamus, which are not protected by the blood–brain barrier. The results of our dual-injection experiments with ketanserin+ WAY100635 provide evidence to support this second hypothesis. While ketanserin blocked the EOD τP2 enhancing effect of 5-HT, it significantly augmented the increase produced by 5HT1AR antagonist WAY100635. WAY100635 passes readily through the blood–brain barrier (Farde et al., 1998), so ketanserin’s action would appear to be on a 5HT2AR in the brain itself, perhaps in a pathway that inhibits the pathway expressing the 5HT1AR.
Activation of 5HT2-like receptors with α-Me-5-HT increased EOD amplitude with no significant effect on EOD τP2. Since α-Me-5-HT has a slightly higher affinity for the 5HT2B receptor than for either the 5HT2A/2C, and because we observed a response at the lowest rather than the highest dose, our agonist challenges suggest the 5HT2B-like receptor has a role in modulating EOD amplitude. However, ketanserin, the antagonist highly selective for the 5HT2A/2C receptor, blocked EOD response to 5-HT. Given the high selectivity of ketanserin for the 5HT2A/2C receptor, the ketanserin block of 5-HT-induced EOD waveform modulations is strong evidence that 5-HT acts specifically via a 5HT2A/2C-like receptor to modulate EOD τP2. While these challenges do not definitively identify which 5HT2-like receptor(s) might be involved in modulating the EOD, they do reveal that the direction of action of a 5HT2-like receptor is to increase EOD amplitude and τP2, opposing the action of a 5HT1A-like receptor.
Our original goal was to clarify mechanisms of 5-HT regulation of the EOD within a social communication context where we assumed that EOD modulations reflect social dominance and subordinance. Recent research conflicts with the prevailing view that 5-HT activity necessarily suppresses aggressive behavior. High 5-HT turnover in parts of the brain has been found in aggressive and dominant individuals (Korzan et al., 2000; Matter et al., 1998) and a “primed” serotonergic system may be necessary to enable an individual to produce an appropriate behavioral response in a complex social context (Sperry et al., 2005). These findings would help explain the apparent paradox we observed in our fish wherein 5-HT injections further masculinized the males’ EODs (Stoddard et al., 2003). If the classical account of the serotonin system as an inhibitor of aggression is accurate and enhancements of the EOD in the presence of conspecifics are aggressive, then the reduction in EOD parameters following activation of the 5HT1AR makes perfect sense, but the increase in these parameters following activation of the 5HT2R is unexpected. Perhaps both very low and very high levels of serotonergic activity augment the EOD, allowing fish to increase the potency of their communication signal both when dominant (low 5-HT enhances the EOD via the 5HT1AR) and when newly challenged (high 5-HT enhances the EOD via a 5HT2R).
If aggression is inhibited by the 5HT1AR, as some studies suggest (Joppa et al., 1997; Sanchez, 1997; Simon et al., 1998; Sperry et al., 2005), then this receptor type may reside in a circuit that rapidly inhibits enhanced EOD response in weakly electric fish, perhaps after a social encounter terminates or in response to confrontation with a more dominant individual. Activating the 5HT1AR autoreceptor inhibits neuronal firing and decreases 5-HT synthesis (Perreault et al., 2003; Sperry et al., 2005). Our data show that activating 5HT1AR with 8-OH-DPAT causes a dampening of both EOD parameters, although we have not yet determined the detailed social significance of specific patterns of enhancements and reductions in the EOD waveform. We expect to find that the social significance of a rise or fall in EOD waveform parameters is more complicated than linear social rank.
Modulations in the EOD, particularly enhancements of τP2, have been proposed to function as mate attracting signals (Hopkins, 1972, 1974a, b). The enhancements seen from serotonergic pathways commonly involved in regulation of aggression and dominance behavior are consistent with a role in intrasexual competition as well. Differential regulation of amplitude and τP2 suggests that the EOD is a multicomponent signal that conveys different information such as body condition and motivation to male or female receivers. Because the expression of EOD parameters are intricately tied to each other, it would be easy to see how the mechanisms that control one social signal, e.g., aggression, could be co-opted to regulate another social signal, e.g., mate attraction.
Melanocortins modulate both EOD amplitude and τP2 by direct action on electrocytes (Markham and Stoddard, 2005), and ongoing experiments indicate that melanocortins are both necessary and sufficient for short-term modulation of these parameters. Thus, the variability in response of amplitude and τP2 between serotonergic drugs and between sets of trials is hard to explain. Our current understanding does not account for how αMe-5-HT might increase amplitude without increasing τP2. Nor do we understand why our first injection trials with 5-HT (Stoddard et al., 2003) and with WAY100635 (Fig. 2) increased both EOD amplitude and τP2 whereas the second sets (Fig. 3) increased only τP2. Neither seasonal nor maturational differences can explain these differences, leading us to suspect that particular social environments in our outdoor breeding pools may somehow affect responsiveness of these parameters through the actions of steroids or other neuromodulators.
Acknowledgments
Financial support and equipment were provided by NIH grants MBRS GM08205 (PKS) and K01MH064550 (MRM). This paper is contribution number 135 to the program in Tropical Biology at Florida International University. We thank David Berman, Ana Hernandez, Jennifer Herrick, Vance Hodge, and Maikel Couto for their assistance with this project. We also thank Brian Dias, Lynne McAnelly, and the two anonymous reviewers for their thoughtful and constructive comments on earlier versions of this manuscript.
References
- Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res. 1996;73:277. doi: 10.1016/0166-4328(96)00112-x. [DOI] [PubMed] [Google Scholar]
- Baxter G, Kennett G, Blaney F, Blackburn T. 5-HT2 receptor subtypes: a family re-united. Trends Pharmacol Sci. 1995;16:105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- Bell AM, Backstrom T, Huntingford FA, Pottinger TG, Winberg S. Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol Behav. 2007;91:15–25. doi: 10.1016/j.physbeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E, Shanahan A, Muller CP, Huston JP. Evidence that the 5-HT1A autoreceptor is an important pharmacological target for the modulation of cocaine behavioral stimulant effects. Brain Res. 2005;1034:162–171. doi: 10.1016/j.brainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology. 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Deckel AW, Fuqua L. Effects of serotonergic drugs on lateralized aggression and aggressive displays in Anolis carolinensis. Behav Brain Res. 1998;95:227–232. doi: 10.1016/s0166-4328(98)00048-5. [DOI] [PubMed] [Google Scholar]
- Dietl M, Palacios JM. Autoradiographic studies of serotonin receptors. In: Sanders-Bush E, editor. The Serotonin Receptors. The Humana Press; Clifton, NJ: 1988. pp. 89–138. [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- Dunnett CW. New tables for multiple comparisons with a control. Biometrics. 1964;20:482–491. [Google Scholar]
- Eison AS, Mullins UL. Regulation of central 5-HT2A receptors: a review of in vivo studies. Behav Brain Res. 1996;73:177. doi: 10.1016/0166-4328(96)00092-7. [DOI] [PubMed] [Google Scholar]
- Farde L, Ito H, Swahn CG, Pike VW, Halldin C. Quantitative analyses of carbonyl-carbon-11-WAY-100635 binding to central 5-hydro-xytryptamine-1A receptors in man. J Nucl Med. 1998;39:1965–1971. [PubMed] [Google Scholar]
- Ferrari MB, McAnelly ML, Zakon HH. Individual variation in and androgen-modulation of the sodium current in electric organ. J Neurosci. 1995;15:4023–4032. doi: 10.1523/JNEUROSCI.15-05-04023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Franchina CR, Stoddard PK. Plasticity of the electric organ discharge waveform of the electric fish Brachyhypopomus pinnicaudatus I. Quantification of day–night changes. J Comp Physiol, A. 1998;183:759–768. doi: 10.1007/s003590050299. [DOI] [PubMed] [Google Scholar]
- Franchina CR, Salazar VL, Volmar CH, Stoddard PK. Plasticity of the electric organ discharge waveform of male Brachyhypopomus pinnicaudatus. II Social effects. J Comp Physiol, A. 2001;187:45–52. doi: 10.1007/s003590000176. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Dukat M, Westkaemper RB. Serotonin receptor subtypes and ligands. Psychopharmacology: the Fourth Generation of Progress. Am Coll Neuropsychopharmacol 2000 [Google Scholar]
- Gozlan H, El-Mestikawy S, Pichat L, Glowinski J, Hamon M. Identification of presynaptic serotonin autoreceptors using a new ligand: 3H-PAT. Nature. 1983;305:140–142. doi: 10.1038/305140a0. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Zelick R. Relative dominance among males is expressed in the electric organ discharge characteristics of a weakly electric fish. Anim Behav. 1989;38:520–525. [Google Scholar]
- Hoglund E, Balm PHM, Winberg S. Stimulatory and inhibitory effects of 5-HT1A receptors on adrenocorticotropic hormone and cortisol secretion in a teleost fish, the Arctic charr (Salvelinus alpinus) Neurosci Lett. 2002;324:193–196. doi: 10.1016/s0304-3940(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Hopkins CD. Sex differences in electric signalling in an electric fish. Science. 1972;176:1035–1037. doi: 10.1126/science.176.4038.1035. [DOI] [PubMed] [Google Scholar]
- Hopkins CD. Electric communication in the reproductive behavior of Sternopygus macrurus (Gymnotidae) Z Tierpsychol. 1974a;35:518–535. doi: 10.1111/j.1439-0310.1974.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Hopkins CD. Electric communication: functions in the social behavior of Eigenmannia virescens. Behaviour. 1974b;50:270–306. [Google Scholar]
- Hopkins CD. Hypopomus pinnicaudatus (Hypopomidae), a new species of gymnotiform fish in French Guiana. Copeia. 1991:151–161. [Google Scholar]
- Hoyer D. 5-Hydroxytryptamine receptors and effector coupling mechanisms in peripheral tissues. In: Fozard JR, editor. Peripheral Actions of 5-HT. Oxford University Press; Oxford: 1989. pp. 72–99. [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Carli M, Di Clemente A, Samanin R. Administration of 8-hydroxy-2-(Di-n-propylamino)tetralin in raphe nuclei dorsalis and medianus reduces serotonin synthesis in the rat brain: differences in potency and regional sensitivity. J Neurochem. 1991;56:243–247. doi: 10.1111/j.1471-4159.1991.tb02587.x. [DOI] [PubMed] [Google Scholar]
- Jerman JC, Brough SJ, Gager T, Wood M, Coldwell MC, Smart D, Middlemiss DN. Pharmacological characterisation of human 5-HT2 receptor subtypes. Eur J Pharmacol. 2001;414:23–30. doi: 10.1016/s0014-2999(01)00775-0. [DOI] [PubMed] [Google Scholar]
- Joppa MA, Rowe RK, Meisel RL. Effects of serotonin 1A and 1B receptor agonists on social aggression in male and female Syrian hamsters. Pharmacol Biochem Behavior. 1997;58:349–353. doi: 10.1016/s0091-3057(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Knigge U, Kjaer A, Vadsholt T, Warberg J. Serotonergic involvement in stress-induced ACTH release. Brain Res. 1998;811:10–20. doi: 10.1016/s0006-8993(98)00901-9. [DOI] [PubMed] [Google Scholar]
- Korzan WJ, Summers TR, Summers CH. Monoaminergic activities of limbic regions are elevated during aggression: influence of sympathetic social signalling. Brain Res. 2000;870:170–178. doi: 10.1016/s0006-8993(00)02420-3. [DOI] [PubMed] [Google Scholar]
- Markham MR, Stoddard PK. Adrenocorticotropic hormone enhances the masculinity of an electric communication signal by modulating the waveform and timing of action potentials within individual cells. J Neurosci. 2005;25:8746–87543. doi: 10.1523/JNEUROSCI.2809-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter JM, Ronan PJ, Summers CH. Central monoamines in free-ranging lizards: differences associated with social roles and territoriality. Brain Behav Evol. 1998;51:23–32. doi: 10.1159/000006526. [DOI] [PubMed] [Google Scholar]
- McAnelly ML, Zakon HH. Coregulation of voltage-dependent kinetics of Na+ and K+ currents in electric organ. J Neurosci. 2000;20:3408–3414. doi: 10.1523/JNEUROSCI.20-09-03408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MD, Walsh PJ. Dogmas and controversies in the handling of nitrogenous wastes: 5-HT2-like receptors are involved in triggering pulsatile urea excretion in the gulf toadfish, Opsanus beta. J Exp Biol. 2004;207:2003–2010. doi: 10.1242/jeb.00957. [DOI] [PubMed] [Google Scholar]
- Michiels M, Monbaliu J, Meuldermans W, Hendriks R, Geerts R, Woestenborghs R, Heykants J. Pharmacokinetics and tissue distribution of ketanserin in rat, rabbit and dog. Arzneimittel-Forschung. 1988;38:775–784. [PubMed] [Google Scholar]
- Millan MJ, Rivet JM, Canton H, Lejeune F, Bervoets K, Brocco M, Gobert A, Lefebvre de Ladonchamps B, Le Marouille Girardon S, Verriele L, Laubie M, Lavielle G. S 14671: a naphtylpiperazine 5-hydroxytryptamine1A agonist of exceptional potency and high efficacy possessing antagonistic activity at 5-hydroxytryptamine1C/2 receptors. J Pharmacol Exp Ther. 1992;262:451–463. [PubMed] [Google Scholar]
- Mills A, Zakon HH. Chronic androgen treatment increases action potential duration in the electric organ of Sternopygus. J Neurosci. 1991;11:2349–2361. doi: 10.1523/JNEUROSCI.11-08-02349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overli O, Harris CA, Winberg S. Short-term effects of fights for social dominance and the establishment of dominant–subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav Evol. 1999;54:263–275. doi: 10.1159/000006627. [DOI] [PubMed] [Google Scholar]
- Parent A, Piotras D, Dube L. Comparative anatomy of central monoaminergic systems. In: Bjorklund A, Hokefelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier; Amsterdam: 1984. pp. 409–439. [Google Scholar]
- Peroutka SJ, Howell TA. The molecular evolution of G protein-coupled receptors: focus on 5-hydroxytryptamine receptors. Neuropharmacology. 1994;33:319–324. doi: 10.1016/0028-3908(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Perreault H, Semsar K, Godwin J. Fluoxetine treatment decreases territorial aggression in a coral reef fish. Physiol Behav. 2003;79:719–724. doi: 10.1016/s0031-9384(03)00211-7. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Bhagwagar Z, Gunn RN, Cowen PJ, Grasby PM. Preferential 5-HT1A autoreceptor occupancy by pindolol is attenuated in depressed patients: effect of treatment or an endophenotype of depression. Neurospsychopharmacology. 2004;29:1688–1698. doi: 10.1038/sj.npp.1300472. [DOI] [PubMed] [Google Scholar]
- Ramage A. Problems of drug selectivity and dose — pharmacology. J Physiol. 2005;569:711. doi: 10.1113/jphysiol.2005.569007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proceedings of the National Academy of Sciences. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C. Interaction studies of 5-HT1A receptor antagonists and selective 5-HT reuptake inhibitors in isolated aggressive mice. Eur J Pharmacol. 1997;334:127–132. doi: 10.1016/s0014-2999(97)01199-0. [DOI] [PubMed] [Google Scholar]
- Saphier D, Farrar GE, Welch JE. Differential inhibition of stress-induced adrenocortical responses by 5-HT1A agonists and by 5-HT2 and 5-HT3 antagonists. Psychoneuroendocrinology. 1995;20:239–257. doi: 10.1016/0306-4530(94)00056-g. [DOI] [PubMed] [Google Scholar]
- Schechter LE, Simansky KJ. 1-(2,5-Dimethoxy-4-iodophenyl)-2-amino-propane (DOI) exerts and anorexic action that is blocked by 5-HT2 antagonists in rats. Psychopharmacology. 1988;94:342–346. doi: 10.1007/BF00174687. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Sharma HS, Westman J, Navarro JC, Dey PK, Nyberg F. Probable involvement of serotonin in the increased permeability of the blood–brain barrier by forced swimming. An experimental study using Evans blue and 131I-sodium tracers in the rat. Behav Brain Res. 1995;72:189–196. doi: 10.1016/0166-4328(96)00170-2. [DOI] [PubMed] [Google Scholar]
- Sharp T, Bramwell SR, Graham-Smith DG. 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br J Pharmacol. 1989;96:283–290. doi: 10.1111/j.1476-5381.1989.tb11815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- Simon NG, Cologer-Clifford A, Lu S, McKenna SE, Hu S. Testosterone and its metabolites modulate 5HT1A and 5HT1B agonist effects on intermale aggression. Neurosci Biobehav Rev. 1998;23:325–336. doi: 10.1016/s0149-7634(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Carolo C, Petit N, Zwingeststein C, Bourson A. Identification of 5-hydroxytryptamine7 receptor binding sites in rat hypothalamus: sensitivity to chronic antidepressant treatment. Mol Pharmacol. 1995;47:99–103. [PubMed] [Google Scholar]
- Smythe GA, Gleeson RM, Stead BM. Mechanisms of 5-hydroxy-L-tryptophan-induced adrenocorticotropin release: a major role for central noradrenergic drive. Neuroendocrinology. 1988;47:389–397. doi: 10.1159/000124944. [DOI] [PubMed] [Google Scholar]
- Sperry TS, Moore IT, Meddle SL, Brenowitz-Fredericks ZM, Wingfield JC. Increased sensitivity of the serotonergic system during the breeding season in free-living American tree sparrows. Behav Brain Res. 2005;157:119–126. doi: 10.1016/j.bbr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Stoddard PK, Markham MR, Salazar VL. Serotonin modulates the electric waveform of the gymnotiform electric fish Brachyhypopomus pinnicaudatus. J Exp Biol. 2003;206:1353–1362. doi: 10.1242/jeb.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, Winberg S. Interactions between the neural regulation of stress and aggression. J Exp Biol. 2006;209:4581–4589. doi: 10.1242/jeb.02565. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang YH, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijngaarden I, Tulp MT, Soudijn W. The concept of selectivity in 5-HT receptor research. Eur J Pharmacol. 1990;188:301–312. doi: 10.1016/0922-4106(90)90190-9. [DOI] [PubMed] [Google Scholar]
- VanderMaelen CP, Matheson GK, Wilderman RC, Patterson LA. Inhibition of serotonergic dorsal raphe neurons by systemic and iontophoretic administration of buspirone, a non-benzodiazepine anxiolytic drug. Eur J Pharmacol. 1986;129:123–130. doi: 10.1016/0014-2999(86)90343-2. [DOI] [PubMed] [Google Scholar]
- Welch JE, Farrar GE, Dunn AJ, Saphier D. Central 5-HT1A receptors inhibit adrenocortical secretion. Neuroendocrinology. 1993;57:272–281. doi: 10.1159/000126369. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson GE. Time course of changes in brain serotonergic activity and brain tryptophan levels in dominant and subordinate juvenile Arctic charr. J Exp Biol. 1993;179:181–195. [Google Scholar]
- Winberg S, Nilsson GE. Multiple high-affinity binding sites for [3H] serotonin in the brain of a teleost fish, the Arctic charr (Salvelinus alpinus) J Exp Biol. 1996;199:2429–2435. doi: 10.1242/jeb.199.11.2429. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson GE, Olsen KH. Changes in brain serotonergic activity during hierarchic behavior in Arctic charr, Salvelinus alpinus (L.) are socially induced. J Comp Physiol, A Sens Neural Behav Physiol. 1992;170:93–99. doi: 10.1007/BF00190404. [DOI] [PubMed] [Google Scholar]
- Winberg S, Nilsson A, Hylland P, Soderstom V, Nilsson GE. Serotonin as a regulator of hypothalamic–pituitary–interrenal activity in teleost fish. Neurosci Lett. 1997a;230:113–116. doi: 10.1016/s0304-3940(97)00488-6. [DOI] [PubMed] [Google Scholar]
- Winberg S, Winberg Y, Fernald RD. Effect of social rank on brain monoaminergic activity in a cichlid fish. Brain Behav Evol. 1997b;49:230–236. doi: 10.1159/000112994. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Brenner S. Molecular cloning of 5-hydroxytryptamine (5-HT) type 1 receptor genes from the Japanese puffer fish, Fugu rubripes. Gene. 1997;191:219–223. doi: 10.1016/s0378-1119(97)00064-4. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]