Abstract
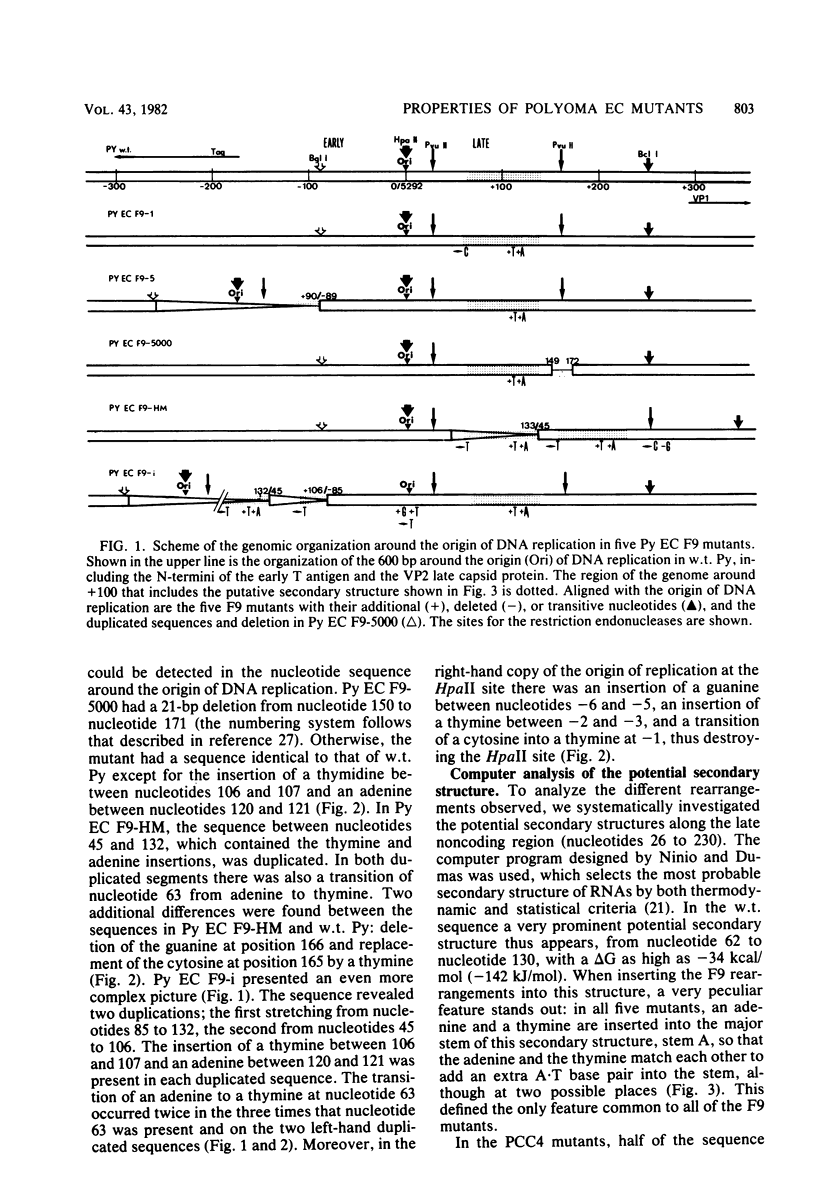
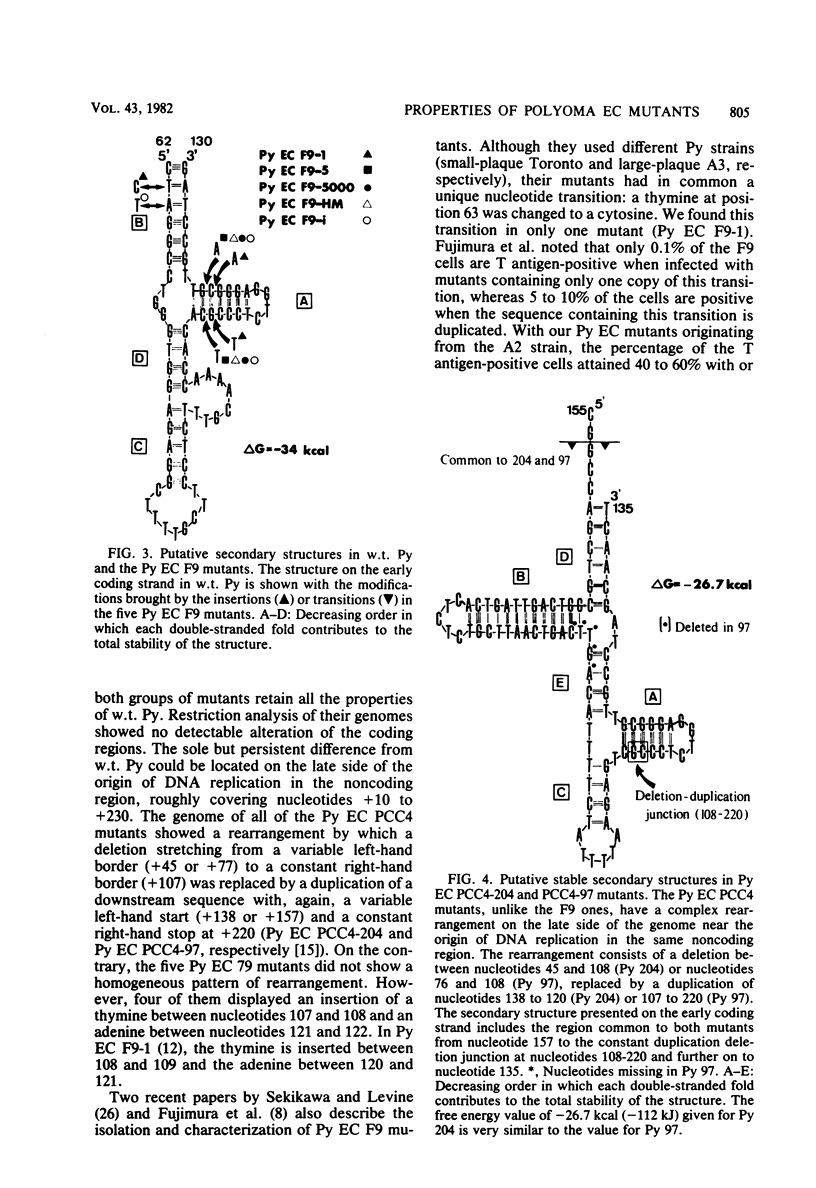
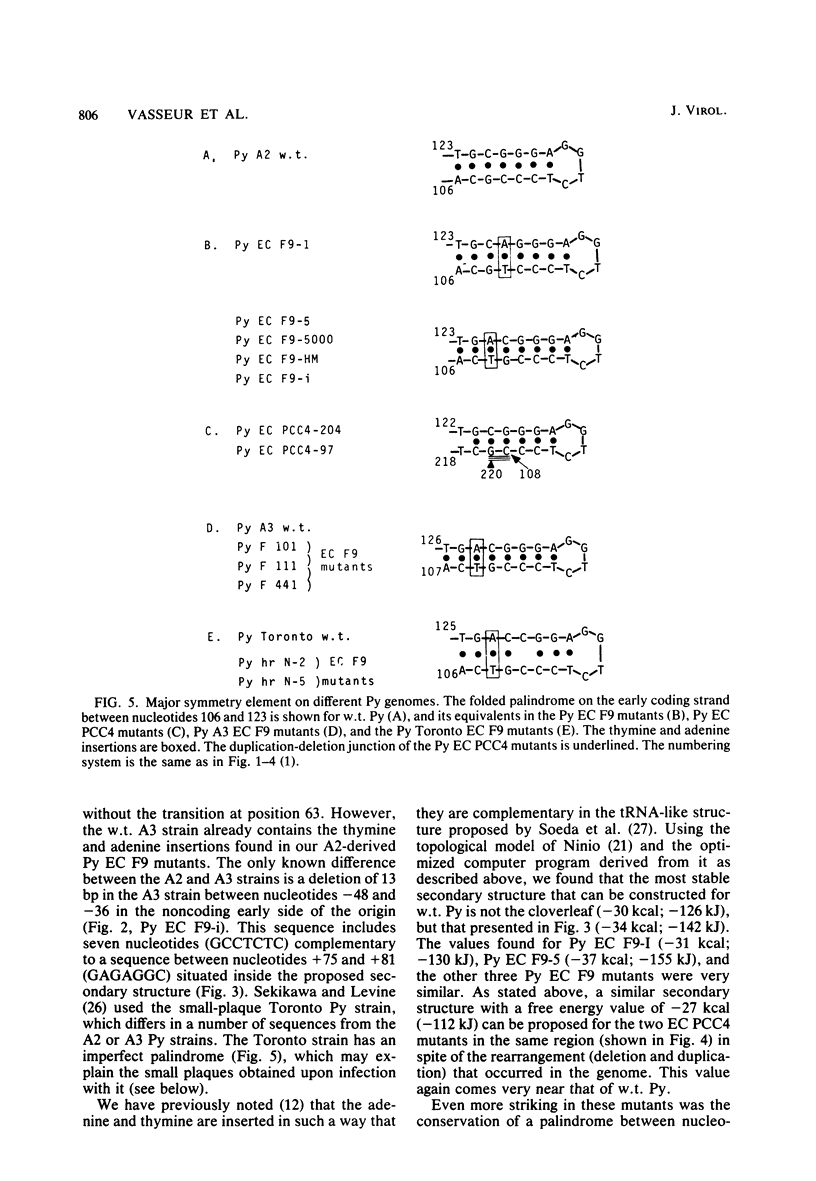
Three new polyoma mutants were selected for their ability to grow on the embryonal carcinoma cell line F9. These mutants share in common an insertion of two nucleotides, a thymine and an adenine, in the noncoding region located on the late side of the origin of replication. We have found that these insertions exist in all of the other polyoma virus mutants able to grow on F9 cells (Fujimura et al., Cell 23:809-814, 1981; Katinka et al., Nature (London) 290:720-722, 1981; K. Sekikawa and A. J. Levine, Proc. Natl. Acad. Sci. U.S.A. 78:1100-1104, 1981). The region containing these insertions could be folded into a stable secondary structure which included a guanine plus cytosine (G + C)-rich stem. The adenine and thymine were inserted in such a way that they maintained the palindrome in the G + C-rich stem and were complementary in the putative secondary structure that we present here. Another class of polyoma virus mutants selected on a multipotential carcinoma cell line (PCC4-Aza) were characterized by a more complex rearrangement (deletion and duplication) which occurred in the same region. This arrangement preserved the G + C-rich palindrome and also yielded a sequence which still allowed the folding of another type of stable secondary structure. The significance of these findings is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. R., Soeda E., Walsh J. E., Smolar N., Griffin B. E. Polyoma virus DNA: Sequence from the late region that specifies the leader sequence for late mRNA and codes for VP2, VP3, and the N-terminus of VP1. J Virol. 1980 Feb;33(2):606–618. doi: 10.1128/jvi.33.2.606-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artzt K., Jacob F. Letter: Absence of serologically detectable H-2 on primitive teratocarcinoma cells in culture. Transplantation. 1974 Jun;17(6):632–634. doi: 10.1097/00007890-197406000-00015. [DOI] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Boccara M., Kelly F. Etude de la sensibilité au virus du polyome et à SV40 de plusieurs lignées cellulaires de tératocarcinome. Ann Microbiol (Paris) 1978 Feb-Mar;129(2):227–238. [PubMed] [Google Scholar]
- Boccara M., Kelly F. Expression of polyoma virus in heterokaryons between embryonal carcinoma cells and differentiated cells. Virology. 1978 Oct 1;90(1):147–150. doi: 10.1016/0042-6822(78)90342-2. [DOI] [PubMed] [Google Scholar]
- Dewey M. J., Filler R., Mintz B. Protein patterns of developmentally totipotent mouse teratocarcinoma cells and normal early embryo cells. Dev Biol. 1978 Jul;65(1):171–182. doi: 10.1016/0012-1606(78)90188-4. [DOI] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Saragosti S., Blangy D., Yaniv M. Fine structure of the origin-proximal DNAase I-hypersensitive region in wild-type and EC mutant polyoma. Cell. 1981 Sep;25(3):651–658. doi: 10.1016/0092-8674(81)90172-0. [DOI] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. W. Clonal lines of teratocarcinoma cells in vitro: differentiation and cytogenetic characteristics. J Cell Physiol. 1976 Nov;89(3):441–455. doi: 10.1002/jcp.1040890310. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Nicolas J. F., Avner P., Gaillard J., Guenet J. L., Jakob H., Jacob F. Cell lines derived from teratocarcinomas. Cancer Res. 1976 Nov;36(11 Pt 2):4224–4231. [PubMed] [Google Scholar]
- Ninio J. Prediction of pairing schemes in RNA molecules-loop contributions and energy of wobble and non-wobble pairs. Biochimie. 1979;61(10):1133–1150. doi: 10.1016/s0300-9084(80)80227-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Stevens L. C., Varnum D. S. The development of teratomas from parthenogenetically activated ovarian mouse eggs. Dev Biol. 1974 Apr;37(2):369–380. doi: 10.1016/0012-1606(74)90155-9. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Rodriguez R. L., West R. W., Jr The rapid purification of T4 DNA ligase from a lambda T4 lig lysogen. J Biol Chem. 1980 Feb 10;255(3):813–815. [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Sundin O., Bohn M. A stretch of "late" SV40 viral DNA about 400 bp long which includes the origin of replication is specifically exposed in SV40 minichromosomes. Cell. 1979 Feb;16(2):453–466. doi: 10.1016/0092-8674(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Kress C., Montreau N., Blangy D. Isolation and characterization of polyoma virus mutants able to develop in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1068–1072. doi: 10.1073/pnas.77.2.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W. A small segment of polyoma virus DNA enhances the expression of a cloned beta-globin gene over a distance of 1400 base pairs. Nucleic Acids Res. 1981 Dec 11;9(23):6251–6264. doi: 10.1093/nar/9.23.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]



