Abstract
Objective
Up to 25% of patients experience subtle declines in post-operative neurocognitive function following, otherwise uncomplicated, carotid endarterectomy (CEA). We sought to determine if post-CEA neurocognitive deficits are associated with cerebral blood flow (CBF) abnormalities on post-operative MR perfusion brain scans.
Methods
We enrolled 22 CEA patients to undergo a battery of neuropsychometric tests preoperatively and on post-operative day 1 (POD 1). Neurocognitive dysfunction was defined as a two standard deviation decline in performance in comparison to a similarly aged control group of lumbar laminectomy patients. All patients received MR perfusion brain scans on POD 1 that were analysed for asymmetries in CBF distribution. One patient experienced a transient ischemic attack within 24 hours before the procedure and was excluded from our analysis.
Results
Twenty-nine percent of CEA patients demonstrated neurocognitive dysfunction on POD 1. One hundred percent of those patients with cognitive deficits demonstrated CBF asymmetry, in contrast to only 27% of those patients without cognitive impairment. Post-CEA cognitive dysfunction was significantly associated with CBF abnormalities (RR=3.75, 95% CI: 1.62−8.67, p=0.004).
Conclusion
Post-CEA neurocognitive dysfunction is significantly associated with post-operative CBF asymmetry. These results support the hypothesis that post-CEA cognitive impairment is caused by cerebral hemodynamic changes. Further work exploring the relationship between CBF and post-CEA cognitive dysfunction is needed.
Keywords: Carotid endarterectomy, neurocognitive, magnetic resonance perfusion
INTRODUCTION
Carotid endarterectomy (CEA) effectively reduces the risk of future stroke in appropriately selected patients with carotid artery stenosis1,2,4,9. Although the incidence of major perioperative stroke is low (1−2%), up to 25% of CEA patients experience subtle declines in postoperative neurocognitive function, as detected by changes in performance on a battery of neuropsychometric tests (NPMTs)6,7.
This post-CEA neurocognitive dysfunction is increasingly well documented in literature and is believed to be caused by subtle cerebral ischemia, resulting from either the dislodgement of microemboli or hemodynamic changes induced during the procedure6,7. To test the hypothesis that abnormalities in cerebral blood flow (CBF) contribute to post-CEA cognitive deficits, we utilized analysis of MR perfusion (MRP) scans to quantify the amount of CBF asymmetry in each cerebral hemisphere. We sought to determine whether post-CEA cognitive dysfunction is associated with post-operative CBF abnormalities.
METHODS
Study population
We prospectively enrolled 22 patients undergoing CEA for carotid artery stenosis who agreed to receive postoperative MRP scans in this institutional review board-approved study. All CEA patients had at least 60% stenosis of the operative carotid artery. Patients with new onset neurological deficits before the procedure were excluded. One patient was excluded based on this criterion. After written informed consent was obtained, patients were assessed with a battery of NPMTs immediately before surgery and 1 day post-operatively. All patients received MRP scans within 24 hours following surgery.
Anesthesia and surgery
No patients were pre-medicated. All patients received general anesthesia with routine hemodynamic and temperature monitoring, as previously described7. For patients undergoing CEA, blood pressure was continuously monitored with a radial artery catheter and an eight-channel encephalographic monitor was utilized during the course of surgery (Neurotrac II; Moberg Medical Inc., Ambler, PA, USA). Sedation before induction consisted of fentanyl and midazolam. General anesthesia was induced with fentanyl, midazolam and either vercuronium or rocuronium, and was maintained with isoflurane. Heparin (5000 or 6000 units) was administered intravenously before vessel occlusion. All CEAs were performed by members of either the neurovascular service or the vascular service. All patients were extubated in the operating room and recovered in a post-operative care or neurological intensive care unit.
Neuropsychometric evaluation
Patients were assessed before surgery and 1 day postoperatively with a battery of five NPMTs. All examinations were administered by a research assistant, trained to administer and score these NPMTs under the supervision of a neuropsychologist. Ten raw scores were generated from this battery of five NPMTs, which were chosen to represent a range of cognitive domains. The Boston Naming test was administered to evaluate patients' ability to verbally identify objects pictured on a series of cards. Halstead–Reitan Trails parts A and B evaluated visual conceptual and visuomotor tracking by timing how long it took a subject to connect consecutively numbered circles with a single line (part A) and then connect the same number of consecutively numbered and lettered circles by alternating between the two sequences (part B). The Controlled Oral Word Association test evaluated verbal fluency and provided information on dominant hemisphere function. During this test, patients were asked to generate as many words as possible within 60 seconds that begin with a certain letter. Three separate trials were performed, one each with the letters C, F and L. The sum of all words generated over the three trials constituted the raw score. The copy portion of the Rey Complex Figure test was administered to evaluate visuospatial organization, providing information on the functioning of the non-dominant hemisphere. Patients were asked to copy the figure and a standardized scoring system was used to evaluate the presence of specific design features and the accuracy of their location11.
Each pre-operative and post-operative test was scored individually for both the enrolled CEA patients and a control group of 20 contemporaneous lumbar laminectomy (LL) patients, who were matched for age and cardiovascular risk factors and received a similar anesthetic regimen as the CEA group. As previously described7, the change in individual test scores were converted to z scores as follows z score=(change score – mean change scoreLL)/standard deviation of change scoreLL z scores for each test were transformed into a point system as follows: z scores greater than or equal to −0.05: 0 points; less than −0.5 to −1.0: 1 point; less than −1.0 to −1.5: 2 points; less than −1.5 to −2.0: 3 points; less than −2.0 to −2.5: 4 points; less than −2.5 to −3.0: 5 points; less than −3.0: 6 points. The higher the test score, the greater the patient's test performance deviated from performance of the control population in the direction of decline. These test scores were summed to generate a total deficit score (TDS), measuring a patient's level of global cognitive decline. By definition, a CEA patient was determined to have experienced neurocognitive decline when the patient's TDS was greater then two standard deviations above the mean total cognitive change score of the control group. Neuropsychometric outcome was expressed as a dichotomous variable (‘injured’ or ‘uninjured’).
Post-operative MRP scans and image analysis
All 21 non-excluded CEA patients received MRP brain scans within 24 hours following surgery. Additionally, for non-statistical comparison, two LL (control) patients also received MRP brain scans within 24 hours following surgery. To analyse these scans, we utilized a previously described algorithm that quantifies the degree of relative difference in CBF between corresponding regions in the ipsilateral and contralateral cerebral hemispheres10. This algorithm constructs a relative difference map (RDM) representing this CBF quantities in two- and three-dimension. An axis of symmetry, a straight line drawn along the AP axis through the septum pellucidum equally dividing the brain into two symmetric hemispheres, is computed automatically. The average intensities of pixels from one hemisphere are subtracted from those of the contralateral hemisphere and this absolute difference is divided by the intensity value on the side where the CBF reading was relatively larger (‘relatively normal hemisphere’). The resulting RDM reflects the amount of CBF asymmetry. The degree of relative CBF differences in both hemispheres was analysed and represented as a histogram, demonstrating the distribution of relative differences in CBF between one brain region and its contralateral counterpart.
RESULTS
Cohort characteristics
Demographic and intraoperative variables for the CEA patients are shown in Table 1. All 21 CEA patients completed the NPMT battery pre-operative on postoperative day 1, and received brain MRP scans within 24 hours following surgery. Thirty-eight percent of CEA patients presented with symptomatic carotid stenosis, while 62% had asymptomatic disease. On post-operative
Table 1.
Demographic and intraoperative parameters of carotid endarterectomy patients
| Parameter | CEA patients |
|---|---|
| No. of patients (%) | 21 |
| Age (years) | 69.1 ± 7.6 |
| Male (%) | 16 (76%) |
| Smoker (%) | 16 (76%) |
| Diabetes mellitus (%) | 3 (14%) |
| Hypertension (%) | 17 (81%) |
| Hypercholesterolemia | 18 (86%) |
| Previous MI (%) | 8 (38%) |
| Symptomatic (%) | 8 (38%) |
| Previous contralateral CEA (%) | 6 (29%) |
| Right operative side (%) | 12 (57%) |
| Duration of surgery (minute) | 198.5 (65) |
| Cross-clamp time (minute) | 49.4 (18) |
| Shunt placement | 1 (5%) |
| Fentanyl (μg/kg) | 2.44 (0.6) |
| Midazolam (mg/kg) | 0.039 (0.01) |
Hypertension is defined as systolic blood pressure>140 mmHg or use of antihypertensive medication; hypercholesterolemia is defined as blood cholesterol>200 mmHg or use of anti-cholesterol medication. Continuous data are expressed as mean ± standard deviation. CEA=carotid endarterectomy; MI=myocardial infarction.
MRP image analysis
Each CEA (n=21) MRP scan and the two LL control group MRP scans were analysed for each of the six major vascular territories. CBF asymmetry was demonstrated in 11 CEA (52%) patients on post-operative day 1. All six (100%) CEA patients with cognitive dysfunction demonstrated CBF asymmetry. One example is demonstrated in Figure 1. Of the 15 CEA patients without post-operative cognitive deficits, 11 (73%) displayed symmetric CBF patterns (Figure 2) and four (27%) demonstrated asymmetric CBF abnormalities. Post-CEA cognitive dysfunction was significantly associated with CBF abnormalities (RR=3.75, 95% CI: 1.62−8.67, p=0.004). Both LL control patients who received MRP scans demonstrated symmetric CBF.
Figure 1.
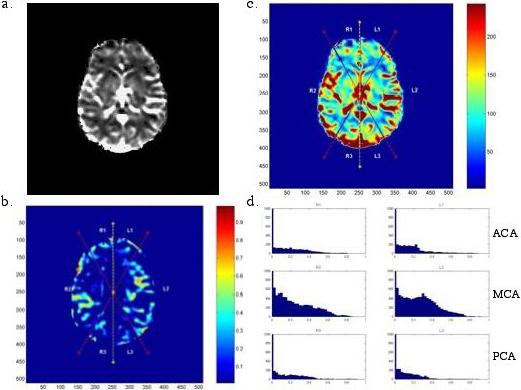
Analysis of MR brain scan of a patient demonstrating post-CEA neurocognitive dysfunction. The original MR perfusion gray-scale image (A) is converted to a color scale and divided into six vascular territories (B). A relative difference map is generated (C). High intensity in the RDM represents regions of relative CBF asymmetry. In this case, CBF asymmetry is pronounced in the MCA territories. Histograms depicting the distribution of RDM intensities are generated (D): the CBF asymmetry being represented by the broad distribution of the MCA histogram
Figure 2.
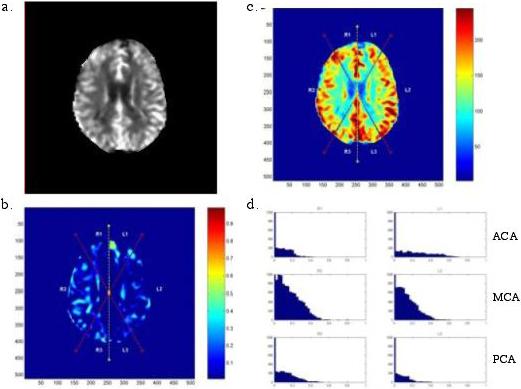
Analysis of MR brain scan of a patient without post-CEA neurocognitive dysfunction. The original MR perfusion gray-scale image (A) is converted to a color scale and divided into six vascular territories (B). A relative difference map is generated (C). The lack of high intensity in the RDM suggests relative CBF symmetry. In this case, CBF symmetry is demonstrated by the narrow downward sloping histograms in each vascular territory (D)
DISCUSSSION
In recent years, neurocognitive dysfunction following CEA has been increasingly recognized. Works now suggest that up to 25% of CEA patients experience declines in post-operative NPMT performance6,7,13. Our previous work identifies a number of risk factors for post-CEA cognitive deficits on post-operative day 1, including advanced age13, elevated pre-operative monocyte counts and the APOE e4 polymorphism8,12. This is the first study to demonstrate an association between post-CEA neurocognitive dysfunction and postoperative CBF abnormalities detected on MRP brain scans.
Although the incidence of post-CEA cognitive decline is well established, the precise mechanism underlying this phenomenon is uncertain. Impaired NPMT performance following CEA is believed to be ischemic in nature, either due to microemboli dislodged or cerebral hemodynamic changes induced during the procedure6. This ischemic hypothesis is supported by elevated postoperative levels of serum S100b, a marker of glial cell death, in patients experiencing declines in postoperative NPMT performance3. Our current findings suggest that hemodynamic dysregulation plays a role in post-CEA cognitive dysfunction. Patients with impaired cerebral autoregulation may be particularly vulnerable to hemodynamic shifts during the period of carotid artery cross-clamping14. Such CBF changes may cause subtle ischemic injury and cognitive decline.
While conventional MRP scan analysis relies on the averaging of CBF values over predetermined regions of interest, our algorithm compares a neighborhood of voxels with its contralateral counterpart over a reflexional axis of symmetry10. This method offers more sensitivity in detecting subtle CBF asymmetry, and has shown promise in several clinical applications, including the detection of CBF abnormalities following subarachnoid hemorrhage that predict delayed cerebral vasospasm10.
In this study, 29% of CEA patients experienced postoperative cognitive dysfunction, consistent with rates of post-CEA cognitive impairment determined in previous works6,7. Using a previously described algorithm, we demonstrate that CBF abnormalities are associated with post-CEA cognitive dysfunction on post-operative day 1 (RR=3.75, p=0.004). Our analysis of CBF asymmetry identified post-CEA cognitive dysfunction with a sensitivity of 100% and a specificity of 73%. While all CEA patients with post-operative cognitive decline demonstrated CBF asymmetry, of the 15 CEA patients without cognitive deficits, four also demonstrated CBF abnormalities. These four cases of MRP abnormalities without cognitive decline may be explained by slight hemodynamic changes that failed to manifest in impaired performance on the NPMT battery, or could represent inherent limitations in our technique. We obtained MRP brain scans on two of our control patients, which both showed CBF symmetry. While our true comparison was between injured and non-injured CEA patients, it would also have been helpful to obtain MRP brain scans on more of these control patients to establish a baseline rate of post-operative CBF asymmetry.
The association between post-operative CBF asymmetry and post-CEA cognitive dysfunction is robust. However, our study remains limited by its small sample size. Furthermore, while our analysis of post-operative MRP brain scans sheds light on the mechanisms underlying post-operative cognitive impairment, a study including pre-operative scans would be beneficial in determining whether the CBF abnormalities are induced by the procedure or whether a subset of high-risk patients displays baseline CBF asymmetry. Owing to the design of this study, we can only show an association between neurocognitive decline and asymmetric postoperative CBF, and further work is necessary to determine if a causal relationship exists. With over 100,000 CEAs performed annually, many for borderline indications5, our findings call for continued investigations into the relationship between CBF and post-CEA cognitive dysfunction .
Table 2.
Risk factors for neurocognitive decline on post-operative day 1
| Injured | Uninjured | OR (95% CI) | p | |
|---|---|---|---|---|
| No. of patients (%) | 33 (18%) | 153 (82%) | — | — |
| Age (years, OR/decade) | 73.2 ± 7.7 | 69.1 ± 8.5 | 1.93 (1.15−3.25) | 0.01* |
| Male (%) | 22 (67%) | 107 (70%) | — | — |
| BMI≥30 (%) | 6 (18%) | 33 (22%) | — | — |
| Smoker (%) | 19 (58%) | 83 (54%) | — | — |
| Diabetes mellitus (%) | 11 (33%) | 36 (24%) | — | — |
| Hypertension (%) | 21 (64%) | 97 (63%) | — | — |
| Hypercholesterolemia | 16 (48%) | 83 (54%) | — | — |
| Statin medication | 13 (39%) | 84 (55%) | — | — |
| Previous MI (%) | 10 (30%) | 43 (28%) | — | — |
| Symptomatic (%) | 16 (48%) | 61 (40%) | — | — |
| Previous contralateral CEA (%) | 6 (18%) | 19 (12%) | — | — |
| Right operative side (%) | 21 (64%) | 76 (50%) | — | — |
| Duration of surgery (minute) | 153.3 ± 46.6 | 153.7 ± 18.2 | — | — |
| Cross-clamp time (minute) | 47.5 ± 18.2 | 45.2 ± 19.0 | — | — |
| Shunt placement | 2 (6%) | 3 (2%) | — | — |
| Fentanyl (μg/kg) | 2.2 ± 1.3 | 2.2 ± 1.2 | — | — |
| Midazolam (mg/kg) | 0.03 ± 0.01 | 0.03 ± 0.01 | — | — |
OR is expressed in units of decades for age. No other variables met univariate
p<0.10 for inclusion in multivariate analysis. Hypertension is defined as systolic blood pressure>140 mmHg or use of antihypertensive medication; hypercholesterolemia is defined as blood cholesterol>200 mmHg or use of anti-cholesterol medication. Continuous data are expressed as mean ± standard deviation. OR=odds ratio; BMI=body mass index; MI=myocardial infarction; CEA=carotid endarterectomy.
ACKNOWLEDGEMENTS
Dr J. Mocco was supported in part by the Congress of Neurological Surgeons Wilder Penfield Clinical Research Fellowship. D. A. Wilson, C. P. Kellner and D. K. Hahn were supported in part by the Doris Duke Clinical Research Fellowship. Dr R. J. Komotar was supported in part by an NIH Research Training Fellowship. Dr E. J. Heyer was supported in part by a grant from the NIA (RO1 AG17604-02).
REFERENCES
- 1.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.Endarterectomy for asymptomatic carotid artery stenosis Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 3.Connolly ES, Jr, Winfree CJ, Rampersad A, et al. Serum S100B protein levels are correlated with subclinical neurocognitive declines after carotid endarterectomy. Neurosurgery. 2001;49:1076–1082. doi: 10.1097/00006123-200111000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 5.Halm EA, Chassin MR, Tuhrim S, et al. Revisiting the appropriateness of carotid endarterectomy. Stroke. 2003;34:1464–1471. doi: 10.1161/01.STR.0000072514.79745.7D. [DOI] [PubMed] [Google Scholar]
- 6.Heyer EJ, Adams DC, Solomon RA, et al. Neuropsychometric changes in patients after carotid endarterectomy. Stroke. 1998;29:1110–1115. doi: 10.1161/01.str.29.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyer EJ, Sharma R, Rampersad A, et al. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heyer EJ, Wilson DA, Sahlein DH, et al. APOE-epsilon4 predisposes to cognitive dysfunction following uncomplicated carotid endarterectomy. Neurology. 2005;65:1759–1763. doi: 10.1212/01.wnl.0000184579.23624.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobson RW, 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221–227. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 10.Imielinska C, Liu X, Rosiene J, et al. Toward objective quantification of perfusion-weighted computed tomography in subarachnoid hemorrhage: Quantification of symmetry and automated delineation of vascular territories. Acad Radiol. 2005;12:874–887. doi: 10.1016/j.acra.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 11.Lu PH, Boone KB, Cozolino L, et al. Effectiveness of the Rey–Osterrieth Complex Figure Test and the Meyers and Meyers recognition trial in the detection of suspect effort. Clin Neuropsychol. 2003;17:426–440. doi: 10.1076/clin.17.3.426.18083. [DOI] [PubMed] [Google Scholar]
- 12.Mocco J, Wilson DA, Ducruet AF, et al. Elevations in preoperative monocyte count predispose to acute neurocognitive decline after carotid endarterectomy for asymptomatic carotid artery stenosis. Stroke. 2006;37:240–242. doi: 10.1161/01.STR.0000195183.04978.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mocco J, Wilson DA, Komotar RJ, et al. Predictors of neurocognitive decline after carotid endarterectomy. Neurosurgery. 2006;58:844–850. doi: 10.1227/01.NEU.0000209638.62401.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]


