Abstract
Rhodopsin is the photosensitive pigment in the rod photoreceptor cell. Upon absorption of a photon, the covalently bound 11-cis retinal isomerizes to the all-trans form enabling rhodopsin to activate transducin, its G protein. All-trans retinal is then released from the protein and reduced to all-trans retinol. It is subsequently transported to the retinal pigment epithelium where it is converted to 11-cis retinol and oxidized to 11-cis retinal before it is transported back to the photoreceptor to regenerate rhodopsin and complete the visual cycle. In this study, we have measured the effects of all-trans and 11-cis retinals and retinols on the opsin’s ability to activate transducin to ascertain their potentials for activating the signaling cascade. Only 11-cis retinal acts as an inverse agonist to the opsin. All-trans retinal, all-trans retinol, and 11-cis retinol are all agonists with all-trans retinal being the most potent agonist and all-trans retinol being the least potent. Taken as a whole, our study is consistent with the hypothesis that the steps in the visual cycle are optimized such that the rod can serve as a highly sensitive dim light receptor. All-trans retinal is immediately reduced in the photoreceptor to prevent back reactions and to lower its effectiveness as an agonist before it is transported out of the cell; oxidation of 11-cis retinol occurs in the retinal pigment epithelium and not the rod photoreceptor cell because 11-cis retinol can act as an agonist and activate the signaling cascade if it were to bind an opsin, effectively light-adapting the cell.
Rods are exquisitely sensitive to dim light. Rhodopsin is the photosensitive pigment that initiates the visual signaling cascade and is comprised of a protein part (opsin) and the 11-cis form of vitamin A aldehyde (11-cis retinal) covalently bound to the opsin via a Schiff base linkage. Rhodopsin belongs to the superfamily of G protein-coupled receptors and uses the 11-cis retinal not only as its chromophore but also as an inverse agonist holding it in an inactive conformation (1, 2). Light converts the 11-cis bond to all-trans changing the inverse agonist to an agonist to enable the opsin to activate its G protein transducin. In a competing reaction, rhodopsin kinase phosphorylates the light-activated rhodopsin, and arrestin binds to the phosporylated cytoplasmic surface to initiate the inactivation process. Eventually, rhodopsin is further deactivated when the chromophore is hydrolyzed from the opsin as all-trans retinal, reduced to all-trans retinol, and transported to the retinal pigment epithelium (RPE). In the RPE, it is isomerized to 11-cis retinol, oxidized to 11-cis retinal, and then shuttled back to the photoreceptor cell to regenerate rhodopsin (Figure 1). The regeneration of rhodopsin whereby the retinoid is recycled through the RPE is called the visual cycle (3) [see also recent reviews (4, 5)].
Figure 1.
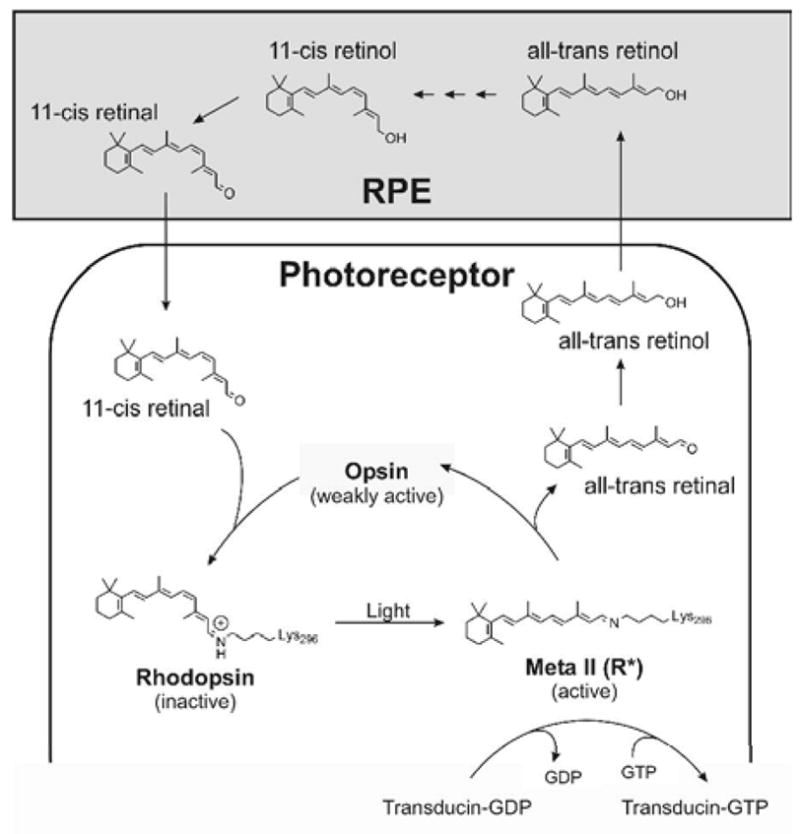
Schematic of the visual cycle. Photoactivation of rhodopsin isomerizes the 11-cis chromophore to the all-trans form after which rhodopsin releases its chromophore, all-trans retinal, which is reduced to all-trans retinol. This is then transported to the RPE where it is converted to 11-cis retinol via several steps that includes esterification, isomerization, and hydrolysis by at least two enzymes (not shown) [e.g., see reviews (4, 5)]. 11-cis retinol is oxidized to 11-cis retinal in the RPE before it is transported back to the photoreceptor cell to bind an opsin to reform rhodopsin. The light-activated rhodopsin, Meta II, is the intermediate that activates transducin by catalyzing a GDP/GTP exchange.
Why would vertebrates evolve to include steps that convert an aldehyde to an alcohol for export and then import the reisomerized chromophore into the photoreceptor as an aldehyde? We address this question by examining the effects of 11-cis and all-trans vitamin A aldehydes and alcohols on the rod opsin’s ability to activate transducin. Our results lead us to propose that the visual cycle is a process that helps to maximize the rod’s sensitivity to dim light.
EXPERIMENTAL PROCEDURES
Materials
All-trans retinal and retinol were purchased from Sigma (St. Louis, MO); 11-cis retinal was synthesized as described previously (6, 7); 11-cis retinol was synthesized by reduction of 11-cis retinal as described previously (8). Purity of retinoids was confirmed by high performance liquid chromatography (9). Retinoid crystals were dissolved in ethanol and quantified by absorption spectroscopy using extinction coefficients as summarized previously (10). The rhodopsin 1D4 antibody was from National Cell Culture Center (Minneapolis, MN). Bovine retinas were from W.L. Lawson (Lincoln, NE). GTPγS-35 (1250 Ci/mmol) was from PerkinElmer Life and Analytical Sciences (Waltham, MA), and the nonradioactive GTPγS was from Roche Applied Science (Indianapolis, IN).
Protein expression
Bovine rod opsin was expressed in COS cells as described previously (11). Cells were harvested 3 days after tranfection and frozen at −80 °C. For membrane preparations, a discontinuous sucrose gradient was used to isolate the plasma membrane fraction (12–14). The amount of opsin in the membrane preparations was determined by slot blot analysis as described previously (13). Briefly, membrane preparation aliquots and known amounts rhodopsin from a bovine rod outer segment preparation were solubilized and loaded into the wells of the slot blot apparatus in triplicate. The blot was probed with the rhodopsin 1D4 antibody (15) and detected by chemiluminescence (13).
Transducin activation assay
Opsin activity was determined by its ability to activate bovine rod transducin using a radioactive filter binding assay essentially as described previously with a few modifications (12, 13). Bovine rod transducin was purified from bovine retinas as described previously (16–18). To assay the ability of the opsin to activate transducin, we followed the increase of radioactive GTPγS bound to transducin with time after addition of retinoid in the dark or after illumination. Typically, our reaction mixture contained 4 nM opsin (unless otherwise stated), 2.5 μM transducin, 3.0 μM GTPγS-35 (~5 nCi/μL), 20 μM retinoid, 10 mM 2-(N-morpholino)ethanesulfonic acid (pH 6.4), 100 mM NaCl, 1 mM dithiothreitol, and 5 mM MgCl2, pH 6.4 at a final volume of 50, 100, or 150 μL. The buffer, opsin membrane, and transducin were first mixed. Retinoids were added from a 50x stock dissolved in ethanol 1 min prior to addition of GTPγS, at which time the clock was started. At specified times, we transferred a 10 μL aliquot onto a nitrocellulose filter membrane attached to a vacuum manifold and washed three times with 4 mL ice-cold buffer; the amount of bound radioactivity was determined by scintillation counting. For measuring light-dependent activation, we began measuring the dark points, illuminated the sample with > 495 nm light from a slide projector with a 300 W bulb passed through a long-pass filter for 12 s at a specified time, and then continued measuring 10 μL aliquots. We did not correct for the basal background activity of transducin itself at this lower pH nor contributions from any endogenous COS cell receptors as they add little to the total activity and shown previously to overlap with activity of rhodopsin in membranes in the dark (1, 19). Because ligand-dependent activation of transducin by opsins is linear, we also measured the amount of transducin activated at a single 5 min time point in order to quickly screen activation under a variety of conditions as described in the text and figure legends.
RESULTS
We tested the ability of bovine rod opsin to activate transducin in a ligand-dependent manner. Consistent with earlier results (19), 11-cis retinal deactivates the opsin, which itself is weakly active (Figure 2A). The reaction pH used here is 6.4 because above neutral pH, the constitutive activity of opsin is difficult to resolve (1). In addition to increasing the relative activity of the opsin relative to rhodopsin in the dark, the lower pH also increases the basal activity of transducin itself (1, 19). We find that all-trans retinal is an agonist to the rod opsin, increasing the activity 8-fold over opsin alone (Figure 2A). This result is consistent with earlier studies (20–25). Interestingly, the alcohols of the 11-cis and all-trans retinoids are also agonists, but they are not as robust an agonist as all-trans retinal (5-fold over opsin alone for all-trans retinol and 7-fold for 11-cis retinol) (Figure 2A).
Figure 2.
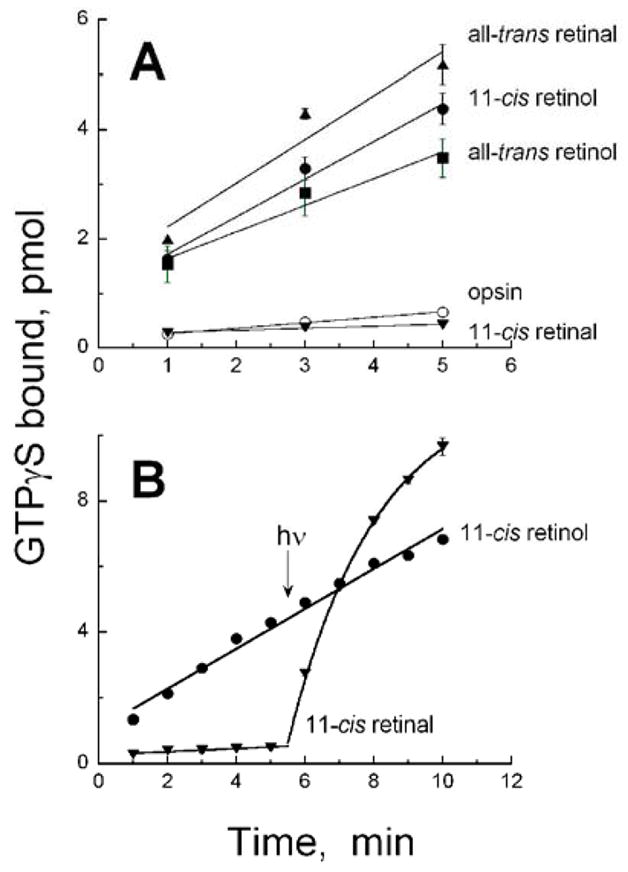
Activation of transducin by bovine rod opsin in the presence or absence of different retinoids and dark/light conditions. (A) Ligand-dependent transducin activation by rod opsin in the dark in the presence of all-trans retinal (triangles), 11-cis retinol (filled circles), all-trans retinol (squares), and 11-cis retinal (upside-down triangles). Also shown is the activation in the absence of ligand by the opsin itself (open circles). (B) Dark- and light-dependent activation of opsin incubated with 11-cis retinal (upside-down triangles) and 11-cis retinol (circles). At 5.5 min, samples were illuminated with > 495 nm light for 12 s as described in the Methods section.
To ensure that activity was not lowered due to oxidation of some 11-cis retinol to retinal and formation of a pigment, we assayed for transducin activation by opsin incubated with 11-cis retinol in the dark and after illumination with a 12 s pulse of > 495 nm light. While this sample activated transducin at a rate higher than opsin alone, the activity did not change after exposure to light (Figure 2B) suggesting that a long-wavelength absorbing pigment did not form with 11-cis retinol. Illumination with ultraviolet light from a hand-held UV lamp also did not alter tranducin activation by opsin in the presence of 11-cis retinol (not shown). Not surprisingly, the sample with 11-cis retinal is able to activate transducin in a light-dependent manner because 11-cis retinal formed a pigment absorbing maximally at 500 nm that can undergo cis/trans photoisomerization (Figure 2B). Under our conditions, light-dependent activation of transducin by rhodopsin is about 30 times higher than transducin activated by opsin alone and about 4 times higher than by opsin with exogenously added all-trans retinal.
The most effective agonist is the covalently bound all-trans retinylidene formed from photoisomerization of the 11-cis chromophore in rhodopsin. The next effective agonist is all-trans retinal, followed by 11-cis retinol and then all-trans retinol. To ensure we were at a saturating level of retinoids, we measured transducin activation by opsin incubated with varying concentrations of these agonists (Figure 3). All three agonists can activate the opsin in a concentration-dependent manner. The aldehyde is able to activate the opsin at much lower concentrations than the alcohols with an EC50 value of about 53 nM for all-trans retinal, while the EC50 values of the two alcohols are about 6 μM. The reaction kinetics shown in Figure 2 was conducted in the presence of 20 μM ligand. One striking feature of this result is that the concentration dependence curves from the alcohols are steeper (fit with n = 2) than that from the aldehyde (fit with n = 1), suggesting cooperativity with the retinols.
Figure 3.

Agonist concentration dependence of transducin activation by rod opsin. The relative rates of transducin activation by 4 nM rod opsin (filled symbols) are plotted against different concentrations of (A) all-trans retinal, (B) all-trans retinol, and (C) 11-cis retinol. The abilities of double the amount of opsin membranes (8 nM) to activate transducin in the presence of all-trans retinal (A, open triangles) or all-trans retinol (B, open squares) are also shown.
We measured transducin activation by doubling the amount of opsin membranes (opsin concentration, 8 nM) in the presence of different concentrations of all-trans retinal and all-trans retinol. When these data are normalized, they (open symbols, Figure 3A and 3B) are essentially indistinguishable from those collected with 4 nM opsin (filled symbols, Figures 3A and 3B). We also show that transducin activation by opsin alone and opsin in the presence of 20 μM all-trans retinal increases linearly with increasing amounts of opsin (Figure 4). Thus, the opsin concentration used in Figures 2 and 3 are in the linear range of activation.
Figure 4.

Activation of transducin as a function of opsin concentration in the absence (open circles) and presence of 20 μM all-trans retinal. The reaction is performed as described in the Methods section, but the amount of COS cell membranes containing the expressed opsin is varied.
DISCUSSION
Low opsin activity is necessary for rods to function under conditions of dim light. An opsin with a high basal level of activity would initiate the transduction cascade in the absence of light and desensitize the cells, compromising their high sensitivity. Such a mechanism is likely the cause for some forms of congenital night blindness. For example, the G90D rhodopsin mutant (26) opsin is constitutively active (27). A rod containing G90D rhodopsin seems to have a significant amount of apoprotein present in the dark (28), and its phenotype is that they appear as if they were light-adapted in the dark (26, 29).
Likewise, a prolonged presence of agonists in rods would be problematic because if they were able to bind an opsin, it would activate the signaling cascade and desensitize the rod independent of light. The first step in retinoid recycling after photoactivation of the pigment is release of all-trans retinal from the opsin (30, 31). All-trans retinal is then reduced to all-trans retinol (32). There are a number of reasons why this step is advantageous to the photoreceptor cell. First, it eliminates the reassociation of an active complex with opsin (33, 34). Secondly, free all-trans retinal is potentially toxic as both a visible light photosensitizer (35) and source for A2E precursors (36, 37); all-trans retinol is not. We now find that all-trans retinol is an agonist to opsin, but it is a less potent agonist than all-trans retinal in terms of lower maximal amplitude of activation and the two orders of magnitude difference of EC50s. These would add additional levels of inactivation of the opsin on top of chromophore release and opsin phosphorylation and arrestin binding.
Exogenous all-trans retinal and retinol have been shown not to compete with 11-cis retinal for rhodopsin pigment regeneration (21). However, Schädel et al. (38) showed that these two all-trans retinoids do bind the rod opsin suggesting that the binding site might differ from where 11-cis retinal binds. An unexpected result in this study is the apparent cooperativity of the all-trans and 11-cis retinol concentration dependence curves that is not seen with all-trans retinal (Figure 3). We have no current explanation for this result; however, it might be related to alternate retinoid binding sites in the opsin.
Previous studies as well as this one have shown all-trans retinal to be a partial agonist (20–25), and we now show that all-trans retinol can also be a partial agonist. Regardless of whether these retinoids are interacting with opsin at the same site as 11-cis retinal or at a secondary site, the released retinal and its reduced product retinol need to be removed from the photoreceptor because otherwise their interaction with the opsin would continue to desensitize the rod cell. As mentioned above, reduction of all-trans retinal to all-trans retinol increases the EC50 from about 53 nM to 6 μM, and this alone would lower the ligand-dependent activation of opsin. The upper limit of free retinoids in the absence of removal from the outer segment will be in the millimolar range because the rhodopsin concentration in the rod outer segment is about 3 mM (39). This makes the concentration ranges of retinoids that activate opsin in this study physiologically possible after photobleaching rhodopsin, and their removal helpful in limiting opsin activity especially under high bleaching conditions.
Similarly, the oxidation of 11-cis retinol outside of the rod photoreceptor and its subsequent transport back to the opsin as the aldehyde is advantageous because 11-cis retinol is an even stronger agonist than all-trans retinol. If 11-cis retinol were to be transported to the rod cell, any opsin that were to interact with the retinol before its oxidation would be activated and consequently desensitize the rod. This action could explain the conclusion that 11-cis retinol was “toxic” to bleached rods as described by Jones et al. (8) from studies on isolated salamander rods. Addition of 11-cis retinol to bleached rods did not restore sensitivity and actually reduced sensitivity. Their results are consistent with rods not oxidizing 11-cis retinol, and the 11-cis retinol interacting with the rod opsin to activate the signaling cascade by activating transducin, which we show is possible in this study. However, the higher EC50 of the 11-cis retinol concentration dependence data indicates that a significantly higher amount of 11-cis retinol would be required for the rod opsin to activate transducin to the same level as with all-trans retinal.
Among the retinoids in this study, only 11-cis retinal behaves as an inverse agonist to rod opsin, and it is used to dark adapt the rod as the rhodopsin pigment is generated. Photoisomerization of the bound ligand to the all-trans form activates the signal cascade. Hydrolysis of the chromophore and subsequent reduction to all-trans retinol reduces the retinoid’s efficacy as an agonist in addition to preventing the potential harmful effects of all-trans retinal (35–37) from taking place. Its removal from the photoreceptor cell to the RPE prevents it from interacting with opsin. The generation of 11-cis retinol and its oxidation to 11-cis retinal occurs in the RPE, and the absence of 11-cis retinol in the rod photoreceptor cell avoids the possibility of its interaction with opsin and causing spurious activation of the signal cascade in the dark. Thus, the visual cycle appears to be exquisitely designed to optimize the rods’ function as a dim light photoreceptor.
Acknowledgments
We would like to thank Yiannis Koutalos for his helpful comments and suggestions, John Oatis for synthesis of 11-cis retinol, and Luanna Bartholomew for critical reading of the manuscript.
Abbreviations
- RPE
Retinal pigment epithelium
Footnotes
This work was supported by the National Institutes of Health grants (EY013748, EY014793, EY04939), Research to Prevent Blindness, Foundation Fighting Blindness, and MUSC Institutional Research Funds.
References
- 1.Cohen GB, Oprian DD, Robinson PR. Mechanism of activation and inactivation of opsin: role of Glu113 and Lys296. Biochemistry. 1992;31:12592–12601. doi: 10.1021/bi00165a008. [DOI] [PubMed] [Google Scholar]
- 2.Melia TJ, Jr, Cowan CW, Angleson JK, Wensel TG. A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin. Biophys J. 1997;73:3182–3191. doi: 10.1016/S0006-3495(97)78344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald G. Carotenoids and the visual cycle. J Gen Physiol. 1935;19:351–371. doi: 10.1085/jgp.19.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepperberg DR, Crouch RK. An illuminating new step in visual-pigment regeneration. Lancet. 2001;358:2098–2099. doi: 10.1016/S0140-6736(01)07231-2. [DOI] [PubMed] [Google Scholar]
- 5.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retinal Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad R, Wheedon BCL. Carotenoids and related compounds. Part III. The synthesis of bisnorcrocetin, a pentane degradation product of azafrin, and other polyenes. J Chem Soc. 1953;1953:3299–3315. [Google Scholar]
- 7.Das J, Crouch RK, Ma J-x, Oprian DD, Kono M. Role of the 9-methyl group of retinal in cone visual pigments. Biochemistry. 2004;43:5532–5538. doi: 10.1021/bi036097u. [DOI] [PubMed] [Google Scholar]
- 8.Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci USA. 1989;86:9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr, Redmond TM, Ma J-x. Retinyl esters are the substrate for isomerohydrolase. Biochemistry. 2003;42:2229–2238. doi: 10.1021/bi026911y. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard R, Brown PK, Bownds D. Methodology of vitamin A and visual pigments. Methods Enzymol. 1971;18C:615–653. [Google Scholar]
- 11.Oprian DD. Expression of opsin genes in COS cells. Methods Neurosci. 1993;15:301–306. [Google Scholar]
- 12.Robinson PR. Assays for the detection of constitutively active opsins. Methods Enzymol. 2000;315:207–218. doi: 10.1016/s0076-6879(00)15845-8. [DOI] [PubMed] [Google Scholar]
- 13.Kono M. Constitutive activity of a UV cone opsin. FEBS Lett. 2006;580:229–232. doi: 10.1016/j.febslet.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kono M, Crouch RK, Oprian DD. A dark and constitutively active mutant of the tiger salamander UV pigment. Biochemistry. 2005;44:799–804. doi: 10.1021/bi047898f. [DOI] [PubMed] [Google Scholar]
- 15.Molday RS, MacKenzie D. Monoclonal antibodies to rhodopsin: Characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- 16.Baehr W, Morita EA, Swanson RJ, Applebury ML. Characterization of bovine rod outer segment G-protein. J Biol Chem. 1982;257:6452–6460. [PubMed] [Google Scholar]
- 17.Wessling-Resnick M, Johnson GL. Allosteric behavior in transducin activation mediated by rhodopsin. J Biol Chem. 1987;262:3697–3705. [PubMed] [Google Scholar]
- 18.Yu H, Kono M, McKee TD, Oprian DD. A general method for mapping tertiary contacts between amino acid residues in membrane-embedded proteins. Biochemistry. 1995;34:14963–14969. doi: 10.1021/bi00046a002. [DOI] [PubMed] [Google Scholar]
- 19.Cohen GB, Yang T, Robinson PR, Oprian DD. Constitutive activation of opsin: influence of charge at position 134 and size at position 296. Biochemistry. 1993;32:6111–6115. doi: 10.1021/bi00074a024. [DOI] [PubMed] [Google Scholar]
- 20.Yoshizawa T, Fukada Y. Activation of phosphodiesterase by rhodopsin and its analogues. Biophys Struct Mech. 1983;9:245–258. doi: 10.1007/BF00535660. [DOI] [PubMed] [Google Scholar]
- 21.Jäger S, Palczewski K, Hofmann KP. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35:2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- 22.Sachs K, Maretzki D, Hofmann KP. Assays for activation of opsin by all-trans-retinal. Methods Enzymol. 2000;315:238–251. doi: 10.1016/s0076-6879(00)15847-1. [DOI] [PubMed] [Google Scholar]
- 23.Buczylko J, Saari JC, Crouch RK, Palczewski K. Mechanisms of opsin activation. J Biol Chem. 1996;271:20621–20630. doi: 10.1074/jbc.271.34.20621. [DOI] [PubMed] [Google Scholar]
- 24.Han M, Sakmar TP. Assays for activation of recombinant expressed opsins by all-trans-retinals. Methods Enzymol. 2000;315:251–267. doi: 10.1016/s0076-6879(00)15848-3. [DOI] [PubMed] [Google Scholar]
- 25.Surya A, Knox BE. Enhancement of opsin activity by all-trans-retinal. Exp Eye Res. 1998;66:599–603. doi: 10.1006/exer.1997.0453. [DOI] [PubMed] [Google Scholar]
- 26.Sieving PA, Richards JE, Naarendorp F, Bingham EL, Scott K, Alpern M. Dark-light: Model for nightblindness from the human rhodopsin Gly-90 --> Asp mutation. Proc Natl Acad Sci USA. 1995;92:880–884. doi: 10.1073/pnas.92.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao VR, Cohen GB, Oprian DD. Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature. 1994;367:639–642. doi: 10.1038/367639a0. [DOI] [PubMed] [Google Scholar]
- 28.Jin S, Cornwall MC, Oprian DD. Opsin activation as a cause of congenital night blindness. Nature Neurosci. 2003;6:731–735. doi: 10.1038/nn1070. [DOI] [PubMed] [Google Scholar]
- 29.Sieving PA, Fowler ML, Bush RA, Machida S, Calvert PD, Green DG, Makino CL, McHenry CL. Constitutive “light” adaptation in rods from G90D rhodopsin: a mechanism for human congential nightblindness without rod cell loss. J Neurosci. 2001;21:5449–5460. doi: 10.1523/JNEUROSCI.21-15-05449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubbard R, Kropf A. The action of light on rhodopsin. Proc Natl Acad Sci USA. 1958;44:130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews RG, Hubbard R, Brown PK, Wald G. Tautomeric forms of metarhodopsin. J Gen Physiol. 1963;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wald G, Hubbard R. The reduction of retinene1 to vitamin A1 in vitro. J Gen Physiol. 1949;32:367–389. doi: 10.1085/jgp.32.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saari JC, Garwin GG, Van Hooser JP, Palczewski K. Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vision Res. 1998;38:1325–1333. doi: 10.1016/s0042-6989(97)00198-3. [DOI] [PubMed] [Google Scholar]
- 34.Palczewski K, Jäger S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 35.Harper WS, Gaillard ER. Studies of all-trans-retinal as a photooxidizing agent. Photochem Photobiol. 2001;73:71–76. doi: 10.1562/0031-8655(2001)073<0071:soatra>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Katz ML, Gao CL, Rice LM. Formation of lipofuscin-like fluorophores by reaction of retinal with photoreceptor outer segments and liposomes. Mech Ageing Dev. 1996;92:159–174. doi: 10.1016/s0047-6374(96)01817-9. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem. 2000;275:29354–29360. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- 38.Schädel SA, Heck M, Maretzki D, Filipek S, Teller DC, Palczewski K, Hofmann KP. Ligand channeling within a G-protein-coupled receptor: the entry and exit of retinals in native opsin. J Biol Chem. 2003;278:24896–24903. doi: 10.1074/jbc.M302115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hárosi F. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975;66:357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]


