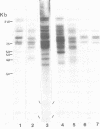
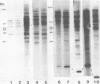
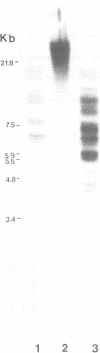
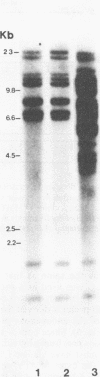
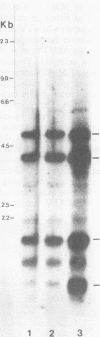
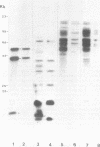
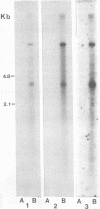
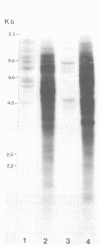
Abstract
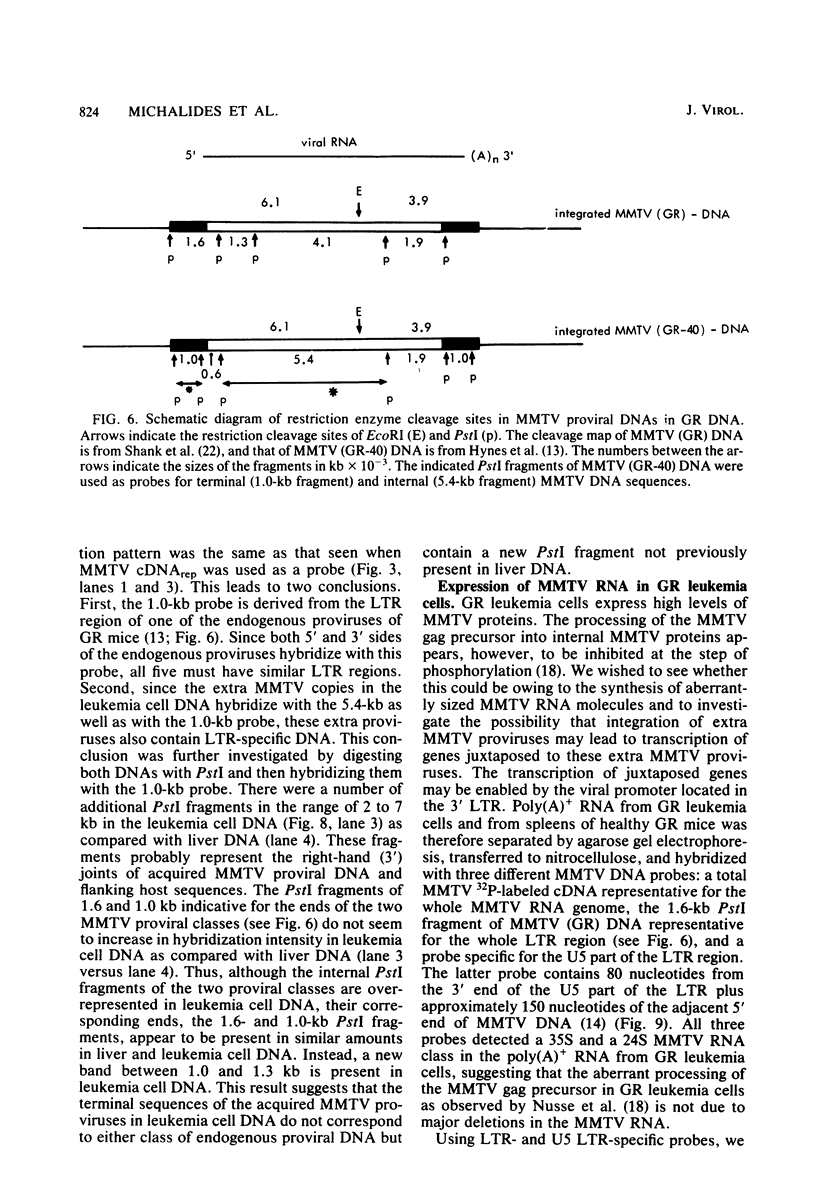
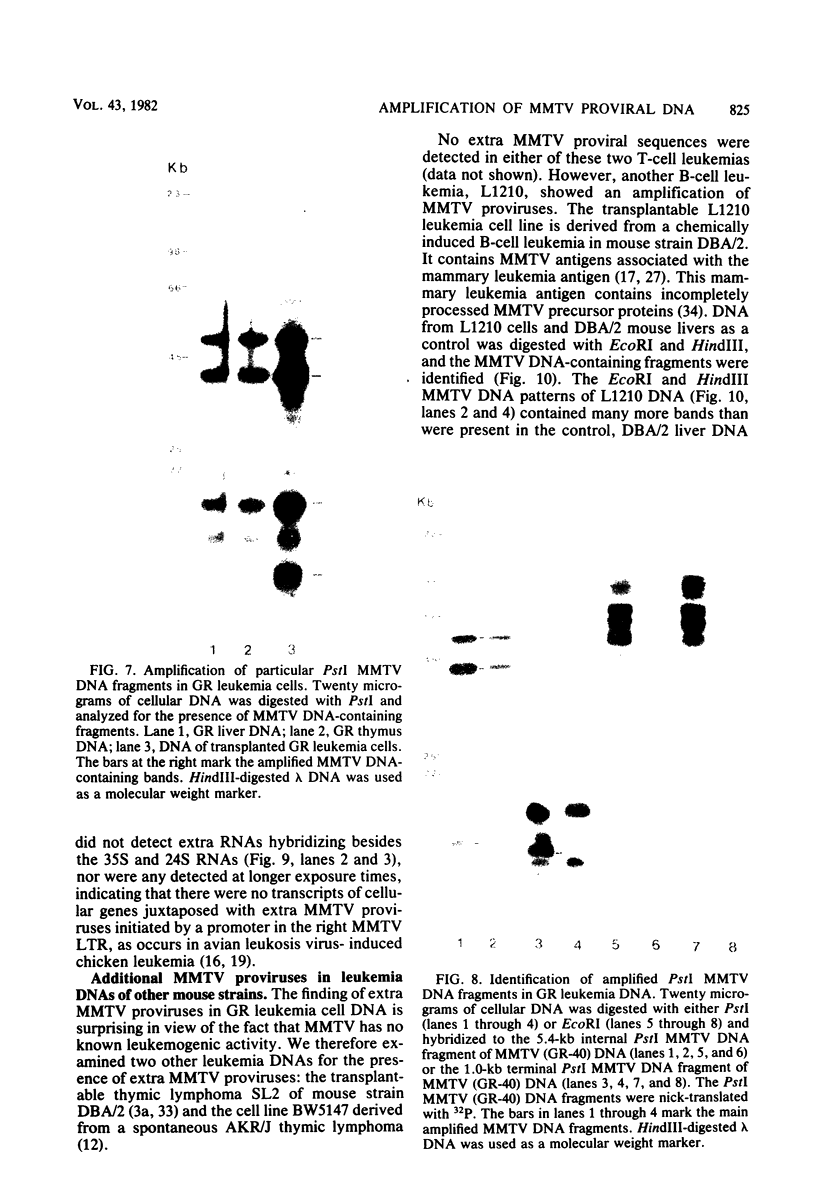
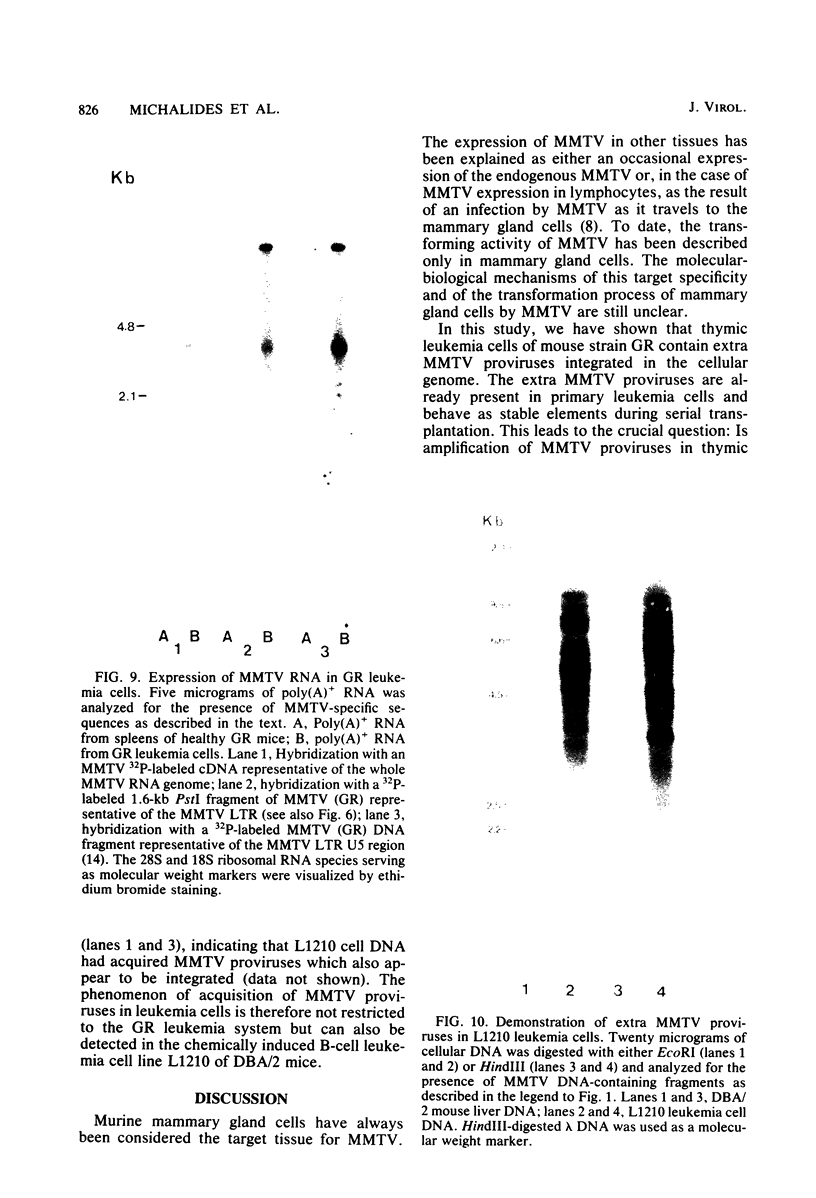
Male mice of strain GR develop T-cell leukemia at a low frequency late in life. These leukemia cells contain large amounts of mouse mammary tumor virus (MMTV) RNA and MMTV proteins in a precursor form (Nusse et al., J. Virol. 32:251-258, 1979). We used restriction enzyme analysis and molecular hybridization to identify MMTV proviruses in the DNA of these leukemia cells. GR leukemia cells contained additional integrated MMTV proviruses at various sites in the genome. This amplification of MMTV proviruses in GR leukemia cells is not restricted to one particular endogenous MMTV provirus of strain GR. The number and location of the extra MMTV proviruses present in transplants of GR leukemia cells did not change upon serial transplantation of the leukemia cells. Acquisition of MMTV proviruses was also found in a similar leukemia, L1210 of the DBA/2 mouse strain, but not in three other leukemias, SL2 of DBA/2, BW5147 of AKR, and a spontaneous thymoma of BALB/c. The two main classes of MMTV RNA, 35S and 24S, were present in the cytoplasmic RNA of GR leukemia cells, indicating that the aberrant processing of MMTV precursor proteins is not due to anomolously sized RNAs. We could not detect extra RNAs in GR leukemia cells which would represent read-through transcripts of cellular genes adjacent to the extra MMTV proviruses, initiated by a promoter signal in the right MMTV long terminal repeat sequence. These data suggest that acquisition of MMTV proviruses may coincide with the onset of leukemogenesis in GR male mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Dullens H. F., Hilgers J., Spit B. J., De Heer E., De Weger R. A., Van Basten C. D., Den Otter W. Staging, growth properties and metastatic behaviour of a transplantable murine T-cell lymphoma. Int J Tissue React. 1982;4(1):15–25. [PubMed] [Google Scholar]
- Fine D. L., Plowman J. K., Kelley S. P., Arthur L. O., Hillman E. A. Enhanced production of mouse mammary tumor virus in dexamethasone-treated, 5-iododeoxyuridine-stimulated mammary tumor cell cultures. J Natl Cancer Inst. 1974 Jun;52(6):1881–1886. doi: 10.1093/jnci/52.6.1881. [DOI] [PubMed] [Google Scholar]
- Groner B., Buetti E., Diggelmann H., Hynes N. E. Characterization of endogenous and exogenous mouse mammary tumor virus proviral DNA with site-specific molecular clones. J Virol. 1980 Dec;36(3):734–745. doi: 10.1128/jvi.36.3.734-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Diggelmann H. Identification of mouse mammary tumor virus-specific mRNA. J Virol. 1979 Apr;30(1):417–420. doi: 10.1128/jvi.30.1.417-420.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hilgers J., van Blitterswijk W. J., Bont W. S., Theuns G. J., Nusse R., Haverman J., Emmelot P. Distribution and antibody-induced redistribution of a mammary tumor virus-induced and a normal antigen on the surface of mouse leukemia cells. J Natl Cancer Inst. 1975 Jun;54(6):1335–1342. doi: 10.1093/jnci/54.6.1335. [DOI] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Kennedy N., Rahmsdorf U., Groner B. Hormone-responsive expression of an endogenous proviral gene of mouse mammary tumor virus after molecular cloning and gene transfer into cultured cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2038–2042. doi: 10.1073/pnas.78.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Nie R., Nusse R., Hynes N. E., Groner B. Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell. 1981 Jan;23(1):165–173. doi: 10.1016/0092-8674(81)90281-6. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Moore D. H., Geering G., Boyse E. A. A soluble antigen of the mammary tumor virus. Virology. 1967 Jan;31(1):1–14. doi: 10.1016/0042-6822(67)90002-5. [DOI] [PubMed] [Google Scholar]
- Nusse R., van der Ploeg L., van Duijn L., Michalides R., Hilgers J. Impaired maturation of mouse mammary tumor virus precursor polypeptides in lymphoid leukemia cells, producing intracytoplasmic A particles and no extracellular B-type virions. J Virol. 1979 Oct;32(1):251–258. doi: 10.1128/jvi.32.1.251-258.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Yamamoto K. R. Production of unintegrated mouse mammary tumor virus DNA in infected rat hepatoma cells is a secondary action of dexamethasone. J Virol. 1978 Apr;26(1):93–101. doi: 10.1128/jvi.26.1.93-101.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- STUECK B., BOYSE E. A., OLD L. J., CARSWELL E. A. ML: A NEW ANTIGEN FOUND IN LEUKAEMIAS AND MAMMARY TUMOURS OF THE MOUSE. Nature. 1964 Sep 5;203:1033–1034. doi: 10.1038/2031033a0. [DOI] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. H. Evidence for a precursor-product relationship between intracytoplasmic A particles and mouse mammary tumour virus cores. J Gen Virol. 1978 Oct;41(1):193–200. doi: 10.1099/0022-1317-41-1-193. [DOI] [PubMed] [Google Scholar]
- Spira J., Wiener F., Ohno S., Klein G. Is trisomy cause or consequence of murine T cell leukemia development? Studies on Robertsonian translocation mice. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6619–6621. doi: 10.1073/pnas.76.12.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Long C. A., Sheffield J. B., Tamura A., Tanaka H. Murine mammary tumor virus deficient in the major glycoprotein: biochemical and biological studies on virions produced by a lymphoma cell line. Virology. 1980 Jul 30;104(2):279–293. doi: 10.1016/0042-6822(80)90333-5. [DOI] [PubMed] [Google Scholar]
- Van Nie R., Verstraeten A. A., De Moes J. Genetic transmission of mammary tumour virus by GR mice. Int J Cancer. 1977 Mar 15;19(3):383–390. doi: 10.1002/ijc.2910190316. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- Wolf A., Barfoot R. K., Johnson R. A. Xenogeneic recognition of tumour spcific plasma membrane antigens derived from mouse lymphoma cells. Immunology. 1972 Mar;22(3):485–491. [PMC free article] [PubMed] [Google Scholar]
- Zak-Nejmark T., Steuden J., Radzikowski C. Mammary leukaemia (ML) antigen isolated from L 1210 leukaemia cells. Int J Cancer. 1978 Apr 15;21(4):490–495. doi: 10.1002/ijc.2910210415. [DOI] [PubMed] [Google Scholar]
- van Nie R., Verstraeten A. A. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer. 1975 Dec 15;16(6):922–931. doi: 10.1002/ijc.2910160606. [DOI] [PubMed] [Google Scholar]
- van der Putten H., Quint W., van Raaij J., Maandag E. R., Verma I. M., Berns A. M-MuLV-induced leukemogenesis: integration and structure of recombinant proviruses in tumors. Cell. 1981 Jun;24(3):729–739. doi: 10.1016/0092-8674(81)90099-4. [DOI] [PubMed] [Google Scholar]