Abstract
In this study, we have identified, for the first time, the presence of de novo cellular immune reactivity against the transcription factor SOX10, using tumor-infiltrating lymphocytes obtained from a patient who experienced a dramatic clinical response to immunotherapy. SOX10 acts as a critical transactivator of tyrosinase-related protein-2 during melanoblast development and a potent transactivator of micropthalmia-associated transcription factor, which is considered to be a master gene that controls the development and postnatal survival of melanocytes. Mutations in SOX10 result in Waardenburg syndrome type 4. The overlapping epitopes AWISKPPGV and SAWISKPPGV, designated SOX10: 332–340 and SOX10: 331–340, respectively, were recognized by tumor-infiltrating lymphocyte clone M37 in an HLA-A2-restricted fashion.
Introduction
In the past several years, various tumor antigens have been identified and cloned, particularly in the field of melanoma immunotherapy (1, 2). The identification of these antigens has opened new opportunities for and facilitated the development of cancer immunotherapies. Most of the antigens reported thus far belong to the two major groups, the melanoma/melanocyte differentiation antigens and the cancer testis antigens, which are overexpressed in tumors of various histologies but not in normal tissues except for testis. The commonly known melanoma/melanocyte differentiation antigens include gp100, MART-1, tyrosinase, and the TRPs2 TRP-1 and TRP-2. Clinical vaccine trials have been conducted based on these antigens, using peptides, recombinant viruses, or recombinant DNA vaccines. A recent study has demonstrated efficacy of immunotherapy using a modified gp100 antigen together with high-dose IL-2 (3). To identify antigens that can be used to improve the efficacy of immunotherapy, we have focused our attention on patients who had experienced dramatic clinical responses after immune stimulation. By analyzing the immunological reactivity in one of these patients, we were able to identify, for the first time, that the WS4 gene, SOX10, encodes a melanoma/melanocyte differentiation antigen recognized by CTLs.
Materials and Methods
Source of TILs for Cloning
Patient MM’s history and the source of bulk TIL 1790 were described in detail previously (4). Briefly, patient MM was a 63-year-old woman who was referred to the Surgery Branch, National Cancer Institute (Bethesda, MD) in 1998 with metastases refractory to chemoimmunotherapy. At that time, she had developed multiple metastatic melanoma lesions at multiple sites including the lungs, liver, intrapelvic area, left abdominal wall, and left thigh. She was started on a four-peptide vaccination protocol using 1 mg each of gp100: 209–217 (210M), gp100: 280–288 (288V), MART-1: 27–35, and tyrosinase: 368–376 in incomplete Freund’s adjuvant s.c. every 3 weeks. She did not receive IL-2. Most of her tumors completely regressed after two cycles of treatment, including complete resolution of a large tumor in her left thigh, an intrapelvic mass, a liver lesion, and most of the nodules in her lungs. She also developed vitiligo on the dorsal areas of her hand and distal forearm bilaterally. She completed a total of six cycles of vaccinations in October 1998. A year later (October 1999), she developed a frontal lobe metastatic brain lesion and a s.c. nodule on her right chest wall, both of which were resected. Bulk TIL 1790 used in this study was grown from the recurrent chest wall lesion.
Cell Lines
Melanoma-reactive CTLs were derived from bulk TIL cultures grown in Iscove’s Modified Dulbecco’s Medium (Life Technologies, Inc., Gaithersburg, MD) containing 6000 IU/ml of human recombinant IL-2 (Chiron, Emeryville, CA) as described previously (5). CTL clones were derived from bulk TIL cultures by limiting dilution with the addition of anti-CD3 Ab (OKT-3; Ortho Pharmaceuticals, Raritan, NJ). Briefly, 5 × 104 irradiated (3000 cG) PBMCs from three allogeneic donors were plated in round-bottomed, 96-well plates with 0.5 to 90 T cells/well. Cells were cultured in RPMI 1640 (Life Technologies, Inc.) containing 20% heat-inactivated FBS (Life Technologies, Inc.) and 30 ng/ml OKT-3 Ab with 300 IU/ml IL-2. The same dose of IL-2 was added on day 7, and clones were tested for recognition of HLA-A2+ versus HLA-A2− melanoma cell lines 14 days after stimulation. After testing, the remainder of T cells were expanded by plating them in a T25 flask with 2.5 × 107 irradiated PBMCs from three allogeneic donors in 25 ml of RPMI 1640 containing 10% FBS and 30 ng/ml OKT-3 Ab. Subsequent expansion was similarly done but with 1–2 × 106 CTLs and 2.5 × 108 allogeneic PBMCs plated in an upright T162 flask in 150 ml of medium.
The melanoma cell lines 624–28, 624–38, 938, 526, 888, 888A2, F002S, and F002R were established in the Surgery Branch (National Cancer Institute). 624–28 and 624–38 cell lines were derived from the same parental cell line 624-mel and share a similar pattern of antigen and HLA allele expression except for HLA-A0201 antigen, which was expressed by 624–38 cells but not by 624–28 cells because of aberrant pre-mRNA splicing (6). The 888A2-mel cell line was obtained by stable transfection of 888-mel with HLA-A2 cDNA (pCDNA3 plasmid; Invitrogen). T2 cells, deficient in transporter associated protein, were used to test HLA-A2-restricted peptides for CTL activity. The 293 cell line was derived from primary human embryonal kidney cells and was used for transfection purpose. The 293A2 cell line was obtained by stable transfection of 293 cells with HLA-A2 cDNA. All cell lines were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS, 10 mM HEPES buffer, 100 units/ml penicillin-streptomycin (Biofluids), and 2 mm l-glutamine (Biofluids). This medium is referred to as complete medium in this report.
Cytokine Release Assays
CTL cells (5 × 104) were plated with 1 × 105 target cells (tumor cells or T2 cells that had been pulsed with peptides at 1 µm for 2 h at 37°C and washed once) in 96-well round-bottomed plates in 200 µl of complete medium. After 18–24 h incubation at 37°C, the supernatant was harvested for detection of IFN-γ release using ELISA kits (Endogen, Cambridge, MA).
cDNA Library Screening
The preparation and screening of the 624mel expression library were described in detail previously (4). Briefly, poly(A) RNA was purified from total RNA and converted to cDNA using an oligo(dT) primer. The cDNA was ligated to the expression vector pEAK 8 and electro-porated into DH10B cells. Pools containing ~100 cDNA clones were prepared from bacteria and transfected into 293A2 cells with Effectene reagent (Qiagen, Valencia, CA). Cytokine release assay was performed as described above.
DNA Sequencing
Sequencing of the isolated cDNA clone was performed with an ABI Prism 310 automated capillary electrophoresis instrument (Perkin-Elmer, Foster City, CA) using the Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer). Searches for sequence homology were done with the GenBank database using the BLAST algorithm.
Peptide Synthesis
Peptides were synthesized using a solid-phase method based on standard Fmoc chemistry on a multiple peptide synthesizer (Gilson Co., Worthington, OH). Peptide identity was verified by laser desorption mass spectrometry (Bio-Synthesis, Inc., Lewisville, TX). Lyophilized peptides were solubilized in DMSO at 10 mg/ml concentration.
RT-PCR Assay
RT-PCR for expression of SOX10 in normal tissues was performed using the Human Rapid-Scan Plate (OriGene Technologies, Inc., Rockville, MD). qRT-PCR was also performed on normal tissues and fresh tumors. The normal tissues used in qRT-PCR were obtained from Stratagene (Cedar Creek, TX). Total RNA of fresh melanoma samples was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was prepared from total RNA using the First-Strand cDNA Synthesis kit (Amersham Pharmacia Biotech, Inc., Piscataway, NJ). SOX10 was amplified using primer pairs 5′-AACGGCGCCAGCAAAAGCAA-3′ (sense) and 5′-GACTACAAGTACCAGCCCAG-3′ (antisense) or 5′-CTTCGGCAACGTGGACATT-3′ (sense) and 5′-TCAGCCACATCAAAGGTCTCC-3′ (antisense). qRT-PCR was performed using the second primer pair with the probe 5′-FAM-CACGAGGTAATGTCCAACA-TAMRA. For relative quantification, relative expression of SOX10 was calculated using the ΔΔCT method as described previously (7). All measurements were normalized to the SOX10:β-actin ratio for the 624mel cell line, which was used as a reference in all experiments.
Results
Antigen Specificity of TIL Clone M37
In an attempt to identify the antigen(s) recognized by TIL 1790, a number of CTL clones were generated from this TIL line by limiting dilution. CTL clone M37 was selected for further study based on its ability to specifically release cytokine (IFN-γ) in response to HLA-A2 melanoma cell lines but not to cell lines that did not express HLA-A2 and based on the lack of recognition of autologous EBVB pulsed with peptides from MART-1, gp100, tyrosinase, and TRP-2 (Table 1). The fact that non-HLA-A2 melanoma cell lines such as 888 stimulated cytokine release from clone M37 after being stably transfected with the cDNA encoding the HLA-A2 antigen indicated that they expressed the antigen(s) of interest and that M37 recognized a melanoma antigen restricted by HLA-A2 (Table 1). In addition, clone M37 reacted with 624–38, but not the HLA-A2 negative variant, 624–28, confirming that M37 was HLA-A2 restricted. Clone M37 was tested and found negative for recognition of previously described antigens in a coculture assay using 293A2 cells transfected with cDNA encoding these antigens (data not shown). The specificity of clone M37 was tested against a variety of normal and tumor cell lines. No HLA-A2+ non-melanoma/melanocyte cell lines were recognized by TIL M37 (two each for EBVB, fibroblast, colon cancer, glioma, sarcoma, and human umbilical vein endothelial cell).
Table 1. Recognition of melanoma cell lines and peptide-pulsed targets by TIL clone M37.
| Targets | HLA-A2 | Clone M37 IFN-γ (pg/ml) |
|---|---|---|
| Melanoma cell line | ||
| 624–38 mel | + | >1000 |
| 526 mel | + | 655 |
| 888A2 mel | + | >1000 |
| F002R mel | + | >1000 |
| F002S mel | + | >1000 |
| SK23 mel | + | >1000 |
| 1760 mel | + | >1000 |
| 1102 mel | + | >1000 |
| 624–28 mel | − | 32 |
| 938 mel | − | 32 |
| 888 mel | − | 38 |
| 397 mel | − | 120 |
| EBVB pulsed with peptide | ||
| No peptide | + | 58 |
| gp 100: 154–162 | + | 58 |
| gp100: 209–217 | + | 58 |
| gp100: 280–288 | + | 55 |
| MART-1: 27–35 | + | 54 |
| TRP-2: 180–188 | + | 53 |
| Tyrosinase: 368–376 | + | 64 |
| No target | 59 |
Isolation and Sequence Determination of the cDNA Clone Encoding a Protein Recognized by TIL Clone M37
A cDNA library prepared from 624mel cells was next used to screen for a gene encoding reactivity with CTL M37. Plasmid DNA from pools containing ~100 cDNA clones was transiently transfected into 293A2 cells, a human embryonal kidney cell line stably transfected with the cDNA encoding the HLA-A2 antigen. One pool in 400 stimulated IFN-γ release from CTL M37. By subcloning the positive pool, one cDNA clone (cDNA 68.3) was identified that induced a high level of IFN-γ secretion from TIL clone M37 (Table 2). The sequence of cDNA 68.3 was identical to that of the SOX10 gene (GenBank accession no. BC007595) with the exception of a single nucleotide difference at position 839 (G instead of T) that did not change the amino acid sequence.
Table 2. Stimulation of TIL clone M37 by 293A2 cells transfected with cDNA 68.3.
293 cells transfected with cDNA 68.3 or green flourescent protein (GFP) and 293A2 cells transfected with GFP were used as controls.
| Stimulator | IFN-γ(pg/ml) |
|---|---|
| 293 + cDNA 68.3 | 0 |
| 293A2 + cDNA 68.3 | 851 |
| 293 + GFP | 2 |
| 293A2 + GFP | 3 |
| 293 | 2 |
| 293A2 | 0 |
| 624mel | >1000 |
| 624–28mel | 0 |
Identification of the T-Cell Epitope in SOX10 That Was Recognized by TIL Clone M37
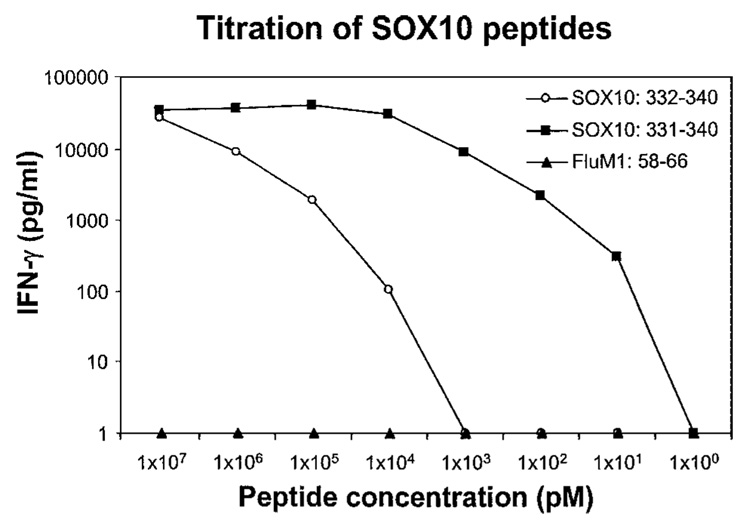
Using a peptide-HLA motif search program, 60 peptides were generated with predicted HLA-A2 binding. These peptides were pulsed on T2 cells and tested for recognition by CTL M37. The decapeptide SAWISKPPGV, designated SOX10: 331–340, was recognized by CTL M37 when pulsed on T2 cells. This peptide was able to sensitize target cells for recognition by CTL M37 at 10 pg/ml (Fig. 1). The nonapeptide AWISKPPGV, designated SOX10: 332–340, was also recognized but at a much lower efficiency (at 10 ng/ml; Fig. 1).
Fig. 1.
IFN-γ release by TIL clone M37 cocultured with T2 cells pulsed with decreasing concentrations of peptides SOX10: 331–340 and 332–340. The flu A matrix protein M1 peptide, FluM1: 58–66, was used as a negative control.
Expression of SOX10 mRNA in Cell Lines and Tissues
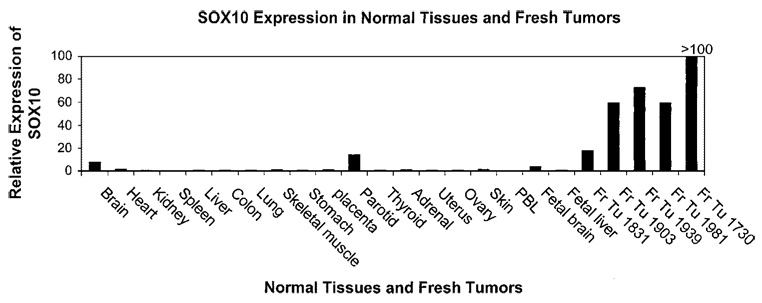
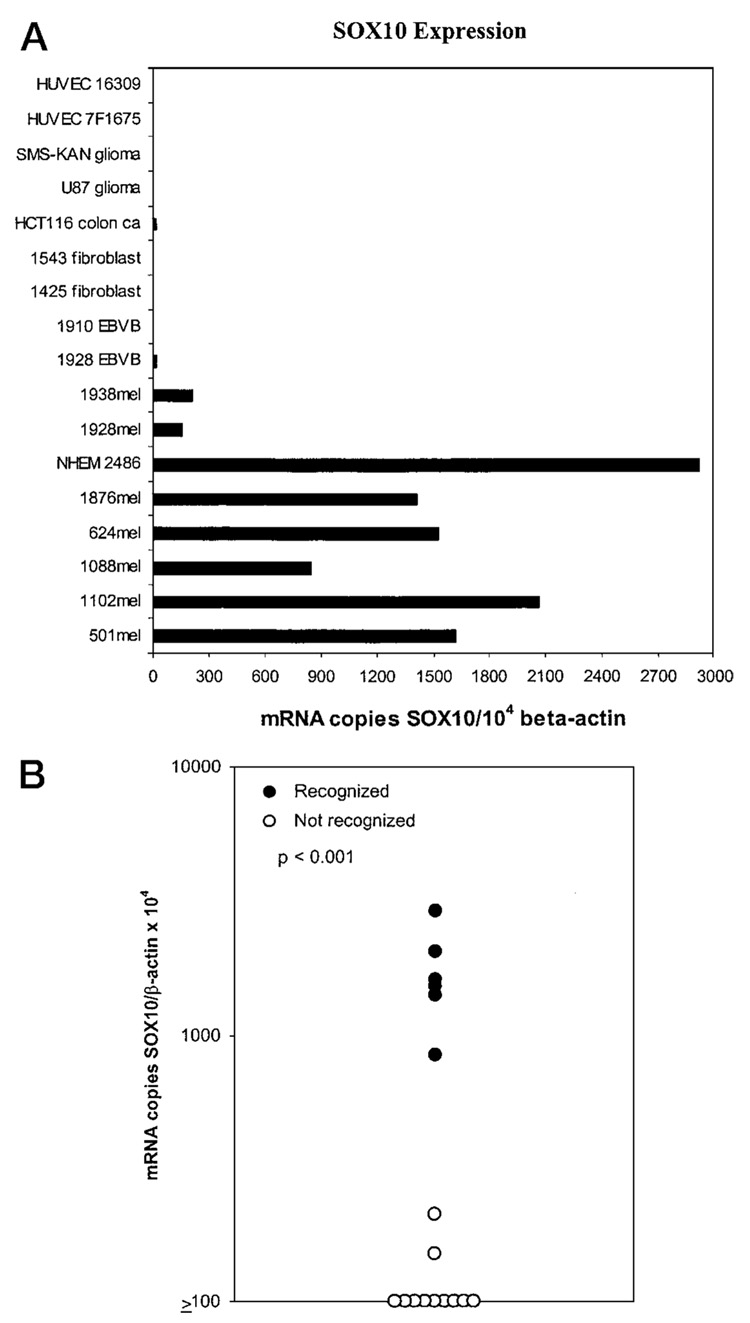
The expression of SOX10 mRNA in various tumor cell lines and normal tissues was analyzed by RT-PCR and by qRT-PCR. Using a highly sensitive RT-PCR assay, SOX10 was found to be expressed in numerous normal tissues, including brain, heart, colon, small intestine, muscle, stomach, testis, salivary, thyroid, adrenal gland, pancreas, uterus, and skin. A real-time relative qRT-PCR assay was then performed on multiple normal tissues as well as fresh melanoma tissues to determine the expression of SOX10 relative to that of the β-actin housekeeping gene. Fresh tumors were found to overexpress SOX10 compared with normal tissues (Fig. 2). To test for a correlation between expression of antigen and recognition by CTL, a panel of HLA-A2+ tumor and normal cell lines of various histologies were used to test for SOX10 mRNA expression in a qRT-PCR (Fig. 3A) and for recognition by TIL clone M37 (Fig. 3B). The non-melanoma/melanocyte cell lines tested were all negative for SOX10 expression. The two melanoma cell lines (1928 and 1938) that were not recognized by TIL clone MB37 expressed low levels of the antigen (<250 SOX10 mRNA copies per 104 β-actin copies), compared with the melanoma and melanocyte cell lines that were recognized (range, 844 to 2921 SOX10 mRNA copies per 104 β-actin copies).
Fig. 2.
Real-time relative qRT-PCR analysis of SOX10 mRNA expression relative to that of β-actin in 19 normal tissues and 5 fresh melanoma tissues. All measurements were normalized to the SOX10:β-actin ratio for the 624mel cell line, which was used as a reference in all experiments.
Fig. 3.
A, real-time qRT-PCR analysis of the mRNA copy number encoding the SOX10 gene normalized to mRNA encoding the β-actin housekeeping gene. Melanocyte and melanomas (mel) had increased expression of SOX10 mRNA compared with other cell lines. HUVEC, human umbilical vein endothelial cells. B, The same cell lines in Fig. 3A were tested for recognition by CTL clone M37. A correlation existed between levels of SOX10 expression and recognition by CTL clone M37 (P < 0.001).
Discussion
In this report, we have identified for the first time the presence of de novo cellular immune reactivity against the transcription factor SOX10 in a patient who experienced a dramatic clinical response to immunotherapy. SOX10 is a member of a new class of genes encoding transcription factors. These genes are characterized by a DNA binding domain similar to the high mobility group domain of sex determining region on the Y chromosome gene (8, 9). SOX10 is first very broadly expressed in migratory neural crest cells during early stages of development, and in adulthood, SOX10 has been identified in numerous tissues including brain, heart, lungs, adrenal glands, salivary glands, colon, small intestine, bladder, pancreas, prostate, and testis (10–12). The identification of SOX10 in visceral tissues is most likely attributable to the presence of glial components of the peripheral nervous system in these tissues.
In the spontaneous mouse mutant Dominant megacolon (Dom), Sox10 is mutated and nonfunctional (13). Dom mice display intestinal aganglionosis and depigmentation. In humans, SOX10 mutations are seen in the WS4, which is characterized by neurosensory deafness, hypopigmentation, and aganglionic megacolon (14).
The depigmentation in Dom mice and in WS4 patients is similar to that of patients with the WS2 syndrome and resulted from mutations of the MITF gene (15, 16). MITF is essential for the development and postnatal survival of melanocytes. MITF controls melanocyte development by regulating expression of a variety of melanocytic genes, in particular tyrosinase, TRP-1, and TRP-2 (15–17). SOX10 was found recently to be a potent transactivator of the MITF promoter (18). This provides the molecular basis for the hypopigmentation and neurosensory deafness seen in WS4 patients. A recent study showed that SOX10 also acted as a critical transactivator of TRP-2 during melanoblast development (19). In the hereditary disorder APS I, vitiligo is common (8–15%, compared with 1% prevalence in the general population). In a recent study of 91 APS I patients, 19 were found to have vitiligo. Of these, 63% had autoantibody directed against SOX10, compared with 11% of patients without vitiligo. In addition, 3 of 93 sera from patients with vitiligo unrelated to APS I showed strong reactivity against SOX10 (20).
Thus, SOX10 is an important transcription factor regulating the development of melanocytes and probably of melanomas. In addition, SOX10 was also recognized by tumor-infiltrating lymphocytes, as reported in the current study. This suggests that SOX10 may be of importance in melanoma immunotherapy because it is an autoantigen that can be targeted by both the humoral and cellular immune responses. Although SOX10 is expressed in various normal tissues, the expression is probably below the threshold for recognition by CTLs.
Acknowledgments
We thank Dr. Maria Parkhurst and John Riley for help with peptide synthesis, Dr. Zhyia Yu for technical assistance with some of the graphics, and Yong Li for help with the automated DNA sequencer.
Footnotes
The abbreviations used are: TRP, tyrosinase-related protein; IL, interleukin; WS4, Waardenburg syndrome type 4; TIL, tumor infiltrating lymphocytes; Ab, antibody; PBMC, peripheral blood mononuclear cell; FBS, fetal bovine serum; RT-PCR, reverse transcription-PCR; qRT-PCR, quantitative RT-PCR; MITF, micropthalmia-associated transcription factor; APS I, autoimmune polyendocrine syndrome type I.
References
- 1.Van den Eynde BJ, Van der Bruggen P. T cell defined tumor antigens. Curr. Opin. Immunol. 1997;9:684–693. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khong HT, Rosenberg SA. Pre-existing immunity to tyrosinase-related protein (TRP)-2, a new TRP-2 isoform, and the NY-ESO-1 melanoma antigen in a patient with a dramatic response to immunotherapy. J. Immunol. 2002;168:951–956. doi: 10.4049/jimmunol.168.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Muul LM, Solomon D, Rosenberg SA. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J. Immunol. Methods. 1987;102:127–141. doi: 10.1016/s0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Marincola FM, Rivoltini L, Parmiani G, Ferrone S. Selective histocompatibility leukocyte antigen (HLA)-A2 loss caused by aberrant pre-mRNA splicing in 624MEL28 melanoma cells. J. Exp. Med. 1999;190:205–215. doi: 10.1084/jem.190.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PE Applied Biosystems: ABI Prism 7700 Sequence Detection System: Relative Quantitation of Gene Expression. User Bulletin #2. Norwalk, CT: Perkin-Elmer Corp.; 1997. pp. 1–36. [Google Scholar]
- 8.Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 2000;16:182–187. doi: 10.1016/s0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- 10.Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J. Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pusch C, Hustert E, Pfeifer D, Sudbeck P, Kist R, Roe B, Wang Z, Balling R, Blin N, Scherer G. The SOX10/Sox10 gene from human and mouse: sequence, expression, and transactivation by the encoded HMG domain transcription factor. Hum. Genet. 1998;103:115–123. doi: 10.1007/s004390050793. [DOI] [PubMed] [Google Scholar]
- 12.Bondurand N, Kobetz A, Pingault V, Lemort N, Encha-Razavi F, Couly G, Goerich DE, Wegner M, Abitbol M, Goossens M. Expression of the SOX10 gene during human development. FEBS Lett. 1998;432:168–172. doi: 10.1016/s0014-5793(98)00843-6. [DOI] [PubMed] [Google Scholar]
- 13.Southard-Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat. Genet. 1998;18:60–64. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- 14.Pingault V, Bondurand N, Kuhlbrodt K, Goerich DE, Prehu MO, Puliti A, Herbarth B, Hermans-Borgmeyer I, Legius E, Matthijs G, Amiel J, Lyonnet S, Ceccherini I, Romeo G, Smith JC, Read AP, Wegner M, Goossens M. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet. 1998;18:171–173. doi: 10.1038/ng0298-171. [DOI] [PubMed] [Google Scholar]
- 15.Bondurand N, Pingault V, Goerich DE, Lemort N, Sock E, Caignec CL, Wegner M, Goossens M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum. Mol. Genet. 2000;9:1907–1917. doi: 10.1093/hmg/9.13.1907. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Goodall J, Verastegui C, Ballotti R, Goding CR. Direct regulation of the microphthalmia promoter by Sox10 links Waardenburg-Shah syndrome (WS4)-associated hypopigmentation and deafness to WS2. J. Biol. Chem. 2000;275:37978–37983. doi: 10.1074/jbc.M003816200. [DOI] [PubMed] [Google Scholar]
- 17.Verastegui C, Bille K, Ortonne JP, Ballotti R. Regulation of the microph-thalmia-associated transcription factor gene by the Waardenburg syndrome type 4 gene, SOX10. J. Biol. Chem. 2000;275:30757–30760. doi: 10.1074/jbc.C000445200. [DOI] [PubMed] [Google Scholar]
- 18.Potterf SB, Furumura M, Dunn KJ, Arnheiter H, Pavan WJ. Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum. Genet. 2000;107:1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- 19.Potterf SB, Mollaaghababa R, Hou L, Southard-Smith EM, Hornyak TJ, Arnheiter H, Pavan WJ. Analysis of SOX10 function in neural crest-derived melanocyte development: SOX10-dependent transcriptional control of dopachrome tautomerase. Dev. Biol. 2001;237:245–257. doi: 10.1006/dbio.2001.0372. [DOI] [PubMed] [Google Scholar]
- 20.Hedstrand H, Ekwall O, Olsson MJ, Landgren E, Kemp EH, Weetman AP, Perheentupa J, Husebye E, Gustafsson J, Betterle C, Kampe O, Rorsman F. The transcription factors SOX9 and SOX10 are vitiligo autoantigens in autoimmune polyendocrine syndrome type I. J. Biol. Chem. 2001;276:35390–35395. doi: 10.1074/jbc.M102391200. [DOI] [PubMed] [Google Scholar]