Summary
Assessment of cutaneous innervation in skin biopsies is emerging as a valuable means of both diagnosing and staging diabetic neuropathy. Immunolabeling, using antibodies to neuronal proteins such as protein gene product 9.5, allows for the visualization and quantification of intraepidermal nerve fibers. Multiple studies have shown reductions in intraepidermal nerve fiber density in skin biopsies from patients with both type 1 and type 2 diabetes. More recent studies have focused on correlating these changes with other measures of diabetic neuropathy. A loss of epidermal innervation similar to that observed in diabetic patients has been observed in rodent models of both type 1 and type 2 diabetes and several therapeutics have been reported to prevent reductions in intraepidermal nerve fiber density in these models. This review discusses the current literature describing diabetes-induced changes in cutaneous innervation in both human and animal models of diabetic neuropathy.
Keywords: intraepidermal nerve fiber, diabetic neuropathy, cutaneous innervation, skin
Introduction
Skin biopsies are emerging as a valuable means of diagnosing and staging peripheral nerve disorders. As a minimally invasive technique, skin biopsies allow for assessment of a variety of fiber types, including the small unmyelinated fibers that are difficult to evaluate by other means. There is particular interest in using this technique to provide an assessment of distal symmetrical neuropathies, such as diabetic neuropathy, both to stage and evaluate progression of neuropathy as well as to assess efficacy of potential therapeutics (Kennedy et al., 1996; Lauria et al., 1998; McArthur et al., 1999). Until recently, clinical studies have been restricted to using electrophysiologic and sensory testing as surrogate markers for nerve pathology, or to the evaluation of sural nerve biopsies, which are invasive and not widely approved as a diagnostic tool.
The innervation of the skin consists of low-threshold mechanoreceptors, thermoreceptors and nociceptors, along with their myelinated and unmyelinated axons (Light and Perl, 1993). Epidermal nerve fibers are predominantly capsaicin-sensitive unmyelinated C-fibers involved in detecting thermal nociceptive pain (Nolano et al., 1999; Malmberg et al., 2004). These fibers originate from dorsal root ganglia neurons and form subepidermal bundles in the papillary dermis, immediately subjacent to the stratum basale of the epidermis. Individual fibers loose their Schwann cell ensheathment as axons cross the dermal-epidermal junction and weave through the keratinocytes of the epidermis (Wang et al., 1990; Kennedy and Wendelschafer-Crabb, 1993). These epidermal nerve fibers can be divided into two subsets, peptidergic and non-peptidergic. The peptidergic neurons are nerve growth factor (NGF)-responsive and express CGRP (calcitonin gene-related peptide), substance P and the trkA receptor. The non-peptidergic nerves are glial cell line-derived neurotrophic factor (GDNF)-responsive and express GDNFRα and the P2X3 receptor (Snider and McMahon, 1998). Quantification of epidermal nerve endings may prove to be a valuable diagnostic tool, particularly for the early detection of ‘dying back’ neuropathies. About half of all diabetic patients will develop neuropathy (Pirart, 1978). Distal symmetric neuropathy, which initially affects the hands and feet, is the most common form (Thomas et al., 1997). The consequences of sensory neuropathy include altered perception of thermal, tactile, and vibratory stimuli and can range from hyperalgesia and allodynia to hypoalgesia. Many patients ultimately experience a complete loss of sensation in their hands and feet, which can increase the risk of trauma and lead to infection and amputation. The small epidermal C-fibers that respond to thermal stimuli are among the most commonly affected (Polydefkis et al., 2003). Hence, their assessment is particularly valuable, not only for diagnostic and staging purposes, but also for the evaluation of treatments for diabetic neuropathy.
Techniques for assessment of intraepidermal nerve fibers
Antibodies
The development of antibodies against a variety of neuronal marker proteins has allowed for the immunohistochemical assessment of intraepidermal nerve fibers (IENFs). The most commonly used antibody is directed at protein gene product 9.5 (PGP9.5), a cytosolic ubiquitin carboxyl-terminal hydroxylase that is found in all neurons and that, in skin biopsies, binds to dermal nerve bundles as well as both peptidergic and non-peptidergic epidermal nerve profiles (Dalsgaard et al., 1989; Wilkinson et al., 1989). PGP9.5 immunoreactivity highlights axons in skin biopsies from a variety of species, including both control and diabetic rats and mice (Fig. 1). Antibodies against components of the cytoskeleton have also been used to identify IENFs. A study comparing anti-unique beta-tubulin, anti-nonphosphorylated microtubule-associated protein-1B, anti-70 and 200 KDa neurofilament, and anti-phosphorylated neurofilament immunoreactivity to PGP9.5 immunoreactivity in skin from healthy subjects and patients with diabetic neuropathy found similar IENF densities in skin labeled with anitbodies against PGP9.5, unique-beta-tubulin and microtubule-associated-protein-1B. IENF densities in the neurofilament-labeled skin were significantly lower than in the PGP9.5-labeled skin (Lauria et al., 2004). Antibodies against neuropeptides, such as calcitonin gene-related peptide (CGRP) and substance P (SP), have also been used to selectively identify peptidergic nerve fibers (Bjorklund et al., 1986). However, in the skin of humans, most SP- and CGRP-immunoreactive profiles terminate in the dermis (Gibbins et al., 1987; Lindberger et al., 1989). Non-peptidergic nerve fibers can be indentified with antibodies against the P2X3 receptor (Bradbury et al., 1998) or through their ability to bind isolectin IB4 (Petruska et al., 1997). In the published literature, antibodies against PGP9.5 are by far the most commonly used for the quantification of IENF density.
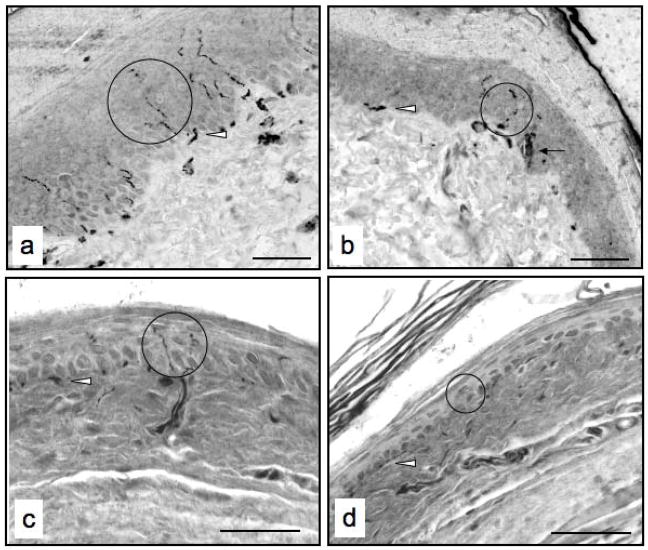
Figure 1.
Micrographs of foot skin from a control rat (a), a streptozotocin-injected rat with diabetes for a duration of two months (b), a control mouse (c) and streptozotocin-injected mouse with diabetes for a duration of one month (d) immunolabeled for binding of an antibody against the pan-axonal marker PGP9.5. Circles identify immunoreactive profiles, the arrow denotes a Langerhans cell and the arrowheads denote subepidermal nerve plexuses. Bar, 40 μm.
Immunolocalization of transient receptor potential vanilloid receptor subtype 1 (TRPV1), the heat-sensitive receptor found on epidermal C-fibers (Caterina et al., 1997), would appear to be another logical marker for IENFs. Although there are several TRPV1 antibodies commercially available, our own experience has been that rat and mouse skin sections typically show high background staining in the epidermis that impedes discrimination of axons. Others have also reported that TPRV1 immunoreactivity is present in keratinocytes (Denda et al., 2001). TRPV1-immunoreactive IENF densities have been observed and quantified in biopsies from breast cancer patients (Gopinath et al., 2005) and patients with painful neuropathies (Lauria et al., 2006). A reduction in TRPV1 immunoreactive epidermal nerve fibers has also been reported in skin from patients with diabetic neuropathy (Facer et al., 2007). Importantly, this reduction was due not only to the destruction of epidermal nerve fibers, but also to a decrease of TRPV1 in surviving fibers. Unfortunately, each of these studies cited the use of an antibody from GlaxoSmithKline that is currently not widely available.
It is important to establish whether the loss of PGP9.5 immunoreactivity in skin from diabetic animals reflects a loss of IENF profiles or a reduction in the PGP9.5 content of intact axons. To explore this possibility, we compared IENF densities in slides labeled for binding of an anti-PGP9.5 antibody to slides labeled for binding of an antibody against high molecular weight tau, a microtubule-associated structural protein selectively expressed in the peripheral nervous system (Oblinger et al., 1991). In a comparative analysis of skin from control rats, we observed similar immunoreactivity and found no significant differences in IENF density between tissue immunolabeled for binding of the anti-PGP9.5 antibody and tissue immunolabeled for binding of the anti-high molecular weight tau antibody (Fig. 2). In contrast to the anti-PGP9.5 antibody, the anti-high molecular weight tau antibody does not obviously label Langerhans cells and processes.
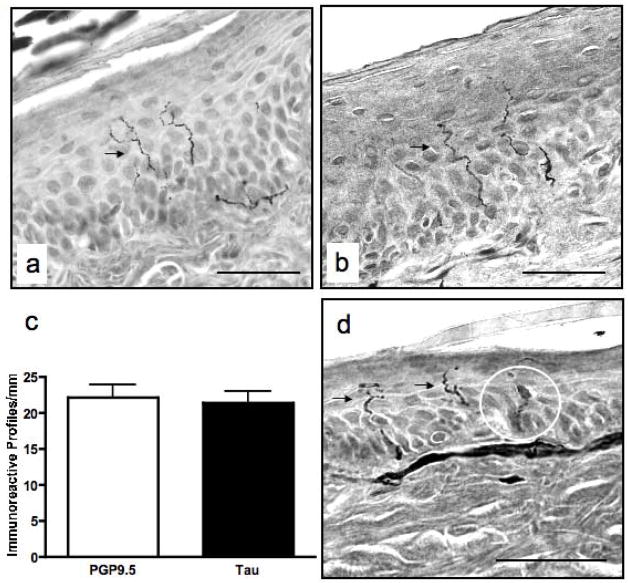
Figure 2.
Micrographs of rat skin immunolabeled for binding of antibodies against PGP9.5 (a) and Tau (b). A comparison of immunoreactive profile densities between control rat skin sections immunolabeled for binding of each antibody revealed no significant differences (c). Langerhans cells (circled) are only prominent in PGP9.5-labeled rat skin (d). Arrows denote immunoreactive profiles. Data are mean+SEM of N=9–10/group. Bar, 40μm.
Protocols
Skin samples can be obtained through either punch biopsies or the blister method. Removal of punch biopsies, the more commonly used technique, is minimally invasive and generally does not require a suture (Kennedy, 2004). The blister method involves applying a suction capsule to the skin, which separates the epidermis from the dermis and creates a blister. The epidermis can then be removed with the epidermal innervation remaining intact (Kennedy et al., 1999).
Once removed, biopsies must be fixed and processed. Commonly used fixatives include paraformyldehyde and Zamboni’s solution (a combination of paraformyldehyde and picric acid diluted with phophate buffer, Kennedy et al., 1996). If processed and embedded in paraffin wax, tissue can be cut at thicknesses ranging from 3 to at least 30 μm. Frozen tissue embedded in OCT (‘optimal cutting temperature’ media) can be cut at thicknesses ranging from 10 to around 100 μm after cryoprotection (Kennedy et al., 1996). Once sectioned, the tissue may then be mounted on glass slides. If thick sections are cut, they can be immunolabeled as ‘free floating’ sections, which allows for antibody penetration deeper into the tissue. Thin tissue sections are appropriate for quantification of immunoreactive profiles. However, for analysis of nerve length, thicker sections are often required (Kennedy et al., 1996).
Visualization and Quantification
Immunoreactive IENF profiles can be visualized using conventional light microscopy, fluorescence microscopy or confocal microscopy (Kennedy et al., 1996; Periquet et al., 1999). Methods for quantifying IENF density include manual counting of immunoreactive profiles (McArthur et al., 1998; Mizisin et al., 1998; Hirai et al., 2000), stereology (McArthur et al., 1998) and color subtractive-computer-assisted analysis (Underwood et al., 2001). Manual determination of linear density, which is perhaps the most commonly used method, has been shown to significantly correlate with the lesser-used stereology method and has a diagnostic efficiency of 88% (McArthur et al., 1998). Combining stereology with confocal microscopy may prove to be a valuable technique for quantification of innervation (Selim et al., 2007).
In the majority of the published literature, quantification of IENF density has involved normalizing counts to section length. However, through the use of grid reticules and point-counting methods, the total area and volume of the epidermis can easily be calculated provided that section thickness is consistent. When profiles are counted throughout the epidermis, normalization to epidermal area may prove to be a more accurate reflection of density. A morphometric analysis of IENF density found a strong correlation between the number of nerves per epidermal area and number of nerves per epidermal length in control subjects and patients with diabetic neuropathy (Koskinen et al., 2005). Another study comparing different methods of epidermal nerve quantification found a strong correlation between nerve profile estimation, nerve fiber fragment estimation and the whole nerve fiber quantification method in which branched or discontinuous nerves are counted as a single fiber (Hilliges and Johansson, 1999). However, the authors cautioned that nerve profile or fragment estimation should only be used if epidermal thickness can be controlled. We have found that the epidermis, measured from the stratum basale to the stratum granulosum, is significantly thinner in both rats and mice with type 1 diabetes (Fig. 3), indicating that differences in epidermal area between non-diabetic and diabetic rodents should be considered when quantifying nerve profiles. When comparing linear density of profiles counted throughout the epidermis between a control group and a diabetic group with a thinner epidermis, a reduction in epidermal innervation expressed as linear IENF density may appear more exaggerated than if epidermal area had been incorporated into the measurement.
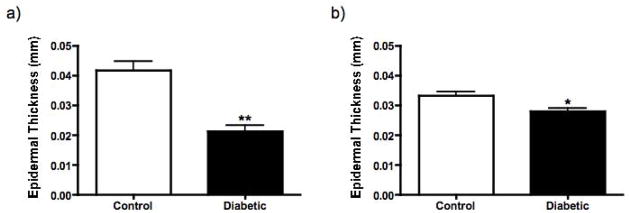
Figure 3.
A comparison of epidermal thickness between control rats and rats with STZ-induced diabetes for a duration of two months (a) and control mice and mice with STZ-induced diabetes for a duration of one month (b). The epidermis from the diabetic group is significantly thinner in both rats and mice (p<0.001 and p<0.01, respectively, unpaired t-test). Data are mean+SEM of N=8–11/group.
Two methods are commonly used to visually quantify IENF density. The first involves counting all immunoreactive profiles that fall within the epidermis, even those that represent nerve fragments. The second method involves counting only the nerve fibers that can be seen crossing the dermal-epidermal border, excluding branches within the epidermis. Both methods have been successfully used to quantify reductions in IENF density. However, a recent study found that the first method is more sensitive to capsaicin-induced fiber loss (Smith et al., 2007). The results of this study are particularly pertinent to the evaluation of therapeutics, as the quantification of immunoreactive profiles throughout the epidermis is more likely to detect not only early signs of degeneration, but also nerve sprouting in the outer layers of the epidermis. However, it should be noted that when fragments are counted, there is a high likelihood that the same nerve will be counted multiple times. These fragments should therefore be referred to as immunoreactive profiles rather than IENFs.
There are other factors that may confound the quantification of IENFs. In addition to nerve fibers, the anti-PGP9.5 antibody also stains Langerhan’s cells (Hamzeh et al., 2000). These antigen-presenting cells are found in the epidermis and possess immunoreactive processes that can be mistaken for IENFs. A higher density of Langerhan’s cells has been observed in the epidermis of type 1 diabetic rats compared to control rats (Lauria et al., 2005). When quantifying IENFs in thinner immunoperoxidase-labeled sections, it is important to exclude immunoreactive profiles that emanate from the bodies of Langerhan’s cells. These processes generally lack the “beaded” appearance of IENF profiles (see Fig. 2d for examples). The use of thicker sections double-immunolabeled for binding of antibodies directed against both PGP9.5 and Langerhan’s cells (Mizoguchi et al., 1992) also remedies this problem. When using immunoperoxidase techniques, accurate quantification of IENFs in the epidermis also depends on the amount of melanin present. Heavy pigmentation can obscure immunoreactive profiles, especially as they cross from the papillary dermis into the epidermis. Melanin-bleaching techniques may remedy this problem (Orchard, 1999).
When compared to other widely used endpoint measures in humans, IENF density measurements were found to be more reproducible than motor and sensory amplitude measurements, with the relative inter-trial variability similar to that of nerve conduction velocity measurements (Smith et al., 2005). As a measure of small fiber neuropathy, IENF density quantification is less invasive and more sensitive than sural nerve biopsy (Herrmann et al., 1999). In diabetic patients, IENF density correlates with warm and cold threshold, heat pain, pressure sense and total neurological disability score (Pittenger et al., 2004; Shun et al., 2004). These findings suggest that measurement of IENF density is a reliable means of evaluating diabetic neuropathy.
Cutaneous innervation in diabetic humans
Initial studies assessing cutaneous innervation in the skin of diabetic human subjects found reduced immunoreactivity to PGP9.5, along with reduced immunoreactivity to the neuropeptides CGRP, SP, vasoactive intestinal polypeptide (VIP) and neuropeptide Y (NPY) (Levy et al., 1989; Lindberger et al., 1989). Multiple subsequent studies have confirmed the decrease in PGP9.5-immunoreactive IENFs (Levy et al., 1992; Properzi et al., 1993; Kennedy et al., 1996; Lauria et al., 1998; Hirai et al., 2000; Gibran et al., 2002; Pittenger et al., 2004; Shun et al., 2004; Koskinen et al., 2005). A reduction in PGP9.5 immunoreactive profiles has been observed in patients suffering from both type 1 (Properzi et al., 1993; Boucek et al., 2005) and type 2 (Shun et al., 2004; Pittenger et al., 2005) diabetes, as well subjects with impaired glucose tolerance (Smith et al., 2005). In a recent study comparing patients with metabolic syndrome to patients with both metabolic syndrome and type 2 diabetes, changes in IENF density were only observed in patients with both afflictions (Pittenger et al., 2005).
In studies examining biopsies from multiple locations, a decrease in IENF density from proximal to distal sites has been observed (Holland et al., 1997; Pittenger et al., 2004), suggesting that loss of small fibers occurs in a length-dependent manner. In addition to the loss of IENFs, diabetic subjects also have a slower rate of nerve regeneration after C-fiber damage induced by topical application of capsaicin compared to non-diabetic subjects, and this impaired regeneration is most marked in patients exhibiting symptomatic neuropathy (Polydefkis et al., 2004).
Correlation with other indices of diabetic neuropathy
Issues of assay reproducibility, sensitivity and the rate of disease progression have impeded clinical trials of agents designed to prevent, halt or reverse indices of diabetic neuropathy. The viability of IENF quantification as an alternative or complimentary biomarker will depend on whether the assay correlates with other measures of neuropathy and provides comparible or enhanced sensitivity and reproducibility. A number of studies have begun to address this issue. There is a significant correlation between reduced nerve length in the dermis and slowing of sural nerve conduction velocity (Hirai et al., 2000) and IENF density in the distal leg shows a significant negative correlation with warm and cold thermal threshold, heat pain, pressure sense and total neurological disability score (Pittenger et al., 2004). A third study of skin biopsies from the distal leg of type 2 diabetic patients found a strong negative correlation between IENF density and warm temperature threshold (Shun et al., 2004). These authors also reported a negative correlation between duration of diabetes and IENF density. A comparison of diabetic patients with and without neuropathic pain found a significant inverse correlation between IENF density and vibration perception threshold, as well as between IENF density and neuropathy status determined with a Michigan Neuropathy Screening Instrument in both groups. However, a significant correlation between IENF density and cold perception threshold was only observed in the group without pain (Sorensen et al., 2006). Most recently, a study comparing assessment of IENF density to corneal nerve fiber density found that both measures correlated with heat pain and cold detection thresholds (Quattrini et al., 2007). These reports provide encouragement for the further evaluation of skin biopsies as an index of diabetic neuropathy.
Correlations between IENF density and other measures of diabetic neuropathy, even those that do not measure small fiber dysfunction specifically, may also be useful for staging neuropathy and provide a means of assessing efficacy of potential therapeutics in clincial trials. Indeed, it has been reported that there is loss of epidermal fibers in patients with impaired glucose tolerance but without overt diabetes (Smith et al., 2001). Another study comparing patients with impaired glucose tolerance (defined by the ADA and WHO criteria as a fasting blood glucose level between 110 and 126 mg/dl or a 2-hour post-glucose challenge level of between 140 and 200 mg/d)l, to a group of diabetic patients (fasting glucose levels exceeding 126 mg/dl or post-glucose challenge levels greater than 200mg/dl) found that the reduction in IENF density in the group with impaired glucose tolerance was less severe (Sumner et al., 2003). Reductions in IENF density have also been reported in diabetic patients that do not yet show clinical or electrophysiological evidence of neuropathy (Umapathi et al., 2007). These studies suggest that skin biopsy may be able to detect neuropathy at its earliest stages.
Animal models
The literature describing cutaneous innervation in non-human primates with diabetes is currently limited to a single paper assessing innervation of glabrous skin from monkeys with naturally occurring type 2 diabetes (Pare et al., 2007). Biopsies from monkeys with short-term hyperglycemia showed a hypertrophy of epidermal nerve fibers. However, in monkeys that were diabetic for duration of eight years or longer, a severe reduction in PGP9.5-immunoreactive IENFs was observed. CGRP- and TRPV1-immunoreactivity was also diminished in the epidermis of longer-term diabetic monkeys.
While rats are the most extensively studied model of diabetic neuropathy, the literature describing immunohistochemical assessment of skin biopsies from diabetic rats is less extensive than the clinical literature. One of the earliest studies (Karanth et al., 1990) assessed SP, CGRP, VIP, NPY and PGP9.5 immunoreactivity in skin from the lip and footpad of rats injected with the β cell toxin streptozotocin (STZ), which induces a model of insulin-deficient type 1 diabetes, and found no change at 2, 4 and 8 weeks of diabetes. However, at 12 weeks of diabetes an increase in CGRP-reactive fibers was observed in both the dermis and epidermis, along with an increase in VIP around sweat glands and blood vessels. An increase in PGP9.5 immunoreactivity was also reported, while dermal or epidermal SP and NPY immunoreactivity was unchanged (Karanth et al., 1990). The early increase in sweat-gland-associated VIP is similar to that seen in humans with diabetes for less than three years (Properzi et al., 1993).
More recent studies have been unable to confirm this apparent increase in epidermal and dermal innervation in diabetic rodents and instead have shown a reduction in epidermal innervation in rat models of diabetic neuropathy (Bianchi et al., 2004; Lauria et al., 2005; Toth et al. 2006; Leonelli et al., 2007; Roglio et al., 2007, Obrosova et al., 2007), which accompanies other nerve disorders and mirrors the reduction observed in human subjects with longer durations of diabetes. Together, these data suggest that rats may be useful for modeling loss of cutaneous innervation in diabetic neuropathy and for investigating the underlying pathophysiolgic mechanisms.
The immunohistochemical assessment of skin biopsies in diabetic mice is also relatively limited to date. In a study examining the cutaneous innervation of insulin-deficient C57Bl/6 mice with STZ-induced diabetes of 7 weeks duration, footpad and flank skin biopsies showed decreased immunoreactivity to CGRP, P2X3, and PGP9.5 when compared to non-diabetic controls (Christianson et al., 2003). However, it should be noted that this quantification included both dermal and epidermal profiles. In another study using STZ-diabetic thy1-YFP mice, which express a yellow fluorescent protein (YFP) in their nerve fibers that allows for noninvasive monitoring of cutaneous innervation, a reduction was observed after 3 months of diabetes (Chen et al., 2005). In our own studies, we have found reductions in PGP9.5-immunoreactive IENF densities in both C57Bl/6 and Swiss Webster mice as early as four weeks after induction of STZ-induced diabetes (Beiswenger et al., 2007). Studies have also been performed in type 2 diabetic mouse models that spontaneously develop insulin resistance before ultimately progressing to insulin deficiency. Compared to their heterozygous non-diabetic littermates, PGP9.5 immunoreactive epidermal fibers were significantly reduced in genetically diabetic C57BL/KsJ-m+/+Leprdb db/db mice, and similar reductions were observed in the skin samples from human subjects evaluated in the same study (Gibran et al., 2002). In C57Bl6/J ob/ob mice, another model of type 2 diabetes, a decrease in IENF density was observed in animals that were aproximately 11 weeks old (Drel et al., 2006; Vareniuk et al., 2007). These results agree with changes observed in both type 1 and type 2 diabetic patients and suggest that mice may also be a valuable model for the studying cutaneous innervation.
Functional consequences of epidermal fiber loss in diabetic rodents
While the obvious prediction is that loss of capsaicin-sensitive TRPV-1 bearing epidermal C-fibers should result in thermal hypoalgeisa, there have been few attempts to date that correlate loss of epidermal fibers in rodents with direct functional consequences. In the STZ-diabetic rat, thermal hypoalgesia was present five weeks after onset of diabetes, but a reduction in IENF density was not observed until eleven weeks (Bianchi et al., 2004). Similarly, in thy1-YFP mice, cutaneous nerve fiber loss was not observed until three months post-STZ injection, although thermal hypoalgesia was established after one month of diabetes (Chen et al., 2005). In our own studies, we have observed thermal hypoalgesia as early as two weeks after onset of hyperglycemia, but did not detect reductions in IENF density until after four weeks (Beiswenger et al., 2007).
Whether these results indicate that functional changes relating to sensory processing precede structural changes in small fiber diabetic neuropathy or that current microscopic techniques are not sufficiently sensitive to detect early structural damage remains to be established. A recent study by Vareniuk et al (2007) found that treatment with assorted peroxynitrite decomposition catalysts partially alleviated thermal hypoalgesia in type 2 diabetic ob/ob mice. However, this type of treatment was unable to prevent reductions in IENF density. These observations further suggest that functional changes may not be as closely linked to structural changes as previously thought.
Therapeutic interventions
The effects of therapeutic intervention on cutaneous innervation in animal models of diabetes remain largely unexplored. In the STZ-diabetic rat, there is a report on the neuroprotective properties of erythropoietin (Bianchi et al., 2004). While the density of PGP9.5 immunoreactive profiles in the epidermis was unchanged after 5 weeks of diabetes, a significant reduction was observed after 11 weeks of diabetes and administration of erythropoietin from week 5 onwards prevented the epidermal nerve fiber deficit from developing. Thermal hypoalgesia, already present at week 5 of diabetes, was also reversed by erythropoietin treatment. Another study examining the effects of intrathecally delivered insulin and IGF-1 in STZ-diabetic rats found an increase in IENF density and IENF length compared to the untreated diabetic group (Toth et al., 2006), although values remained lower than in control rats. In a more recent study, treatment with a poly(ADP-ribose) polymerase (PARP) inhibitor prevented reductions in IENF density and partially alleviated thermal hypoalgesia in STZ-diabetic rats (Obrosova et al., 2007). There is also evidence that steroid hormones or their metabolites may have protective effects against cutaneous nerve fiber loss (Leonelli et al., 2007; Roglio et al., 2007). In the STZ model, treatment with progesterone, dihydroprogesterone or tetrahydroprogesterone was able to prevent a reduction in IENF density and reverse established thermal hypoalgesia (Leonelli et al., 2007). Treatment with testosterone, dihydrotestosterone or 5α-androstan-3α,17β-diol also prevented a reduction in IENF density but only dihydrotestosterone and 5α,17β-diol were able to partially reverse thermal hypoalgesia (Roglio et al., 2007). Taken together, these data serve to futher dissociate functional and structural measures of sensory neuropathy in rats and emphasize that there may be functional loss without overt loss of epidermal fibers.
In STZ-diabetic mice, intrathecal treatment with NGF for two weeks stimulated axonal branching, but failed to improve cutaneous innervation, whereas intrathecal GDNF and neurturin (NTN) treatment increased both axonal branching and cutaneous innervation in diabetic mice (Christianson et al., 2003). Herpes simplex virus-mediated gene transfer of both vascular endothelial growth factor (VEGF) (Chattopadhyay et al., 2005) and neurotrophin-3 (NT-3) (Chattopadhyay et al., 2007) have been shown to prevent reductions in proportional area of footpad skin and subcutaneous tissue occupied by PGP9.5-immunoreactive nerve fibers in STZ-diabetic mice. In the ob/ob mouse model, treatment with an aldose reducatase inhibitor prevented both onset of thermal hypoalgesia and IENF loss (Drel et al., 2006). However, in none of these studies was the therapy used against established loss of epidermal fibers and, while the data are encouraging for the development of prophylactics, whether these or any other agents have the capacity to promote functional re-innervation of the epidermis remains to be established.
Pathogenic mechanisms
In most clinical studies, distinctions have not been made between patients with insulin-dependent type 1 diabetes and patients with insulin-resistant type 2 diabetes. However, whether or not IENF loss is affected by the presence or absence of insulin is an important question. While several studies have reported a reduction in IENF density in rat models of type 1 diabetes (Bianchi et al., 2004; Lauria et al., 2005; Leonelli et al., 2007; Roglio et al., 2007), the effects of hyperglycemia on epidermal innervation in rat models of type 2 diabetes remain unexplored. Fiber loss has been reported in mouse models of type 2 diabetes (Gibran et al., 2002; Drel et al., 2006; Vareniuk et al., 2007), although these models can show insulinopenia in later stages of the disease. Another study of STZ-diabetic mice found evidence of epidermal reinnervation in individuals that spontaneously regained islet cell function (Kennedy and Zochodne, 2005). The role that endogenous insulin plays in the preservation of cutaneous innervation is certainly worthy of further study.
Examination of the effects of various therapeutics on IENF density (see above) may also shed light on the pathogenic mechanisms underlying cutaneous nerve fiber loss. The abillity of erythropoietin (Bianchi et al., 2004) and insulin (Toth et al., 2006), both of which have neurotrophic properties (Konishi et al., 1993; Fernyhough et al., 1993; Xu et al., 2004), as well as GDNF, NTN (Christianson et al., 2003), VEGF (Chattopadhyay et al., 2005) and NT-3 (Chattopadhyay et al., 2007) to prevent cutaneous nerve fiber loss in diabetic rodents suggests that diminished neurotrophic support may contribute to similar losses in patients with diabetes. The efficacy of aldose reductase inhibition (Drel et al., 2006) indicates that increased flux through the polyol pathway may also contribute to the pathogenic mechanisms involved. The therapeutic effects of PARP inhibition in diabetic rats, as well as the resistance to diabetes-induced fiber loss observed in PARP −/− mice, suggest that PARP activation may also be involved in epidermal fiber loss (Obrosova et al., 2007).
Conclusion
Quantification of epidermal innervation is emerging as a reliable and minimally invasive means of diagnosing and staging diabetic neuropathy. Along with the assessment of corneal nerve fiber density using the non-invasive technique of in-vivo corneal confocal microscopy (Malik et al., 2003), which has shown a similar progressive reduction in a group of patients with diabetic neuropathy (Quattrini et al., 2007), quantification of IENF density offers the potential for direct assessment of neuropathy in future clinical trials of therapies designed to prevent or reverse this major clincial concern. The recent demonstration of equivalent diabetes-induced changes to IENF density in rats and mice suggests that experimental diabetes may be valuable for investigating etiologic mechanisms and the evaluation of therapeutics.
Acknowledgments
We would like to thank Drs. Itzack Fisher and Corinne Jolivalt for providing the anti-high molecular weight tau antibody and Joshua A. Gregory for providing a micrograph of PGP9.5-immunolabeled rat skin. Supported by NIH grants DK057629 (NAC) and DK078374 (APM) and the Juvenile Diabetes Research Foundation (APM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beiswenger K, Calcutt NA, Mizisin AP. The time course of structural and functional changes in epidermal nerves of a mouse model of type 1 diabetes. J Peripher Nerv Syst. 2007;12(S1):8–9. [Google Scholar]
- Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, Lauria G, Borgna M, Lombardi R, Cimen B, Comelekoglu U, Kanik A, Tatarolgu C, Cerami A, Ghezzi P. Erythropoetin both protects from and reverses experimental diabetic neuropathy. PNAS. 2004;101:823–828. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund H, Dalsgaard CJ, Jonsson CE, Hermansson A. Sensory and autonomic innervation of non-hairy and hairy human skin. An immunohistochemical study. Cell Tissue Res. 1986;243:51–7. doi: 10.1007/BF00221851. [DOI] [PubMed] [Google Scholar]
- Boucek P, Havrdova T, Voska L, Lodererova A, Saudek F, Lipar K, Janousek L, Adamec M, Sommer C. Severe depletion of intraepidermal nerve fibers in skin biopsies of pancreas transplant recipients. Transplant Proc. 2005;37:3574–5. doi: 10.1016/j.transproceed.2005.09.047. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12(4–5):256–68. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Krisky K, Wolfe D, Glorioso JC, Mata M, Fink DJ. HSV-mediated gene transfer of vascular endothelial growth factor to dorsal root ganglia prevents diabetic neuropathy. Gene Therapy. 2005;12:1377–1384. doi: 10.1038/sj.gt.3302533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Mata M, Goss J, Wolfe D, Huang S, Glorioso JC, Fink DJ. Prolonged preservation of nerve function in diabetic neuropathy in mice by herpes simplex virus-mediated gene transfer. Diabetologia. 2007;50:1550–8. doi: 10.1007/s00125-007-0702-4. [DOI] [PubMed] [Google Scholar]
- Chen YS, Chung SSM, Chung SK. Noninvasive monitoring of diabetes-induced cutaneous nerve fiber loss and hypoalgesia in thy1-YFP transgenic mice. Diabetes. 2005;54:3112–3118. doi: 10.2337/diabetes.54.11.3112. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Riekhof JT, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol. 2003;179:188–99. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- Daalsgard CJ, Rydh M, Haegerstrand A. Cutaneous innervation in man visualized with protein gene product 9.5 (PGP9.5) antibodies. Histochemistry. 1989;92:385–9. doi: 10.1007/BF00492495. [DOI] [PubMed] [Google Scholar]
- Denda M, Fuziwara S, Inoue K, Denda S, Akamatsu H, Tomitaka A, Matsunaga K. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem and Biophys Res Comm. 2001;285:1250–1252. doi: 10.1006/bbrc.2001.5299. [DOI] [PubMed] [Google Scholar]
- Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. The leptin-deficient (ob/ob) mouse: A new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–43. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, Sinisi M, Birch R, Anand P. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurons. Brain Res. 1993;607:117–124. doi: 10.1016/0006-8993(93)91496-f. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Wattchow D, Coventry B. Two immunohistochemically identified populations of calcitonin gene-related peptide (CGRP)-immunoreactive axons in human skin. Brain Res. 1987;414:143–148. doi: 10.1016/0006-8993(87)91335-7. [DOI] [PubMed] [Google Scholar]
- Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108:122–28. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, Bountra C, Anand P. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5:2. doi: 10.1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzeh H, Gaudillere A, Sabido O, Tchou I, Lambert C, Schmitt D, Genin C, Misery L. Expression of PGP9.5 on Langerhans’ cells and their precursors. Acta Derm Venereol. 2000;80:14–16. doi: 10.1080/000155500750012423. [DOI] [PubMed] [Google Scholar]
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- Holland NR, Stocks A, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology. 1997;48:708–11. doi: 10.1212/wnl.48.3.708. [DOI] [PubMed] [Google Scholar]
- Hirai A, Yasuda H, Joko M, Maeda T, Kikkawa R. Evaluation of diabetic neuropathy through quantitation of cutaneous nerves. J Neurol Sci. 2000;172:55–62. doi: 10.1016/s0022-510x(99)00290-7. [DOI] [PubMed] [Google Scholar]
- Karanth SS, Springall DR, Francavilla S, Mirrlees DJ, Polak JM. Early increase in CGRP- and VIP-immunoreactive nerves in the skin of streptozotocin-induced diabetic rats. Histochemistry. 1990;94:659–66. doi: 10.1007/BF00271994. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G. The innervation of the human epidermis. J Neurol Sci. 1993;115:184–90. doi: 10.1016/0022-510x(93)90223-l. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Brelje TC. Innervation and vasculature of human sweat glands: an immunohistochemistry-laser scanning confocal fluorescence microscopy study. J Neurosci. 1994;14:6825–6833. doi: 10.1523/JNEUROSCI.14-11-06825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–48. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Nolano M, Wendelschafer-Crabb G, Johnson TL, Tamura E. A skin blister method to study epidermal nerves in peripheral nerve disease. Muscle Nerve. 1999;22:360–71. doi: 10.1002/(sici)1097-4598(199903)22:3<360::aid-mus9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kennedy WR. Opportunities afforded by the study of unmyelinated nerves in skin and other organs. Muscle Nerve. 2004;29:756–767. doi: 10.1002/mus.20062. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Zochodne DW. Experimental diabetic neuropathy with spontaneous recovery: is there irreparable damage? Diabetes. 2005;54:830–837. doi: 10.2337/diabetes.54.3.830. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Chui DH, Hirose H, Kunishita T, Tabira T. Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res. 1993;609:29–35. doi: 10.1016/0006-8993(93)90850-m. [DOI] [PubMed] [Google Scholar]
- Koskinen M, Hietaharju A, Kylaniemi M, Peltola J, Rantala I, Udd B, Haapasalo H. A quantitative method for the assessment of intraepidermal nerve fibers in small-fiber neuropathy. J Neurol. 2005;252:789–94. doi: 10.1007/s00415-005-0743-x. [DOI] [PubMed] [Google Scholar]
- Lauria G, McArthur JC, Hauer PE, Griffin JW, Cornblath DR. Neuropathological alterations in diabetic truncal neuropathy: evaluation by skin biopsy. J Neurol Neurosurg Psychiatry. 1998;65:762–66. doi: 10.1136/jnnp.65.5.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria G, Borgna M, Morbin M, Lombardi R, Mazzoleni G, Sghirlanzoni A, Pareyson D. Tubule and neurofilament immunoreactivity in human hairy skin: markers for intraepidermal nerve fibers. Muscle Nerve. 2004;30:310–6. doi: 10.1002/mus.20098. [DOI] [PubMed] [Google Scholar]
- Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. Intraepidermal nerve fiber density in rat foot pad: Neuropathologic-neurophysiologic correlation. J Periph Nerv Syst. 2005;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- Lauria G, Morbin M, Lombardi R, Capobianco R, Camozzi F, Pareyson D, Manconi M, Geppetti P. Expression of capsaicin receptor immunoreactivity in human peripheral nervous system and in painful neuropathies. J Peripher Nerv Syst. 2006;11:262–71. doi: 10.1111/j.1529-8027.2006.0097.x. [DOI] [PubMed] [Google Scholar]
- Leonelli E, Bianchi R, Cavaletti G, Caruso D, Crippa D, Garcia-Segura LM, Lauria G, Magnaghi Roglio I, Melcangi RC. Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: A multimodel analysis. Neuroscience. 2007;144:1293–1304. doi: 10.1016/j.neuroscience.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Levy DM, Karanth SS, Springall DR, Polak JM. Depletion of cutaneous nerves and neuropeptides in diabetes mellitus: an immunocytochemical study. Diabetologia. 1989;32:427–433. doi: 10.1007/BF00271262. [DOI] [PubMed] [Google Scholar]
- Levy DM, Terenghi G, Gu XH, Abraham RR, Springall DR, Polak JM. Immunohistochemical measurements of nerves and neuropeptides in diabetic skin: relationship to tests of neurological function. Diabetologia. 1992;35:889–97. doi: 10.1007/BF00399938. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Peripheral sensory systems. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, editors. Peripheral Neuropathy. 3. Philadelphia: Saunders; 1993. pp. 149–165. [Google Scholar]
- Lindberger M, Daa Schroder H, Schultzberg M, Kristensson K, Persson A, Ostman J, Link H. Nerve fibre studies in skin biopsies in peripheral neuropathies. J Neurol Sci. 1989;93:289–96. doi: 10.1016/0022-510x(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Malik RA, Kallinikos P, Abbott CA, van Schie CHM, Morgan P, Efron N, Boulton AJM. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–88. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Mizisin AP, Calcutt NA, von Stein T, Robbins WR, Bley KR. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain. 2004;111:360–7. doi: 10.1016/j.pain.2004.07.017. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: Normative reference range and diagnostic efficiency. Arch Neurol. 1998;55:1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- Mizisin AP, Kalichman MW, Garrett RS, Dines KC. Tactile hyperesthesia, altered epidermal innnervation and plantar nerve injury in the hindfeet of rats housed on wire grates. Brain Res. 1998;788:13–19. doi: 10.1016/s0006-8993(97)01474-1. [DOI] [PubMed] [Google Scholar]
- Mizoguchi S, Takahashi K, Takeya M, Naito M, Morioka T. Development, differentiation, and proliferation of epidermal Langerhans cells in rat ontogeny studied by a novel monoclonal antibody against epidermal Langerhans cells, RED-1. J Leukoc Biol. 1992;52(1):52–61. doi: 10.1002/jlb.52.1.52. [DOI] [PubMed] [Google Scholar]
- Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–45. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- Oblinger MM, Argasinski A, Wong J, Kosik KS. Tau gene expression in rat sensory neurons during development and regeneration. J Neurosci. 1991;11:2453–2459. doi: 10.1523/JNEUROSCI.11-08-02453.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Xu W, Lyzogubov VV, Ilnytska O, Maxhtalir N, Vareniuk I, Pavlov IA, Zhang J, Slusher B, Drel VR. PARP inhibition or gene deficiency counteracts intraepidermal nerve fiber loss and neuropathic pain in advanced diabetic neuropathy. Free Radic Biol Med. 2007 doi: 10.1016/j.freeradbiomed.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard GE. Heavily pigmented melanocytic neoplasms: comparision of two melanin-bleaching techniques and subsequent immunohistochemical staining. Br J Biomed Sci. 1999;56:188–193. [PubMed] [Google Scholar]
- Pare M, Albrecht PJ, Noto CJ, Bodkin NL, Pittenger GL, Schreyer DJ, Tigno XT, Hansen BC, Rice FL. Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J Comp Neurol. 2007;501:543–567. doi: 10.1002/cne.21262. [DOI] [PubMed] [Google Scholar]
- Periquet MI, Novak V, Collins MP, Nagaraja HN, Erdem S, Nash SM, Freimer ML, Sahenk Z, Kissel JT, Mendell JR. Painful sensory neuropathy: prospective evaluation by skin biopsy. Neurology. 1999;53:1641–47. doi: 10.1212/wnl.53.8.1641. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Streit WJ, Johnson RD. Localization of unmyelinated axons in rat skin and mucocutaneous tissue utilizing the isolectin GS-I-IB4. Somatosens Mot Res. 1997;14:17–26. doi: 10.1080/08990229771187. [DOI] [PubMed] [Google Scholar]
- Pirart J, Lauvaux JP, Rey W. Blood sugar and diabetic complications. N Engl J Med. 1978;298:1149. doi: 10.1056/NEJM197805182982020. [DOI] [PubMed] [Google Scholar]
- Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care. 2004;27:1974–9. doi: 10.2337/diacare.27.8.1974. [DOI] [PubMed] [Google Scholar]
- Pittenger GL, Mehrabyan A, Simmons K, Rice A, Dublin C, Barlow P, Vinik AI. Small fiber neuropathy is associated with metabolic syndrome. Metab Syndr Relat Disord. 2005;3:113–121. doi: 10.1089/met.2005.3.113. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Griffin JW, McArthur J. New insights into diabetic neuropathy. JAMA. 2003;290(10):1371–6. doi: 10.1001/jama.290.10.1371. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- Properzi G, Francavilla S, Poccia G, Aloisi P, Gu X, Terenghi G, Polak JM. Early increase precedes a depletion of VIP and PGP9.5 in the skin of insulin-dependent diabetics – correlation between quantitative immunohistochemistry and clinical assessment of peripheral neuropathy. J Pathol. 1993;169:269–277. doi: 10.1002/path.1711690215. [DOI] [PubMed] [Google Scholar]
- Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–54. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- Roglio I, Bianchi R, Giatti S, Cavaletti G, Caruso D, Scurati S, Crippa D, Garcia-Segura LM, Camozzi F, Lauria G, Melcangi RC. Testosterone derivatives are neuroprotective agents in experimental diabetic neuropathy. Cell Mol Life Sci. 2007;64:1158–1168. doi: 10.1007/s00018-007-7002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim MM, Wendelschafer-Crabb G, Redmon B, Khoruts A, Walk D, Kennedy WR. Mucosal gastric nerves: A marker of autonomic neuropathy in type 1 diabetic neuropathy with gastroparesis. J Peripher Nerv Syst. 2007;12(S1):79. [Google Scholar]
- Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004;127:1593–1605. doi: 10.1093/brain/awh180. [DOI] [PubMed] [Google Scholar]
- Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001;57:1701–1704. doi: 10.1212/wnl.57.9.1701. [DOI] [PubMed] [Google Scholar]
- Smith AG, Howard JR, Kroll R, Ramachandran P, Hauer P, Singleton JR, McArthur J. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci. 2005;228:65–69. doi: 10.1016/j.jns.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Smith AG, Pollari D, Tovbias J, Sutton MA, Muhammad N, Babbar S, Chanda S, Bley KR. The effect of counting criteria on intraepidermal nerve fiber density estimates. J Peripher Nerv Syst. 2007;12(S1):82. [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 20:629–32. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care. 2006;29:883–887. doi: 10.2337/diacare.29.04.06.dc05-2180. [DOI] [PubMed] [Google Scholar]
- Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–11. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- Thomas PK. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy - Current State and Perspectives of Diabetes Research: Chronic Complications. Diabetes. 1997;46:S54–57. doi: 10.2337/diab.46.2.s54. [DOI] [PubMed] [Google Scholar]
- Toth C, Brussee V, Zochodne W. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia. 2006;49:1081–88. doi: 10.1007/s00125-006-0169-8. [DOI] [PubMed] [Google Scholar]
- Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve. 2007;35:591–98. doi: 10.1002/mus.20732. [DOI] [PubMed] [Google Scholar]
- Underwood RA, Gibran NS, Muffley LA, Usui ML, Olerud JE. Color subtractive-computer-assisted image analysis for quantification of cutaneous nerves in a diabetic mouse model. J Histochem Cytochem. 2001;49:1285–1291. doi: 10.1177/002215540104901011. [DOI] [PubMed] [Google Scholar]
- Vareniuk I, Pavlov IA, Drel VR, Lyzogubov VV, Ilnytska O, Bell SR, Tibrewala J, Groves JT, Obrosova IG. Nitrosative stress and peripheral diabetic neuropathy in leptin-deficient (ob/ob) mice. Exp Neurol. 2007;205:425–36. doi: 10.1016/j.expneurol.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Wang L, Hilliges M, Jernberg T, Wiegleb-Edstrom D, Johansson O. Protein gene product 9.5-immunoreactive nerve fibres and cells in human skin. Cell Tissue Res. 1990;261:25–33. doi: 10.1007/BF00329435. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP9.5 is a ubitquitin carboxyl-terminal hydrolase. Science. 1989;246:670–73. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Xu QG, Li XQ, Kotecha SA, Cheng C, Sun HS, Zochodne DW. Insulin as an in vivo growth factor. Exp Neurol. 2004;188:43–51. doi: 10.1016/j.expneurol.2004.03.008. [DOI] [PubMed] [Google Scholar]