Abstract
Antagonism of T cell responses by variants of the cognate peptide is a potential mechanism of viral escape from immune responses and may play a role in the ability of HIV to evade immune control. We show here a rarely described mechanism of antagonism by a peptide shorter than the minimum length epitope for an HIV p24-specific CD4+ T cell clone. The shorter antagonist peptide-MHC complex bound the T cell receptor (TCR), albeit with lower affinity than the full-length agonist peptide. Prior work showing the crystal structure of the peptide-MHC complex revealed a unique glycine hinge near the C-terminus of the agonist peptide, allowing the generation of full-length antagonist peptide lacking the hinge. These results confirm the dependence of productive TCR engagement on residues spilling out from the C-terminus of the MHC binding groove and show that partial engagement of the TCR with a truncated, low-affinity ligand can result in T cell antagonism.
1. Introduction
T helper (Th) cells likely play a key role in immune control of HIV replication (Norris and Rosenberg, 2002; Rosenberg et al., 1997), yet the precise targets and determinates of activation for these cells remain poorly defined. It has been demonstrated that sequence variation can decrease Th cell recognition of the virus (Fernandez et al., 1997; Harcourt et al., 1998; Norris et al., 2004; Siliciano et al., 1988). Such altered peptide ligands not only can escape recognition by T cells, but also may antagonize responses to agonist peptide (Alexander et al., 1993; De Magistris et al., 1992; Ostrov et al., 1993; Racioppi et al., 1993). Antagonism of T cell responses by variant viral sequences has been described in HIV (Klenerman et al., 1994) and other viral infections, and antagonism of Th cell responses has been posited as a possible mechanism of HIV vaccine failure (Kent et al., 1997). Understanding the nature of antagonist peptides and the potential effects they have on Th cell responses is likely to be critical for HIV vaccine efforts.
Antagonist peptides block activation induced by stimulation with agonist peptide through mechanisms that have not been completely elucidated. Experiments using T cells expressing dual TCRs with unique specificities have yielded conflicting results. In some but not all cases a peptide antagonistic for one TCR can block activating signals delivered through the second TCR (cross antagonism), and it may be that cross antagonism is only seen in CD4+ T cells (Daniels et al., 1999; Robertson and Evavold, 1999; Stotz et al., 1999; Yang and Grey, 2003). Antagonists may deliver a dominant negative signal (Dittel et al., 1999; Rudolph et al., 2004; Sykulev et al., 1998), though not in all instances. Partial and differential phosphorylation of the TCR ζ chain has been demonstrated after T cell exposure to antagonist peptides (Kersh et al., 1999; Reis e Sousa et al., 1996), as has activity and phosphorylation of Src homology 2 (SH2) domain-containing protein tyrosine phosphatase (Kilgore et al., 2003) and CD4-Lck kinase (Racioppi et al., 1996; Stefanova et al., 2003). Studies using galectin-1 to induce antagonism suggest that disruption of lipid raft formation at TCR contact sites may also play a role in antagonism (Chung et al., 2000), and cells stimulated in the presence of an antagonist show decreased density of MHC in the immunological synapse (Sumen et al., 2004). Antigen presenting cells (APCs) also likely play a role as activated APCs can mitigate the effects of antagonists (Kissler et al., 2002). More recent work has shown that exogenous IL-2 can reverse antagonism in human clones (Gebe et al., 2004). In summary, mechanisms of antagonism likely vary depending on the model system and specific epitope/MHC context, with at least some antagonists able to deliver a dominant negative signal, possibly through differential phosphorylation of intracellular signaling proteins.
The MHC and TCR binding properties of antagonist peptides have also been studied. MHC-peptide complexes of most known antagonists bind the TCR with lower affinity than those of the corresponding agonist peptide, though antagonist peptides that act at lower concentrations than agonist peptides have been described (Sewell et al., 1997). Many antagonist peptides bind MHC as well as the agonist peptide, and crystallization experiments have shown that antagonist peptides can induce little or no conformational change in the peptide/MHC/TCR complex compared to agonist peptide (Ding et al., 1999). HLA-DR1-restricted epitope analogs have been shown to become stronger antagonists the more they resemble the agonist peptide (Alexander et al., 1993). Some antagonist peptides have no discernable agonist activity, while others are partial agonists. A spectrum of partial agonists has been demonstrated wherein stronger antagonists can antagonize the partial agonist activity of weaker antagonists, implying a graded response to altered peptide ligands (Bachmann et al., 1998). The characteristics of peptide/MHC/TCR interaction that lead to antagonism have not been universally defined, but are hypothesized to depend on the strength and duration of binding of the peptide/MHC complex to the TCR (Matsui et al., 1994; Rabinowitz et al., 1996).
We previously described the minimum epitopes and HLA restriction of five HIV-specific Th cell clones generated by natural infection (Norris et al., 2004; Norris et al., 2001). One of the clones, AC-25-1, required a sixteen amino acid minimum epitope, PEVIPMFSALSEGATP (PP16), for induction of proliferation, IFN-γ secretion, and cytolytic activity. Partial response was seen to an N-terminal truncated peptide EVIPMFSALSEGATP, but smaller peptides were inactive (Norris et al., 2004). While the binding groove of MHC class II (MHC-II) molecules is open on each end and can bind long peptides, it was unclear why such a long minimum epitope was required for T cell activation. Co-crystallization studies of MHC-II, peptide, and TCR had suggested that typically only nine to ten residues of a MHC-II restricted epitope are able to contact the MHC molecule, with residues within or immediately flanking this region able to contact TCR (Reinherz et al., 1999). X-ray diffraction analysis of HLA-DR1 bound to PP16 and to a shorter, non-activating peptide that contained the expected MHC-binding region (underlined) PEVIPMFSALSEG (PG13), showed that both peptides bound within the MHC-peptide binding site in essentially identical conformation (Zavala-Ruiz et al.). However, the C-terminal region of PP16 extended outside the MHC-II binding site and adopted a hairpin conformation that allowed it to interact with the TCR. Both PP16 and PG13 bound tightly to HLA-DR1, the restricting class II allele (Kd ~ 3–6 nM) (Zavala-Ruiz et al.).
In this study we dissect the mechanism of antagonism at the level of peptide/MHC/TCR interaction, using HIV-specific CD4+ T cells derived during the course of HIV infection in a patient who had been treated with highly active antiretroviral therapy (HAART) during primary infection (Norris et al., 2001). We show that truncation of the epitope by three C-terminal amino acids rendered the peptide unable to elicit proliferation, IFN-γ release, or serine esterase release, even though it retained strong binding to the MHC molecule. Using MHC II tetramers we demonstrate that the shorter thirteen amino acid peptide complexed to HLA-DR1 bound the TCR without inducing functional activity, and with lower affinity than the full-length peptide. Furthermore, we show here that the truncated peptide acts as an antagonist to the full-length epitope, a rarely described form of antagonism.
2. Materials and methods
2.1. Study subjects
Subject AC-25 was infected with HIV in 1998 and started highly active antiretroviral therapy within three months of seroconversion. A CD4+ T cell clone designated AC-25-1 was derived eighteen months after infection shortly after a structured therapy interruption, when HIV p24-specific proliferative responses were robust A second subject, 161J, has been HIV infected for over 25 years and has consistently maintained a virus load <50 without antiretroviral medication. All study samples were obtained with informed consent, and the studies were approved by the Massachusetts General Hospital Institutional Review Board.
2.2. Peptides
Peptides were generated as free acids using an Advanced ChemTech (Louisville, KY) 396Ω peptide synthesizer and purity was determined by high-pressure liquid chromatography.
2.3. Generation of Th cell clone and B cell lines
The CD4+ T cell clones and autologous EBV-immortalized B cell lines were derived as previously described (Norris et al., 2001). Briefly, the clones were isolated by stimulating PBMC with whole p24 antigen, resting them for two weeks, then restimulating and plating at limiting dilution on round bottom 96 well plates. Responding wells were tested for specificity, and clones were restimulated every two weeks with IL-2 (100 U/ml), 12F6 (anti-CD3 antibody obtained from Johnson Wong, the Massachusetts General Hospital, 0.1 μg/ml), and 106 feeder cells irradiated 30 Gy.(Norris et al., 2001) Clones were fed twice weekly with RPMI media supplemented with penicillin/streptomycin (Mediatech, Herndon, VA), HEPES (Mediatech), and L-glutamine (Mediatech), (R10). B cell lines were Epstein-Barr virus transformed in the presence of cyclosporin A as previously described (Norris et al., 2001).
2.4. IFN-γ ELISPOT assays
ELISPOT plates were coated with anti-IFN-γ antibody (Endogen, Woburn, MA) and incubated overnight at 4°C. The following day plates were washed 6 times with phosphate buffered saline (PBS, Mediatech). B-LCL (5 × 104) and antigen were added in 100 μl R10. T cell clones were added at 100 cells/well in 100 μl R10. After overnight incubation the cells were discarded and plates were washed 6 times with PBS, and biotinylated anti-IFN-γ (Mabtech, Mariemont, OH) was added for 1.5 hours at 25°C. The plates were then washed 6X with PBS. Streptavidin (Mabtech) 100 μl/well was added and plates were incubated 45 minutes at 25°C. After 6 more PBS washes 100 μl/well coloring reagent (NBT/BCIP, Bio-Rad, Hercules, CA) was added. Once dots appeared the coloring reagent was discarded and the wells were incubated with PBS plus 1% Tween for 10 minutes, then plates were washed in tap water thrice. Background responses to wells with no antigen or irrelevant antigen ranged from 0 to 2.5 spots per well. Responses greater than 5 spots per well and 5 times maximum background were considered significant.
2.5. Staining with HLA-DR1 tetramers
Fluorescence-labeled tetramers of HLA-DR1 carrying particular peptides were prepared as previously described (Cameron et al., 2002). Tetramer staining was performed by first centrifuging the clone cells at 1500 rpm for 10 min. The media was aspirated and the cells were resuspended in R10H at 300,000 cells per 10 μl for each condition. The cells were then placed in round bottom 96 well plates and 5 μl of tetramer reagent was added. After 1.5 hours’ incubation at 25°C the cells were washed in 200 μl PBS, fixed with 1% paraformaldehyde, and analyzed on a Becton Dickinson LSR II flow cytometer.
2.6. Proliferation assays
Antigen was presented by autologous B lymphoblastoid cell lines (B-LCL). The B-LCL were irradiated (120 Gy) and resuspended in R10 with the designated antigen at 1 μg/ml unless otherwise noted. T cell clones and B-LCL were then plated in triplicate wells at 50,000 cells/well in 96 well plates in R10. After 48 hours, 1 μCi of 3H-thymidine (Dupont Nen, Boston, MA) in 50 μl R10 was added per well. Plates were harvested onto glass fiber filters after 18 hours. Assays were performed in triplicate. Results were expressed as net CPM, the difference between the counts in the presence of antigen and the counts without antigen present. Significant responses were considered to be net CPM greater than 1000 (Norris et al., 2001).
A newer technique to measure proliferation by dilution of carboxyfluorescein diacetate succinimidyl ester (CFSE) was also used (Bird et al., 1998). With each successive cell division the concentration of CFSE falls two-fold in daughter cells. Clone cells were labeled for 15 minutes at 37C with 0.05 μM CFSE in PBS, then washed twice with PBS. Cells were placed at 125,000 per FACS tube at a 5 degree slant and incubated in media with appropriate antigen as above. B cells were pre-pulsed with antigen at 0.1 μg/ml agonist peptide prior to adding at 125,000 cells/tube with appropriate concentrations of antagonist or control peptide. Negative control conditions included B cells not pulsed with agonist peptide. 50,000 events/condition were collected.
2.7. Lytic granule release assay
To quantify lytic granule release, autologous B-LCL were pulsed with various concentrations of cognate peptide for 1 hour at 37°C and were combined with CD4+ T cells at E:T ratio 1:1 in round-bottom 96-well plate. The density of the T cells in the mixture was 7 × 105 per ml (1.5 × 105 per well). After 4 hours at 37°C the culture supernatant was collected and activity of serine esterase, an essential component of the secreted granules, was measured using the BLT (N-α-benzyloxycarboxyl-L-lysinethiobenzyl ester) substrate in the presence of DTNB by reading the optical density (OD) at 405 nm (Norris et al., 2001). In our experiments DTNB was added prior to the addition of BLT to measure the background OD at 405 nm. The background was then subtracted from the final reading for each sample.
2.8. Flow cytometry
Intracellular cytokine staining was performed using a modification of published techniques (Pitcher et al., 1999). Briefly, 0.5 × 106 cells were incubated with antigen for two hours, then treated with 10 μg/ml brefeldin A (Sigma-Aldrich, St. Louis, MO) for four hours. Cells were surface stained with anti-CD3-PE (Becton Dickinson, San Jose, CA) for 30 minutes, washed in PBS then fixed and permeabilized using Caltag reagents (Caltag Laboratories, Burlingame, CA). Staining for IFN-γ was performed using anti-IFN-γ-APC for 30 minutes. Cells were acquired on an LSR II flow cytometer (Becton Dickinson), 50,000 events/condition. Control conditions included staining unstimulated aliquots for IFN-γ.
2.9. Statistical analysis
Statistical analysis was performed using Student’s paired t-Tests with Microsoft Excel.
3. Results
3.1. Lack of activity of peptide PG13, shorter than the minimum epitope
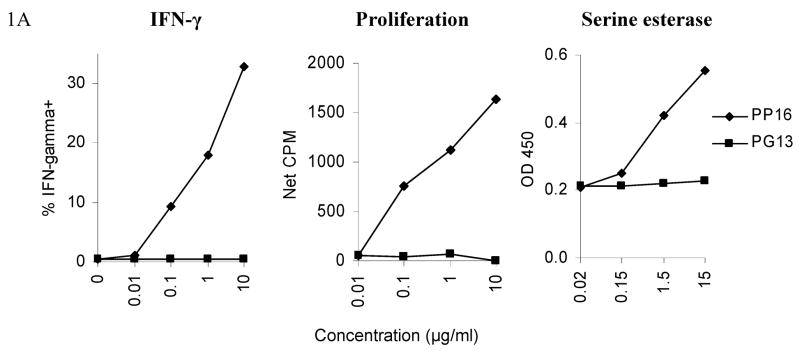
In this study, we investigated the agonist and antagonist properties of the PP16 and PG13 peptides in their interaction with a clone specific for this epitope, designated AC-25-1. As previously reported (Zavala-Ruiz et al.), PP16 but not PG13 was able to induce proliferation of the effector T cells (Figure 1A). Similarly, PP16 was able to induce IFN-γ secretion and serine esterase release by the clone, while PG13 was not (Figure 1A). The response to the PP16 epitope was tested directly ex vivo by stimulating AC-25 PBMC with the 22 residue peptide AFSPEVIPMFSALSEGATPQDL. No response was detected directly ex vivo, but was detected after in vitro expansion for one month, implying that the specific cells are present at low frequency ex vivo and can be expanded (data not shown). As a positive control, PG13 (as well as the shorter ES10 peptide contained within PG13) was able to activate a Th clone derived from another individual (subject 161J) in the context of another MHC-II restricted allele, implying that the peptide could be processed, was able to bind MHC, and was not inherently inhibitory (Figure 1B).
Fig. 1. Lack of agonist activity by PG13.
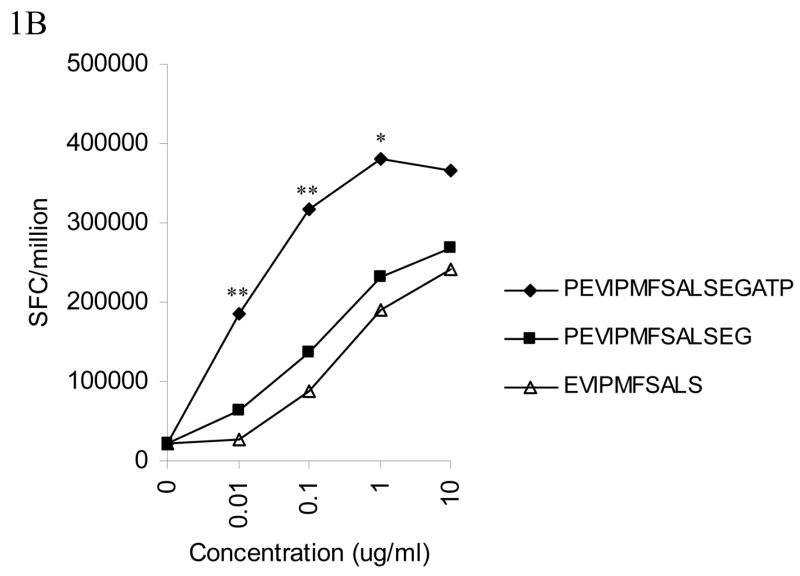
(A)The Th clone from AC-25 was stimulated with varied concentrations of PP16 or PG13. The left panel shows IFN-γ secretion in an intracellular cytokine staining assay with at least 50,000 events collected/condition. The middle panel shows proliferation by 3H-thymidine incorporation, performed in triplicate. The right panel shows serine esterase release in the supernatant 4 hours after stimulation. PP16 elicited responses while PG13 did not. Each of the experiments was repeated at least twice. (B) In a control experiment peptide PG13 was able to stimulate IFN-γ secretion measured by ELISPOT assay from a DR4 restricted clone from subject 161J to a comparable degree to the minimum epitope EVIPMFSALS for the 161J Th cell clone. The data represent the average of seven experiments. Responses to PG13 and EVIPMFSALS were not statistically significantly different (P > 0.05), while PP16 responses were greater: ** p < 0.05, PG13 and EVIPMFSALS vs. PP16; * p < 0.05, EVIPMFSALS vs. PP16.
3.2. TCR binding to PP16 and PG13 peptide-MHC complexes
The above data suggest that peptide PG13 binds the DR1 molecule but this complex cannot activate the cognate T cell. To test this, MHC-II tetramers were constructed consisting of the DR1 molecule bound to PP16 or PG13 (Cameron et al., 2002). Control tetramers were generated using the DR1 molecule and two tight-binding peptides, RVEYHFLSPYVSPKESP (TFR-RP17), a peptide from the transferrin receptor protein, and IPMFSALSEGATP (IP13) another non-activating subpeptide within PP16. These reagents allowed us to assess which portion of the 16 amino acid minimum epitope was essential for binding the MHC molecule and the TCR. The antagonist peptide PG13 bound both the MHC molecule and the TCR of the clone AC-25-1, while IP13 (in association with DR1) did not bind the TCR (Figure 2A). These data imply that the PG13-DR1 complex is able to bind the TCR but cannot activate the cells. A control clone from subject 161J was DR4 restricted and was not stained with any of the tetramers (data not shown).
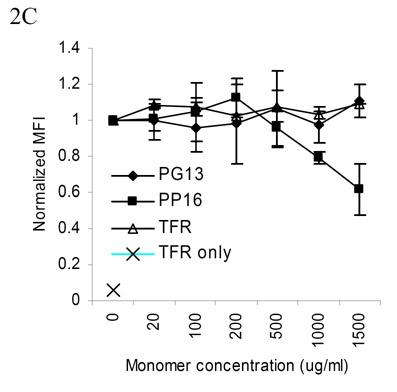
Fig. 2. Tetramer binding to live AC-25 CD4+ T cell clones.
(A) Upper panel: The DR1-tetramer with peptide PG13 but not IP13 or transferrin receptor (TFR) bound to AC-25 T cells. Lower panel: The PG13 tetramer did not bind a control DQ7 restricted clone specific for a different region of Gag. (B) Relative binding of PP16, PG13, and TFR-containing tetramers showing brightest staining with the PP16 tetramer, representative of two replicate experiments. All staining was performed at 25C for 1.5 hours. 50,000 events were collected per condition. MFI = Mean Fluorescence Intensity (C) Competitive inhibition of PG13 tetramer binding by unlabeled DR1-PP16 monomeric complex. AC-25 T cells were stained with low dose PG13 tetramer (40 μg/ml) and monomers were added as noted. TFR only = cells stained with negative control tetramer. Mean fluorescence intensity from two replicate experiments was normalized and averaged. Error bars represent the standard error.
In a second set of experiments we compared the relative binding avidities of the PP16 and PG13 peptide-MHC complexes to AC-25-1 clone TCR (Figure 2B). The PP16-DR1 tetramer bound with higher avidity than the PG13-DR1 tetramer, implying that tighter association of the peptide-MHC complex with the TCR was associated with the extent of T cell activation, consistent with prior work (Sykulev et al., 1994a; Sykulev et al., 1994b). We explored the relative binding affinity of the two peptide-MHC complexes by pre-incubating AC-25-1 T cells with monomeric, unlabeled DR1-peptide complexes followed by staining with low-dose (40 μg/ml) PG13-DR1 tetramer in the presence of the monomeric complexes (Figure 2C). Binding of the PG13-DR1 tetramer was blocked by high concentrations of PP16-DR1 monomer, but not a control TFR-RP17-DR1 monomer or PG13-DR1 monomer, implying higher affinity of the PP16 than the PG13 complex for the TCR. Similar experiments performed after staining with the PP16 tetramer showed that neither monomer could out-compete the PP16 tetramer over the range of monomer concentrations available (data not shown), again supporting higher affinity binding for the PP16-DR1 tetramer.
3.3. Antagonism of Th cell responses by peptide PG13
The ability of the shorter peptide PG13 to bind clone AC-25-1 TCR without inducing Th cell effector activity led us to examine whether the peptide could be an antagonist of T cell responses. Most antagonist peptides described to date have been altered from the native peptide sequence, but two prior reports have suggested antagonism by a peptide shorter than the full-length epitope (Carson et al., 1999; Matsushita and Matsuoka, 1999). We assayed the ability of PG13 to antagonize responses to PP16 in the presence of suboptimal concentrations of PP16. Peptide PG13 antagonized responses to PP16 and abrogated secretion of IFN-γ, proliferation, and serine esterase release (Figure 3). Originally PG13 was added to B cells pulsed for one hour with PP16 peptide and subsequently washed, though no difference in antagonism was seen if the B cells were not washed after pulsing with agonist peptide. These findings demonstrate antagonism by a peptide shorter than minimum length agonist peptides.
Fig. 3. Antagonism by PG13 peptide.
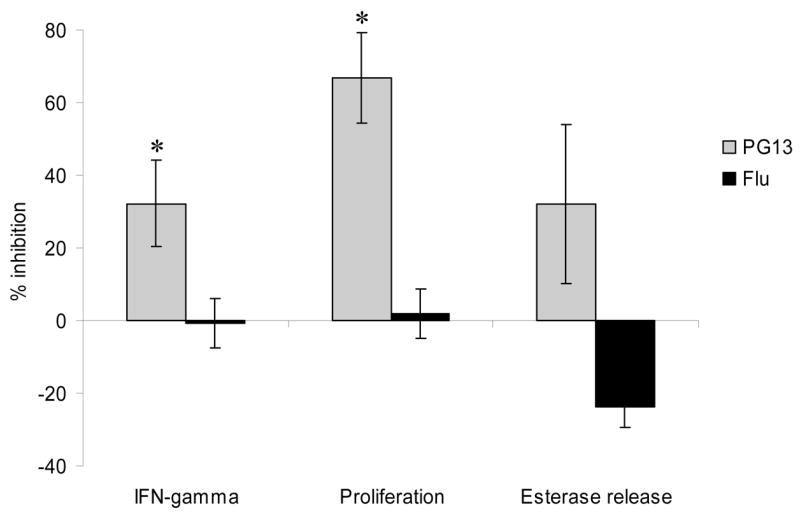
In all of the assays the AC-25 clone was stimulated with autologous B-LCL pulsed for one hour with a suboptimal concentration of agonist peptide PP16, 0.1 μg/ml for IFN-γ secretion and proliferation and 1.6 μg/ml for serine esterase release. Cells were then washed 10 μg/ml of antagonist peptide PG13 or control influenza matrix peptide PLKAEIAQRLEDV (Flu) was added. The control peptide binds DR1 but does not activate the HIV-specific clone. The left panel shows IFN-γ secretion in an intracellular cytokine staining assay with at least 50,000 events collected/condition. The middle panel shows proliferation by CFSE dilution. The right panel shows serine esterase release in the supernatant 4 hours after stimulation. All experiments were performed at least twice. * p < 0.05, PG13 vs. Flu peptide epitope.
3.4. Generation of full-length antagonist peptide
In the crystal structures, both PP16 and PG13 peptides were found to bind DR1 in the same register, with valine at P1, methionine at P4, and serine at P9 (PEVIPMFSALSEGATP) (Zavala-Ruiz et al., 2004). Interestingly, the glycine at P11 acted as a hinge to allow the C-terminal ATP to bend back around to contact the region where the TCR would rest (Figure 4, top panel). To test the importance of the four C-terminal residues we substituted alanine for each of the residues. All three of the substituted peptides showed decreased activity, particularly the T to A substitution (data not shown). We next generated a number of substitutions designed to disrupt the glycine hinge or to provide additional substitutions for the alanine and threonine. Disruption of the glycine hinge with the cyclic residue proline (PEVIPMFSASLEPATP, Figure 4, bottom panel) led to complete loss of activity in the full-length peptide (Zavala-Ruiz et al., 2004). Finally, the substituted peptides were tested for antagonist activity of IFN-γ secretion as described above. The engineered peptide with the G to P substitution not only lost agonist activity, but also became antagonistic (Figure 5). These results imply that the mechanism of antagonism of the shorter peptide depends on the antagonist’s ability to interact with portions of the TCR, but not all of the contact sites bound by the full-length PP16 peptide.
Fig. 4. Generation of full-length peptide lacking a glycine hinge.
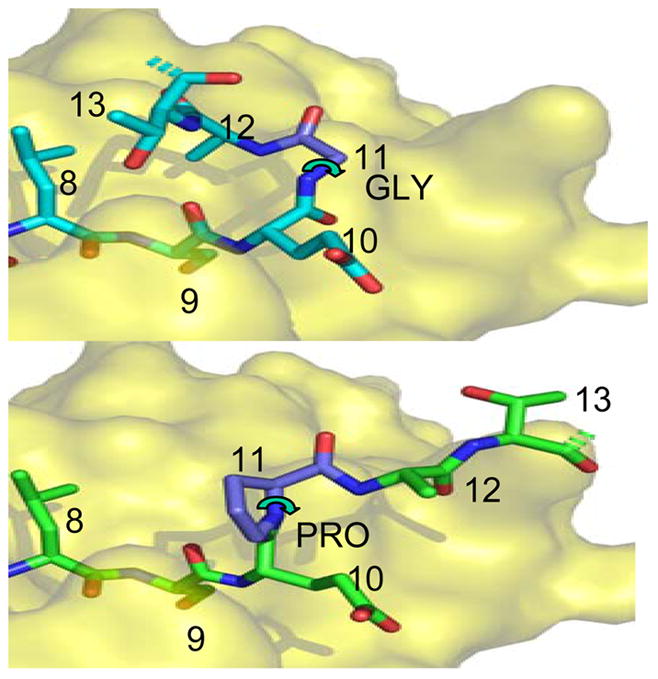
Top: Structure of native gag PP16 peptide bound to HLA-DR1, from the crystal structure of the complex (Zavala-Ruiz et al., 2004). The view shows the C-terminal region of the peptide binding site, viewed from above. The dashed bonds indicate the continuation of the peptide backbone to the terminal proline residue; this was not visualized in the crystal structure. The P9 residue serine is buried in the site, and the other residues are partially or completely exposed. The glycine at P11 allows the peptide backbone to bend sharply, forming a hairpin that orients the P13 residue threonine for interaction with TCR.
Bottom: Hypothetical model of the Pro11 peptide bound into the same site, with the proline at P11 indicated in a common conformation. Note that this peptide cannot assume the hairpin conformation shown above because the proline 5-membered ring severely constrains the phi angle (curved arrow) to ~60 degrees. This angle is free to rotate for glycine.
Fig. 5. Antagonist effects engineered peptide.
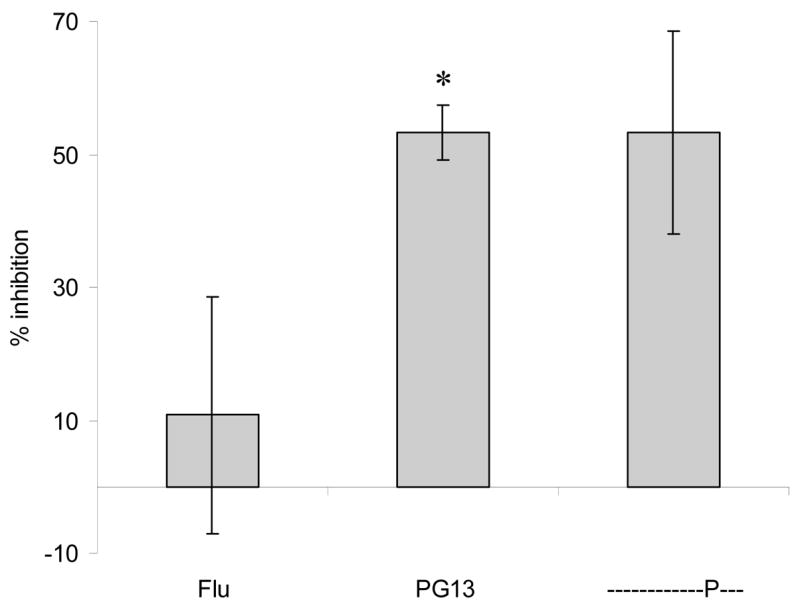
The full-length peptide with a glycine to proline substitution at P11 was tested for antagonist activity in an intracellular cytokine staining assay for IFN-γ secretion. Potential antagonist peptides (10 μg/ml) were added to B-LCL pre-pulsed with a suboptimal concentration of PP16 (0.1 μg/ml). At least 50,000 events were collected per condition, and the null DR1-binding influenza peptide (Flu) was included as a negative control in the antagonist assay. The data represent the average of three independent experiments. * p < 0.05, PG13 vs. Flu peptide epitope.
4. Discussion
We demonstrate an unusual mechanism of antagonism in CD4+ T cells utilizing a peptide shorter than the minimum epitope. The sixteen-residue minimal agonist epitope we studied was particularly long, even for Th cell epitopes, and possessed a unique glycine hinge near the C-terminus (Zavala-Ruiz et al., 2004). The shorter antagonist peptide still bound MHC with high affinity, and the corresponding MHC-peptide complex could bind the TCR, although with lower affinity than the longer, agonist peptide. Based on the crystal structure of the agonist and antagonist peptides with the DR1 molecule, we were able to engineer a full-length antagonist peptide by disrupting the glycine hinge found near the C-terminal portion of the full-length epitope. These findings further our understanding of the nature of antagonist-MHC interaction with the TCR and demonstrate a rarely reported mechanism of antagonism by peptides shorter than the agonist (Carson et al., 1999; Matsushita and Matsuoka, 1999).
The relative affinity of antagonist compared to agonist peptides has been reported to vary widely. Early work showed that strong antagonists could be generated from conservative substitutions of the agonist peptide (Alexander et al., 1993). Many studies demonstrate that antagonist peptides coupled to MHC have decreased affinity for the TCR relative to agonist peptides (Alam et al., 1996; Baker et al., 2000; Ding et al., 1999), while others show that antagonists can act at lower concentrations than the agonist (Sewell et al., 1997) or that antagonists coupled to MHC have a higher affinity for the TCR than a weak agonist (Sykulev et al., 1998). In the present study we used MHC-II tetramers to show that the antagonist peptide-MHC complex bound the TCR with lower affinity than the full-length agonist peptide-MHC, consistent with the majority of prior studies. It may be that the affinity of peptide-MHC for the TCR is not the lone factor determining antagonism. An early study analyzing on and off rates of peptide-MHC from TCR showed that a weaker agonist ligand had similar affinity to wild type peptide-MHC for the TCR but had a faster dissociation rate from the TCR (Matsui et al., 1994). More recent work has shown altered peptide ligands with very weak agonist activity in spite of higher affinity for the TCR than wild type peptide, again with a comparatively shorter half-life of association between peptide-MHC and the TCR (Kersh et al., 2001). Two studies suggest that antagonist peptides coupled to MHC can have slower off-rates from the TCR than agonist peptide-MHC complexes and that affinity was not a determining factor of antagonism (Alam et al., 1996; Kessler et al., 1997). While our work did not address the half-life of the peptide-MHC-TCR complex, the results do suggest that the antagonist peptide-MHC complex had lower affinity for the TCR than the agonist peptide-MHC complex. Thus, several mechanisms are likely to be responsible for antagonism. All of them could triggered by structural changes at the peptide/MHC/TCR interface that could be manifested into distinct patterns of membrane-associated and intracellular molecular events, all leading to a decreased ability of T cells to mount responses. These molecular events may involve differences in the positioning of α,β-TCR relative to CD3 and other accessory and co-stimulatory molecules and the formation of cellular junctions with altered structure that are translated to aberrant signaling leading to impaired T cell responses (Huang et al., 2000; Sumen et al., 2004; Zal et al., 2002).
Antagonists have been described to have multiple effects on T cells, selectively blocking some responses and sometimes behaving as partial agonists. Early studies showed induction of IL-4 secretion only or proliferation only by altered peptide ligands (Sloan-Lancaster et al., 1994; Soloway et al., 1991). Selective antagonism of effector functions such as IL-2 but not IL-3 secretion has been described (Racioppi et al., 1996). In our studies the antagonist peptide acted as a null peptide and did not cause significant effector function including proliferation, IFN-γ secretion, or serine esterase release. Antagonist peptides deliver a negative signal to responding T cells, though the point in the T cell activation pathway at which the signal is delivered is not yet clear. We observed no consistent difference in CD3 downregulation or CD69 upregulation after stimulation with agonist or antagonist peptides. The mechanism of antagonism for the shorter antagonist peptide appears similar to that of altered peptide ligands in two ways. Based on the crystal structure of the PG13 peptide and MHC, conformational changes did occur in the binding (Zavala-Ruiz et al., 2004), and relatively small conformational changes have been previously described in the binding of antagonist peptides (Ding et al., 1999; Reid et al., 1996). Additionally, truncation of the agonist peptide caused loss of a likely TCR contact residue (the penultimate threonine), and loss of TCR contact residues has been described with altered peptide ligand antagonists (Achour et al., 2002).
In summary, our findings demonstrate Th cell antagonism by antagonist peptide shorter than the minimum length epitope. The minimum epitope is unusual in that it employs a complex folded structure for TCR engagement. Using MHC class II tetramers we showed that the antagonist-MHC complex binds the TCR with lower affinity than the agonist-MHC. Further analysis of the thermodynamic binding properties of the agonist and antagonist peptide and of the activation state of the T cell will provide information on the mechanisms of antagonism and shed light on a potentially important obstacle to HIV vaccine development.
Acknowledgments
We thank Tina Nguyen for expert assistance in preparing Figure 4, the model of peptide binding to MHC. The following reagent was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: IL-2, catalog # 136, from Hoffman-La Roche, Inc. This work was supported by the National Institutes of Health grants AI-01698 and RO1 28568. PJN and BDW were supported by the Doris Duke Charitable Foundation.
References
- Achour A, Michaelsson J, Harris RA, Odeberg J, Grufman P, Sandberg JK, Levitsky V, Karre K, Sandalova T, Schneider G. A structural basis for LCMV immune evasion: subversion of H-2D(b) and H-2K(b) presentation of gp33 revealed by comparative crystal structure. Analyses. Immunity. 2002;17:757–68. doi: 10.1016/s1074-7613(02)00478-8. [DOI] [PubMed] [Google Scholar]
- Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–20. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- Alexander J, Snoke K, Ruppert J, Sidney J, Wall M, Southwood S, Oseroff C, Arrhenius T, Gaeta FC, Colon SM, et al. Functional consequences of engagement of the T cell receptor by low affinity ligands. J Immunol. 1993;150:1–7. [PubMed] [Google Scholar]
- Bachmann MF, Speiser DE, Zakarian A, Ohashi PS. Inhibition of TCR triggering by a spectrum of altered peptide ligands suggests the mechanism for TCR antagonism. Eur J Immunol. 1998;28:3110–9. doi: 10.1002/(SICI)1521-4141(199810)28:10<3110::AID-IMMU3110>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Baker BM, Gagnon SJ, Biddison WE, Wiley DC. Conversion of a T cell antagonist into an agonist by repairing a defect in the TCR/peptide/MHC interface: implications for TCR signaling. Immunity. 2000;13:475–84. doi: 10.1016/s1074-7613(00)00047-9. [DOI] [PubMed] [Google Scholar]
- Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–37. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- Cameron TO, Norris PJ, Patel A, Moulon C, Rosenberg ES, Mellins ED, Wedderburn LR, Stern LJ. Labeling antigen-specific CD4(+) T cells with class II MHC oligomers. J Immunol Methods. 2002;268:51–69. doi: 10.1016/s0022-1759(02)00200-4. [DOI] [PubMed] [Google Scholar]
- Carson RT, Desai DD, Vignali KM, Vignali DA. Immunoregulation of Th cells by naturally processed peptide antagonists. J Immunol. 1999;162:1–4. [PubMed] [Google Scholar]
- Chung CD, Patel VP, Moran M, Lewis LA, Miceli MC. Galectin-1 induces partial TCR zeta-chain phosphorylation and antagonizes processive TCR signal transduction. J Immunol. 2000;165:3722–9. doi: 10.4049/jimmunol.165.7.3722. [DOI] [PubMed] [Google Scholar]
- Daniels MA, Schober SL, Hogquist KA, Jameson SC. Cutting edge: a test of the dominant negative signal model for TCR antagonism. J Immunol. 1999;162:3761–4. [PubMed] [Google Scholar]
- De Magistris MT, Alexander J, Coggeshall M, Altman A, Gaeta FC, Grey HM, Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68:625–34. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- Ding YH, Baker BM, Garboczi DN, Biddison WE, Wiley DC. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11:45–56. doi: 10.1016/s1074-7613(00)80080-1. [DOI] [PubMed] [Google Scholar]
- Dittel BN, Stefanova I, Germain RN, Janeway CA., Jr Cross-antagonism of a T cell clone expressing two distinct T cell receptors. Immunity. 1999;11:289–98. doi: 10.1016/s1074-7613(00)80104-1. [DOI] [PubMed] [Google Scholar]
- Fernandez MH, Fidler SJ, Pitman RJ, Weber JN, Rees AD. CD4+ T-cell recognition of diverse clade B HIV-1 isolates. Aids. 1997;11:281–8. doi: 10.1097/00002030-199703110-00004. [DOI] [PubMed] [Google Scholar]
- Gebe JA, Masewicz SA, Kochik SA, Reijonen H, Nepom GT. Inhibition of altered peptide ligand-mediated antagonism of human GAD65-responsive CD4+ T cells by non-antagonizable T cells. Eur J Immunol. 2004;34:3337–45. doi: 10.1002/eji.200425535. [DOI] [PubMed] [Google Scholar]
- Harcourt GC, Garrard S, Davenport MP, Edwards A, Phillips RE. HIV-1 variation diminishes CD4 T lymphocyte recognition. J Exp Med. 1998;188:1785–93. doi: 10.1084/jem.188.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Sugie K, La Face DM, Altman A, Grey HM. TCR antagonist peptides induce formation of APC-T cell conjugates and activate a Rac signaling pathway. Eur J Immunol. 2000;30:50–8. doi: 10.1002/1521-4141(200001)30:1<50::AID-IMMU50>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kent SJ, Greenberg PD, Hoffman MC, Akridge RE, McElrath MJ. Antagonism of vaccine-induced HIV-1-specific CD4+ T cells by primary HIV-1 infection: potential mechanism of vaccine failure. Journal of Immunology. 1997;158:807–15. [PubMed] [Google Scholar]
- Kersh EN, Kersh GJ, Allen PM. Partially phosphorylated T cell receptor zeta molecules can inhibit T cell activation. J Exp Med. 1999;190:1627–36. doi: 10.1084/jem.190.11.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh GJ, Miley MJ, Nelson CA, Grakoui A, Horvath S, Donermeyer DL, Kappler J, Allen PM, Fremont DH. Structural and functional consequences of altering a peptide MHC anchor residue. J Immunol. 2001;166:3345–54. doi: 10.4049/jimmunol.166.5.3345. [DOI] [PubMed] [Google Scholar]
- Kessler B, Hudrisier D, Cerottini JC, Luescher IF. Role of CD8 in aberrant function of cytotoxic T lymphocytes. J Exp Med. 1997;186:2033–8. doi: 10.1084/jem.186.12.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore NE, Carter JD, Lorenz U, Evavold BD. Cutting edge: dependence of TCR antagonism on Src homology 2 domain-containing protein tyrosine phosphatase activity. J Immunol. 2003;170:4891–5. doi: 10.4049/jimmunol.170.10.4891. [DOI] [PubMed] [Google Scholar]
- Kissler S, Anderton SM, Wraith DC. Cross-reactivity and T-cell receptor antagonism of myelin basic protein-reactive T cells is modulated by the activation state of the antigen presenting cell. J Autoimmun. 2002;19:183–93. doi: 10.1006/jaut.2002.0614. [DOI] [PubMed] [Google Scholar]
- Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Koppe B, Rosenberg W, Boyd D, Edwards A. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants [see comments] Nature. 1994;369:403–7. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- Matsui K, Boniface JJ, Steffner P, Reay PA, Davis MM. Kinetics of T-cell receptor binding to peptide/I-Ek complexes: correlation of the dissociation rate with T-cell responsiveness. Proc Natl Acad Sci U S A. 1994;91:12862–6. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita S, Matsuoka T. Peptide length-dependent TCR antagonism on class II HLA-restricted responses of peripheral blood mononuclear cells and T cell clones. Eur J Immunol. 1999;29:431–6. doi: 10.1002/(SICI)1521-4141(199902)29:02<431::AID-IMMU431>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Norris PJ, Moffett HF, Brander C, Allen TM, O'Sullivan KM, Cosimi LA, Kaufmann DE, Walker BD, Rosenberg ES. Fine specificity and cross-clade reactivity of HIV-1 Gag-specific CD4+ T cells. AIDS Res Hum Retroviruses. 2004;20:315–25. doi: 10.1089/088922204322996554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris PJ, Rosenberg ES. CD4(+) T helper cells and the role they play in viral control. J Mol Med. 2002;80:397–405. doi: 10.1007/s00109-002-0337-3. [DOI] [PubMed] [Google Scholar]
- Norris PJ, Sumaroka M, Brander C, Moffett HF, Boswell SL, Nguyen T, Sykulev Y, Walker BD, Rosenberg ES. Multiple effector functions mediated by human immunodeficiency virus-specific cd4(+) t-cell clones. J Virol. 2001;75:9771–9. doi: 10.1128/JVI.75.20.9771-9779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrov D, Krieger J, Sidney J, Sette A, Concannon P. T cell receptor antagonism mediated by interaction between T cell receptor junctional residues and peptide antigen analogues. J Immunol. 1993;150:4277–83. [PubMed] [Google Scholar]
- Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression [see comments] Nature Medicine. 1999;5:518–25. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JD, Beeson C, Lyons DS, Davis MM, McConnell HM. Kinetic discrimination in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:1401–5. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racioppi L, Matarese G, D'Oro U, De Pascale M, Masci AM, Fontana S, Zappacosta S. The role of CD4-Lck in T-cell receptor antagonism: evidence for negative signaling. Proc Natl Acad Sci U S A. 1996;93:10360–5. doi: 10.1073/pnas.93.19.10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racioppi L, Ronchese F, Matis LA, Germain RN. Peptide-major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand-related differences in T cell receptor-dependent intracellular signaling. J Exp Med. 1993;177:1047–60. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SW, McAdam S, Smith KJ, Klenerman P, O'Callaghan CA, Harlos K, Jakobsen BK, McMichael AJ, Bell JI, Stuart DI, Jones EY. Antagonist HIV-1 Gag peptides induce structural changes in HLA B8. J Exp Med. 1996;184:2279–86. doi: 10.1084/jem.184.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz EL, Tan K, Tang L, Kern P, Liu J, Xiong Y, Hussey RE, Smolyar A, Hare B, Zhang R, Joachimiak A, Chang HC, Wagner G, Wang J. The crystal structure of a T cell receptor in complex with peptide and MHC class II [see comments] Science. 1999;286:1913–21. doi: 10.1126/science.286.5446.1913. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C, Levine EH, Germain RN. Partial signaling by CD8+ T cells in response to antagonist ligands. J Exp Med. 1996;184:149–57. doi: 10.1084/jem.184.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Evavold BD. Cutting edge: dueling TCRs: peptide antagonism of CD4+ T cells with dual antigen specificities. J Immunol. 1999;163:1750–4. [PubMed] [Google Scholar]
- Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia [see comments] Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- Rudolph MG, Shen LQ, Lamontagne SA, Luz JG, Delaney JR, Ge Q, Cho BK, Palliser D, McKinley CA, Chen J, Wilson IA, Eisen HN. A peptide that antagonizes TCR-mediated reactions with both syngeneic and allogeneic agonists: functional and structural aspects. J Immunol. 2004;172:2994–3002. doi: 10.4049/jimmunol.172.5.2994. [DOI] [PubMed] [Google Scholar]
- Sewell AK, Harcourt GC, Goulder PJ, Price DA, Phillips RE. Antagonism of cytotoxic T lymphocyte-mediated lysis by natural HIV-1 altered peptide ligands requires simultaneous presentation of agonist and antagonist peptides. Eur J Immunol. 1997;27:2323–9. doi: 10.1002/eji.1830270929. [DOI] [PubMed] [Google Scholar]
- Siliciano RF, Lawton T, Knall C, Karr RW, Berman P, Gregory T, Reinherz EL. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988;54:561–75. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Evavold BD, Allen PM. Th2 cell clonal anergy as a consequence of partial activation. J Exp Med. 1994;180:1195–205. doi: 10.1084/jem.180.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloway P, Fish S, Passmore H, Gefter M, Coffee R, Manser T. Regulation of the immune response to peptide antigens: differential induction of immediate-type hypersensitivity and T cell proliferation due to changes in either peptide structure or major histocompatibility complex haplotype. J Exp Med. 1991;174:847–58. doi: 10.1084/jem.174.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–54. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- Stotz SH, Bolliger L, Carbone FR, Palmer E. T cell receptor (TCR) antagonism without a negative signal: evidence from T cell hybridomas expressing two independent TCRs. J Exp Med. 1999;189:253–64. doi: 10.1084/jem.189.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumen C, Dustin ML, Davis MM. T cell receptor antagonism interferes with MHC clustering and integrin patterning during immunological synapse formation. J Cell Biol. 2004;166:579–90. doi: 10.1083/jcb.200404059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykulev Y, Brunmark A, Jackson M, Cohen RJ, Peterson PA, Eisen HN. Kinetics and affinity of reactions between an antigen-specific T cell receptor and peptide-MHC complexes. Immunity. 1994a;1:15–22. doi: 10.1016/1074-7613(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Sykulev Y, Brunmark A, Tsomides TJ, Kageyama S, Jackson M, Peterson PA, Eisen HN. High-affinity reactions between antigen-specific T-cell receptors and peptides associated with allogeneic and syngeneic major histocompatibility complex class I proteins. Proc Natl Acad Sci U S A. 1994b;91:11487–91. doi: 10.1073/pnas.91.24.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykulev Y, Vugmeyster Y, Brunmark A, Ploegh HL, Eisen HN. Peptide antagonism and T cell receptor interactions with peptide-MHC complexes. Immunity. 1998;9:475–83. doi: 10.1016/s1074-7613(00)80631-7. [DOI] [PubMed] [Google Scholar]
- Yang W, Grey HM. Study of the mechanism of TCR antagonism using dual-TCR-expressing T cells. J Immunol. 2003;170:4532–8. doi: 10.4049/jimmunol.170.9.4532. [DOI] [PubMed] [Google Scholar]
- Zal T, Zal MA, Gascoigne NR. Inhibition of T cell receptor-coreceptor interactions by antagonist ligands visualized by live FRET imaging of the T-hybridoma immunological synapse. Immunity. 2002;16:521–34. doi: 10.1016/s1074-7613(02)00301-1. [DOI] [PubMed] [Google Scholar]
- Zavala-Ruiz Z, Strug I, Walker BD, Norris PJ, Stern LJ. A hairpin turn in a class II MHC-bound peptide orients residues outside the binding groove for T cell recognition. Proc Natl Acad Sci U S A. 2004;101:13279–84. doi: 10.1073/pnas.0403371101. [DOI] [PMC free article] [PubMed] [Google Scholar]