Abstract
Although heat shock proteins have been studied for decades, new intracellular and extracellular functions in a variety of diseases continue to be discovered. Heat shock proteins function within networks of interacting proteins; they can alter cellular physiology rapidly in response to stress without requiring new protein synthesis. This review will focus on the heat shock protein 70 family and consider especially the functions of the inducible member, heat shock protein 72, in the setting of cerebral ischemia. In general, inhibiting apoptotic signalling at multiple points and upregulating survival signaling, heat shock protein 70 has a net pro-survival effect. Heat shock protein 70 has both anti-inflammatory and pro-inflammatory effects depending on the cell type, context, and intracellular or extracellular location. Intracellular effects are often anti-inflammatory with inhibition of Nuclear Factor κB signaling. Extracellular effects can lead to inflammatory cytokine production or induction of regulatory immune cells and reduced inflammation.
Brief Summary
Heat shock protein 70 is induced in cells by stress, but is also released from cells. Intracellular and extracellular heat shock protein 70 have distinct roles as survival proteins and modulators of the immune response.
Introduction
Heat shock proteins (HSP), also called stress proteins, are induced by specific types of stress including heat, and they are highly conserved from bacteria to man.1-4 The HSP70 family facilitates the folding of newly synthesized polypeptides in an adenosine triphosphate (ATP)-dependent manner, plays an important role in maintaining the dynamic stability of protein folding and protein-protein interactions within the cell, and inhibits protein aggregation. 5,6 These are referred to collectively as chaperone functions. By interacting with a range of cochaperones and client proteins, both constitutive and inducible HSPs regulate the functioning of other proteins and indeed whole signaling cascades. These interactions allow a cell to rapidly respond to stresses and changes in its environment without requiring protein synthesis, though induction of stress protein synthesis provides the next line of response. HSPs are divided into families on the basis of molecular weight. HSPs that are present as a single copy in bacteria (e.g., dna K), are generally represented by multiple related genes in eukaryotes (e.g., HSP70 family).
HSP70 family members have long been recognized to have cytoprotective effects. The human HSP70 family consists of at least 12 members.7 The best known members are the heat inducible form Hsp70/Hsp72, the constitutively expressed Hsc70/Hsp73/Hsc73, the endoplasmic reticulum form, Grp78/BiP, and Hsp75/mtHsp70/mortalin that is localized largely to mitochondria. Of these, the cytosolic inducible Hsp72 plays a major role in mediating cytoprotective, antiapoptotic, and immune regulatory effects, and is by far the best studied. Enhanced expression of Hsp72 in experimental models of stroke, sepsis, acute respiratory distress syndrome, renal failure, and myocardial ischemia, has been shown to reduce organ injury and in some cases improve survival.8-11 Deletion of the hsp70.1/3 gene is associated with poorer outcome in mice.12 In addition to their intracellular protective and antiapoptotic role, HSPs also function as extracellular signals.13 We will use HSP70 to refer to the entire family, and Hsp70 in instances where either Hsp72 or 73 is referred to, as some reports and some antibodies do not distinguish between these two cytosolic family members, though the majority of studies focus on the stress inducible Hsp72.
Clinical studies have begun to identify correlations between Hsp70 and outcome in a variety of diseases. A reduced ability to induce Hsp72 in peripheral lymphocytes was noted in patients with sepsis.14 Higher serum Hsp72 levels correlated with improved survival after trauma 15 and severe sepsis.16,17 Several studies have evaluated Hsp70 expression after myocardial infarction and cardiac surgery with bypass and found significant increases in Hsp70 expression in all cases.18-20 Thus increased levels of Hsp70 can indicate tissue damage, but they may also indicate the successful mounting of a stress response that correlates with tissue protection and better outcome.21 Hsp70 appears to participate in protection against organ dysfunction both in critically ill patients and in patients during the perioperative period. Overexpression by gene therapy or chemical induction of a stress response is under investigation as a potential treatment for ischemia in several organ systems, including the use of glutamine to increase Hsp70 in critically ill patients.17,22 We will focus primarily on data from cerebral ischemia in this review.
Hsp72 in Cell Death Signaling Pathways in Cerebral Ischemia
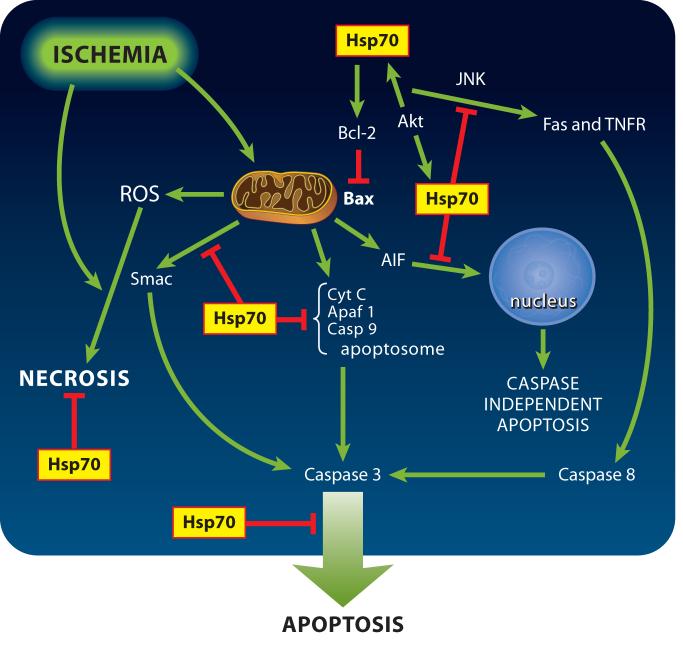
Hsp72 has been shown to provide neuroprotection from cerebral ischemia in animal and cell-culture models of stroke.10,23-25 While the mechanism of this protection was initially attributed to chaperone functions (i.e.; maintaining correct protein folding and blocking aggregation), recent work has shown that Hsp72 may also directly interfere with cell death pathways such as apoptosis and necrosis (see Figure 1), and may modulate inflammation.10,26-34
Figure 1.
Ischemia induces cell death by several distinct pathways, and Hsp70 reduces all of these. Arrows indicate increased activity or amount while the barred ends indicate steps that are blocked or reduced when Hsp70 is overexpressed. These do not indicate direct protein-protein interactions in many cases, see text for details. Fas and the Tumor Necrosis Factor Receptor (TNFR) are transmembrane receptors with intracellular death domains that can induce apoptosis by activating caspase-8 via a pathway including Tumor Necrosis Factor Associated Factor (TRAF). Cyt C = cytochrome c, casp = caspase, JNK = c Jun N terminal Kinase, Akt=PKB, Apaf-1=apoptosis protease activating factor 1, ROS=reactive oxygen species, AIF=apoptosis inducing factor; Smac/DIABLO is a mitochondrial protein which upon release neutralizes the caspase inhibitory effects of inhibitor of apoptosis family proteins. Bcl-2 is an anti-apoptotic protein, Bax a proapoptotic member of the same family.
Programmed cell death occurs by multiple pathways. Apoptosis occurs primarily by one of two pathways.35 The intrinsic pathway responds to stress and intracellular changes; it relies on the release of mitochondrial pro-apoptotic molecules, opening of the mitochondrial permeability transition pore, and activation of caspases.36 The second well described pathway is the extrinsic pathway, which is triggered by the activation of plasma membrane receptors which then signal through their death domains. This signaling activates caspase-8 and can proceed independently of the intrinsic pathway, but it can also lead to activation of the intrinsic pathway.37 In addition, caspase independent forms of cell death have been described,38,39 and depletion of Hsp70 can trigger caspase independent cell death in cancer cells.40,41
Hsp72 reduces mitochondria-dependent apoptosis signaling
Mitochondria are central to both necrotic and apoptotic cell death; the pathway followed often depends on the severity of the insult.42 The resulting death reflects the signaling cascade activated by the stress or apoptotic stimulus.43-46 In most instances, severe cerebral ischemia rapidly renders mitochondria unable to produce ATP, which ensures necrotic cell death. Mitochondrial alterations that occur during both global and focal cerebral ischemia and contribute to cell death include changes in mitochondrial respiratory function,47,48 production of reactive oxygen species,49,50 changes in mitochondrial membrane potential and permeability,51,52 and release of regulatory and signaling molecules from the mitochondrial intermembrane space.53
Activation of the intrinsic mitochondrial pathway in ischemic brain has been demonstrated in both neonatal and adult models by the release of mitochondrial cytochrome c.46,52,54 Cytochrome c translocates from the mitochondria to the cytosol, where it interacts with the CED-4 homologue, apoptosis protease activating factor-1, and dATP, to form the apoptosome and activate caspase-9.35,36 Caspase-9 activates caspase-3, one of the executioner caspases, as well as caspases-2, -6, -8, and -10.55 Caspase-3 also activates caspase-activated DNase, which fragments DNA. In cerebral ischemia, caspases-3 and -9 have been shown to play a key role in neuronal death after both global ischemia56,57 and focal ischemia,58-61 with caspase-3 dependent apoptosis more prominent in neonatal ischemia than adult, and more prominent in global than focal ischemia. In cerebral ischemia the downstream caspases cleave many substrate proteins, including poly(ADP-ribose) polymerase (PARP).56,57,62 With cleavage of multiple targets within the cell and DNA fragmentation, apoptotic cell death results.63-67
Hsp70 affects several different steps in the apoptosis cascade (see Figure 1). Hsp72 interacts with components of the programmed cell death machinery upstream 68,69 and potentially downstream70 of mitochondrial events. Hsp72 can inhibit cytochrome c release in both neonatal and adult ischemia,54,71,72 and inhibit apoptosis inducing factor (AIF) translocation to the nucleus34,73 while reducing ischemic brain injury in both adult and neonatal models. Several of the studies on effects of Hsp72 in cerebral ischemia have been performed in transgenic mice overexpressing this gene These findings in cerebral ischemia are consistent with observations in other systems where Hsp72 has been shown to interfere with recruitment of procaspase-9 into the apoptosome, and to sequester AIF.74 Hsp72 also inhibited release of the proapoptotic protein Smac/DIABLO from myocyte mitochondria.75
Mitochondrial Hsp70/Hsp75/mortalin helps maintain mitochondrial membrane potential, which may contribute to the preservation of mitochondrial function76 and mitochondrial protein import.77,78 Several authors have postulated an involvement of Hsp75 in preventing electron leak between complexes III and IV, by binding and consequently reducing cytochrome c loss from mitochondrial membranes, thereby averting an increase in state IV respiration rates and induction of cytochrome c-linked apoptosis.79 Overexpression of Hsp75 in astrocytes reduced their vulnerability to oxygen glucose deprivation, an in vitro model of ischemia, and maintained higher ATP levels in stressed cells.80 Overexpression of Hsp72 in astrocytes was associated with reduced reactive oxygen species formation and better maintained mitochondrial membrane potential following ischemia in vitro81 and with better preservation of glutathione levels.27 In myocardial cells, overexpression of Hsp72 was shown to increase the activity of the mitochondrial antioxidant enzyme manganese superoxide dismutase.82
Hsp72 and the Bcl-2 family regulators of apoptosis
Viral vector-mediated Hsp72 overexpression was associated with increased levels of Bcl-2 protein in brain cells.83 Bcl-2 is a key anti-apoptotic protein; its increased expression blocks release of cytochrome c and AIF, and reduces caspase activation. The balance between pro- and anti-apoptotic members of the large Bcl-2 family determine whether cells undergo apoptosis by regulating the mitochondrial membrane permeability transition.84,85 Transgenic overexpression of Bcl-2 decreased infarction after focal cerebral ischemia,86 whereas, Bcl-2 knockout mice had increased infarct area..87 Thus increased Hsp72 expression can reduce induction of apoptosis upstream of mitochondria in cerebral ischemia both directly and via increased Bcl-2 levels. Hsp72 blocks heat-induced apoptosis primarily by inhibiting translocation of the proapoptotic Bcl-2 family member Bax, thereby preventing the release of proapoptotic factors from mitochondria.69 Hsp72 also interferes with the activity of apoptosis protease activating factor-1 (Apaf-1), which is required for formation of the apoptosome and activation of caspase-9 54,74,88 but also see Steel et al. who demonstrated lack of direct interaction with apoptosis protease activating factor-1 68.
Hsp72 and regulation of transcription factors in cell death signaling
Hsp72 interacts with pathways leading to activation of transcription factors important in regulating cell death. Hsp72 has been shown to inhibit c-Jun N-terminal kinase (JNK) dephosphorylation thereby blocking its activation.89-91 Activated JNK phosphorylates the transcription factor c-JUN to upregulate a specific group of proteins.91 JNK activation plays both direct and indirect roles in neuronal apoptosis 92 and it is a proposed target for stroke therapy.93,94 JNK is implicated in apoptosis triggered by Fas, a member of the TNF superfamily of membrane receptors,95 as well as figuring prominently in the apoptosis of neurons induced by growth factor withdrawal.92 JNK is one of the mitogen-activated protein kinases. These kinases constitute one of the central signaling pathways in intracellular response,96 often determining whether a cell responds with apoptosis or differentiation and survival. JNK signaling in the nervous system is not solely for promoting apoptosis. There is a high level of basal JNK signaling activity in the nervous system compared to other tissues, suggesting normal physiological functions.94 Increasing evidence suggests that the downstream events of JNK activation leading to apoptosis involve both transcription97,98 and mitochondrial mechanisms.92,93
In ischemic stroke, increased c-JUN phosphorylation co-localized with TUNEL-labeling in the penumbral area in an experimental model of focal ischemia.99 Subsequent studies showed that Jnk3-deficient mice have increased resistance to global ischemia-hypoxia.94 JNK3 deficiency causes reduced Bim and Fas expression after stroke, and Jnk3-null hippocampal neurons released less cytochrome c following oxygen-glucose deprivation.94 Furthermore, mice lacking the JNK signaling scaffold protein JIP1 have increased resistance to glutamate excitotoxicity 100 and reduced infarct volume in a focal ischemia model of stroke.101 These studies suggest that JNK signaling may play an important role in determining cell death or survival for neurons-at-risk in the ischemic penumbra.
Hsp72 also interacts with topoisomerase 1, which is also implicated as a regulator of apoptosis.102,103 These interactions were shown to be independent of the ATP binding domain.102 Hsp72 is also an effector for the important antiapoptotic prosurvival kinase Akt/PKB,104,105 and acts upstream of the transcription factor nuclear factor κB (NFκB) reducing its activation, as discussed below.
Hsp72 and Inflammation
Hsp72 also plays a role in modulating inflammation caused by cerebral ischemia. Inflammation can contribute to the damage resulting from stroke.106-109 Inflammatory responses include the activation of resident microglia and astrocytes, as well as recruitment of peripheral inflammatory cells. Inflammation and the concomitant release of reactive oxygen species and reactive nitrogen species by inflammatory cells exacerbate damage caused by direct ischemic production of reactive oxygen species. Blocking the neutrophil integrin CD11/CD18 with an antibody reduced injury in focal ischemia in association with a marked reduction of neutrophil infiltration.110 Recruitment of peripheral leukocytes weakens the blood brain barrier, leading to further damage. HSP70 family members play a crucial role in modulating these responses.33,111
Hsp72 and inflammatory cytokines
Intracellular Hsp72 has a range of anti-inflammatory actions. It can prevent responses to inflammatory cytokines such as tumor necrosis factor alpha (TNF) and interleukin-1 (IL-1). Mice subjected to heat shock are protected from normally lethal inflammatory shock after systemic administration of high doses of TNF, whereas mice missing the hsp70.1 gene are no longer protected.112 Liposomally delivered Hsp72 protein protected rats from IL-1-induced impaired pancreatic beta cell function in a diabetes model.113 However, such protection from inflammatory responses may come at a price, as Hsp72 can actually make cells more liable to undergo apoptosis in response to TNF.114,115 In addition to modulating the response to inflammatory cytokines, Hsp72 also down regulates their production (see Figure 2). Overexpression of Hsp72 in human macrophages blocked LPS-induced increases in the production of TNF, IL-1, IL-10 and IL-12.116 In the setting of focal cerebral ischemia overexpression of Hsp72 was associated with reduced production of TNF and IL-1b,111 likely a reflection of reduced NFκB activation.
Figure 2.

Intracellular Hsp70 blocks activation of the transcription factor NFκB reducing production of downstream inflammatory mediators. Three mechanisms have been described, inhibition of activation of IKK, inhibition of ubiquitination of TRAF6 and stabilization of the inhibitory complex with IκB. TRAF=Tumor Necrosis Factor Associated Factor 6; ubi=ubiquitination; IKK= IκB kinase; IκB= inhibitor of NFκB, p50-p65 are two of the NFκB subunits which after release move to the nucleus to act as a transcription factor resulting in activation of inflammatory genes. MMP=matrix metalloproteinase, iNOS=inducible nitric oxide synthase. The Y shaped bracket from Hsp70 to the IκB:NFκB complex is meant to indicate binding and stabilization of the complex.
Hsp72, iNOS, NADPH oxidase, and matrix metalloproteinases
Hsp72 may limit production of reactive oxygen species via several routes. Inflammation leads to the production of reactive oxygen species by activation of both the inducible form of nitric oxide synthase (iNOS) and NADPH oxidase. Induction of iNOS occurs in response to cytokine release.117 Mice lacking the iNOS gene are protected from cerebral ischemia relative to wild-type mice. At high levels of production, nitric oxide reacts with superoxide to produce the highly toxic strong oxidant, peroxynitrite.117 However, iNOS can be beneficial in facilitating neurogenesis in ischemia.118,119 Hsp72 suppresses iNOS activation in glial cells exposed to bacterial lipopolysaccharide.120
NADPH oxidase is one source of superoxide induced by inflammation. NADPH oxidase produces the oxidative burst of phagocytic leukocytes.121 Recent work suggests that it may be activated in neurons as well as in microglia. That neuronal NADPH oxidase plays a role in aging and hypoglycemic injury was also suggested.122,123 Heat shock induction of Hsp72 reduces NADPH oxidase activity in neutrophils and increases superoxide dismutase, which scavenges superoxide, in phagocytes.124,125 Hsp72 has also been linked to regulation of matrix metalloproteinases. Matrix metalloproteinases are involved in remodeling of the extracellular matrix; they are associated with breakdown of the blood brain barrier and hemorrhage following cerebral ischemia.126 Hsp72 overexpressing astrocyte cultures downregulated matrix metalloproteinase-9 after oxygen glucose deprivation, compared to wild-type cell cultures,127 consistent with involvement of Hsp72 in regulation of this aspect of inflammation.
Hsp72 and NFκB
Much, if not most, of intracellular Hsp72’s modulatory effects on inflammation can be attributed to its regulation of the nuclear factor κB (NFκB) pathway (see Figure 2). Transcription factors of the NFκB family are key players in the initiation of the inflammatory response.128 NFκB is comprised of four related proteins that function as dimers. The most well studied of these is the p50/p65 heterodimer that is normally sequestered in the cytoplasm by its interaction with Inhibitor of κB (IκB). Phosphorylation of IκB by the IκB kinase complex (IKK) leads to ubiquitination and degradation of IκB, freeing the NFκB dimer to translocate to the nucleus, where it induces the expression of a multitude of genes involved in inflammatory and immune responses, including TNF, IL-1, iNOS and matrix metalloproteinase-9.128,129
Induction of Hsp72 inhibits the nuclear translocation of NFκB in response to inflammatory cytokines or other stimuli.130 Mice overexpressing Hsp72 showed reduced NFκB activation following stroke.111 This reduced activation may be accomplished through direct interaction of Hsp72 with NFκB proteins, or by interactions with other proteins in the NFκB regulatory pathway. Guzhova et al. 130 were able to coimmunoprecipitate Hsp72 with three members of the NFκB family (p65, p50, and c-Rel) after heat shock. IκB, however did not coprecipitate. Feinstein et al 120 demonstrated that heat shock or Hsp72 expression decreased the accumulation of NFκB p65 in the nucleus. Wong et al 131 found that heat shock prevented degradation of IκB, thereby preventing activation of NFκB. Later studies identified interactions between Hsp72 and the gamma subunit of the IKK complex.114 Hsp72 may also interact directly with upstream inducers of the NFκB pathway. Another recent study found that Hsp72 directly associated with the IκB-NFκB complex and suggested stabilization of the complex as another mechanism.111 Chen et al 132 found a direct interaction between Hsp72 and tumor necrosis factor receptor-associated protein 6 (TRAF6). Ubiquitination of TRAF6 is a crucial step in the activation of the NFκB pathway by bacterial lipopolysaccharide and IL-1.133-135 Hsp72 prevents this ubiquitination, which in turn prevents activation of the IKK complex. It is likely that Hsp72 can operate at many levels of the NFκB pathway to inhibit or dampen its activation. Likely independent of its effects on inflammation, NFκB has frequently been associated with cell survival, acting downstream of the kinases AKT and RIP-1. Although there is also a report that NFκB may be involved in induction of apoptosis by ceramide, the majority of reports find it to have antiapoptotic actions.136
Extracellular HSP70s
Although most experiments to date address the intracellular functions of HSP70s, studies have now clearly demonstrated that Hsp72/Hsc73 can be released from cells. The mechanisms of release and the extracellular effects of HSP70 are growing areas of study. One of the first observations suggesting extracellular release of Hsp70 was made in the nervous system; exposure to heat caused an increase in production of heat-shock like proteins in the glial sheath surrounding the squid giant axon (reviewed by Tytell).137 These proteins were transferred from the glial sheath to the interior of the axon. Work from several laboratories now suggests that Hsp72/Hsc73 is released from astrocytes or Schwann cells and can be transferred to and affect neighboring neurons/axons.138-142 Hsp70 release has been documented from a variety of non-neuronal cell types including epithelial cells,143 rat embryo cells,144 B lymphocytes and dendritic cells,145,146 maturing erythrocytes,147 and tumor cells.148 Hsp70 and anti-Hsp70 antibodies have been identified in human serum.149 Since then, numerous studies have examined levels of extracellular Hsp70 in relation to diseases and pathological states, as mentioned above in the introduction, though in some instances Hsp72 and Hsc73 were not distinguished. Current thinking suggests that HSPs are released physiologically, as well as by dying cells, and can act on a variety of receptors.13,150
Mechanism of release of extracellular HSP70
Since Hsp72/Hsc73 do not contain a leader sequence for membrane targeting or localization to membrane vesicles of the secretory pathway, several alternative mechanisms for extracellular release have been proposed. One hypothesis is release from lysosomes. Lysosomal inhibitors were shown to block Hsp72 release and release correlated with increased expression of the intralysosomal protein LAMP1 on cell surfaces,151 though others found little effect with lysosomal inhibitors. Release of Hsp72 by exosomes is the mechanism supported by the most evidence at this point.145-148,152 Exosomes are membrane-bound vesicles containing various cytosolic proteins including Hsp72/Hsc73 as well as peripheral and integral membrane proteins.146 Some investigators found that lipid rafts, which are sphingolipid-cholesterol-rich micro-domains in cell membranes, play a role in HSP70 release.143,153,154 In contrast, others saw no effect on Hsp72 release when either lipid rafts or the classical secretory pathway were disrupted.152
Effects of extracellular Hsp72
If there are physiological mechanisms for the release of HSP70, then there must also be physiological functions for these extracellular proteins. Although HSP release from dying cells can serve as a danger signal, release from live cells can signal a successful stress response,21 and suggests a modulatory or signaling role. Several reports demonstrated that extracellular Hsp72 could induce release of cytokines including TNF, interleukin-6 (IL-6), and IL-1β from monocytes.155-158 Other reports cast doubt on those conclusions, suggesting that at least in some cases, the response is due to contamination with lipopolysaccharide, a potent inducer of cytokine release.159-161 Extracellular Hsp72- induced cytokine release was found to be mediated through toll-like receptor 2 (TLR2), TLR4, and downstream activation of NFκB (see Figure 3).155 This contrasts with the inhibition of NFκB activation observed in mice overexpressing Hsp72 following cerebral ischemia 111 discussed above, which is likely due to intracellular effects.
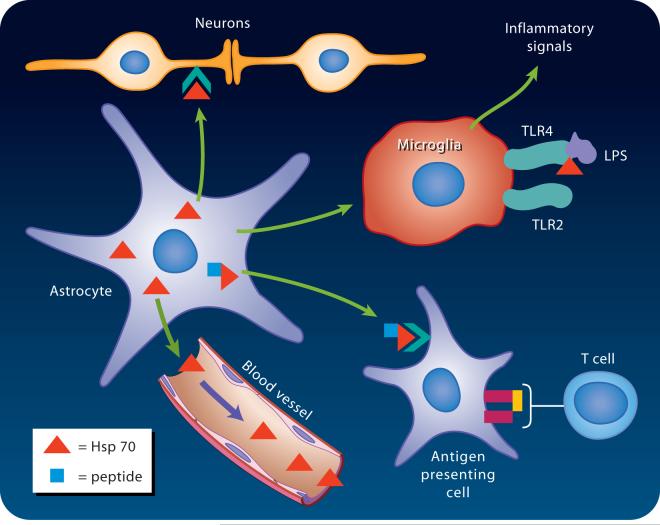
Figure 3.
Extracellular Heat Schock Protein 70 (Hsp70) can be released by astrocytes within the central nervous system and bind to a variety of cells, especially neurons and microglia. Several cell surface receptors are implicated in Hsp70 binding to monocytes such as microglia. Hsp70 plays an important role in antigen presentation, and can be present in serum. LPS= lipopolysaccharide, TLR = toll-like receptor.
TLR4 initiates the signaling cascade triggered by lipopolysaccharide from gram-negative bacteria, whereas TLR2 mediates the signaling cascade triggered by bacterial lipoproteins, gram-positive bacteria, mycoplasma, yeast, and spirochetes. A role for HSP70 in the response to lipopolysaccharide has been identified. The details of the activation complex induced by lipopolysaccharide are still being worked out, but elegant studies of the mobility of lipopolysaccharide and some of the relevant receptors in the plasma membrane suggest that Hsp70 and 90 can be immobilized in the plasma membrane and colocalize with lipopolysaccharide and TLR4, following an initial transient interaction of lipopolysaccharide with CD14.159 Lipopolysaccharide signaling is thus mediated by a large complex which can include Hsp70. The composition of the complex determines whether signaling results in induction or inhibition of immune response.162,163 There is still discussion in the literature on the extent to which Hsp70 binding is directly mediated by TLR2 or 4, and whether the interaction of these receptors with Hsp70 is of high affinity, as overexpression of either receptor alone does not increase binding of Hsp70 to cells that previously did not bind Hsp70.150
Arispe and colleagues showed a direct interaction of Hsp72/Hsc73 with lipid components. Hsc73 was shown to incorporate into the lipid bilayer and create an ATP-dependent cation channel.164 These investigators also showed that Hsp72 and Hsc73 are able to aggregate liposomes by interacting with phosphatidylserine.165 Although phosphatidylserine is generally found on the cytosolic side of the plasma membrane, it is present on the surface of apoptotic cells. Hsp72 and Hsc73 appear to accelerate cell death by interacting with phosphatidylserine on the surfaces of apoptotic cells.165
Internalization of extracellular Hsp72 is thought to be via cell surface receptors. Hsp72 was found to interact with two main families of cell surface proteins: the scavenger receptor family members LOX-1 and SR-A,166 and the C-type lectins of the NK family. These proteins could mediate internalization of Hsp72 protein from the extracellular space.167 Extracellular Hsp72 has been extensively studied for its role in antigen presentation via the major histocompatibility complex pathway, a function important for recognition of tumor cells.168 Extracellular Hsp70 is important in triggering the activity of Natural Killer (NK cells). Multhoff et al. identified an N-terminal 14 amino acid peptide of Hsp70 that was as active in stimulating NK cell cytolytic activity as full-length Hsp70 protein.169 The activation of the cytolytic activity of NK cells by Hsp70 is mediated through C-type lectin receptor CD94 and the adhesion molecule CD56.170
Interestingly, administration of Hsp70 in vivo promoted wound healing by stimulating macrophage phagocytic activity,171 and in some chronic inflammatory diseases it is now appreciated that HSPs can prevent or arrest inflammatory damage, and promote production of anti-inflammatory cytokines.172 Pretreatment with Hsp70 has also been shown to reduce the inflammatory response of monocytes to a subsequent challenge with lipopolysaccharide.173 Thus several different functions have already been described for extracellular HSP70, including protection of neurons and modulation of immune cell function.
Extracellular Hsp70 and Cardiovascular Disease
As the importance of inflammation in cardiovascular disease is increasingly recognized, the likelihood that immunomodulatory effects of HSP70 may be relevant increases. A significant correlation between elevated levels of serum Hsp70 and reduced progression of atherosclerosis assessed as carotid intima-media thickness was found.174 A study of coronary artery disease patients observed significantly higher serum Hsp70 levels in patients found not to have coronary artery disease on angiogram, and disease severity was inversely correlated with serum Hsp70 levels.175
Although higher serum Hsp72 levels were associated with reduced risk of atherosclerosis, Hsp72 is released with myocardial infarction; serum levels after acute myocardial infarction were higher than in patients with angina.18 Levels of extracellular Hsp72 also correlated with levels of IL-6 and IL-8. In atherosclerosis endothelial cells are activated and macrophages release inflammatory cytokines. Oxidized low-density-lipoproteins (LDL) accumulate in macrophages. Svensson and colleagues found that oxidized LDL-treated macrophages released increased amounts of Hsp72, and this released Hsp72 induced IL-1β and IL-12 production by naive macrophages.156 While elevated serum Hsp72 was associated with slower progression of carotid intimal thickening, it may also have some pro-inflammatory effects. The role of Hsp72 in cerebral atherosclerosis and stroke is thus complex.
Conclusions
Many studies support the protective effect of Hsp72 in cerebral ischemia. These studies employed transgenic overexpression of Hsp72 in neonatal and adult models of ischemia,54,72,73,176 the use of mice in which the Hsp70.1 gene was knocked out,71 and transfection or viral vector mediated overexpression.10,34 While each method has its own caveats, the consistent result strongly suggests Hsp72 is efficacious at reducing cerebral ischemic injury. However, in evaluating the different mechanisms discussed above, much work remains to define the relative contributions of each to protection in the setting of cerebral ischemia, and differences between different models should be expected. While there is already strong evidence for both anti-inflammatory and anti-cell death effects of Hsp72 in cerebral ischemia, the relative importance of these mechanisms remains to be determined. In marked contrast, the role of extracellular Hsp72 in stroke has not yet been studied in animal models, and at this moment we are in the curious position of having more data on the association of serum Hsp70 with ischemic disease in patients than in animal models. Future studies should address this issue.
Hsp70 has many physiological roles, both intracellular and extracellular, and participates in the regulation of many intracellular processes. Hsp70 holds great promise as a potential therapeutic approach to many diseases involving abnormalities of protein folding or increased aggregation as found in both acute and chronic neurodegenerative diseases. Hsp70 is also an important immune modulator, and is now appreciated to play a role as an extracellular signaling molecule. Current understanding suggests active release of HSP from live cells to modulate the function of other cells as well as release from dying cells as a danger signal. The use of serum Hsp70 as a marker in diverse disease states, and its possible use in prognosis are just being investigated. Thus Hsp70 holds promise as both a therapeutic strategy and as a biomarker for severity of stress.
Acknowledgement
The authors thank Jenny Hu*, Erin Reiland* and Jessica Howard* for help in preparing the manuscript and figures.
*Secretary, Department of Anesthesia, Stanford University School of Medicine, Stanford, California, USA
Financial Support: This work was supported in part by the National Institutes of Health, Bethesda, Maryland, USA, grants GM49831, NS053898, NS37520, NS014543 and GM 062188 and the Departments of Anesthesia at Stanford University, Stanford, California, USA, and Beijing Tiantan Hospital, Beijing, China.
Footnotes
Submitted from the Department of Anesthesia, Stanford University School of Medicine, Stanford, California, USA
References
- 1.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 2.Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–81. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- 4.Cotto JJ, Morimoto RI. Stress-induced activation of the heat-shock response: cell and molecular biology of heat-shock factors. Biochem Soc Symp. 1999;64:105–18. [PubMed] [Google Scholar]
- 5.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–8. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 6.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–51. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–8. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002;110:801–6. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HW, Hsu C, Lu TS, Wang SJ, Yang RC. Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis. Shock. 2003;20:274–9. doi: 10.1097/00024382-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Jo SK, Ko GJ, Boo CS, Cho WY, Kim HK. Heat preconditioning attenuates renal injury in ischemic ARF in rats: role of heat-shock protein 70 on NF-kappaB-mediated inflammation and on tubular cell injury. J Am Soc Nephrol. 2006;17:3082–92. doi: 10.1681/ASN.2005101077. [DOI] [PubMed] [Google Scholar]
- 12.Singleton KD, Wischmeyer PE. Effects of HSP70.1/3 gene knockout on acute respiratory distress syndrome and the inflammatory response following sepsis. Am J Physiol Lung Cell Mol Physiol. 2006;290:L956–61. doi: 10.1152/ajplung.00466.2005. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder S, Lindemann C, Hoeft A, Putensen C, Decker D, von Ruecker AA, Stuber F. Impaired inducibility of heat shock protein 70 in peripheral blood lymphocytes of patients with severe sepsis. Crit Care Med. 1999;27:1080–4. doi: 10.1097/00003246-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52:611–7. doi: 10.1097/00005373-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Bruemmer-Smith S, Stuber F, Schroeder S. Protective functions of intracellular heat-shock protein (HSP) 70-expression in patients with severe sepsis. Intensive Care Med. 2001;27:1835–41. doi: 10.1007/s00134-001-1131-3. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer PE. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med. 2005;31:1079–86. doi: 10.1007/s00134-005-2690-5. [DOI] [PubMed] [Google Scholar]
- 18.Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–90. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt JP, Schunkert H, Birnbaum DE, Aebert H. Kinetics of heat shock protein 70 synthesis in the human heart after cold cardioplegic arrest. Eur J Cardiothorac Surg. 2002;22:415–20. doi: 10.1016/s1010-7940(02)00327-5. [DOI] [PubMed] [Google Scholar]
- 21.Ganter MT, Ware LB, Howard M, Roux J, Gartland B, Matthay MA, Fleshner M, Pittet JF. Extracellular heat shock protein 72 is a marker of the stress protein response in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L354–61. doi: 10.1152/ajplung.00405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singleton KD, Wischmeyer PE. Glutamine’s protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1839–45. doi: 10.1152/ajpregu.00755.2006. [DOI] [PubMed] [Google Scholar]
- 23.Hoehn B, Ringer TM, Xu L, Giffard RG, Sapolsky RM, Steinberg GK, Yenari MA. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J Cereb Blood Flow Metab. 2001;21:1303–9. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 24.van der Weerd L, Lythgoe MF, Badin RA, Valentim LM, Akbar MT, de Belleroche JS, Latchman DS, Gadian DG. Neuroprotective effects of HSP70 overexpression after cerebral ischaemia--an MRI study. Exp Neurol. 2005;195:257–66. doi: 10.1016/j.expneurol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–91. [PubMed] [Google Scholar]
- 26.Papadopoulos MC, Sun XY, Cao J, Mivechi NF, Giffard RG. Over-expression of HSP-70 protects astrocytes from combined oxygen-glucose deprivation. Neuroreport. 1996;7:429–32. doi: 10.1097/00001756-199601310-00013. [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Giffard RG. HSP70 protects murine astrocytes from glucose deprivation injury. Neurosci Lett. 1997;224:9–12. doi: 10.1016/s0304-3940(97)13444-9. [DOI] [PubMed] [Google Scholar]
- 28.Vayssier M, Polla BS. Heat shock proteins chaperoning life and death. Cell Stress Chaperones. 1998;3:221–7. doi: 10.1379/1466-1268(1998)003<0221:hspcla>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–53. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- 30.Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261–71. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- 31.Kelly S, Yenari MA. Neuroprotection: heat shock proteins. Curr Med Res Opin. 2002;18:s55–60. [PubMed] [Google Scholar]
- 32.Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22:9041–7. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- 33.Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Ouyang YB, Xu L, Chow AM, Anderson R, Hecker JG, Giffard RG. The carboxyl-terminal domain of inducible Hsp70 protects from ischemic injury in vivo and in vitro. J Cereb Blood Flow Metab. 2006;26:937–50. doi: 10.1038/sj.jcbfm.9600246. [DOI] [PubMed] [Google Scholar]
- 35.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–98. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 36.Gogvadze V, Orrenius S. Mitochondrial regulation of apoptotic cell death. Chem Biol Interact. 2006;163:4–14. doi: 10.1016/j.cbi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–44. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–30. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 39.Culmsee C, Landshamer S. Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res. 2006;3:269–83. doi: 10.2174/156720506778249461. [DOI] [PubMed] [Google Scholar]
- 40.Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jaattela M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor- specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci U S A. 2000;97:7871–6. doi: 10.1073/pnas.97.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nylandsted J, Wick W, Hirt UA, Brand K, Rohde M, Leist M, Weller M, Jaattela M. Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res. 2002;62:7139–42. [PubMed] [Google Scholar]
- 42.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J.Exp.Med. 1997;185:1481–6. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ankarcrona M. Glutamate induced cell death: apoptosis or necrosis? Prog Brain Res. 1998;116:265–72. doi: 10.1016/s0079-6123(08)60442-2. [DOI] [PubMed] [Google Scholar]
- 44.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 45.Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, Chan PH. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci. 2000;20:2817–24. doi: 10.1523/JNEUROSCI.20-08-02817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem Res. 2004;29:1943–9. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- 47.Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–26. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 48.Dugan LL, Kim-Han JS. Astrocyte mitochondria in in vitro models of ischemia. J Bioenerg Biomembr. 2004;36:317–21. doi: 10.1023/B:JOBB.0000041761.61554.44. [DOI] [PubMed] [Google Scholar]
- 49.Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36:347–52. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- 50.Christophe M, Nicolas S. Mitochondria: a target for neuroprotective interventions in cerebral ischemia-reperfusion. Curr Pharm Des. 2006;12:739–57. doi: 10.2174/138161206775474242. [DOI] [PubMed] [Google Scholar]
- 51.Iijima T. Mitochondrial membrane potential and ischemic neuronal death. Neurosci Res. 2006;55:234–43. doi: 10.1016/j.neures.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto S, Friberg H, Ferrand-Drake M, Wieloch T. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J.Cereb.Blood Flow Metab. 1999;19:736–41. doi: 10.1097/00004647-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Plesnila N. Role of mitochondrial proteins for neuronal cell death after focal cerebral ischemia. Acta Neurochir Suppl. 2004;89:15–9. doi: 10.1007/978-3-7091-0603-7_3. [DOI] [PubMed] [Google Scholar]
- 54.Matsumori Y, Northington FJ, Hong SM, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70. Stroke. 2006;37:507–12. doi: 10.1161/01.STR.0000199057.00365.20. [DOI] [PubMed] [Google Scholar]
- 55.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–92. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J.Neurosci. 1998;18:4914–28. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugawara T, Noshita N, Lewen A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci. 2002;22:209–17. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–39. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- 59.Manabat C, Han BH, Wendland M, Derugin N, Fox CK, Choi J, Holtzman DM, Ferriero DM, Vexler ZS. Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain. Stroke. 2003;34:207–13. doi: 10.1161/01.STR.0000047101.87575.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–36. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 61.Gill R, Soriano M, Blomgren K, Hagberg H, Wybrecht R, Miss MT, Hoefer S, Adam G, Niederhauser O, Kemp JA, Loetscher H. Role of caspase-3 activation in cerebral ischemia-induced neurodegeneration in adult and neonatal brain. J Cereb Blood Flow Metab. 2002;22:420–30. doi: 10.1097/00004647-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Kang SJ, Wang S, Hara H, Peterson EP, Namura S, Amin-Hanjani S, Huang Z, Srinivasan A, Tomaselli KJ, Thornberry NA, Moskowitz MA, Yuan J. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149:613–22. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kauppinen TM, Swanson RA. The role of poly(ADP-ribose) polymerase-1 in CNS disease. Neuroscience. 2007;145:1267–72. doi: 10.1016/j.neuroscience.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 64.Garnier P, Ying W, Swanson RA. Ischemic preconditioning by caspase cleavage of poly(ADP-ribose) polymerase-1. J Neurosci. 2003;23:7967–73. doi: 10.1523/JNEUROSCI.23-22-07967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kauppinen TM, Swanson RA. Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J Immunol. 2005;174:2288–96. doi: 10.4049/jimmunol.174.4.2288. [DOI] [PubMed] [Google Scholar]
- 66.Koh DW, Dawson TM, Dawson VL. Poly(ADP-ribosyl)ation regulation of life and death in the nervous system. Cell Mol Life Sci. 2005;62:760–8. doi: 10.1007/s00018-004-4508-y. [DOI] [PubMed] [Google Scholar]
- 67.Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006;103:18308–13. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, Anderson RL. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem. 2004;279:51490–9. doi: 10.1074/jbc.M401314200. [DOI] [PubMed] [Google Scholar]
- 69.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 Inhibits Heat-induced Apoptosis Upstream of Mitochondria by Preventing Bax Translocation. J Biol Chem. 2005;280:38729–39. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 70.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–43. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 71.Lee SH, Kwon HM, Kim YJ, Lee KM, Kim M, Yoon BW. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke. 2004;35:2195–9. doi: 10.1161/01.STR.0000136150.73891.14. [DOI] [PubMed] [Google Scholar]
- 72.Tsuchiya D, Hong S, Matsumori Y, Shiina H, Kayama T, Swanson RA, Dillman WH, Liu J, Panter SS, Weinstein PR. Overexpression of rat heat shock protein 70 is associated with reduction of early mitochondrial cytochrome C release and subsequent DNA fragmentation after permanent focal ischemia. J Cereb Blood Flow Metab. 2003;23:718–27. doi: 10.1097/01.WCB.0000054756.97390.F7. [DOI] [PubMed] [Google Scholar]
- 73.Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:899–910. doi: 10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]
- 74.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat.Cell Biol. 2000;2:469–75. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 75.Jiang B, Xiao W, Shi Y, Liu M, Xiao X. Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells. Cell Stress Chaperones. 2005;10:252–62. doi: 10.1379/CSC-124R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geissler A, Rassow J, Pfanner N, Voos W. Mitochondrial import driving forces: enhanced trapping by matrix Hsp70 stimulates translocation and reduces the membrane potential dependence of loosely folded preproteins. Mol Cell Biol. 2001;21:7097–104. doi: 10.1128/MCB.21.20.7097-7104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geissler A, Krimmer T, Bomer U, Guiard B, Rassow J, Pfanner N. Membrane potential-driven protein import into mitochondria. The sorting sequence of cytochrome b(2) modulates the deltapsi-dependence of translocation of the matrix-targeting sequence. Mol Biol Cell. 2000;11:3977–91. doi: 10.1091/mbc.11.11.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strub A, Lim JH, Pfanner N, Voos W. The mitochondrial protein import motor. Biol Chem. 2000;381:943–9. doi: 10.1515/BC.2000.115. [DOI] [PubMed] [Google Scholar]
- 79.Samali A, Orrenius S. Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones. 1998;3:228–36. doi: 10.1379/1466-1268(1998)003<0228:hspros>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Voloboueva LA, Duan M, Ouyang Y, Emery JF, Stoy C, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600600. advance online publication, doi: 10.1038/sj.jcbfm.9600600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ouyang YB, Xu LJ, Sun YJ, Giffard RG. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones. 2006;11:180–6. doi: 10.1379/CSC-182R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suzuki K, Murtuza B, Sammut IA, Latif N, Jayakumar J, Smolenski RT, Kaneda Y, Sawa Y, Matsuda H, Yacoub MH. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106:I270–6. [PubMed] [Google Scholar]
- 83.Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, Yenari MA, Steinberg GK. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol. 2002;52:160–7. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
- 84.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 85.Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–67. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- 86.Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–30. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 87.Hata R, Gillardon F, Michaelidis TM, Hossmann KA. Targeted disruption of the bcl-2 gene in mice exacerbates focal ischemic brain injury. Metab Brain Dis. 1999;14:117–24. doi: 10.1023/a:1020709814456. [DOI] [PubMed] [Google Scholar]
- 88.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat.Cell Biol. 2000;2:476–83. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 89.Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. Embo J. 2001;20:446–56. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yaglom JA, Gabai VL, Meriin AB, Mosser DD, Sherman MY. The function of HSP72 in suppression of c-Jun N-terminal kinase activation can be dissociated from its role in prevention of protein damage. J Biol Chem. 1999;274:20223–8. doi: 10.1074/jbc.274.29.20223. [DOI] [PubMed] [Google Scholar]
- 91.Lee JS, Lee JJ, Seo JS. HSP70 deficiency results in activation of c-Jun N-terminal Kinase, extracellular signal-regulated kinase, and caspase-3 in hyperosmolarity-induced apoptosis. J Biol Chem. 2005;280:6634–41. doi: 10.1074/jbc.M412393200. [DOI] [PubMed] [Google Scholar]
- 92.Becker EB, Howell J, Kodama Y, Barker PA, Bonni A. Characterization of the c-Jun N-terminal kinase-BimEL signaling pathway in neuronal apoptosis. J Neurosci. 2004;24:8762–70. doi: 10.1523/JNEUROSCI.2953-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuan CY, Burke RE. Targeting the JNK signaling pathway for stroke and Parkinson’s diseases therapy. Curr Drug Targets CNS Neurol Disord. 2005;4:63–7. doi: 10.2174/1568007053005145. [DOI] [PubMed] [Google Scholar]
- 94.Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, Davis RJ, Rakic P. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–9. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kitamura C, Ogawa Y, Nishihara T, Morotomi T, Terashita M. Transient co-localization of c-Jun N-terminal kinase and c-Jun with heat shock protein 70 in pulp cells during apoptosis. J Dent Res. 2003;82:91–5. doi: 10.1177/154405910308200203. [DOI] [PubMed] [Google Scholar]
- 96.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 97.Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:665–70. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–43. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 99.Herdegen T, Claret FX, Kallunki T, Martin-Villalba A, Winter C, Hunter T, Karin M. Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury. J Neurosci. 1998;18:5124–35. doi: 10.1523/JNEUROSCI.18-14-05124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whitmarsh AJ, Kuan CY, Kennedy NJ, Kelkar N, Haydar TF, Mordes JP, Appel M, Rossini AA, Jones SN, Flavell RA, Rakic P, Davis RJ. Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 2001;15:2421–32. doi: 10.1101/gad.922801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Im JY, Lee KW, Kim MH, Lee SH, Ha HY, Cho IH, Kim D, Yu MS, Kim JB, Lee JK, Kim YJ, Youn BW, Yang SD, Shin HS, Han PL. Repression of phospho-JNK and infarct volume in ischemic brain of JIP1-deficient mice. J Neurosci Res. 2003;74:326–32. doi: 10.1002/jnr.10761. [DOI] [PubMed] [Google Scholar]
- 102.Ciavarra RP, Goldman C, Wen KK, Tedeschi B, Castora FJ. Heat stress induces hsc70/nuclear topoisomerase I complex formation in vivo: evidence for hsc70-mediated, ATP-independent reactivation in vitro. Proc Natl Acad Sci U S A. 1994;91:1751–5. doi: 10.1073/pnas.91.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kroeger PE, Rowe TC. Interaction of topoisomerase 1 with the transcribed region of the Drosophila HSP 70 heat shock gene. Nucleic Acids Res. 1989;17:8495–509. doi: 10.1093/nar/17.21.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barati MT, Rane MJ, Klein JB, McLeish KR. A proteomic screen identified stress-induced chaperone proteins as targets of Akt phosphorylation in mesangial cells. J Proteome Res. 2006;5:1636–46. doi: 10.1021/pr0502469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rafiee P, Theriot ME, Nelson VM, Heidemann J, Kanaa Y, Horowitz SA, Rogaczewski A, Johnson CP, Ali I, Shaker R, Binion DG. Human esophageal microvascular endothelial cells respond to acidic pH stress by PI3K/AKT and p38 MAPK-regulated induction of Hsp70 and Hsp27. Am J Physiol Cell Physiol. 2006;291:C931–45. doi: 10.1152/ajpcell.00474.2005. [DOI] [PubMed] [Google Scholar]
- 106.Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001;14:89–94. doi: 10.1097/00019052-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 107.Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–92. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- 108.Wang X, Feuerstein GZ. The Janus face of inflammation in ischemic brain injury. Acta Neurochir Suppl. 2004;89:49–54. doi: 10.1007/978-3-7091-0603-7_6. [DOI] [PubMed] [Google Scholar]
- 109.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 110.Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, Whitaker S, Steinberg GK. Hu23F2G, an antibody recognizing the leukocyte CD11/CD18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153:223–33. doi: 10.1006/exnr.1998.6876. [DOI] [PubMed] [Google Scholar]
- 111.Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2007;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- 112.Van Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16:685–95. doi: 10.1016/s1074-7613(02)00310-2. [DOI] [PubMed] [Google Scholar]
- 113.Margulis BA, Sandler S, Eizirik DL, Welsh N, Welsh M. Liposomal delivery of purified heat shock protein hsp70 into rat pancreatic islets as protection against interleukin 1 beta-induced impaired beta-cell function. Diabetes. 1991;40:1418–22. doi: 10.2337/diab.40.11.1418. [DOI] [PubMed] [Google Scholar]
- 114.Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, Sharp FR. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18:1466–81. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng X, Bonni S, Riabowol K. HSP70 induction by ING proteins sensitizes cells to tumor necrosis factor alpha receptor-mediated apoptosis. Mol Cell Biol. 2006;26:9244–55. doi: 10.1128/MCB.01538-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ding XZ, Fernandez-Prada CM, Bhattacharjee AK, Hoover DL. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16:210–9. doi: 10.1006/cyto.2001.0959. [DOI] [PubMed] [Google Scholar]
- 117.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–9. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 119.Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–9. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J.Biol.Chem. 1996;271:17724–32. doi: 10.1074/jbc.271.30.17724. [DOI] [PubMed] [Google Scholar]
- 121.Robinson JM, Badwey JA. The NADPH oxidase complex of phagocytic leukocytes: a biochemical and cytochemical view. Histochem Cell Biol. 1995;103:163–80. doi: 10.1007/BF01454021. [DOI] [PubMed] [Google Scholar]
- 122.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–8. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–18. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 124.Maridonneau-Parini I, Clerc J, Polla BS. Heat shock inhibits NADPH oxidase in human neutrophils. Biochem Biophys Res Commun. 1988;154:179–86. doi: 10.1016/0006-291x(88)90667-5. [DOI] [PubMed] [Google Scholar]
- 125.Polla BS, Stubbe H, Kantengwa S, Maridonneau-Parini I, Jacquier-Sarlin MR. Differential induction of stress proteins and functional effects of heat shock in human phagocytes. Inflammation. 1995;19:363–78. doi: 10.1007/BF01534393. [DOI] [PubMed] [Google Scholar]
- 126.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–91. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 127.Lee JE, Kim YJ, Kim JY, Lee WT, Yenari MA, Giffard RG. The 70 kDa heat shock protein suppresses matrix metalloproteinases in astrocytes. Neuroreport. 2004;15:499–502. doi: 10.1097/00001756-200403010-00023. [DOI] [PubMed] [Google Scholar]
- 128.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–4. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 129.Matthews JR, Hay RT. Regulation of the DNA binding activity of NF-kappa B. Int J Biochem Cell Biol. 1995;27:865–79. doi: 10.1016/1357-2725(95)00071-v. [DOI] [PubMed] [Google Scholar]
- 130.Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2:132–9. doi: 10.1379/1466-1268(1997)002<0132:msphiw>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wong HR, Ryan M, Wispe JR. The heat shock response inhibits inducible nitric oxide synthase gene expression by blocking I kappa-B degradation and NF-kappa B nuclear translocation. Biochem Biophys Res Commun. 1997;231:257–63. doi: 10.1006/bbrc.1997.6076. [DOI] [PubMed] [Google Scholar]
- 132.Chen H, Wu Y, Zhang Y, Jin L, Luo L, Xue B, Lu C, Zhang X, Yin Z. Hsp70 inhibits lipopolysaccharide-induced NF-kappaB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett. 2006;580:3145–52. doi: 10.1016/j.febslet.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 133.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 134.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 135.Qian Y, Commane M, Ninomiya-Tsuji J, Matsumoto K, Li X. IRAK-mediated translocation of TRAF6 and TAB2 in the interleukin-1-induced activation of NFkappa B. J Biol Chem. 2001;276:41661–7. doi: 10.1074/jbc.M102262200. [DOI] [PubMed] [Google Scholar]
- 136.Seegers H, Grillon E, Trioullier Y, Vath A, Verna JM, Blum D. Nuclear factor-kappa B activation in permanent intraluminal focal cerebral ischemia in the rat. Neurosci Lett. 2000;288:241–5. doi: 10.1016/s0304-3940(00)01245-3. [DOI] [PubMed] [Google Scholar]
- 137.Tytell M. Release of heat shock proteins (Hsps) and the effects of extracellular Hsps on neural cells and tissues. Int J Hyperthermia. 2005;21:445–55. doi: 10.1080/02656730500041921. [DOI] [PubMed] [Google Scholar]
- 138.Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 139.Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci. 2005;25:9735–45. doi: 10.1523/JNEUROSCI.1912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol. 2007;67:1815–29. doi: 10.1002/dneu.20559. [DOI] [PubMed] [Google Scholar]
- 141.Edbladh M, Ekstrom PA, Edstrom A. Retrograde axonal transport of locally synthesized proteins, e.g., actin and heat shock protein 70, in regenerating adult frog sciatic sensory axons. J Neurosci Res. 1994;38:424–32. doi: 10.1002/jnr.490380408. [DOI] [PubMed] [Google Scholar]
- 142.Sheller RA, Smyers ME, Grossfeld RM, Ballinger ML, Bittner GD. Heat-shock proteins in axoplasm: high constitutive levels and transfer of inducible isoforms from glia. J.Comp Neurol. 1998;396:1–11. doi: 10.1002/(sici)1096-9861(19980622)396:1<1::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 143.Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278:21601–6. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- 144.Hightower LE, Guidon PT., Jr. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–66. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- 145.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–8. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 146.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mathew A, Bell A, Johnstone RM. Hsp-70 is closely associated with the transferrin receptor in exosomes from maturing reticulocytes. Biochem J. 1995;308(Pt 3):823–30. doi: 10.1042/bj3080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–47. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–77. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- 150.Calderwood SK, Mambula SS, Gray PJ, Jr., Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–94. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 151.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–57. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 152.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–55. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 153.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100:5813–8. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li N, Shaw AR, Zhang N, Mak A, Li L. Lipid raft proteomics: analysis of in-solution digest of sodium dodecyl sulfate-solubilized lipid raft proteins by liquid chromatography-matrix-assisted laser desorption/ionization tandem mass spectrometry. Proteomics. 2004;4:3156–66. doi: 10.1002/pmic.200400832. [DOI] [PubMed] [Google Scholar]
- 155.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 156.Svensson PA, Asea A, Englund MC, Bausero MA, Jernas M, Wiklund O, Ohlsson BG, Carlsson LM, Carlsson B. Major role of HSP70 as a paracrine inducer of cytokine production in human oxidized LDL treated macrophages. Atherosclerosis. 2006;185:32–8. doi: 10.1016/j.atherosclerosis.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–12. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 158.Lehner T, Wang Y, Whittall T, McGowan E, Kelly CG, Singh M. Functional domains of HSP70 stimulate generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem Soc Trans. 2004;32:629–32. doi: 10.1042/BST0320629. [DOI] [PubMed] [Google Scholar]
- 159.Triantafilou K, Triantafilou M, Ladha S, Mackie A, Dedrick RL, Fernandez N, Cherry R. Fluorescence recovery after photobleaching reveals that LPS rapidly transfers from CD14 to hsp70 and hsp90 on the cell membrane. J Cell Sci. 2001;114:2535–45. doi: 10.1242/jcs.114.13.2535. [DOI] [PubMed] [Google Scholar]
- 160.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J Biol Chem. 2003;278:174–9. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 161.Bausinger H, Lipsker D, Ziylan U, Manie S, Briand JP, Cazenave JP, Muller S, Haeuw JF, Ravanat C, de la Salle H, Hanau D. Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur J Immunol. 2002;32:3708–13. doi: 10.1002/1521-4141(200212)32:12<3708::AID-IMMU3708>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 162.Triantafilou M, Lepper PM, Briault CD, Ahmed MA, Dmochowski JM, Schumann C, Triantafilou K. Chemokine receptor 4 (CXCR4) is part of the lipopolysaccharide “sensing apparatus”. Eur J Immunol. 2008;38:192–203. doi: 10.1002/eji.200636821. [DOI] [PubMed] [Google Scholar]
- 163.Triantafilou M, Brandenburg K, Kusumoto S, Fukase K, Mackie A, Seydel U, Triantafilou K. Combinational clustering of receptors following stimulation by bacterial products determines lipopolysaccharide responses. Biochem J. 2004;381:527–36. doi: 10.1042/BJ20040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Arispe N, De Maio A. ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J Biol Chem. 2000;275:30839–43. doi: 10.1074/jbc.M005226200. [DOI] [PubMed] [Google Scholar]
- 165.Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. Faseb J. 2004;18:1636–45. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- 166.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 167.Theriault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J Immunol. 2006;177:8604–11. doi: 10.4049/jimmunol.177.12.8604. [DOI] [PubMed] [Google Scholar]
- 168.Binder RJ, Blachere NE, Srivastava PK. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem. 2001;276:17163–71. doi: 10.1074/jbc.M011547200. [DOI] [PubMed] [Google Scholar]
- 169.Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones. 2001;6:337–44. doi: 10.1379/1466-1268(2001)006<0337:amhpsn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gross C, Schmidt-Wolf IG, Nagaraj S, Gastpar R, Ellwart J, Kunz-Schughart LA, Multhoff G. Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones. 2003;8:348–60. doi: 10.1379/1466-1268(2003)008<0348:hspria>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kovalchin JT, Wang R, Wagh MS, Azoulay J, Sanders M, Chandawarkar RY. In vivo delivery of heat shock protein 70 accelerates wound healing by up-regulating macrophage-mediated phagocytosis. Wound Repair Regen. 2006;14:129–37. doi: 10.1111/j.1743-6109.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 172.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–30. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 173.Aneja R, Odoms K, Dunsmore K, Shanley TP, Wong HR. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J Immunol. 2006;177:7184–92. doi: 10.4049/jimmunol.177.10.7184. [DOI] [PubMed] [Google Scholar]
- 174.Pockley AG, Georgiades A, Thulin T, de Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42:235–8. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- 175.Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:1055–9. doi: 10.1161/01.ATV.0000074899.60898.FD. [DOI] [PubMed] [Google Scholar]
- 176.Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2007;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]