Abstract
We used a continuous recognition procedure that included multiple presentations of test items, along with high-resolution functional magnetic resonance imaging (fMRI), to investigate the relationship between item novelty and recognition-related activity in the medial temporal lobe (MTL). In several regions of hippocampus and parahippocampal cortex, activity elicited by new items exceeded that for old items, whereas no MTL regions exhibited greater activity for old items. Critically, anatomically-distinct regions of MTL were engaged by item novelty in two different ways, as evidenced by statistically-dissociable profiles of activity. In bilateral medial hippocampus and left posterior parahippocampal cortex, activity followed a categorical profile in which it was greater for new than old items but did not differ further with additional presentations of old items. By contrast, effects in adjacent regions of right lateral hippocampus and left parahippocampal cortex were graded, whereby activity declined linearly with respect to each successive item presentation. These findings suggest that the relationship between hippocampal (and parahippocampal) activity and continuous psychological dimensions, such as item novelty, cannot be captured by a unitary function.
Keywords: novelty, familiarity, hippocampus, fMRI, high-resolution
Consistent with human and animal lesion data implicating MTL in recognition memory (for reviews, see Brown & Aggleton, 2001; Eichenbaum et al., 2007; Squire et al., 2007), fMRI and intracranial (local field potential and single-neuron) recording studies have demonstrated that hippocampal activity is modulated by the study status of recognition test items (e.g., Grunwald et al., 1998; Heit et al., 1988; for a review of fMRI findings, see Henson, 2005). Among event-related fMRI studies employing separate study and test phases, it has occasionally been reported that correctly-recognized (old) items elicit greater hippocampal activity than correctly-rejected (new) items (e.g., Donaldson et al., 2001; Stark & Squire, 2001). More frequently, enhanced hippocampal activity has been reported for recognition test items that are accompanied by either “remember” or accurate source memory judgments, relative to when items are designated with “know” responses or lack source information (e.g., Cansino et al., 2002; Eldridge et al., 2000; Johnson & Rugg, 2007; Wheeler & Buckner, 2004; Woodruff et al., 2005; Yonelinas et al., 2005). The latter findings are commonly taken to imply that enhanced hippocampal activity is associated with the retrieval (recollection) of qualitative information about a study episode, rather than an acontextual sense of familiarity (Rugg & Yonelinas, 2003; but see Squire et al., 2007). Of significance, however, is that other fMRI studies of recognition memory have reported greater hippocampal activity for new compared to old items (e.g., Duzel et al., 2003; Rombouts et al., 2001; Rugg et al., 2003). Such findings have been interpreted as evidence that the hippocampus is sensitive to stimulus novelty, with elevated activity reflecting an allocation of processing resources toward the encoding of novel items (e.g., Duzel et al., 2003; Stark & Okado, 2003; also see Stern et al., 1996). To further complicate matters, activity in the same hippocampal region can seemingly demonstrate enhancement to both recollected and new test items relative to items that are merely familiar, suggesting that recollection and encoding may be subserved by common or closely adjacent neuronal populations (Woodruff et al., 2005; Yonelinas et al., 2005).
Enhanced hippocampal activity for new relative to old items has also been observed in studies employing continuous recognition memory tests (where old/new status of items is manipulated within a single list; e.g., Brozinsky et al., 2005; Viskontas et al., 2006). Using fMRI, Brozinsky et al. (2005) reported greater hippocampal activity for new items compared to old items that were repeated after short (2 or 8 intervening items) but not longer (16 or 32) inter-item lags. Analogous findings were reported in a human single-neuron recording study of continuous recognition: Relative to the baseline firing rate, most of the hippocampal cells responsive to new test items showed enhanced firing, whereas cells responsive to old items largely showed a reduction in firing rate (Viskontas et al., 2006; see Rutishauser et al., 2006, for similar recognition-related reductions in hippocampal single-neuron activity). Together, these findings suggest that, in the context of continuous recognition tasks in which the lag between first and second presentations is relatively short, old items are associated with a net reduction in hippocampal neural activity relative to the level of activity elicited by new items. One possible explanation of these findings is that the demands of the continuous recognition task place a premium on detecting and encoding new items, such that the corresponding hippocampal response overshadows any retrieval-related activity elicited by old items (cf. Stark & Okado, 2003).
Here, we build on these prior observations of hippocampal activity during continuous recognition by employing four presentations of test items rather than the two presentations employed in previous studies. Our aims were two-fold: to replicate and generalize previous findings of a recognition-related reduction in hippocampal activity, and to gain insight into the relationship between hippocampal activity and stimulus novelty (or familiarity)1 by investigating how activity varies with additional presentations of old items. In this regard, three theoretically distinct scenarios—based in part on the findings described above, but extrapolated to additional (> 2) item presentations—can be envisaged. First, old/new effects might be categorical, such that the activity elicited by old items is diminished relative to new items, but does not differ with additional old-item presentations. Alternatively, the effects might be graded, with reductions in activity continuing with each successive presentation. Finally, the function mapping hippocampal activity to multiple item presentations might be U-shaped, with an enhancement of activity both for new and later (relative to intermediate) presentations. The first of these scenarios would be consistent with a threshold-like (all-or-none) process associated with the detection of item novelty, the second would suggest that the hippocampus responds according to the relative novelty of the eliciting item, and the final scenario would be most consistent with the proposal that hippocampal activity during recognition is an amalgam of encoding- and retrieval-related processing, as has been previously suggested (Stark & Okado, 2003; Woodruff et al., 2005; Yonelinas et al., 2005).
We employed a continuous recognition task in combination with high-resolution fMRI of the hippocampus and surrounding MTL, so as to optimize the ability to localize and discriminate between different patterns of recognition-related activity (similar high-resolution fMRI methods have been employed by Kirwan et al., 2007). Sixteen subjects (8 males; age range = 18–29 years; mean age = 20 years; for exclusion criteria, see Johnson & Rugg, 2007) undertook six continuous recognition runs, three which were comprised of concrete words and three of colored pictures of objects. Each run was accompanied by fMRI acquisition and consisted of 105 item trials [presentation of an item for 500 ms followed by a fixation (+) for 1900 ms] intermixed with 35 null trials (fixation for 2400 ms). Items within a run were presented between 1 and 4 times—hereafter referred to as the new, old1, old2, and old3 repetition conditions. Subjects indicated, via a binary button response, whether each item was being shown for the first time or had already been presented (disregarding the number of prior presentations). Trials in each run were organized into a series of 7 sub-blocks (though viewed by subjects as a continuous series of items) that provided a lead-in period to establish multiple item presentations and also allowed control over the number of intervening items (range = 3–34; mean = 16) between consecutive repetitions of a given item. Each of the final 4 sub-blocks per run consisted of a 1:1 ratio of new and old items, and included items from every repetition condition. All behavioral and fMRI results reported here are based on data obtained from these final 4 sub-blocks, eliminating the potential confound caused by items from the different conditions being sampled disproportionately from disparate portions of a run.
MRI data were acquired with a Philips Intera Achieva 3T MR scanner (Philips Medical Systems, Bothell, WA) equipped with an 8-channel SENSE head coil. Functional data consisted of partial brain volumes (175 per run), each comprising 26 T2*-weighted images (FE-EPI pulse sequence; 70° flip angle; 1.5-mm thick slices with.5-mm gap; FOV = 240×180 mm; 1.5-mm2 in-plane resolution; SENSE factor = 2.5; 2 sec TR; 25 ms TE) positioned parallel to the hippocampal axis to fully cover the MTL. Whole-brain anatomical data (T1-weighted; sagittal acquisition; FOV = 240×180 mm;.75-mm3 voxel size; SENSE factor = 1.5) were collected with a 3-D MP-RAGE pulse sequence. All data were processed and analyzed with SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). Functional data were spatially realigned to the first volume of the series and then to the across-run mean volume, after which they were co-registered with the anatomical data. The anatomical data were normalized MNI space using a unified segmentation procedure (Ashburner & Friston, 2005), the resulting deformation parameters from which were also applied to the functional data.2 The functional data were then resampled into 1.5-mm3 voxels and smoothed with a 4-mm FWHM Gaussian kernel. Separate regressors modeled the onsets of correctly-identified items for each repetition condition and type of material (collapsing over the 3 runs of each type), with an additional regressor modeling all other items. Other aspects of the subject- and group-level analyses were as described previously (see Johnson & Rugg, 2007).
The fMRI results reported here were restricted, by a group-based mask, to hippocampus and surrounding MTL (based on standard anatomical landmarks; Insausti et al., 1998). The mask was created by manually tracing (with MRIcro software; www.mricro.com) the MTL on coronal slices of the across-subjects mean normalized anatomical image and then smoothing the result with a 4-mm FWHM Gaussian kernel. The mask was used in combination with a small volume correction (SVC) procedure to identify significant effects. Clusters were accepted as significant if the number of contiguous activated voxels (thresholded at p < .005) exceeded a corrected cluster-level threshold of p < .05 (amounting to approximately 30 voxels in the present case). Preliminary analyses indicated that none of the effects of interest (see below) in MTL were modulated by the type of material (words/pictures). Thus, our analyses collapsed over this factor and further minimized its influence by exclusively masking the across-material effects with the bidirectional (F) repetition × material interaction contrast (p < .05).
Accuracy rates and response times (RTs; see Table 1) were submitted to separate two-way ANOVAs, with factors of repetition (new/old1/old2/old3) and material (words/pictures). For response accuracy, ANOVA gave rise solely to a main effect of repetition [F(1.5, 22.1) = 27.97, p < .001], with pair-wise t-tests indicating that accuracy rates were lowest for old1 (all ps <.001) and highest for old3 items (all ps <.05). For RTs, ANOVA gave rise to main effects of repetition [F(2.1, 31.1) = 6.02, p < .01] and material [F(1, 15) = 14.14, p < .005], and to an interaction [F(2.4, 35.3) = 21.05, p < .001]. Separate material-wise ANOVAs of RTs revealed a repetition main effect for words only [F(1.9, 28.6) = 16.60, p < .001], with t-tests indicating shorter RTs for old3 than old2 words (p < .001), which were in turn shorter than for old1 and new words (both ps < .025).
Table 1.
Means (and SEMs) of accuracy rates and RTs (in ms) for word and picture test items in each repetition condition
| Repetition Condition | |||||
|---|---|---|---|---|---|
| Material | New | Old1 | Old2 | Old3 | |
| Accuracy rate | Word | .92 (.02) | .80 (.03) | .94 (.02) | .98 (.01) |
| Picture | .97 (.01) | .82 (.04) | .94 (.02) | .98 (.01) | |
| RT | Word | 888 (37) | 878 (36) | 822 (35) | 747 (25) |
| Picture | 855 (35) | 857 (36) | 871 (37) | 876 (36) | |
Analysis of the fMRI data was first directed at MTL regions exhibiting categorical old/new effects (see above). These effects were identified with bidirectional contrasts of the activity elicited by new items and that elicited by old items, giving equal weight to items from the old1, old2, and old3 conditions (i.e., contrast weights of −3 +1 +1 +1 and +3 −1 −1 −1 for new, old1, old2, and old3, respectively). No regions were identified where activity was greater for old than for new items. However, reversed (new > old) effects were evident in three regions: left and right anteromedial hippocampus and left posterior parahippocampal cortex (see Figure 1).
1.
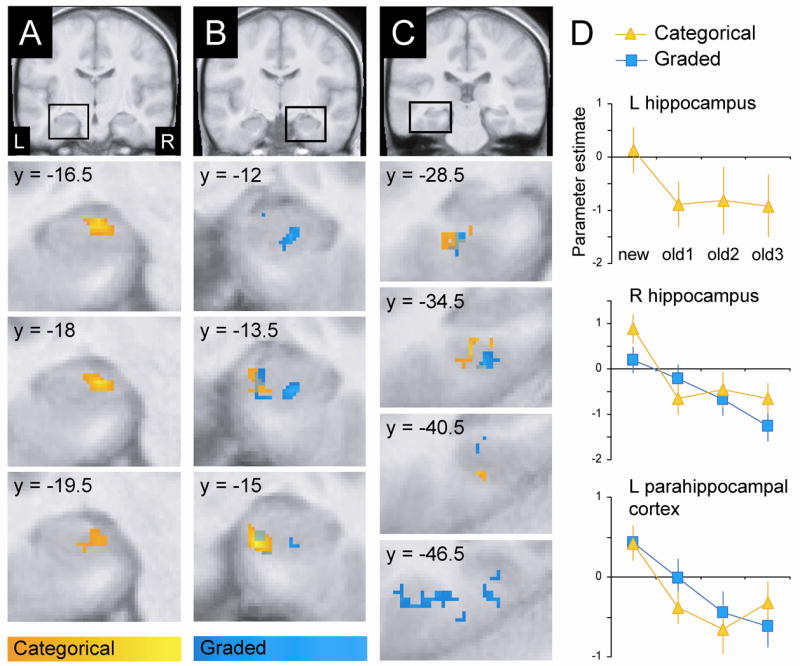
MTL regions exhibiting categorical (orange) and graded (blue) changes in activity according to multiple presentations of continuous recognition test items. All activations exceeded a cluster-wise significance level of p < .05 and are overlaid on across-subjects mean normalized anatomical images. A: Categorical effects in left anteromedial hippocampus (42 voxels, peak MNI coordinates = −22.5 −18 −16.5, peak Z = 4.24). B: Categorical effects in right anteromedial hippocampus (29 voxels, 19.5 −15 −22.5, Z = 4.60) and graded effects in right anterolateral hippocampus (47 voxels, 27 −13.5 −22.5, Z = 4.20). C: Categorical and graded effects in left posterior parahippocampal cortex (categorical: 106 voxels, −28.5 −36 −16.5, Z = 3.99; graded: 2 clusters, 61 and 35 voxels, −31.5 −30 −18 and −30 −43.5 −12, Z = 3.76 and 3.70). D: Graphs indicate the mean parameter estimates (across subjects and voxels; in arbitrary units; with SEMs) corresponding to the four repetition conditions (collapsed over words and pictures) from voxels uniquely exhibiting categorical and graded effects.
Graded fMRI effects were identified with bidirectional linear contrasts (−3 −1 +1 +3 and +3 +1 −1 −3) of the activity elicited by new, old1, old2, and old3 items. As with the categorical analyses, no MTL regions were identified where activity increased with additional item presentations. By contrast, graded decreases in activity were revealed in right anterior hippocampus and left posterior parahippocampal cortex. As is illustrated in Figure 1, the graded effects in these two regions overlapped somewhat with the categorical effects described above. Importantly, however, the graded effects in hippocampus extended to regions lateral and anterior to those exhibiting categorical effects. Graded effects in parahippocampal cortex extended more posteriorly than the categorical effects.
Bidirectional quadratic contrasts (+1 −1 −1 +1 and −1 +1 +1 −1), employed to identify regions where activity followed a U-shaped response profile, failed to reveal any significant effects. To further test whether any MTL regions exhibited enhanced activity for old3 items, pair-wise contrasts were conducted comparing this condition to the remaining three item conditions. As with the results of the quadratic analyses, no significant effects were revealed by these additional contrasts.
Figure 1 also illustrates the mean (across voxels and subjects) parameter estimates for MTL clusters uniquely associated with categorical or graded response profiles. To determine whether the two different response profiles seemingly evident within right hippocampus and left parahippocampal cortex were statistically dissociable, the parameter estimates from clusters uniquely exhibiting the different profiles within each region were submitted to ANOVAs [factors of repetition, material, and profile (categorical/graded)]. For both regions, ANOVA gave rise to a significant repetition × profile interaction [right hippocampus: F(2.8, 41.9) = 5.66, p < .005; left parahippocampal cortex: F(2.6, 39.2) = 6.46, p < .005].
Consistent with previous findings from continuous recognition tasks (Brozinsky et al., 2005; Viskontas et al., 2006), hippocampal old/new effects took the form of relative reductions in activity for old compared to new items. In a significant extension to those previous findings, we found that regions which would have exhibited indistinguishable effects in recognition memory tasks employing only two presentations of each test item can actually be dissociated with additional presentations. That is, the new > old effects observed here followed two distinct recognition-related activity profiles that were, to a large extent, anatomically segregated (see Figure 1). Whereas categorical (threshold-like) effects were localized bilaterally to the medial aspect of anterior hippocampus (possibly in the vicinity of the CA3 field and dentate gyrus, according to Duvernoy, 2005), graded effects were most evident in right anterolateral hippocampus (possibly corresponding to the CA1 field).3 This anatomical separation of the two response profiles raises the possibility that different regions of the hippocampus support distinct novelty-sensitive processes—one reflecting discrete changes in old/new status, and the other corresponding to relative variations in stimulus novelty.
The functional significance of the two different profiles of hippocampal activity is unclear. It is tempting, however, to relate these results to recent evidence indicating that the CA3 field exhibits more abrupt activity changes in response to changes in stimulus input than does CA1—a distinction that is believed to correspond to the theoretical constructs of pattern separation versus completion, respectively (Bakker et al., 2008; Leutgeb et al., 2004; Vazdarjanova & Guzowski, 2004). From this perspective, the present findings reflect the tendency of CA3 to categorize input (in this case, new versus old items) in an ‘all or none’ fashion, while CA1 demonstrates a more continuous (less differentiated) response to the same change in input (Bakker et al., 2008). This conjecture is, of course, predicated on the assumption that the respective fMRI response profiles do indeed map anatomically onto the proposed hippocampal sub-regions; we would be among the first to admit that this assumption requires further support.
Regardless of the validity of the foregoing conjecture, we doubt that the present findings reflect a significant on-line contribution of the hippocampus to the associated continuous recognition judgments. Patients with lesions limited to the hippocampus often perform well above chance on recognition memory tasks (e.g., Holdstock et al., 2002; Manns et al., 2003; Wais et al., 2006), and recognition (delayed-nonmatch-to-sample) performance in primates with hippocampal lesions is impaired only after study-test delays of several minutes (and then only moderately), in striking contrast to the debilitating effects of perirhinal lesions (for reviews, see Brown & Aggleton, 2001; Eichenbaum et al., 2007; Squire et al., 2007). Thus, extra-hippocampal MTL regions such as perirhinal cortex are sufficient to support simple recognition memory judgments. Moreover, single-neuron activity in primate perirhinal cortex is strongly modulated by the study status of recognition memory test items (for review, see Brown & Xiang, 1998). The onset latency of these modulations (~90 ms) is considerably shorter than the latency of recognition-sensitive single-neuron effects in human hippocampus which, so far as one can estimate from published figures, onset only after several hundred milliseconds (e.g., Rutishauser et al., 2006; Viskontas et al., 2006). Together, these findings lead us to propose that the present hippocampal new > old effects reflect new-item encoding (as also suggested by Duzel et al., 2003, and Okado & Stark, 2003, among others), and likely played a minimal (if any) role in supporting recognition judgments that largely depended on faster-acting extra-hippocampal neuronal populations.4 Of course, this is not to say that the hippocampus never contributes directly to continuous recognition performance. We conjecture that the hippocampus is necessary to the extent that accurate recognition performance cannot be attained solely by relying on item familiarity.
Interpretation of our findings as evidence for qualitatively distinct novelty-sensitive neural response profiles is predicated on the assumption that the regions exhibiting the two different profiles do not differ in their hemodynamic transfer functions (that is, the parameters governing the coupling of hemodynamic and neural activity). Since these transfer functions are not necessarily linear, and can differ across brain regions (Logothetis & Wandell, 2004), this assumption can be questioned. Thus, it is possible that the two different response profiles described above merely reflect the fact that some medial temporal regions demonstrate a relatively linear transfer function, whereas in others the function is nonlinear. A very similar argument was advanced recently by Squire et al. (2007) to account for the finding that perirhinal cortex appears to demonstrate graded changes in hemodynamic activity only when memory ‘strength’ is low, whereas the hippocampus demonstrates enhanced activity only for especially strong memories. Given current ignorance about the form of the transfer functions associated with different regions of the hippocampus (and parahippocampal cortex), we cannot rule out the possibility that the present findings reflect a dissociation at the hemodynamic rather than the neural level. (A single neuron recording study employing the present experimental procedure would be helpful in this regard.) Regardless of how this issue is resolved, however, our findings indicate that the relationship between hippocampal activity and continuously varying psychological variables (here, item novelty/familiarity) cannot be characterized by a single function (cf. Squire et al., 2007).
Acknowledgments
Grant sponsor: National Institutes of Health
Grant number: R01-MH072966
Footnotes
We employ the terms novelty and familiarity interchangeably, given that they are essentially reciprocals of one another.
Normalization using this segmentation method provided a substantial improvement in across-subject alignment compared to normalization based on deformation parameters derived solely from warping the anatomical data without segmentation (Ashburner & Friston, 1999). Preliminary analyses showed an approximate 30% difference between the methods in the number of anterior hippocampal voxels that overlapped across all 16 subjects.
Segregation of the categorical and graded fMRI response profiles within the medial versus lateral aspects of hippocampus, respectively, was evident not only on the across-subjects mean of normalized anatomical images (shown in Figure 1), but also at the level of each subject’s normalized anatomical image (available on request from the first author). In several subjects, the categorical effect in right medial hippocampus appeared to extend slightly into the subiculum.
Our failure to detect significant recognition-related effects in perirhinal cortex may be attributed to magnetic susceptibility artifact in this region, which prevents us from drawing any conclusions about its involvement in the present task. It is noteworthy, however, that both Brozinsky et al. (2005) and Yassa and Stark (in press) reported perirhinal new > old effects during a continuous recognition task.
References
- Ashburner J, Friston K. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brozinsky CJ, Yonelinas AP, Kroll NEA, Ranganath C. Lag-sensitive repetition suppression effects in anterior parahippocampal gyrus. Hippocampus. 2005;15:557–561. doi: 10.1002/hipo.20087. [DOI] [PubMed] [Google Scholar]
- Brown MW, Xiang J-Z. Recognition memory: Neuronal substrates of the judgement of prior occurrence. Prog Neurobiol. 1998;55:149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL. Dissociating memory retrieval processes using fMRI: evidence that priming does not support recognition memory. Neuron. 2001;31:1047–1059. doi: 10.1016/s0896-6273(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human hippocampus: functional anatomy, vascularization, and serial sections with MRI. 3. New York: Springer; 2005. p. 232. [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J Neurosci. 2003;23:9439–9444. doi: 10.1523/JNEUROSCI.23-28-09439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci. 2000;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald T, Lehnertz K, Heinze HJ, Helmstaedter C, Elger CE. Verbal novelty detection within the human hippocampus proper. Proc Natl Acad Sci USA. 1998;95:3193–3197. doi: 10.1073/pnas.95.6.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit G, Smith ME, Halgren E. Neural encoding of individual words and faces by the human hippocampus and amygdala. Nature. 1988;333:773–775. doi: 10.1038/333773a0. [DOI] [PubMed] [Google Scholar]
- Henson R. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q J Exp Psychol B. 2005;58:340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O’Reilly RC, Norman KA. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cereb Cortex. 2007;17:2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CEL. High-resolution fMRI investigation of the medial temporal lobe. Hum Brain Mapp. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser M, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Witter MP, Machielsen WC, Scheltens P. Anterior medial temporal lobe activation during attempted retrieval of encoded visuospatial scenes: an event-related fMRI study. NeuroImage. 2001;14:67–76. doi: 10.1006/nimg.2001.0799. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA, Robb WGK. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41:40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Yonelinas AP. Human recognition memory: a cognitive neuroscience perspective. Trends Cogn Sci. 2003;7:313–319. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Schuman EM. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron. 2006;49:805–813. doi: 10.1016/j.neuron.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. Simple and associative recognition memory in the hippocampal region. Learn Mem. 2001;8:190–197. doi: 10.1101/lm.40701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: Evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, Knowlton BJ, Steinmetz PN, Fried I. Differences in mnemonic processing by neurons in the human hippocampus and parahippocampal regions. J Cogn Neurosci. 2006;18:1654–1662. doi: 10.1162/jocn.2006.18.10.1654. [DOI] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49:459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remember and knowing. NeuroImage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Multiple signal of recognition memory in the medial temporal lobe. Hippocampus. doi: 10.1002/hipo.20452. In press. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


