Abstract
Background
Secretory IgA prevents pathogen adherence at mucosal surfaces to prevent infection. Polymeric immunoglobulin receptor (pIgR), located on the basolateral surface of mucosal cells, binds dimeric IgA produced by B cells with the cooperation of T cells in the lamina propria. This IgA-pIgR complex is transported apically, where it is exocytosed as secretory IgA to the mucosal surface. Our prior work shows that parenteral nutrition (PN) impairs both airway and small intestine mucosal immunity by reducing T & B cells and IgA levels. This work examines intestinal and respiratory tissue-specific pIgR responses to PN.
Methods
Cannulated male ICR mice were randomized to Chow (n=10) or PN (n=10). After 5 days animals were sacrificed and lavages obtained from the small intestine (SIL), lung (BAL=bronchoalveolar lavage), and nasal airways (NAL). Small intestine, lung and nasal passage tissues were also collected. Lavage and tissue homogenate IgA levels were quantified by ELISA and pIgR by Western blot.
Results
PN group SIL and NAL IgA levels dropped significantly compared to Chow. PN significantly reduced pIgR levels in the SI while no pIgR change was noted in nasal passages and lung pIgR actually increased with PN. Tissue homogenate IgA levels did not change with PN in the SI while levels in the nasal passage and lung decreased.
Conclusions
PN impairs airway mucosal immunity by reduction in IgA available for transport rather than via a reduction in pIgR levels. In the small intestine diminished pIgR is implicated in the deterioration of antibody-mediated mucosal immunity.
Introduction
Parenteral nutrition (PN) increases infectious morbidity due to pneumonia and intra-abdominal abscesses in severely injured patients compared to enteral feeding [15, 20-22]. Increasing evidence suggests that a PN-induced mucosal immune deficiency is at least partly responsible for this difference in clinical outcome. The common mucosal immune hypothesis describes an integrated immune system linking intestinal and extra-intestinal mucosal sites together to provide diffuse protection from potential pathogens at mucosal surfaces throughout the respiratory and digestive tracts [14].
The mucosa-associated lymphoid tissue (MALT) provides the immunologic infrastructure for this system. It collectively constitutes one of the largest and most active secondary immune organs. The murine small intestine contains more than 80% of all immunoglobulin-secreting cells and more than 70% of these cells produce IgA [5, 30]. A mucosal immune response begins as naïve lymphocytes are activated by exposure to antigen in Peyer's patches within the gut-associated lymphoid tissue (GALT) (Figure 1). Further maturation and development of memory ensues while the cells migrate to the systemic circulation by way of lymph nodes and the thoracic duct [24]. Mature cells are distributed to the lamina propria underlying mucosal surfaces to produce immunoglobulin (predominately IgA) for transport by the polymeric immunoglobulin receptor (pIgR) to the mucosal surfaces. The mucosal surfaces are linked via this system; exposure to antigen at one mucosal surface provides specific immunity to that antigen at other mucosal sites [14, 19, 29].
Figure 1. Secretory Immunoglobulin A (IgA) production through the mucosal immune system.
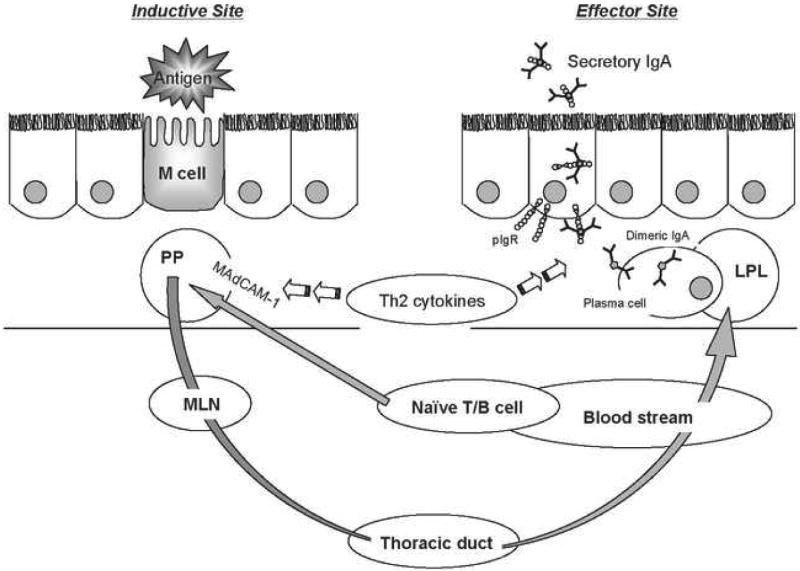
Luminal antigen taken up by M cells sensitized naïve T and B lymphocytes attracted into the Peyer's patches (PP) by mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) The lymphocytes migrate through mesenteric lymph nodes (MLN), the thoracic duct and into the blood stream. Mature lymphocytes home to the lamina propria (LPL) of effector site organs to produce IgA. IgA produced by plasma cell is transported to the mucosal surfaces by polymeric immunoglobulin receptor (pIgR) and secreted as a secretory IgA, which binds to bacteria to prevent invasive infection.
Our previous experimental work demonstrates PN-induced mucosal immune defects. PN feeding with lack of enteral stimulation results in reduced IgA levels at intestinal and respiratory mucosal surfaces as well changes in GALT cell phenotype. Specifically, PN reduces lamina propria and Peyer's patch T & B lymphocyte mass [17], intestinal Th-2 type IgA-stimulating cytokines [31] and intestinal pIgR levels [26]. Each alteration would appear to negatively impact luminal IgA levels by reducing production capacity (reduced cell mass & altered cytokines) or transport (reduced pIgR) of IgA from the site of production. In a model of IgA-mediated antiviral defense however, the capacity of IgA transport in the upper respiratory tract of PN fed animals appeared at least adequate to provide protection when exogenous viral-specific IgA was provided [25]. Therefore, we hypothesized that PN induces tissue specific pIgR (transport) protein alterations in the upper and lower respiratory tract and the intestine.
Materials and Methods
Animals
All protocols were approved by the Animal Care and Use Committee of the University of Wisconsin-Madison, and the Middleton Veterans Administration Hospital, Madison. Male Institute of Cancer Research (ICR) mice were purchased from Harlan (Indianapolis, IN) and housed in an American Association for Accreditation of Laboratory Animal Care-accredited conventional facility. Mice were housed in an environment controlled for temperature, humidity and light (12 hour light: dark). Mice were housed 5 per covered/filtered box and fed ad libitum chow (LabDiet, PMI Nutrition International, St. Louis, MO) and water for 1 week prior to initiation of study protocol. After study entry mice were individually housed in metal cages with wire grid floors to prevent coprophagia.
Experimental Protocol
Twenty mice, 6 to 8 weeks-old, were cannulated via the external jugular vein (0.012″ I.D./0.25″ O.D.; Helix Medical, Inc., Carpinteria, CA) under general anesthesia (ketamine 100 mg/kg and acepromazine maleate 10 mg/kg mixture). Catheters were tunneled subcutaneously over the back and exited mid tail. Mice were partially immobilized by tail restraint to protect the catheter during infusion, which has been shown not to induce significant physical or biochemical stress [28].
Following catheterization mice were connected to infusion pumps and recovered for 48 hours while receiving 4 mL/day of 0.9% saline as well as chow and water ad libitum. After the recovery period the animals were randomized to continue on chow (n=10) or to receive PN (n=10). Chow fed animals received 0.9% saline at 4 mL/day as well as chow and water ad libitum throughout the study. PN fed mice received solution at 4 mL/day (day 1), 7 mL/day (day 2) and 10 mL/day (days 3-5). The PN solution contains 6.0% amino acids, 34.9% dextrose (6002 kJ/L), electrolytes, and multivitamins, with a non-protein calorie/nitrogen ratio of 535.8 kJ/g Nitrogen. The PN feeding meets the calculated nutrient requirements of mice weighing 25 to 30 g.
After 5 days of feeding mice were anesthetized and exsanguinated by cardiac puncture. The small intestine was removed by dissection and cleaned of mesenteric fat and vasculature, and the lumen flushed with 20 mL of Hank's balanced salt solution (HBSS, Bio Whittaker, Walkersville, MD) to obtain small intestinal lavage (SIL) specimens. The SIL were spun at 3,000 rpm for 10 min at 4°C and the clear supernatants were stored at -80°C until analysis. The intestine was divided into three equal length segments approximating the duodenum, jejunum and ileum. Each section was again divided into 2 equal lengths and one of these ‘half-lengths’ from each section were combined and constituted the small intestinal sample for each animal.
A midline incision made over the ventral aspect of the trachea slightly anterior to the thoracic inlet allowed access to the trachea. A tracheotomy was made and 1 mL of saline was slowly injected cephalad through the upper respiratory tract via an 18 gauge angiocath attached to a tuberculin syringe. The fluid was collected as it exited the nasal passages. The angiocath was then redirected distally and a bronchoalveolar lavage (BAL) performed with 1 mL saline. Left lung tissue was excised and washed in HBSS. The head of the animal was processed to obtain nasal passages: the facial skin, cheek muscles, bones and palate were removed according to the method of Asanuma et al. [3, 4]. The remaining sections, consisting of nasal turbinates, septa, and lateral walls were obtained as nasal passage tissue. Nasal airway lavage (NAL) and BAL were stored at -80°C until assayed. The small intestine, nasal passage and lung tissues were frozen in liquid N2 and stored at -80°C.
Preparation of tissue homogenates
The small intestine, nasal passage and lungs were homogenized in 1 mL of 1X RIPA lysis buffer (Upstate, Lake Placid, NY) plus 10% proteases inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The crude homogenate was kept on ice for 1 h and spun at 16,100 rpm for 10 min at 4°C in a bench top Eppendorf 5415 centrifuge. The supernatant was transferred to a clean tube, the protein content was quantified with the dye-binding Bradford method, and the samples stored at −20°C until analyzed.
IgA Antibody Quantitative Analysis
Total IgA in SIL, NAL, BAL and tissue protein homogenates {small intestine (SIH), nasal passage (NPH), lung (LH)} was measured using a sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates (BD Biosciences, Bedford, MA) were coated with 50 μl of a 10 μg/mL goat anti-mouse IgA, α-chain specific (Sigma-Aldrich, St Louis, MO) in 0.1 mol/L carbonate-bicarbonate coating buffer, pH 9.6, and incubated overnight at 4 °C. Plates were washed 3 times and blocked with 100 μl of 1% bovine serum albumin in Tris-buffered saline with 0.5% Tween-20 solution (TBS-Tween) for 1 h at room temperature. Due to differing content of IgA, each sample was diluted as follows: lavages, SIL 1: 100; NAL 1:2; BAL 1:5; tissue homogenates, SIH 1:5,000, NPH 1:2, and LH 1:10 with 5% non-fat dry milk prepared in TBS-Tween (blotto). One hundred microliters of each sample or IgA standards (seven 2-fold dilutions, from 1,000 → 7.8 ng/mL, Sigma-Aldrich, St Louis, MO) were added, and plates were incubated for 1 h at room temperature. Plates were washed 3 times and 100 μL of a 1:500 dilution of the secondary antibody, goat anti-mouse IgA, α-chain specific-HRP conjugate (Sigma-Aldrich, St Louis, MO) were added and incubated for 1 h at room temperature. Plates were washed 5 times, and 100 μL of the substrate solution (H2O2 and o-phenylenediamine, Sigma-Aldrich, St Louis, MO) were added and incubated for 12 min at room temperature. The reaction was stopped by the addition of 50 μL of 2N H2SO4 and the absorbance read at 490 nm in a Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). Mass amounts of IgA in the samples were calculated by plotting their absorbance values on the IgA standard curve, which was calculated using a 4-parameter logistic fit with SOFTmax PRO software (Molecular Device, Sunnyvale, CA).
To determine the amount of luminal IgA remaining following the first lavage (which would likely be measured with the ‘tissue’ sample) we performed 4 sequential washes of the intestinal (20mL/wash) and lung (1mL/wash) lumens in a separate cohort of chow fed mice and measured IgA in each sequential sample. The amount of IgA in the 1st wash was calculated as a percent of IgA captured by all 4 washes. The mass amount of luminal IgA remaining after the first wash was also calculated as a percentage of IgA measured in the tissue samples. This analysis was not reliable for the nasal passages due to small sample mass and extremely low protein content.
Western blot for pIgR Expression
Solubilized protein was denatured at 95 °C for 10 minutes with sodium dodecylsulfate and β-mercaptoethanol, and protein in each specimen (SIH: 15 μg, NPH: 6 μg and LH: 20 μg) was separated in a denaturing 10% polyacrylamide gel by electrophoresis at 150V for 1 h at room temperature. The proteins were transferred to a polyvinylidinefluoride (PVDF) membrane using a Tris-glycine buffer plus 20% methanol at 80 V for 50 min at 4°C. The membrane was blocked with blotto for 1 h at room temperature with constant agitation. Membranes were incubated with the primary antibody, rabbit anti-mouse secretory component (SC) IgG diluted in blotto (1: 20,000 for SIH, and 1: 1,000 for NPH and LH) for 3 h at room temperature with constant agitation. Membranes were washed and incubated with stabilized goat anti-rabbit-IgG HRP conjugate (Pierce Biotechnology, Rockford, IL) diluted 1: 5,000 for 1.5 h at room temperature with agitation. After the last wash, the membrane was incubated for 5 min with the substrate for HRP (Supersignal West Femto Maximum Sensitivity Substrate, Pierce Biotechnology, Rockford, IL) and bands were detected using photographic film. Densitometric measurements of protein bands were analyzed and quantified with the NIH Image J software. The anti-SC antibody used in this Western blot detected 2 bands at ∼120 kDa and ∼94 kDa representing pIgR and secretory component (SC), respectively [2, 27]. The combined value of these two bands was determined for the quantitation of the pIgR protein expression in each case [1]. pIgR values were expressed as a percentage of the Chow case with the strongest band (100%) on each gel.
Statistical Analysis
All values (including pIgR percentages) are expressed as mean ± SE. Statistical analysis was performed by unpaired Student's t-test. Significance was set at p < 0.05.
Results
There were no significant differences in initial body weights and no significant differences in body weight change during feeding between Chow and PN groups (Table 1). PN significantly reduced both intestinal and respiratory tract IgA levels confirming our previous observations and assuring that the conditions to interpret the pIgR data existed in this experiment.
Table 1. Mice body weight and weight change.
Values are expressed as mean ± SE.
There were no significant differences in initial body weight or weight change after feeding between Chow and PN groups.
| Group | n | Body weight (g) | Weight change (g) |
|---|---|---|---|
| Chow | 10 | 33.17 ± 1.00 | - 1.99 ± 0.39 |
| PN | 10 | 30.81 ± 0.97 | - 3.30 ± 0.59 |
The sequential washing experiment revealed that the initial washing captured 89% and 68% of the total luminal IgA collected from the small intestinal and lung lumens, respectively, in chow fed mice. However, the mass amounts of IgA left in the lumen were < 5% and <2% of tissue IgA measured in the small intestine and lung respectively and thus extremely unlikely to significantly influence these results.
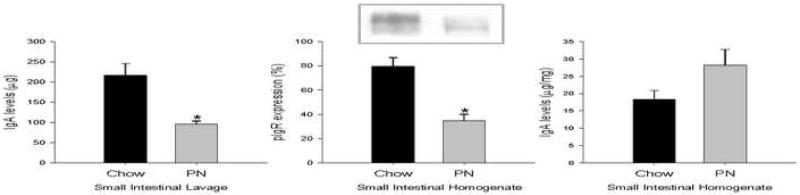
In the intestine, both IgA levels in SIL and pIgR expression in SIH decreased significantly with PN feeding compared to Chow. There were no significant differences in homogenate IgA levels between the two groups (Figure 2). These results implicate impaired transport as a mechanism for decreases in luminal IgA.
Figure 2. Immunoglobulin A (IgA) and polymeric immunoglobulin receptor (pIgR) in the small intestine.
Parentreral nutrition (PN) fed mice displayed significantly decreased small intestinal lavage IgA (a) and small intestinal homogenate pIgR (b) levels. There was no significant difference in small intestinal homogenate IgA levels (c). Two bands ∼120 (pIgR) and ∼94 (secretory component) kDa were detected by Western blot (b). Values are expressed as mean ± SE. *p<0.05 vs. Chow.
In nasal passages, IgA levels in NAL and in the NPH significantly dropped with PN feeding compared to Chow. pIgR expression in the NPH did not significantly change between the two groups (Figure 3), implying intact transport but reduced production in the upper respiratory tract.
Figure 3. Immunoglobulin A (IgA) and polymeric immunogloblin receptor (pIgR) in the nasal passages.
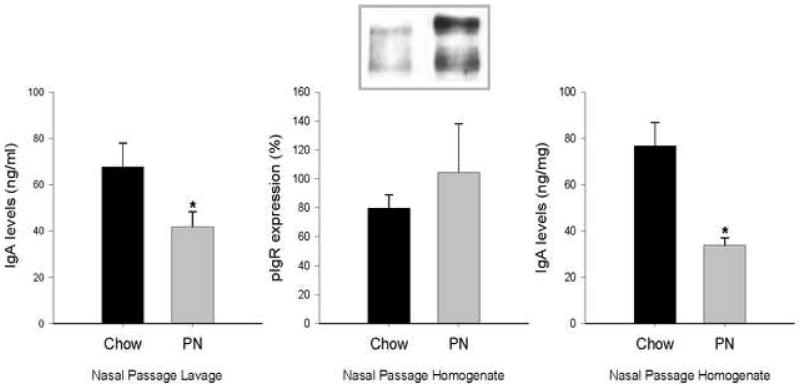
Nasal passage lavage (a) and nasal passage homogenate (c) IgA levels were significantly decreased in parenteral nutrition (PN) groups. Nasal passage homogenate pIgR levels (b) did not change with PN treatment. Two bands ∼120 (pIgR) and ∼94 (secretory component) kDa were detected by Western blot (b). Values are expressed as mean ± SE. *p<0.05 vs. Chow.
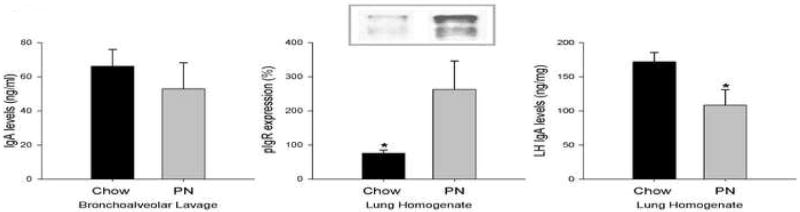
In the lung, BAL IgA levels were similar between Chow and PN groups. Homogenate IgA levels however were significantly decreased in the PN group (Figure 4) while pIgR levels were significantly higher in the PN group compared to Chow. These results imply intact or possibly up regulated transport, but reduced production in the lower respiratory tract.
Figure 4. Immunoglobulin A (IgA) and polymeric immunoglobulin receptor (pIgR) in the lung.
Parenteral nutrition (PN) fed mice had significantly decreased lung homogenate IgA (c) levels compared to Chow. There was no significant difference in bronchoalveolar lavage IgA (a) levels between Chow and PN fed mice. Lung homogenate pIgR levels were increased in PN animals. Two bands ∼120 (pIgR) and ∼94 (secretory component) kDa were detected by Western blot (b). Values are expressed as mean ± SE. *p<0.05 vs. Chow
Discussion
Significant clinical and experimental evidence shows that severely injured patients fed enterally incur less infectious morbidity compared to patients fed parenterally or starved. In studies of severely injured trauma patients, individuals randomized to enteral feeding sustained significantly fewer pneumonias and intraabdominal abscesses than an injured group fed parenterally [15, 20-22]. Experimentally, our work and that of others show that route and type of nutrition influence established mucosal immunologic defenses [13, 16], intraperitoneal cytokine and immunologic responses [18], and systemic inflammatory responses to injury [6-8]. This work focuses on PN induced alterations in mucosal immunity.
Mucosal immune responses are initiated when luminal antigen is taken up by M cells on the surface of Peyer's patches and processed within this structure leading to activation of T & B cells. Naïve T and B lymphocytes are attracted to the Peyer's patches by a specific adhesion molecule, mucosal addressin cellular adhesion molecule-1, and are sensitized to the processed antigen. The lymphocytes migrate through mesenteric lymph nodes, the thoracic duct and back into the blood stream and home to the lamina propria in the respiratory tract and intestine (as well as all other mucosal sites) where the mature lymphocytes produce dimeric IgA [14] The IgA binds to pIgR located on the basolateral surface of epithelial cells. pIgR has 7 domains: 5 extracellular, 1 transmembrane, and 1 cytoplasmic. This pIgR-IgA complex is endocytosed by epithelial cells, and transported to the apical cell surface. The complex is released into the lumen as secretory IgA by cleavage of the extracellular part of pIgR, also called secretory component [12, 23]. Secretory IgA prevents invasive infection by blocking pathogen attachment to mucosal surfaces.
Our work consistently shows that chow feeding maintains normal mucosal defenses but alterations in route and type of nutrition significantly affect MALT and GALT with direct effects on the main antigen-specific mucosal immune defense, IgA. Parenteral feeding (with no enteral stimulation) significantly reduces intestinal and respiratory defenses by decreasing levels of molecules which are responsible for directing T & B cells into and through the mucosal immune network [9, 10]. Such changes contribute to impaired mucosal immune function and integrity through several means including (among others) IgA production and IgA transport. We documented that PN decreases the number of T & B cells in the intestine [17] and lung (unpublished data), reduces the number antibody-forming cells in the upper respiratory tract (reduced production) in response to an acute infection [11], reduces levels of Th2-type IgA stimulating cytokines (reduced production), such as IL-4 and IL-10 [31], and reduces levels of pIgR protein within the intestinal mucosa (reduced transport) [26]. The overall effect of these alterations reduce available IgA production (reduced T & B cells and Th2-type cytokines) and transport (lowered intestinal pIgR levels) to impair or eliminate established anti-viral and anti-bacterial defenses in our murine model [13, 16]. In one previous experiment, however, we documented that respiratory protection against the A/PR8 (H1N1) influenza virus was preserved in PN-fed mice when influenza-specific monoclonal polymeric IgA was provided exogenously [25]. This suggested that airway transport was still adequate to provide protection if adequate IgA was provided and led to our hypothesis that PN induces unique tissue-specific effects on transport and production.
The present work confirms this hypothesis by quantifying levels of IgA and pIgR in tissue homogenates, and luminal IgA in the intestine, lung and upper respiratory nasal passages. Lumen lavage specimens quantify the levels of IgA secreted onto mucosal surfaces by the epithelial cells while tissue homogenate IgA reflects IgA available for transport by pIgR, and pIgR protein levels measure an important component of the transport process. IgA levels in lavage specimens from the nasal passages and intestine dropped significantly with PN compared to chow fed mice replicating prior observations in regard to this particular outcome [25, 31] and assuring that the model functioned and reliably reproduced conditions to interpret the new findings. Uniquely, this work demonstrates organ specific effects of enteral stimulation - or lack of enteral stimulation - on tissue (not luminal) IgA levels and the critical IgA transport protein, pIgR. Tissue homogenate IgA levels drop significantly in lung and nasal passages of PN-fed mice, but levels are maintained in the small intestine. PN-fed mice preserve pIgR expression in the nasal passage and seem to augment pIgR levels in the lung consistent with our observations that adequate residual transport is present in the upper respiratory tract when IgA is provided exogenously [25]. Our data implies that PN has less effect on respiratory transport (although we limited our measurement to only one component of transport) than on production. This is consistent with our observations that acute infection leads to lower numbers of IgA producing cells in the nasal passages of PN-fed mice [11] and the low levels of tissue homogenate IgA found in our specimens. Conversely, intestinal pIgR levels drop with PN, while SI tissue homogenate IgA stays unchanged. The result of decreased pIgR expression in small intestine after PN feeding remains consistent with our hypothesis because small intestinal levels of IL-4 (a Th-2 cytokine that stimulates pIgR production) decreases in PN-fed mice [31]. This almost certainly implicates impaired transport as a main factor in the decrease of SI mucosal surface IgA levels with PN.
There are several limitations to this study. First, the IgA compartments (luminal by lavage, and tissue by homogenization) cannot be completely separated. We investigated the effect of the initial lavage on levels of IgA remaining in the lumen by measuring IgA in sequential washings of chow fed mice to address this problem. The initial lavage captured the majority of the IgA collected by this method in the intestine and lung with the amount of remaining luminal IgA small compared to levels measured in the tissues (< 5% in intestine and < 2% in lung). This residual surface IgA is unlikely to significantly falsely elevate such measurements particularly in PN fed mice that have even lower luminal IgA levels than the chow fed mice. Secondly, we cannot sub-localize “tissue” IgA to the lamina propria (production) or to the epithelial cell layer (in transit) with current techniques. Without such an assay, the intestinal tissue homogenate IgA results should be interpreted with caution but we are reluctant to believe that IgA production would trend towards increase in PN animals given our previous data showing dramatic, 50% reductions in T & B cell numbers in the gut lamina propria [17] and decreases in IL-4 and IL-10, two important IgA stimulating cytokines, with PN [31]. Such data would suggest that IgA production is profoundly affected in the small intestine with PN. We believe this trend towards increased tissue IgA may be partially an artifact since our results are expressed as IgA mass/mg protein. We recently noted in a pilot study that total small intestinal protein dropped by almost 40% in PN vs. chow-fed mice (unpublished data). Lavage IgA results are not subject to such influences since they are measured in mass/volume of lavage effluent and the lavage volume is constant for all animals. A specific ELISA for detecting murine secretory component could potentially aid in solving this problem of IgA “sub-localization” but currently such an assay is unavailable since the anti-SC antibody used for Western blotting does not work in an ELISA. However, we acknowledge the potential value of immunohistochemical studies to complement the current work and are developing such assays after quantifying this process using whole tissue samples. Traditional cross sectional immunohistochemical analysis may be problematic to study events in the lamina propria [32] and we are approaching such analysis with caution.
In the context of previously published work, these results continue to develop mechanistic explanations for the primary end effects of PN feeding on respiratory immunity; namely, decreased mucosal IgA levels and an inability to generate new immunity or utilize previously acquired responses in the respiratory tract. This work also provides evidence that alterations in type and route of nutrition alter the mucosal immune components of respiratory and intestinal organ systems in different ways. Clinically this may prove an important distinction and future efforts will be designed to discern the basic mucosal immune alterations of IgA production and transport that are organ/tissue specific. While mucosal immune lymphocytes are thought to be educated in the gut before being distributed throughout the body, local signals at their final destination likely effect their terminal function. We previously showed that chemokine mRNA and protein levels are altered in a tissue specific manner with PN [33]. Cytokine profiles have also been shown to be tissue specific [34] which likely influences immune cell function within those tissues.
In summary, reduction in pIgR expression appears primarily responsible for reduced secretory IgA levels in the small intestine of PN-fed mice. On the other hand, PN appears to reduce IgA production rather than transport (at least via pIgR) in the respiratory tract.
Acknowledgments
The authors thank the Yakult Central Institute for Microbiological Research for providing the SC antibody.
Source of support: NIH grant R01 GM53439 and also based upon work supported in part by the Office of Research and Development, Biomedical Laboratory R&D Service, Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackermann LW, Denning GM. Nuclear factor-kappaB contributes to interleukin-4- and interferon-dependent polymeric immunoglobulin receptor expression in human intestinal epithelial cells. Immunology. 2004;111:75–85. doi: 10.1111/j.1365-2567.2003.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahi Y, Yoshikawa T, Watanabe I, Iwasaki T, Hasegawa H, Sato Y, Shimada S, Nanno M, Matsuoka Y, Ohwaki M, Iwakura Y, Suzuki Y, Aizawa C, Sata T, Kurata T, Tamura S. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol. 2002;168:2930–2938. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- 3.Asanuma H, Inaba Y, Aizawa C, Kurata T, Tamura S. Characterization of mouse nasal lymphocytes isolated by enzymatic extraction with collagenase. J Immunol Methods. 1995;187:41–51. doi: 10.1016/0022-1759(95)00165-7. [DOI] [PubMed] [Google Scholar]
- 4.Asanuma H, Thompson AH, Iwasaki T, Sato Y, Inaba Y, Aizawa C, Kurata T, Tamura S. Isolation and characterization of mouse nasal-associated lymphoid tissue. J Immunol Methods. 1997;202:123–131. doi: 10.1016/s0022-1759(96)00243-8. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg P, Farstad IN, Johansen FE, Morton HC, Norderhaug IN, Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukatsu K, Kudsk KA, Zarzaur BL, Sabek O, Wilcox HG, Johnson CD. Increased ICAM-1 and beta2 integrin expression in parenterally fed mice after a gut ischemic insult. Shock. 2002;18:119–124. doi: 10.1097/00024382-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Fukatsu K, Lundberg AH, Hanna MK, Wu Y, Wilcox HG, Granger DN, Gaber AO, Kudsk KA. Increased expression of intestinal P-selectin and pulmonary E-selectin during intravenous total parenteral nutrition. Arch Surg. 2000;135:1177–1182. doi: 10.1001/archsurg.135.10.1177. [DOI] [PubMed] [Google Scholar]
- 8.Fukatsu K, Lundberg AH, Hanna MK, Wu Y, Wilcox HG, Granger DN, Gaber AO, Kudsk KA. Route of nutrition influences intercellular adhesion molecule-1 expression and neutrophil accumulation in intestine. Arch Surg. 1999;134:1055–1060. doi: 10.1001/archsurg.134.10.1055. [DOI] [PubMed] [Google Scholar]
- 9.Fukatsu K, Zarzaur BL, Johnson CD, Wu Y, Wilcox HG, Kudsk KA. Decreased MAdCAM-1 expression in Peyer patches: a mechanism for impaired mucosal immunity during lack of enteral nutrition. Surg Forum. 2000;51:211–214. [Google Scholar]
- 10.Ikeda S, Kudsk KA, Fukatsu K, Johnson CD, Le T, Reese S, Zarzaur BL. Enteral feeding preserves mucosal immunity despite in vivo MAdCAM-1 blockade of lymphocyte homing. Ann Surg. 2003;237:677–85. doi: 10.1097/01.SLA.0000064364.40406.EA. discussion 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson CD, Kudsk KA, Fukatsu K, Renegar KB, Zarzaur BL. Route of nutrition influences generation of antibody-forming cells and initial defense to an active viral infection in the upper respiratory tract. Ann Surg. 2003;237:565–573. doi: 10.1097/01.SLA.0000059991.89316.B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 13.King BK, Kudsk KA, Li J, Wu Y, Renegar KB. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. 1999;229:272–278. doi: 10.1097/00000658-199902000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183:390–398. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 15.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, Kuhl MR, Brown RO. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–11. doi: 10.1097/00000658-199205000-00013. discussion 511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996;223:629–35. doi: 10.1097/00000658-199606000-00001. discussion 635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–51. doi: 10.1097/00005373-199507000-00006. discussion 51-2. [DOI] [PubMed] [Google Scholar]
- 18.Lin MT, Saito H, Fukushima R, Inaba T, Fukatsu K, Inoue T, Furukawa S, Han I, Muto T. Route of nutritional supply influences local, systemic, and remote organ responses to intraperitoneal bacterial challenge. Ann Surg. 1996;223:84–93. doi: 10.1097/00000658-199601000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 20.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma--a prospective, randomized study. J Trauma. 1986;26:874–881. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, Kellum JM, Jr, Welling RE, Moore EE. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989;29:916–22. doi: 10.1097/00005373-198907000-00003. discussion 922-3. [DOI] [PubMed] [Google Scholar]
- 23.Mostov KE, Friedlander M, Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984;308:37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- 24.Parrott DM. The gut as a lymphoid organ. Clin Gastroenterol. 1976;5:211–228. [PubMed] [Google Scholar]
- 25.Renegar KB, Kudsk KA, Dewitt RC, Wu Y, King BK. Impairment of mucosal immunity by parenteral nutrition: depressed nasotracheal influenza-specific secretory IgA levels and transport in parenterally fed mice. Ann Surg. 2001;233:134–138. doi: 10.1097/00000658-200101000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano Y, Gomez FE, Kang W, Lan J, Maeshima Y, Hermsen JL, Ueno C, Kudsk KA. Intestinal Polymeric Immunoglobulin Receptor is Affected by Type and Route of Nutrition. JPEN J Parenter Enteral Nutr. 2007;31:351–357. doi: 10.1177/0148607107031005351. [DOI] [PubMed] [Google Scholar]
- 27.Shimada S, Kawaguchi-Miyashita M, Kushiro A, Sato T, Nanno M, Sako T, Matsuoka Y, Sudo K, Tagawa Y, Iwakura Y, Ohwaki M. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol. 1999;163:5367–5373. [PubMed] [Google Scholar]
- 28.Sitren HS, Heller PA, Bailey LB, Cerda JJ. Total parenteral nutrition in the mouse: development of a technique. JPEN J Parenter Enteral Nutr. 1983;7:582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 29.Underdown BJ, Schiff JM. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- 30.van der Heijden PJ, Stok W, Bianchi AT. Contribution of immunoglobulin-secreting cells in the murine small intestine to the total ‘background’ immunoglobulin production. Immunology. 1987;62:551–555. [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229:662–7. doi: 10.1097/00000658-199905000-00008. discussion 667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald KG, Newberry RD. Whole-mount techniques to evaluate subepithelial cellular populations in the adult mouse intestine. Biotechniques. 2007;43:50–56. doi: 10.2144/000112514. [DOI] [PubMed] [Google Scholar]
- 33.Hermsen JL, Gomez FE, Sano Y, Maeshima Y, Kang W, Kudsk KA. Decreased enteral stimulation alters mucosal immune chemokines. JPEN J Parenter Enteral Nutr. doi: 10.1177/014860710803200136. in press. [DOI] [PubMed] [Google Scholar]
- 34.Perl M, Gebhard F, Knoferl MW, Bachem M, Gross HJ, Kinzel L, Strecker W. The pattern of preformed cytokines in tissues frequently affected by blunt trauma. Shock. 2003;19:299–304. doi: 10.1097/00024382-200304000-00001. [DOI] [PubMed] [Google Scholar]