Abstract
Scientists have used numerous techniques to measure organismal metabolic rate, including assays of oxygen (O2) consumption and carbon dioxide (CO2) production. Relatively few studies have directly compared estimates of metabolic rate on the same groups of animals as determined by different assay methods. This study directly compared measures of the metabolic rate of three lines of Drosophila simulans as determined either from direct measures of CO2 production using infrared gas analysis (IRGA), or from estimates of O2 consumption based on manometeric techniques. Determinations of metabolic rate of the same cohorts of flies using these two methods produced results that often differed widely. Typically metabolic rate as determined by the manometric method was significantly greater than that determined by CO2 output. These differences are difficult to explain by simple biotic or abiotic factor/s. Because of the idiosyncratic nature of these differences it is not possible to use a simple factor to convert from metabolic rate measurements done using manometric techniques to those expected from direct measures of CO2 output or O2 consumption. Although manometric devices are simple to construct and use, measurements of metabolic rate made with this method can vary significantly from measurements made by directly assaying CO2 production or O2 consumption.
Keywords: Drosophila, metabolic rate, oxygen consumption, IRGA
Introduction
The rate of metabolism of an organism integrates fundamental aspects of its physiology, perhaps more so than most other physiological parameters (Bartholomew, in Gordon et al. 1972). Lavoisier, along with Laplace, first recognized that metabolism was a combustion process similar to that which takes place when a candle burns, and that both animals and burning candles required oxygen (O2) (Poirier, 1996). Experimenting with assays of metabolism, these authors measured heat production of a guinea pig (Cavia sp.) using an ice calorimeter, and indirectly quantified metabolism of the same species by assessing its O2 consumption (Kleiber 1975). These vanguard measurements of O2 consumption were novel, yet imprecise for several reasons, most notably because they failed to account for production of water (H2O) vapor from respiratory passages and skin of the guinea pig in their calculations (Kleiber 1975).
Since these seminal determinations of metabolism, a plethora of scientists have employed a variety of techniques to measure metabolic rate, including direct assessment of heat production, assays of O2 consumption, often by sophisticated electronic instrumentation, carbon dioxide (CO2) production by IRGA, and food consumption. Although these methods have been used in thousands of determinations of metabolic rate, relatively few studies have examined potential errors in their determinations or compared estimates of metabolic rate on the same groups of animals as estimated by different assay methods.
For arthropods, a standard manometric assay for O2 consumption uses a constant pressure “microrespirometer” originally devised by Engelmann (1963), and subsequently modified by other investigators (Conrradi-Larsen, 1974; Lee and Baust, 1982; Lee, 1995). These devices are low in cost, simple to construct, and are thought to provide relatively sensitive measurements of O2 consumption (Lee, 1995). The microrespirometer is typically fashioned from a disposable plastic syringe to which has been glued a micropipette at one end. Animals are placed in the barrel of the syringe, the plunger inserted to form a seal, and then the entire assembly placed in a constant temperature chamber. The total pressure of gases in the system is Pt = pN2 + pO2 + p CO2 + pH2O. Measurements of O2 are based on the stoichiometric principle that for each molecule of O2 consumed, one molecule of CO2 is released when carbohydrate is the primary energy source. Because the partial pressure of CO2 is thought to be held near zero by placing an absorbent, such as KOH or NaOH, inside the syringe, or in some cases, within the barrel of the micropipette, decreases in overall pressure within the syringe are said to be the result of O2 consumption, as monitored by movement of fluid in the micropipette (Dixon, 1951; Lee, 1995). An empty microrespirometer often serves as a control for minor fluctuations in temperature and pressure. Like experiments of Lavoisier and Laplace, one underlying assumption contained within the above method is that pressure changes attributable to respiratory and cutaneous H2O losses from the animal are negligible, or that the CO2 absorbent maintains a constant H2O vapor pressure within the syringe during an assay period. However, since the overall reaction of CO2 with NaOH or KOH produces H2O, [NaOH + CO2 → Na2CO3 + H2O], the later alternative is likely not true. Manometric methods are still commonly used to measure metabolic rates of arthropods, particularly in relatively small insects such as Drosophila, but we have been unable to find a validation of their efficacy for measuring O2 consumption (Sohal et al., 1993; Ross, 2000; Hulbert et al., 2004).
In this study, we tested the accuracy of the manometric method for determining metabolic rate of small arthropods. We compared the metabolic rate of Drosophila simulans as determined either from direct measures of CO2 production using IRGA, or from estimates of O2 consumption based on manometeric techniques. To determine if either genetic differences or age influenced the relationship between metabolic rate as measured by manometric or IRGA methods, we compared metabolic rates between three different lines of flies at four different ages. The inclusion of several different fly lines at numerous different ages gives us confidence in the generality of our results. The main advantage of IRGA is that it is a highly sensitive technique that can be used to accurately measure the quantity of CO2 produced by small organisms (± 1ppm CO2), such as by an individual Drosophila, in real time (Dickinson and Lighton, 1995; Berrigan and Partridge, 1997; Van Voorhies, Khazaeli and Curtsinger, 2004), but it also requires expensive equipment.
Our results suggest that measurements of metabolic rate as determined by the microrespirometry-manometric method significantly overestimated metabolism compared to IRGA. Estimates of O2 consumption by the manometric method deviated by as much as 50% from those based on CO2 measurements, a lack of concordance that is difficult to explain. Because we could not detect systematic errors in our manometric determinations, construction of simple conversion factors to adjust data from our manometric method was not possible. We conclude that although manometric devices are simple to construct and use, measures of metabolic rate taken with this method can vary widely from measures of metabolic rate taken by directly assaying for changes in CO2 or O2 levels. Therefore in any study using the microrespirometer method, we recommend that tests of assumptions be performed prior to publishing results.
Materials and Methods
Fly Lines
We measured rates of metabolism of non-virgin males from three D. simulans fly lines; HW09 was collected from Hawaii in 1998, NC48 from New Caledonia in 1991, and MD106 from Madagascar in 1998 (Ballard, 2000). In 2004, lines were sibling-mated for 5 generations to reduce genetic heterogeneity. Fly lines were treated with tetracycline to eliminate Wolbachia infection >5 generations before the study commenced (James and Ballard, 2003).
Flies used in the study were raised as a single cohort at University of Iowa except for the group of flies used to simultaneously compare metabolic rates using manometric and IRGA methods in New Mexico. Two generations prior to the study, flies were maintained at low density on instant Drosophila media (Carolina Biological, Burlington, NC) in 250-ml glass bottles. Parents of the flies used in the study were released into population cages and allowed to acclimate for 2 to 3d. Oviposition resources were provided for 4-h periods to collect eggs. Larvae were reared at a consistent density by transferring ∼200 eggs onto instant Drosophila medium in 250-ml bottles (Clancy and Kennington, 2001). Four days after emerging from pupae, flies were immobilized on ice, sorted by sex, and non-virgin males were placed in 1-L plastic population cages at an initial density of about 100 flies per cage (Tu, Epstein and Tatar, 2002). During our studies, fly lines were maintained at 23±1°C, 50% RH, and 12h light: 12h dark daily cycle. Media was changed and dead flies were removed every 2d. Flies assayed at New Mexico State University were raised at University of Iowa, transported via overnight delivery as pupae or adult flies to New Mexico, and maintained as described above.
Infrared CO2 gas analysis
The real time metabolic rate of a single Drosophila can easily be measured using modern flow-through respiratory methods (Dickinson and Lighton, 1995; Lighton and Schilman, 2007; Lighton, 2007). However, we did not use flow-through respiratory to measure fly metabolic rate because we wanted to keep the conditions under which IRGA based metabolic rates were determined as similar as possible to those used for manonmeteric based methods.
After immobilizing them by cooling on crushed ice, we placed individual flies into 2.2-ml glass chambers, sealed with a rubber stopper. Chambers were flushed for 15s at a flow rate of 90ml/min with CO2-free, water-saturated (100% RH) room air introduced through a 22-guage needle with a second needle inserted to vent the chamber. After removing both needles, we allowed each fly to respire for 1 h. The gas stream used to flush the chambers was hydrated by flowing it through a series of glass syringes filled with sterile H2O and cotton wool. The water and syringes were autoclaved and after cooling a low flow of pure nitrogen was continuously run thorough the syringes for several days previous to their being used to fill the fly metabolic chamber. The water vapor content of the air stream entering the metabolic chamber was at essentially 100% R.H (18.7 mg H2O /l) and contained 1-2 ppm of CO2 as assayed with a Li-Cor 6262 CO2/H2O analyzer (Li-Cor, Lincoln, Nebraska). The amount of CO2 was a small fraction of the final CO2 level produced by a fly at the end of the sample period (typically between 500-1000 ppm CO2). Thereafter we removed a 1-ml (stpd) sample of the chamber gas using a Hamilton SampleLock syringe (Hamilton, Reno, Nevada) and injected it directly into a 150 ml/min (±1%) stpd, CO2-free carrier air stream scrubbed of water with a magnesium perchlorate (MgClO4) filter located before a Li-Cor 6251 CO2 gas analyzer, but after the site of sample injection. The flow rate of the carrier air stream was determined on dry (dew point <-60°C) air. The measurement chamber was flushed again with CO2-free air, and 1h later a second sample was taken. The quantity of CO2 produced by an individual fly was calculated from the second CO2 measurement using Datacan software (Sable Systems International, Las Vegas, NV).
The quantity of CO2 produced was converted to O2 consumption by directly determining the respiratory quotient (RQ) on groups of flies and using this value to calculate O2 consumption. Several empty chambers were included in the sampling to control for background CO2, presence of CO2 in the air used for flushing, and to check for gas leakage in the assay chambers. Analysis of these chambers indicated that background CO2 levels or chamber leakage was typically <10 ppm. Over a 1 hour period a single fly typically produced between 2-3 μl of CO2. The reading from an empty chamber over this time was <5% of this amount and was consistent within and between sets of measurements. We found that the small amount of CO2 in the blank was very close to the amount the remained adsorbed to the metabolic chamber at the end of a sample interval. For this reason we did not subtract out the blank CO2 reading from that of the fly.
After the second infrared CO2 gas analyzer measurement, individual flies were frozen at - 80°C and thereafter weighed on a Sartorius M2P microbalance to the nearest 0.01mg (Sartorius, Goettingen, Germany).
The CO2 gas analysis system was zeroed daily against CO2-free air, and spanned regularly against a 51 ppm certified gas standard (Air Products, Long Beach, CA). The zero CO2 standard was obtained by pumping room air through a Pure-Gas generator, (MTI, Denver Colorado), for initial removal of CO2 and water vapor and then running the outflow through an Ascarite filter. Direct comparison showed that an air stream scrubbed of CO2 in this manner was equivalent to zeroing the analyzer with pure nitrogen. The accuracy of the CO2 analysis system was also checked by injecting in gas samples containing a known CO2 concentration into the gas sampling system. The CO2 concentration of a sample was approximately equal to that produced by a fly in a typical metabolic measurement. This method of calibration checks the accuracy of the total metabolic analysis system, including the mass flow meter and sampling syringe. When tested by this method the calculated CO2 value was within 2% of the expected reading.
Oxygen consumption and respiratory quotient determination
To compare the relationship between CO2 production and O2 consumption and to determine the RQ of the three fly lines, we placed 4d or 14 d old post-emergent flies into 50 ml glass chambers for 1h. To increase the signal for our instrument that measured oxygen concentration, we placed 10 flies in the chamber for each measurement. The chambers were kept in an illuminated, custom-designed environmental chamber at 23°C. At the end of the sample interval, air in the chamber was flushed into a Li-Cor 6251 CO2 analyzer linked in series to a Sable Systems Oxzilla oxygen analyzer. The Li-Cor analyzer has a sensitivity of <0.1ppm and an accuracy of <1ppm while the Oxzilla analyzer has a sensitivity of 0.0001% and an accuracy of 0.1%. The sample was scrubbed of water vapor with MgClO4 before entering the analyzers. CO2 production and O2 consumption were determined using equations in the Sable Systems data analysis software. Means ± 1SEM were determined for 18 groups of 14 day post-emergent flies, and 9 groups of 4 day post-emergent flies.
Water Loss
To measure water loss from flies exposed to a low water vapor pressure, we exposed three groups of 10 flies each to dried air. Flies were placed in 50 ml glass chambers that were sealed with stainless steel Swagelock valves and custom machined Viton O-ring sealed plugs. The chambers were flushed six times for 30s with a MgClO4 dried airstream at a flow of 150ml/min. The flies were then sealed in the chamber for 30min and the total amount of water vapor in the sample was calculated by running the outflowing airstream into a LiCor 6262 CO2/H2O analyzer.
Manometers
This study utilized a single manometric procedure using manometers that were constructed following recently published protocols (Poirier, 1996; Ross, 2000; Hulbert et al., 2004). Five replicate manometers were prepared for each D. simulans line on each experimental day. Ten male flies were immobilized on ice, transferred into manometer chambers, and a small cotton plug inserted to confine flies. Flies were chilled <10min and became active in <1min after transfer to manometers. We chilled flies for such a short time because slow recovery from chilling depresses metabolic rate.
CO2 was absorbed by 100μl of saturated KOH solution (Sigma, St. Louis, MO, USA) applied to a 1cm2 piece of Whatman No. 1 filter paper (Whatman, Middlesex, UK), which was inserted behind the cotton plug of our microrespirometer (Chadwick and Gilmour, 1940; Dixon, 1951; Umbreit, 1972). Saturated KOH-soaked papers were chosen because they apparently absorb CO2 rapidly and efficiently (Dixon, 1951). This an advantage over other methods in which only a small drop of KOH solution is placed in the chamber, providing a more limited surface area available for CO2 to react with. A KOH solution in a closed chamber filled with air would be expected to establish and maintain a constant, low relative humidity (approximately 8%) environment (Winston and Bates, 1960).
Flies were acclimated in the chamber for 30 min at 23°C before we measured their respiration rate. Measurements of O2 consumption were initiated by placing a small drop of 50% glycerol containing bromophenol blue into the tip of the microcapillary pipette. After 30min the position of the top edge of the drop was marked and the distance moved in millimeters was recorded. The change in volume due to O2 consumption was calculated and values were adjusted to stpd (Schmidt, 1997).
To express metabolic rate as μl O2/ h×fly, the standardized volume of O2 consumed was divided by assay time and by the number of flies in the manometer. Manometers containing no flies and manometers containing flies and a 1cm2 piece of filter paper soaked in distilled water were included to control for external changes in temperature and pressure during the assay. No flies died during our measurements.
After assays, manometers were placed on ice, and when flies were immobilized, we transferred them to pre-weighed 1.5ml microcentrifuge tubes, and weighed them on an Ohaus Adventurer AR2140 balance to the nearest 0.1mg (Ohaus, Pine Brook, NJ). Weight of one fly was calculated by dividing the total weight of flies by the number of flies in the manometer.
We measured metabolic rate of individual flies by IRGA and by manometers at 4, 11, 18, and 25d post emergence. All IRGA gas analysis was conducted at New Mexico State University. We determined metabolic rate by manometers at the University of Iowa, except for one set of measurements that we made in New Mexico. To determine if transportation and/or assay site affected CO2 production, simultaneous IRGA and manometric metabolic assays were conducted on the same cohort of 4d-old flies in New Mexico. For this experiment, we divided cohorts of flies from the three lines into two groups, one for manometric determination of metabolic rate, the other for IRGA measurement of metabolic rate.
Relative humidity within chambers was likely much lower for flies in our manometers than for flies in glass vials used for IRGA analysis. To determine the effect of humidity level on metabolic rate of flies, 11d- old post-emergent flies were individually placed in 2 ml glass metabolic chambers. These chambers were then flushed with CO2-free air either at 100% relative humidity or 0% RH. The 100% relative humidity gas stream was hydrated using the previously described method for producing high humidity/low CO2-air. For IRGA measurements, we determined CO2 twice for each fly.
A factor potentially influencing the fly metabolic rate as well as the relationship between IRGA and manometric determinations of metabolic rate is the period that the flies were acclimated in the laboratory prior to the measurements. To compare the effect of acclimation time on metabolic rate groups of flies were maintained at New Mexico State University for 14d. The metabolic rate of these flies was then compared to that of members of the same cohorts of flies that were reared at the University of Iowa for 14d and shipped to New Mexico on the day of the metabolic measurement. Metabolic rate measurements of the flies shipped from Iowa were started within 30min of the arrival of the flies in New Mexico. Flies were 18d post-emergent at the time of the measurements.
Statistical Analysis
Data were analyzed using either StatView 5.0.1 or Systat 5.2.1 statistical analysis programs with statistical tests that included ANOVA, Students t-test, and linear regression analysis. Post hoc tests were done using Fisher's LSD.
Results
Manometric vs IRGA determinations of Metabolic Rate
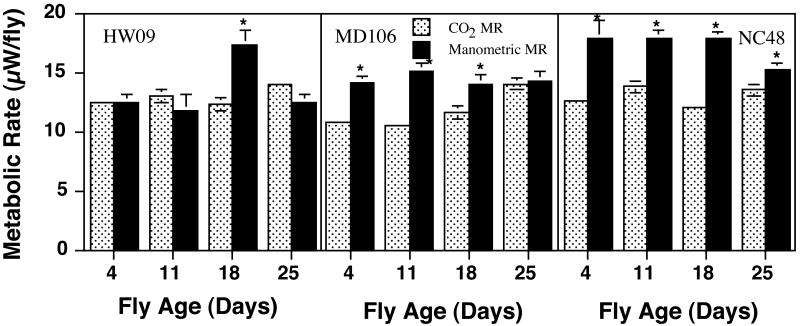
We found significant differences between estimates of fly metabolism generated by the manometric method compared with IRGA (Figure 1). Differences in apparent metabolic rate are difficult to explain without invoking a complex line by age by method interaction. ANOVA showed significant effects of method (F1, 329 = 10.1, P < 0.001) on metabolic rate. Age or method × age interactions were not significant factors affecting metabolic rate ((F1, 329 = 0.21, P = NS), (F1, 329 = 0.66, P = NS). Body mass was not a significant covariate affecting metabolic rate in any of the lines measured.
Figure 1.
Comparison of metabolic rates as measured by CO2 production using IRGA or O2 consumption with manometric methods. Metabolic rates were measured on cohorts of flies from 3 different lines at 4 different ages. Metabolic rates for each line are plotted separately. The hatched bars represent metabolic rate as determined from CO2 flux while the solid bars represent metabolic rate as determined by manometric based assays. Means ± 1SEM are plotted for each group. Sample size varied from 18 to 33 individual flies for the CO2 measurements. Manometric reading were determined on groups of 10 flies with five samples done per line for each age. Asterisks indicate significant differences between the lines at P<0.05.
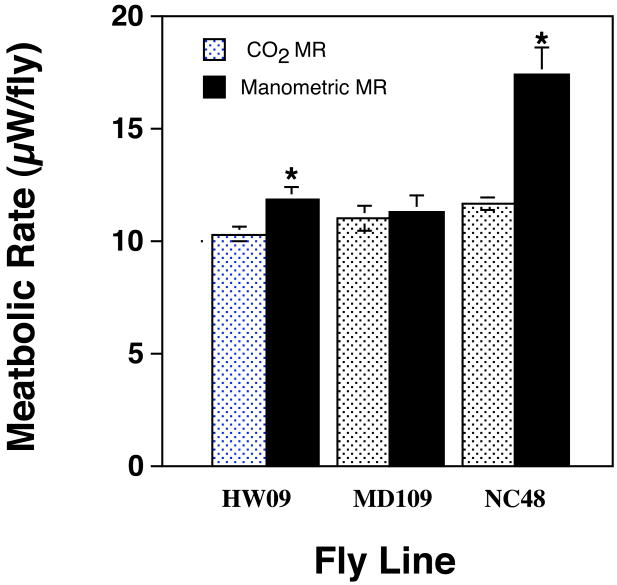
Our experiments also indicated significant differences in metabolic rate when we compared flies of the same cohort measured simultaneously in the same laboratory (Fig. 2). There was a significant difference in metabolic rate between the 6 groups (ANOVA F1, 103, P≪0.001). Post hoc (Fisher's LSD) comparisons of metabolic rates between these groups shows that manometeric estimates of metabolic rate were significantly greater than measurements taken with an IRGA in two of the three lines (Line HW09, P=0.006; Line MD106, P=0.152; Line NC48, P≪0.001).
Figure 2.
Comparison of metabolic rate as estimated by directly measuring CO2 output or from manometric methods on cohorts of flies simultaneously measured in the same location. Sample size was 19-20 individual flies for the CO2 measurements. Manometric readings were determined on groups of 10 flies with 15 samples per line. Asterisks indicate significant differences between the lines at P<0.05.
Oxygen consumption and RQ determination
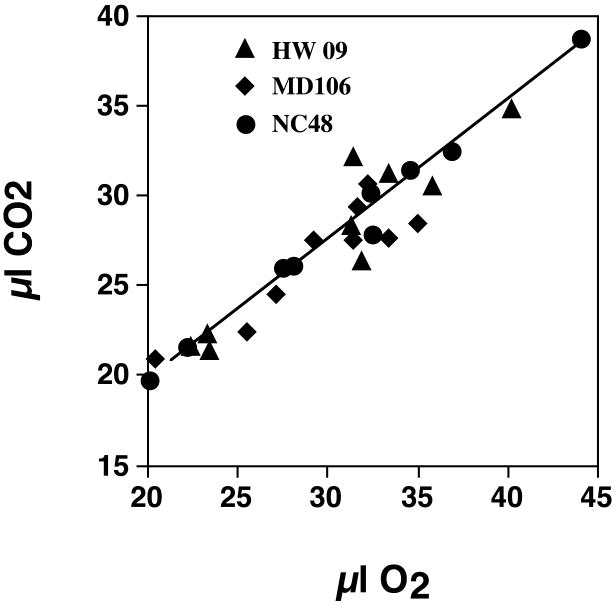
We found a significant correlation between CO2 production and O2 consumption (Fig. 3; r2= 0.91, p<0.001, n=27), but no significant interline differences in RQ of 14 day old flies (ANOVA, F2, 15 =0.91, P=0.42). Because of limited sample size, no statistical comparisons were made between RQ's of 4 day old flies. The RQ of lines averaged 0.91±0.01, n=27. This value was used to convert IRGA derived CO2 values to μW using an energy equivalent of 20.1 kJ/l (Kleiber, 1975).
Figure 3.
Relationship between O2 consumption and CO2 output for male D. simulans lines collected from three different geographic locations (Hawaii, New Caledonia and Madagascar).
Relative Humidity, Water Loss and Metabolic Rate
After a 30 min exposure to air with 0% RH, flies lost an average of 0.035 mg H2O/fly. Because these measurements were done in a closed chamber, in which flies gradually increased water vapor pressure, we think that our estimate of water loss is likely an underestimate. At the end of the sample period, flies had increased the RH of the chamber from 0% RH to approximately 40% RH.
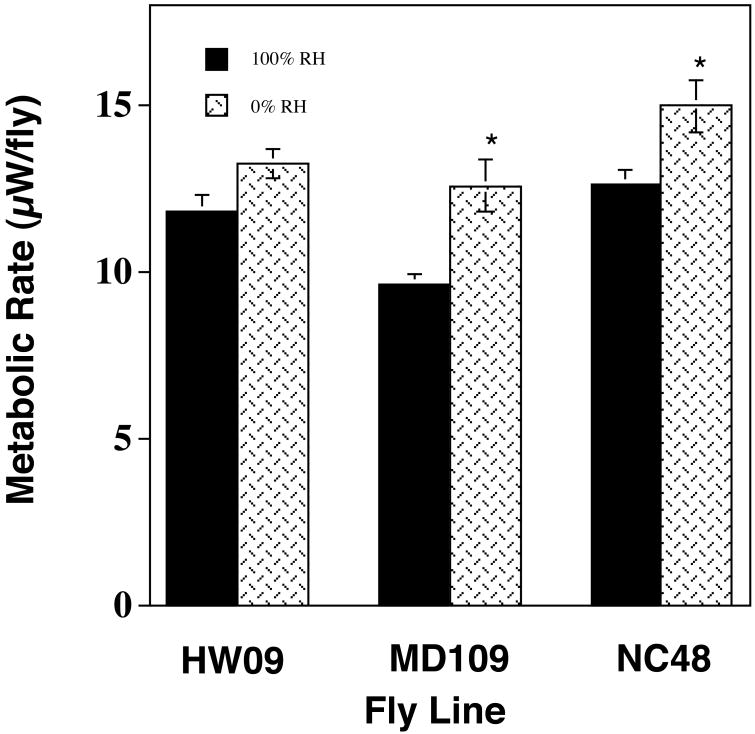
Even under these conditions, relative humidity of the chamber could have a significant effect on fly metabolic rate (Figure 4). Metabolic rates of flies in dry air were often elevated compared to that of flies placed in water saturated air. This difference became more pronounced with the amount of time that the fly was in the chamber. The second metabolic reading were significantly higher in dry air for two of the lines (Student's t-test, Line MD106, t27=4.2, P≪0.001; Line NC48, t28=2.8 P<0.001) but not significant different for the third line (Line HW09, t28=1.6, P=0.12).
Figure 4.
The effect of chamber relative humidity on D. simulans metabolic rate. Individual flies were placed in 2ml glass metabolic chambers which were then flushed with either 100% RH or 0% RH CO2 free air. Since the measurements were done on flies in a closed chamber the actual RH of the flies in 0% RH air would gradually increased over the time due to water loss from the fly. Metabolic comparisons were done between 20 flies exposed to 100% RH air and 10 flies exposed to 0% RH air. Means ± 1SEM are plotted. Asterisks indicate significant differences between the lines at P<0.05.
Transportation and Acclimatization Effects
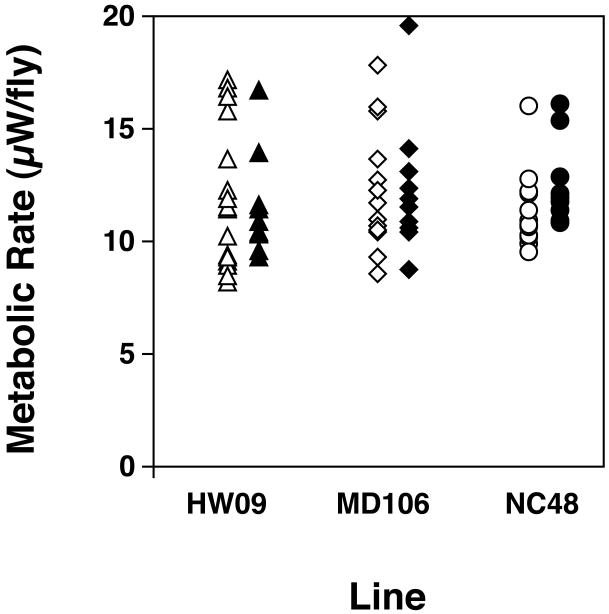
The metabolic rate of flies that had been housed in an incubator in New Mexico for 14 days previous to a determination of metabolic rate were not significantly different from that of flies from the same cohort which were reared in Iowa for 2 weeks and sent via overnight shipping (Figure 5) (Student's t-test, Line HW09, t26=0.2, P=0.83; Line MD106, t27=0.2, P=0.87; Line NC48, t23=0.65 P=0.52).
Figure 5.
Comparison of the metabolic rates of flies that where acclimated in the laboratory in New Mexico for 2 wk (open or closed symbols) verses flies that were shipped overnight from the Iowa lab (open or closed symbols) and were in NM for 30 m before being analyzed. Metabolic rates of flies from both groups were measured at the same time. The flies were sent at one of the hottest times of year in NM (air temperature was 40C on the day the flies arrived). Each symbol plots the metabolic rate of an individual fly. There were no significant differences in metabolic rates between the lines.
Discussion
Although manometric devices are simple to construct and easy to use, we have shown that they can produce an overestimate of metabolic rate that is significantly different than that obtained by directly measuring CO2 production. Of the 15 comparisons done in this study, metabolic rate as estimated by manometric methods was significantly higher than the IRGA estimate in 9 cases and never significantly less than the IRGA based method. Relative differences between manometric and IRGA methods did not follow a consistent pattern, both within and between lines tested, making it impossible to devise a simple conversion factor to correct measurements made by the manometric method. The differences in metabolic rate estimates between manometric and IRGA also do not appear to be due to the assays being conducted at different locations. Simultaneous metabolic measurements done on the same cohort of flies at the same location also found that the manometric based methods typically produced higher estimates of metabolic rate than CO2 based estimates. The fact that the manometric measurements were conducted on groups of 10 flies while the IRGA measurements were done on single flies also appears unlikely to be the factor responsible for the differences in metabolic rate. A study that directly examined the effect of group size on metabolic rate in Drosophila found no significant effect of group size on Drosophila metabolic rate (Van Voorhies et al. 2004).
The most widely accepted measure of metabolic rate is based on the determination of an organisms' oxygen consumption. The argument that CO2 production may not be accurately measuring oxygen consumption is not valid in this study. Because the ratio of CO2 produced to O2 consumed depends on factors such as metabolic substrate utilization and acid base balance, care must be taken when extrapolating from metabolic rate measurements based on CO2 production to International System of Units measures such as Joules or Watts (Frayn, 1983; Gessaman and Nagy, 1988; Walsberg and Wolf, 1995; Schmidt-Nielsen, 1997). In this study direct, simultaneous measures of both CO2 production and O2 consumption indicated that there is an excellent correlation between the two parameters (r2 = 0.914). The correlation coefficient would likely have been even higher had there been a wider range of values in the parameters measured. A previous study comparing the correlation between CO2 production and O2 consumption in Drosophila melanogaster across a wider range of values of CO2 production and O2 consumption found a near perfect correlation (r2 = 0.99) between these two parameters with an average RQ of 0.95 (Van Voorhies et al., 2004).
This study could not identify an abiotic factor that is responsible for the lack of correlation between manometric and IRGA based methods of determining metabolic rate. This makes it likely that an intrinsically variable factor, such as a humidity dependent change in activity level is responsible for the differences in metabolic measurements. When determining metabolic rates using manometric methods the hydroscopic nature of the KOH used to absorb CO2 potentially exposes the animal to a low humidity environment during the measurement. In contrast, in direct measures of gas exchange such as used in CO2/O2 based methods of metabolic determination, chamber humidity can be maintained up to 100% relative humidity levels as long as the water vapor is removed from the air stream before it enters the analyzers. This may be one factor responsible for the relative elevation of metabolic rates of flies measured in manometers compared to IRGA. The relatively small size of Drosophila means that a low humidity environment would provide a desiccation stress for the animal. In two of the lines tested, desiccation stress induced a significant increase in metabolic rate compared to flies in chambers with fully water-saturated air. The hypothesis that Drosophila exposed to a low humidity environment would show an increased metabolic rate relative to flies in high water vapor content air is supported by data showing that Drosophila melanogaster exposed to desiccation stress show an increase in both activity level and metabolic rates (Gibbs, 2002).
The metabolic rate of Drosophila appears to be relatively insensitive to transport effects. In this study metabolic rate of flies that were acclimated for 2 weeks in the laboratory in New Mexico was indistinguishable from members of the same cohorts of flies that were reared several thousand kilometers away, sent by overnight express mail during the middle of summer, and whose metabolic rate was assayed within 30min of arriving in the laboratory. This indicates that it is feasible to send flies over long distances to measure their metabolic rates in laboratories that can make direct measurements. This option may be more desirable than using manometric methods for measuring metabolic rates.
In conclusion investigators who use manometric methods to determine metabolic rate should take care before assuming that estimates of oxygen consumption determined by this method are the same as those determined by more direct measures of metabolic rate such as assay of CO2 or O2 flux.
Acknowledgments
Funds were provided by National Science Foundation Grant DEB-0444766, and National Institutes of Health RO1 grant GM067862-01 to JWOB and a National Cancer Institute MSI CCP NCI U56 CA96286 to WVV. Funds were provide to JBW by NSF grant IBN 0212092. Two anonymous reviewers supplied very helpful comments on the original manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berrigan D, Partridge L. Influence of temperature and activity on the metabolic rate of adult Drosophila melanogaster. Comparative Biochemistry Physiology: A Physiology. 1997;118:1301–1307. doi: 10.1016/s0300-9629(97)00030-3. [DOI] [PubMed] [Google Scholar]
- Chadwick L, Gilmour D. Respiration during flight in Drosophila repleta wollaston: the oxygen consumption considered in relation to the wing-rate. Physiology Zoology. 1940;13:398–410. [Google Scholar]
- Clancy DJ, Kennington WJ. A simple method to achieve consistent larval density in culture bottles. Drosophila Information Service. 2001;84:168–169. [Google Scholar]
- Conrradi-Larsen EM. A constant pressure microrespirometer for measurements of O2 consumption in Collembola. Norsk Entomologisk Tidsskrift. 1974;21:187–190. [Google Scholar]
- Dickinson MH, Lighton JR. Muscle efficiency and elastic storage in the flight motor of Drosophila. Science. 1995;268:87–90. doi: 10.1126/science.7701346. [DOI] [PubMed] [Google Scholar]
- Dixon M. Manometric methods as applied to the measurement of cell respiration and other processes. Cambridge University Press; Cambridge: 1951. [Google Scholar]
- Engelmann M. A constant pressure respirometer for small arthropods. Entomological News. 1963;24:181–186. [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of Applied Physiology. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Gessaman JA, Nagy KA. Energy metabolism: errors in gas-exchange conversion factors. Physiological Zoology. 1988;61:507–513. [Google Scholar]
- Gibbs AG. Water balance in desert Drosophila: lessons from non-charismatic microfauna. Comparative Biochemistry and Physiology. 2002;133:781–789. doi: 10.1016/s1095-6433(02)00208-8. [DOI] [PubMed] [Google Scholar]
- Gordon MS, Bartholomew GA, Grinnell AD, Jorgensen CB, White FN. Animal Physiology: Principles and Adaptations. 2nd. Macmillian Company; NY: 1972. [Google Scholar]
- Hulbert AJ, Clancy DJ, Mair W, Braeckman BP, Gems D, Partridge L. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Experimental Gerontology. 2004;39:1137–1143. doi: 10.1016/j.exger.2004.04.006. [DOI] [PubMed] [Google Scholar]
- James AC, Ballard JW. Mitochondrial genotype affects fitness in Drosophila simulans. Genetics. 2003;164:187–194. doi: 10.1093/genetics/164.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber M. The Fire of Life: An Introduction to Animal Energetics. 2nd. Robert E. Keieger Publ Co.; Huntington, NY: 1975. [Google Scholar]
- Lee RE. Using microrespirometers to measure O2 consumption by insects and small invertebrates. The American Biology Teacher. 1995;57:284–285. [Google Scholar]
- Lee RE, Baust JG. Respiratory metabolism of the Antarctic tick, Ixodes uriae. Comparative Biochemistry Physiology and Physiology. 1982;72A:167–171. [Google Scholar]
- Lighton JR, Schilman PE. Oxygen reperfusion damage in an insect. PLoS ONE. 2007;2:e1267. doi: 10.1371/journal.pone.0001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighton JRB. Hot hypoxic flies: Whole organism interactions between hypoxic and thermal stressors in Drosophila melanogaster. Journal of Thermal Biology. 2007;32:134–143. [Google Scholar]
- Poirier J. Lavoisier: Chemist, Biologist, Economist. University of Pennsylvania Press; Philadelphia: 1996. [Google Scholar]
- Ross RE. Age-specific decrease in aerobic efficiency associated with increase in oxygen free radical production in Drosophila melanogaster. Journal of Insect Physiology. 2000;46:1477–1480. doi: 10.1016/s0022-1910(00)00072-x. [DOI] [PubMed] [Google Scholar]
- Schmidt WD. Metabolism: Oxygen consumption: E E B 5157 Animal Physiology Laboratory Manual. University of Minnesota; St Paul: 1997. [Google Scholar]
- Schmidt-Nielsen . Animal Physiology. Vol. 5. Cambridge Un. Press; Cambridge: 1997. [Google Scholar]
- Sohal RS, Agarwal S, Dubey A, Orr WA. Protein oxidative damage is associated with life expectancy of houseflies. Proceeding National Academy of Sciences. 1993;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu MP, Epstein D, Tatar M. The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homologue chico. Aging Cell. 2002;1:75–80. doi: 10.1046/j.1474-9728.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- Umbreit WW. Manometric techniques and related methods for the study of tissue metabolism. Burgess Publishing Company; Minneapolis: 1972. [Google Scholar]
- Van Voorhies WA, Khazaeli AA, Curtsinger JW. Testing the “Rate of Living” model: Further evidence that longevity and metabolic rate are not inversely correlated in Drosophila melanogaster. Journal of Applied Physiology. 2004;97:1915–1922. doi: 10.1152/japplphysiol.00505.2004. [DOI] [PubMed] [Google Scholar]
- Walsberg GE, Wolf BO. Variation in the respiratory quotient of birds and implications for indirect calorimetry using measurements of carbon dioxide production. Journal of Experimental Biology. 1995;198:213–219. doi: 10.1242/jeb.198.1.213. [DOI] [PubMed] [Google Scholar]
- Winston PW, Bates DH. Saturated solutions for the control of humidity in biological research. Ecology. 1960;41:232–237. [Google Scholar]