Abstract
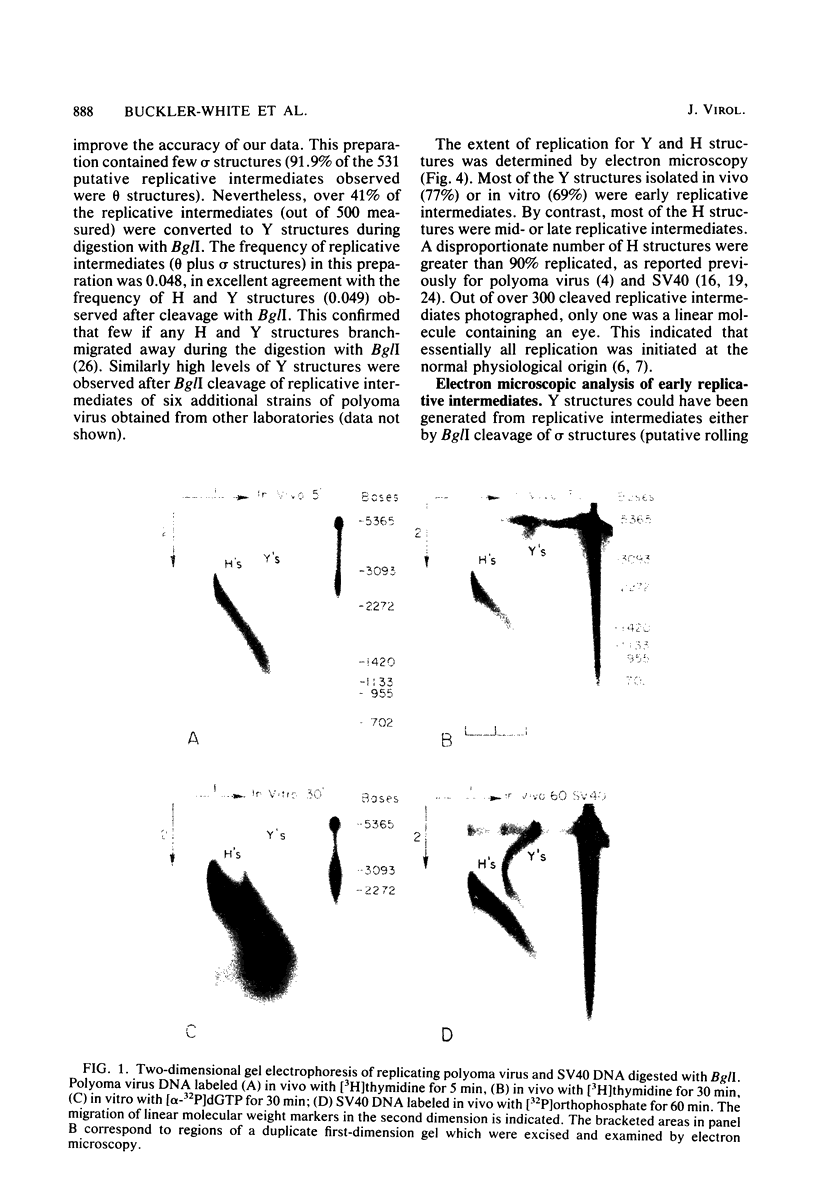
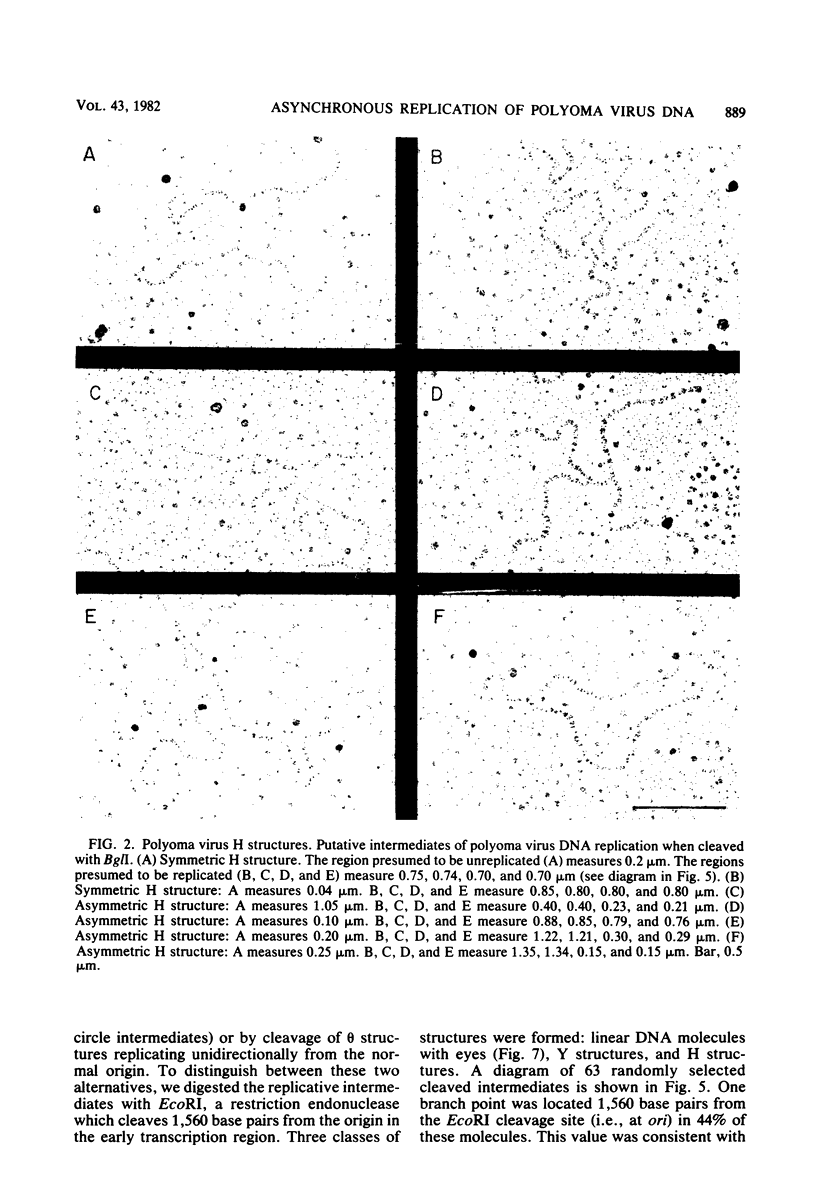
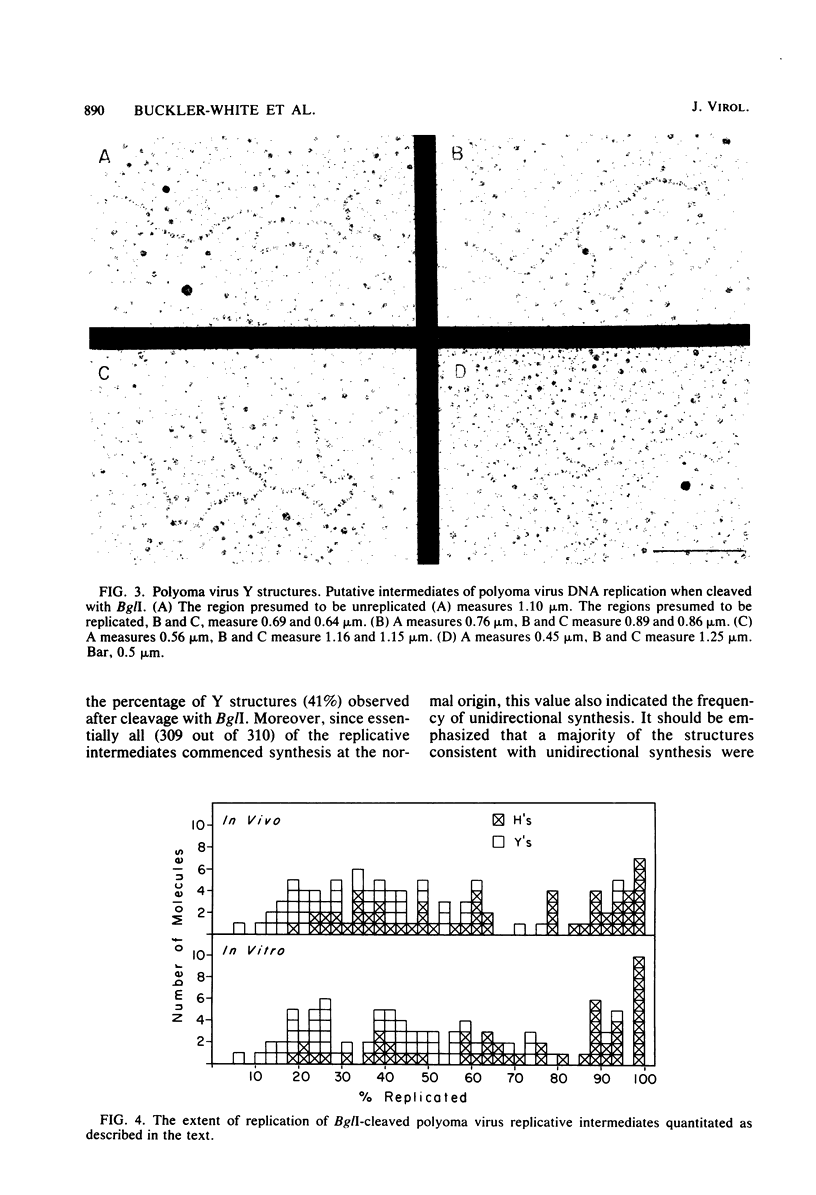
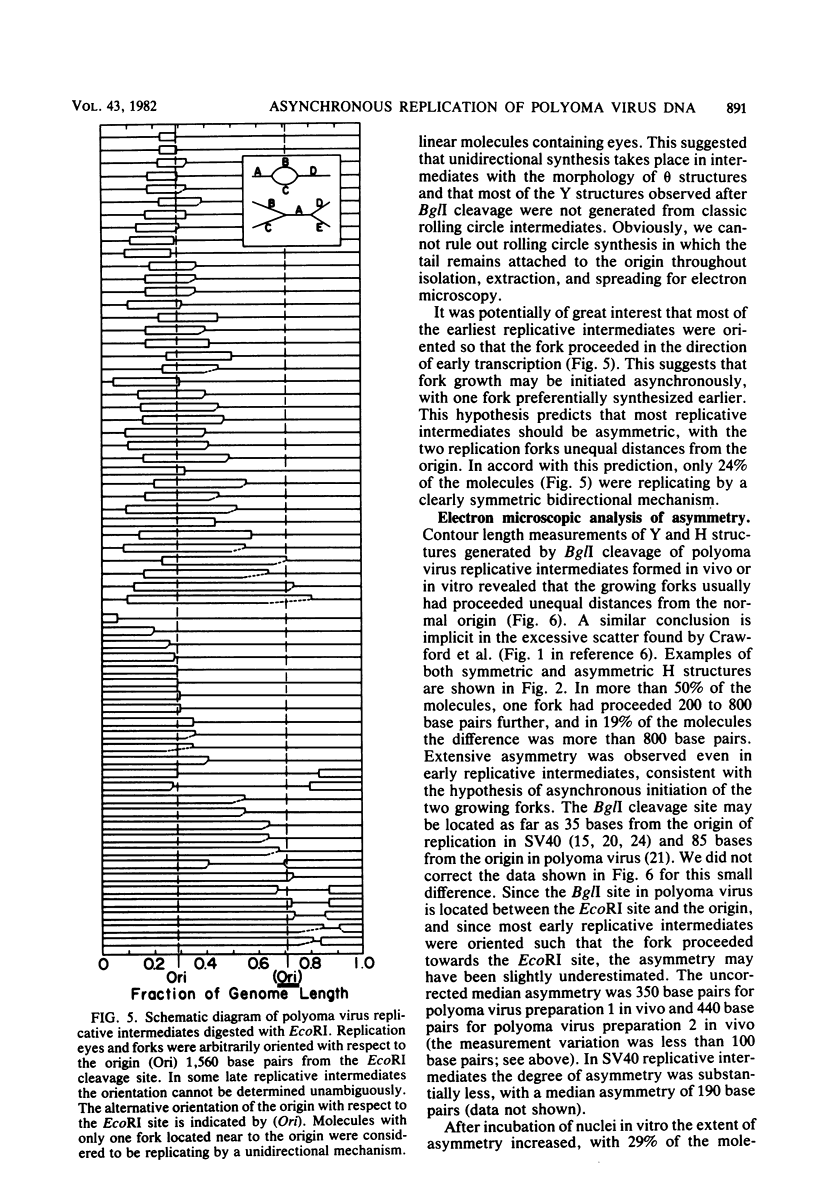
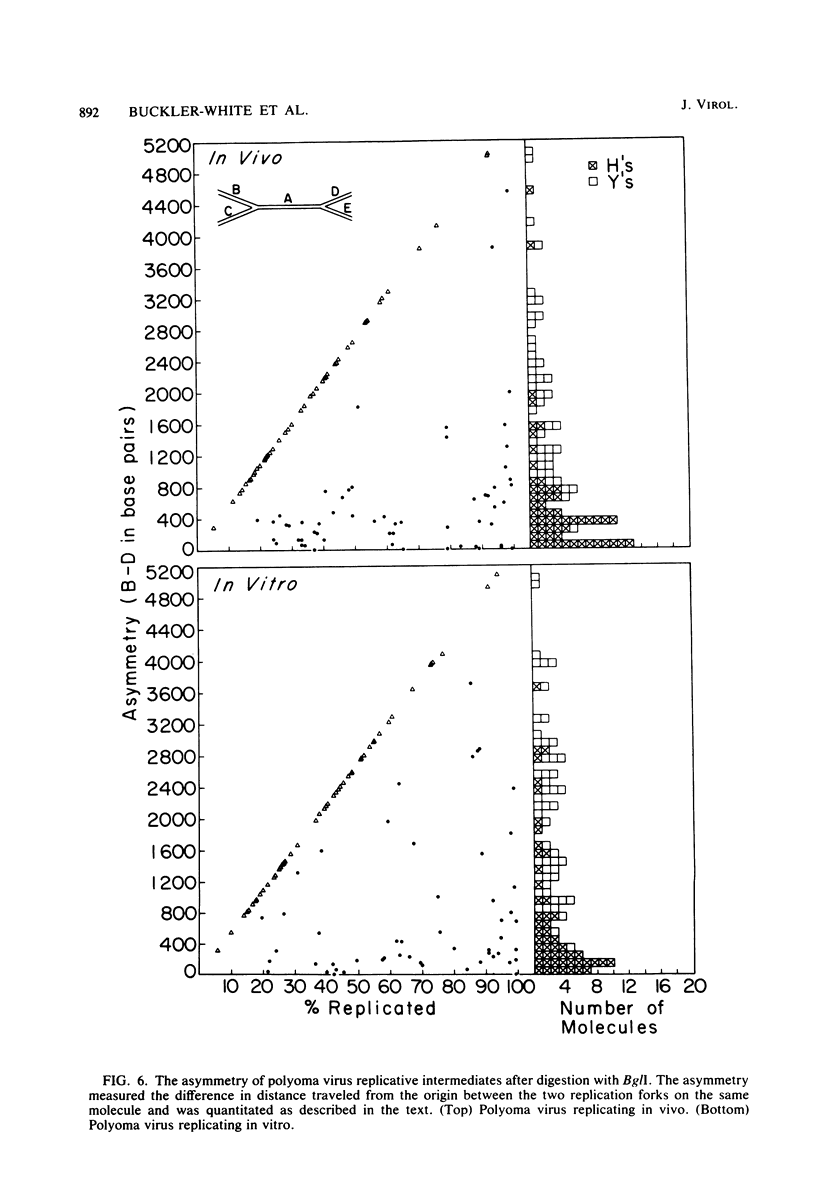
The structure of polyoma virus replicative intermediates isolated from infected 3T6 cells was analyzed by two-dimensional agarose gel electrophoresis (Sundin and Varshavsky, Cell 21:103-114, 1980) and quantitative electron microscopy (Krauss and Benbow, J. Virol. 38:815-825, 1981). DNA replication was initiated at a single site (ori) in essentially all of the replicative intermediates. Most of the early replicative intermediates were formed by unidirectional synthesis in the direction of early transcription. Most mid- and late replicative intermediates contained two replication forks which had traveled unequal distances from the origin. Asynchronous initiation of the two growing forks was postulated to account for these observations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjursell G. Effects of 2'-deoxy-2'-azidocytidine on polyoma virus DNA replication: evidence for rolling circle-type mechanism. J Virol. 1978 Apr;26(1):136–142. doi: 10.1128/jvi.26.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell G., Munck V., Therkelsen A. J. Polyoma DNA synthesis in isolated nuclei: evidence for defective replication forks. J Virol. 1979 Jun;30(3):929–932. doi: 10.1128/jvi.30.3.929-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D., Seiler P. The replication of the ring-shaped DNA of polyoma virus. II. Identification of molecules at various stages of replication. J Mol Biol. 1971 Jul 14;59(1):195–206. doi: 10.1016/0022-2836(71)90421-9. [DOI] [PubMed] [Google Scholar]
- Buckler-White A. J., Humphrey G. W., Pigiet V. Association of polyoma T antigen and DNA with the nuclear matrix from lytically infected 3T6 cells. Cell. 1980 Nov;22(1 Pt 1):37–46. doi: 10.1016/0092-8674(80)90152-x. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Robbins A. K., Nicklin P. M. Location of the origin and terminus of replication in polyoma virus DNA. J Gen Virol. 1974 Oct;25(1):133–142. doi: 10.1099/0022-1317-25-1-133. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Syrett C., Wilde A. The replication of polyoma DNA. J Gen Virol. 1973 Dec;21(3):515–521. doi: 10.1099/0022-1317-21-3-515. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Chan H. W., Rowe W. P., Martin M. A. Molecular cloning of polyoma virus DNA in Escherichia coli: plasmid vector system. Science. 1979 Mar 2;203(4383):883–887. doi: 10.1126/science.217087. [DOI] [PubMed] [Google Scholar]
- Krauss M. R., Benbow R. M. Polyoma virus minichromosomes: associated DNA molecules. J Virol. 1981 Jun;38(3):815–825. doi: 10.1128/jvi.38.3.815-825.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laipis P. J., Sen A., Levine A. J., Mulder C. DNA replication in SV40 infected cells X. The structure of the 16 S gap circle intermediate in SV40 DNA synthesis. Virology. 1975 Nov;68(1):115–123. doi: 10.1016/0042-6822(75)90153-1. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P. The initiation of SV40 DNA synthesis is not unique to the replication origin. Cell. 1980 Jun;20(2):381–391. doi: 10.1016/0092-8674(80)90624-8. [DOI] [PubMed] [Google Scholar]
- Mayer A., Levine A. J. DNA replication in SV40-infected cells. 8. The distribution of replicating molecules at different stages of replication in SV40-infected cells. Virology. 1972 Nov;50(2):328–338. doi: 10.1016/0042-6822(72)90384-4. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Crawford L. V., Syrett C., James A. W. Unidirectional replication of a minority of polyoma virus and SV40 DNAs. J Gen Virol. 1975 Jan;26(1):59–69. doi: 10.1099/0022-1317-26-1-59. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Salzman N. P. Late replicative intermediates are accumulated during simian virus 40 DNA replication in vivo and in vitro. J Virol. 1979 May;30(2):600–609. doi: 10.1128/jvi.30.2.600-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Regulatory mutants of simian virus 40: constructed mutants with base substitutions at the origin of DNA replication. J Mol Biol. 1979 Jul 15;131(4):801–817. doi: 10.1016/0022-2836(79)90202-x. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Griffin B. E. Sequence from early region of polyoma virus DNA containing viral replication origin and encoding small, middle and (part of) large T antigens. Cell. 1979 Jun;17(2):357–370. doi: 10.1016/0092-8674(79)90162-4. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. Simian virus 40 DNA replication in isolated replicating viral chromosomes. J Virol. 1978 Oct;28(1):53–65. doi: 10.1128/jvi.28.1.53-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Preferred DNA sites are involved in the arrest and initiation of DNA synthesis during replication of SV40 DNA. Cell. 1980 Nov;22(1 Pt 1):97–108. doi: 10.1016/0092-8674(80)90158-0. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Studies on cells rendered neoplastic by polyoma virus: the problem of the presence of virus-related materials. Virology. 1962 Jan;16:41–51. doi: 10.1016/0042-6822(62)90200-3. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Persico M., Martin R. G. The remarkable instability of replication loops provides a general method for the isolation of origins of DNA replication. Cell. 1981 Nov;27(1 Pt 2):155–163. doi: 10.1016/0092-8674(81)90369-x. [DOI] [PubMed] [Google Scholar]