Abstract
The COMT (catechol-O-methyltransferase) gene has been linked to a spectrum of human phenotypes, including cognition, anxiety, pain sensitivity and psychosis. Doubts about its clinical impact exist, however, because of the complexity of human COMT polymorphism and clinical variability. We generated transgenic mice overexpressing a human COMT-Val polymorphism (Val-tg), and compared them with mice containing a null COMT mutation. Increased COMT enzyme activity in Val-tg mice resulted in disrupted attentional set-shifting abilities, and impaired working and recognition memory, but blunted stress responses and pain sensitivity. Conversely, COMT disruption improved working memory, but increased stress responses and pain sensitivity. Amphetamine ameliorated recognition memory deficits in COMT-Val-tg mice but disrupted it in wild types, illustrating COMT modulation of the inverted-U relationship between cognition and dopamine. COMT-Val-tg mice showed increased prefrontal cortex (PFC) calcium/calmodulin-dependent protein kinase II (CaMKII) levels, whereas COMT deficiency decreased PFC CaMKII but increased PFC CaMKKβ and CaMKIV levels, suggesting the involvement of PFC CaMK pathways in COMT-regulated cognitive function and adaptive stress responses. Our data indicate a critical role for the COMT gene in an apparent evolutionary trade-off between cognitive and affective functions.
Keywords: working memory, attentional set shifting, genes, mice, stress, calcium/calmodulin-dependent kinase
Introduction
Catechol-O-methyltransferase (COMT) (Axelrod and Tomchick, 1958) methylates catechol structures, including dopamine (DA), norepinephrine (NE), epinephrine, caffeine, and catechol estrogens. In humans and rodents, COMT is abundantly expressed in pyramidal neurons of the prefrontal cortex (PFC) and hippocampus (Matsumoto et al., 2003) and plays a specific role in the catabolism of cortical DA but not cortical NE (Gogos et al., 1998; Tunbridge et al., 2004; Yavich et al., 2007), likely because of the scarcity of cortical DA transporters (Sesack et al., 1998) and the abundance of cortical NE transporters (Morón et al., 2002). DA signaling in the PFC is critical for modulating higher-order cognitive functions, impacting many domains of human behavior, thought, and emotion (Goldman-Rakic, 1998). Moreover, in humans as well as in monkeys, rats, and mice, cortical DA signaling affects cognitive performance through a U-shaped relationship, with too little or too much DA having deleterious effects (Mattay et al., 2003; Vijayraghavan et al., 2007).
Human studies have primarily focused on a Val/Met functional single nucleotide polymorphism (Lachman et al., 1996). The COMT-Val form leads to higher COMT protein levels and enzymatic activity compared with COMT-Met (Chen et al., 2004). However, at least three different COMT common haplotypes impacting its enzymatic activity and protein levels have been discovered (Nackley et al., 2006), increasing the complexity of COMT genetic variation and its association with clinical phenotypes. In general, genetic variations in human COMT have been associated with physiological functions (Egan et al., 2001; Winterer et al., 2006), and behavioral phenotypes related to PFC and hippocampal information processing, including cognition (Egan et al., 2001; Blasi et al., 2005; Bertolino et al., 2006), anxiety (Drabant et al., 2006), obsessive-compulsive disorder (OCD) (Pooley et al., 2007), and pain sensitivity (Nackley et al., 2006). However, aspects of the data remain controversial (McGrath et al., 2004; Ho et al., 2005), probably because of the complexity of human behavior and to the complex set of interacting genetic variants within the human COMT gene (Tunbridge et al., 2006).
Mutant mice bearing targeted mutations of COMT are unique tools to elucidate the role of COMT in cognitive function, emotional arousal, and the neurobiological basis of the behavioral associations in humans. Genetically altered mice provide a level of molecular specificity that is not possible in human studies, where potential interactions between functional loci within the gene and with genetic background are difficult to control. We studied genetically engineered mice lacking functional COMT and a new transgenic mouse overexpressing the human COMT-Val variant, and compared them to normal wild-type COMT-Leu mice. This approach allowed us to contrast life-long effects of genetic variation resulting in relatively low and relatively high COMT activity, respectively. Furthermore, in an effort to identify molecular mechanisms of cognitive effects, we examined the impact of COMT genetic modifications on the expression of components of the Ca2+/calmodulin-dependent protein kinase superfamily (CaMK), which have been linked to learning and memory processes (Runyan et al., 2005).
Materials and Methods
Mutation of the Kozak sequence in human COMT gene and in vitro translation.
The Kozak sequence of mouse COMT gene was introduced into the Kozak sequence of human COMT gene by PCR with primers GTGCTTTGAAGATGCCGGAGGC (forward primer for wild-type human COMT) or GTGCTgacgAGATGCCGGAGGC (forward primer for mutant human COMT) and GTGTGCTTTGCATTTAGGACACA (backward primer). The PCR product containing the wild-type or mutant human COMT gene was used as template for in vitro translation analysis as described previously (Chen et al., 2004). The membrane bound (MB)-COMT and soluble (S)-COMT proteins labeled with 35S-methionine were quantified by densitometry and the ratio of MB-COMT versus S-COMT was calculated.
Construction and development of human COMT-Val-tg mice.
Human COMT-Val cDNA (GenBank accession number BC011935) plasmid (pOTB7-COMT-Val) was purchased from American Type Culture Collection. The human COMT-Val cDNA insert was released by digestion with EcoRI and XhoI, and the ends were blunted by T4 DNA polymerase. The COMT-Val cDNA was subcloned to the SmaI site of the tetracycline-regulated pTet-splice vector, where the COMT-Val is under the control of a tetracycline responsive promoter. The new plasmid was designated as pTet-COMT-Val. The sequences and the orientation of COMT-Val were confirmed by DNA sequencing. A DNA fragment, containing the tetracycline responsive promoter, human COMT-Val, a small SV40 intron and a SV40 poly(A) signal, was released from the pTet-COMT-Val by digestion with XhoI and NotI and purified by gel purification with the QIAGEN Gel Extraction kit. The DNA was filtered with a 0.22 μm Millipore filter and diluted with 1 mm TrisHCl, pH7.5, 0.05 mm EDTA buffer to 10 μg/ml. The DNA was microinjected into the pronuclei of 200 fertilized embryos from the mating of C57BL/6J mice. The microinjected embryos were implanted into 10–15 pseudopregnant recipients. The founder mice were identified by PCR genotyping with specific primers and confirmed in separated experiments performed three times. The COMT-Val transgenic mice were crossbred with neuron-specific enolase (NSE)-tetracycline transactivator (tTA) transgenic mice to bring the COMT-Val and tTA transgenes together and achieve tissue-specific expression because of the NSE promoter. The NSE-tTA transgenic mice were on a CD-1 genetic background, thus the resulting COMT-Val-tg mice were in a mixture C57BL/6JxCD-1 genetic background. Single transgenic COMT-Val, NSE-tTA, and mice carrying neither transgene were pooled together in the control group because we saw no COMT-Val expression or effect of single transgene in any experiment.
COMT-deficient mice were originally generated at the Rockefeller University (New York, NY) as described previously (Gogos et al., 1998). The mutated COMT allele was introduced into a mixed 129/JxC57BL/6J genetic background and by 10-generation backcrossing the mutation was introduced into a more homogenous C57BL/6J genetic background.
Subjects.
All procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and followed the National Institutes of Health Using Animals in Intramural Research. COMT-Val-tg mice were mated with control littermates. COMT null mutant mice (COMT−/−) and their heterozygous (COMT+/−) and wild-type (COMT+/+) littermates were bred by +/− mating. Mice were identified by PCR analysis of tail DNA. Mice were group housed (two to four per cage) in a climate-controlled animal facility (22 ± 2°C) and maintained on a 12 h light/dark cycle with ad libitum access to food and water, unless specified in particular experiments. Testing was conducted in male mice, 3 to 7 months old, during the light phase. Experimenters were blind to the genotype during behavioral testing. Mice were handled by the experimenter on alternate days during the week preceding the tests. At least 1 h before any test manipulation, mice were habituated in a room adjacent to the testing room. For immunoblotting, COMT activity assay, and real-time quantitative PCR (RT-qPCR), see the supplemental Methods (available at www.jneurosci.org as supplemental material).
Physical health.
Measures of general health and neurological reflexes were assessed in the mice as described previously (Crawley, 2007). General health measures included posture, physical condition of the fur, observations for unusual home cage behaviors, empty cage observations for 3 min, and irregular spontaneous behaviors such as excessive grooming, digging, rearing, or stereotypy. Neurological reflexes examined included trunk curl, forepaw reaching, corneal reflex, ear twitch, whisker twitch, and the righting reflex. The wire hang test to evaluate limb strength was performed by placing the mouse on one set of wire bars and then inverting the lid for a cutoff time of 60 s.
Locomotor activity and novel object recognition test.
The experimental apparatus consisted of four Plexiglas Digiscan automated open fields (Accuscan; 42 × 42 × 30 cm), evenly illuminated by overhead white lighting (50 ± 2 lux) and equipped with photobeam sensors to quantify exploratory and locomotor activities.
The novel object recognition test was used to evaluate recognition memory, which is generally defined as the ability to discriminate the familiarity of things encountered previously (Mumby, 2001). Cognitive performance in this relatively unstressful task does not depend on the retention of a rule and does not involve positive or negative reinforcers, but rather exploits the natural tendency of mice to explore a novel object more than a familiar one. Finally, it has been suggested that this task is hippocampus independent when the novel object is encountered in the same context where the acquisition phase occurred (O'Brien et al., 2006).
The novel object recognition test procedure consisted of three phases: habituation, acquisition, and retention. The day before the test, group-housed mice were individually habituated for 1 h in the empty open field arena. The next day, during the acquisition session, each mouse was introduced for 10 min into the open field, which contained two identical copies of the same object (two rectangular boxes, 4 × 4 × 7 cm, or two laboratory flasks, 4 × 7 cm, each either black or white). The objects were placed in two corners of the open field apparatus, 8 cm from side walls. The 5 min retention test took place 1 h after the acquisition trial. The mice were placed back into the same arena with a duplicate of the old object and the new object of the opposite color and shape. Testing sessions were videotaped with the experimenter absent from the room during the test. Time spent exploring each object was subsequently scored from the videotapes as the number of seconds when each subject was facing the object and <1 cm away. An exploratory preference index, the ratio of the amount of time spent exploring the novel object over total time spent exploring both objects, was used to measure the recognition memory.
Another cohort of control and COMT-Val-tg littermates were tested in the novel object recognition test as described above. Immediately after the 10 min acquisition session, one-half of the mice of each genotype were treated with vehicle, and one-half with d-amphetamine sulfate (1.5 mg/kg, i.p.; Sigma-Aldrich) that was dissolved in physiological saline and injected in a volume of 10 ml/kg. Vehicle mice were injected with the same volume of saline.
Attentional set-shifting test.
A new “stuck-in-set” perseveration paradigm, modified from previous studies (Owen et al., 1993; Garner et al., 2006) was used. For details on the apparatus used, see supplemental Figure S1 (available at www.jneurosci.org as supplemental material). Metal bowls were used to hold the digging medium. The outer surfaces of the bowls were covered with a texture, and the bowls were filled with a digging medium, which could be scented (for stimulus exemplars used, see Table 1). Thus, the bowls could be varied by their odor, the texture of their outer surface, or the digging medium in which the food bait was hidden. The bait was a 14 mg food pellet (5TUL, dust-free purified rodent tablets; TestDiet).
Table 1.
Stimulus exemplars used in the attentional set-shifting task
| Dimension | Exemplar pairs | ||||
|---|---|---|---|---|---|
| Outer texture | |||||
| Set A | Smooth plastic | Smooth cardboard | Aluminum foil | Coarse sandpaper | Regular paper |
| Set B | Bubble wrap | Ridged cardboard | Pink cloth | Fine sandpaper | Waxed paper |
| Digging medium | |||||
| Set 1 | Aspen bedding a | Moss | Repti Bark | CareFresh | Crystalit |
| Set 2 | Aquarium gravel | Kay-Kob | Alpha-dri | Ultra White CareFresh | PaperChip |
| Odor | |||||
| Set ■ | Sage | Pepper | Cumin | Thyme | Nutmeg |
| Set ○ | Coriander | Cinnamon | Ginger | Oregano | Cloves |
Compound discriminations were based on fixed combinations of pairs of exemplars. The exemplar pairs from different dimensions were presented in random combination, but each sequence performed by a control mouse was matched by that of a COMT-Val-tg littermate. Neutral stimuli for the different dimensions were: no outer texture; sawdust bedding (Harlan Teklad); digging medium; and no odor. The digging medium was changed often so the mice would not use odor cues to locate the bowls baited with food pellets. For 500 ml of each medium, 10 ml of crushed food pellets (about 6 g) were mixed with the medium to mask the smell of the hidden food pellet. For scented mixtures, an odor (0.7g) was mixed with 500 ml of the medium and crushed pellets.
aAspen bedding, Aquarium gravel, Moss, Kay-Kob, Repti Bark (PetSmart); Alpha-dri (Shepherd Specialty Papers); CareFresh, Ultra White CareFresh (International Absorbents); Crystalit (Pharmacal Research Laboratories); PaperChip (Shepherd Specialty Papers).
After a week of single housing, on each of 3 consecutive days a baseline of their ad libitum body weight and 24 h food intake was recorded. Mice were then food restricted throughout the experiment to maintain 85% of their ad libitum body weight. Two days of habituation followed. During the first day, animals were placed in the apparatus and exposed for 15 min to two baited bowls (one pellet for each bowl) that did not contain a medium. After both pellets were retrieved, the bowls were rebaited again. Then, for 30 min, the bowls were filled with sawdust and baited again. Once again, when both pellets were retrieved, the bowls were rebaited. On the second day of habituation, the mice were exposed for 30 min to two sawdust-filled bowls that were rebaited every 2 min. When the mouse was reliably digging to retrieve the rewards, he was trained on a series of two simple discriminations (SDs) between digging media, such as a medium of Crystalit versus PaperChip and an odor of oregano versus thyme, to a criterion of 8 of 10 consecutive trials. These exemplar scents were not used again. On the third day, the testing paradigm started. Each trial was initiated by raising the two dividers to give the mouse access to the two digging bowls, only one of which was baited. We took care to raise the doors simultaneously and only when the mouse was not sniffing at or facing a door. The first four trials were discovery trials: the mouse was permitted to dig in both of the bowls, but only one was baited. An error was recorded if the mouse dug first in the unbaited bowl. On subsequent trials, if the mouse started to dig in the unbaited bowl, an error was recorded, and the trial was terminated. The mice had to reach a criterion of 8 correct choices of 10 consecutive trials to complete each stage. The time until the first dig and time to finish each stage were also recorded. If a mouse made three consecutive incomplete trials (no dig after 2 min), the session was terminated and the test was continued the next day. The order of the discriminations was always the same, but the dimensions and the pairs of exemplars were equally represented within groups and counterbalanced between groups.
The sequence of stages to complete comprised a SD, compound discrimination (CD), compound discrimination reversal (CDRe), intradimensional shift (IDS), intradimensional shift reversal (IDSRe), intradimensional shift 2 (IDS2), intradimensional shift reversal 2 (IDSRe2), extradimensional shift (EDS), and extradimensional reversal (EDSRe). The mice were exposed to the tasks in this order so that they could develop a set, or bias, toward discriminating between the baited bowls. In the SD, the mice were introduced to a dimension that was relevant throughout the tasks until the EDS, in which the mouse had to find the bait after a new stimulus dimension and the previously relevant dimension became irrelevant. An example of the entire task is diagrammed in supplemental Table S1 (available at www.jneurosci.org as supplemental material).
Continuous spatial alternation T-maze task and reversal.
A group of naive control and COMT-Val-tg mice was tested in this T-maze task as described previously (Brito et al., 1987; Green and Stanton, 1989). The T-maze apparatus was constructed from transparent Plexiglas (0.5 cm thick; dimensions of the alleys: 40 × 10.2 × 17.5 cm; for the COMT-Val-tg mice and their control littermates: 50 ± 2 lux in the main alley, 25 ± 2 lux in the side alleys; for the COMT+/+, COMT+/− and COMT−/− mice: 20 ± 2 lux in the main alley, 10 ± 2 lux in the side alleys). A recessed cup at the end of each side alley concealed the food reinforcement from view. In addition, care was taken to not provide visual cues that could be used by the animal to guide their response: behavioral studies were conducted in a room without any visual landmarks (for example windows, etc.) and the two goal arms presented the same cues symmetrically disposed. Mice were singled housed, food restricted, and habituated to the food reinforcer (14 mg, 5TUL; TestDiet) as reported in the attentional set-shifting test. Successively, each mouse was habituated to the T-maze apparatus and shaped to retrieve the food reinforcement for 10 min/d. Ten training trials per day were then initiated. After the mouse made its first arm entry in the T-maze, it was subsequently rewarded for entering the arm opposite to the one entered on the previous trial. On the first trial of each day, one food reinforcer pellet was presented in both goal arms. Each day during the next nine trials, the arm opposite to the one the animal had entered on the previous trial was baited, except when the animal had gone to the empty arm on the last trial. In that case, the food was left in place and the baited side was changed only after the animal had alternated. When the mouse made the correct choice, it was given time to consume the reinforcer pellet, and then returned to the home cage for a 10 s intertrial interval. Incorrect choices were not rewarded or punished. Number of errors in arm selection and number of days to criterion were recorded. This training continued until a criterion of 80% correct choices in 10 daily trials on 3 consecutive days was achieved. A maximum of 20 training days was given. Starting from the day after reaching the learning criteria for the continuous alternation task, mice were tested daily switched from an alternation rule to a same arm rule. That is, after the mouse made its first arm entry in the T-maze, it was subsequently rewarded for entering the same arm to the one entered on the previous trial during a succession of 10 trials. This training continued until a criterion of 80% correct choices (in 10 trials) on 3 consecutive days was achieved.
Discrete paired-trial variable-delay T-maze task.
Two new cohorts of naive control and COMT-Val-tg mice and COMT+/+, COMT+/−, and COMT−/− mice were tested in this T-maze task. Animals were presented with a sequence of randomly chosen forced runs, each followed by a choice run so that they were required to integrate information held online (the forced run) with the learned rule (nonmatch to sample). Mice were tested in the same T-maze apparatus using the same habituation procedure as in the continuous spatial alternation T-maze task. After this, mice were exposed to 1 d of 10 forced-alternation runs. Specifically, the mouse was placed in the T-maze with one goal arm closed off and had up to 2 min to run and eat the food reinforcer (14 mg, 5TUL; TestDiet) in the open arm. After consuming the food pellet and an intertrial period of at least 20 min in the home cage, the mouse was placed back in the maze for another forced run. Beginning on the following day, the discrete-trial delayed alternation training began. After a randomly chosen forced run and a 4 s delay interval in the home cage, the animal was placed back in the maze with access to both arms. The food reinforcer was located in the opposite arm entered in the previous forced trial. After an intertrial period of at least 20 min, the animal was placed back in the maze for another forced run/choice run paired trial. A different pseudorandomly chosen pattern of forced runs (e.g., R-R-L-R-L-L-R-L-R-L) was used every day, but on a given day the same pattern was used for all animals. Animals were trained at a 4 s intratrial delay and 20 min intertrial delay for 20 d, or until an animal successfully performed 8 of 10 daily trials (80% choice accuracy) for 3 consecutive days. Animals that did not reach this criterion were eliminated. Once the mouse performed consistently at the 4 s intratrial delay, training at three additional intratrial delays (30, 60, and 240 s presented in a random manner) and 20 s intertrial delay began. Mice were given four trials of each delay on 3 consecutive days of testing for a total of 12 trials for each delay. To limit potential confounding factors related to differences in the number of days required to reach the learning criteria in the COMT-Val-tg and control mice cohort, after each mouse reached criteria, it was returned to ad libitum feeding conditions for 7 consecutive days. Each mouse was then retested for 3 consecutive days at the same conditions as the training phase, and then tested at intratrial delays of 4, 30, 60, and 240 s, with an intertrial delay of 20 s.
Hot plate and tail flick tests.
The analgesia tests examined the subject's pain threshold and sensitivity. The hot plate and tail flick tests assess centrally mediated and spinal pain reflexes, respectively, and were performed as described previously (Crawley, 2007). For more details, see the supplemental Methods (available at www.jneurosci.org as supplemental material).
Acoustic startle response and prepulse inhibition.
Acoustic startle response and prepulse inhibition (PPI) were measured using four SR-Lab Systems (San Diego Instruments). Test sessions began by placing the mouse in the Plexiglas holding cylinder for a 5 min acclimation period. Over the next 10.5 min, mice were presented with each of seven trial types across six discrete blocks of trials for a total of 42 trials. The order in which trial types were presented was randomized within each block. The interval between trials was 10–20 s. One trial type measured the response to no stimulus (baseline movement), and another presented the startle stimulus alone (acoustic amplitude), which was a 40 ms, 120 dB sound burst. The other five were acoustic prepulse plus acoustic startle stimulus trials. Prepulse tones were 20 ms at 74, 78, 82, 86, and 90 dB, presented 100 ms before the startle stimulus. The maximum startle amplitude was the dependent variable. A background level of 70 dB white noise was maintained over the duration of the test session.
Naive COMT+/+, COMT+/− and COMT−/− mice were tested for the startle amplitude to different acoustic startle stimuli. As above, each test session began by placing a subject in the Plexiglas cylinder where it was left undisturbed for 5 min. After the 5 min acclimation, each subject received 36 trials over the 9 min test session. There were six different sound levels (in decibels) presented: 0, 82, 90, 100, 110, and 120. Each stimulus was 40 ms and presented four times in pseudorandom order such that each sound level was presented within a block of six trials. The interval between trials was 10–20 s. The startle response was recorded for 65 ms (measuring the response every 1 ms) starting with the onset of the startle stimulus. The maximum startle amplitude recorded during the 65 ms sampling window was used as the dependent variable.
Stress-induced hyperthermia paradigm.
The stress-induced hyperthermia (SIH) paradigm reflects a physiological response to mild stress exposure and is sensitive to treatment with anxiolytic drugs (Adriaan Bouwknecht et al., 2007). In this procedure, mice were single housed in a clean cage with nesting material 1 week before the test. The stressor consisted of measuring temperature T with a rectal probe (digital thermometer, type 871A, 1Tegam; Geneva) dipped in KY Jelly (Johnson and Johnson) and gently inserted ∼2 cm into the rectum. For the COMT-Val-tg and control mice, a supplemental stress consisting of placing them in a novel clean cage (same dimension as the home cage) after the first temperature measurement (T1) was also used. Ten minutes after the T1, the temperature was measured again (T2, stressed temperature). For the manipulation using the supplemental clean-cage stress, a 25 min interval between T1 and T2 was used. The stress manipulations caused a hyperthermic response. All tests were performed between 11:30 A.M. and 2:30 P.M.
Elevated plus maze.
The elevated plus maze paradigm was used as a well validated animal model of anxiety-like traits that is based on the natural aversion of rodents to height and open spaces (Pellow et al., 1985; Lister, 1987). The maze consisted of two open and two enclosed (by 15-cm-high transparent Plexiglas walls) arms of the same size (30 × 5 cm) extending from a central platform (5 × 5 cm). It was elevated 40 cm above the ground. An indirect light provided a 14 ± 2 lux (for the COMT-Val-tg mice and their control littermates) and 2 ± 1 lux (for the COMT+/+, COMT+/− and COMT−/− mice) light level in the maze. A camera mounted above the apparatus allowed recording test sessions for later scoring of the animal's behavior. The subjects were individually tested in 5 min sessions. Each mouse was placed onto the center platform facing an open arm to initiate the test session. Behaviors scored were the number of open and closed arm entries and time spent on the various sections of the maze. Arm entry was defined as entry of all four paws into the arm. The number of entries into and the time spent on the open arms were expressed as a percentage of the total number of arm entries and test duration, respectively. Total (open plus closed) arm entries were taken as an index of general exploratory activity.
Statistical analysis.
Results are expressed as mean ± SEM throughout. Student's t test was used to compare COMT-Val-tg versus control littermates on some general health parameters. Two-tailed Fisher exact analyses were used to compare genotypes for the other general health parameters. Student's t test was used to compare COMT-Val-tg versus control littermates on COMT enzyme activity, tyrosine hydroxylase (TH), dopamine-β-hydroxylase (DβH), and GAD67, CaMKII immunoreactivity, the days required to reach the criteria in the T-maze, the time spent exploring the two objects during the acquisition session, the novel object exploration index during the retention trial of the first object recognition task, and the elevated plus maze paradigms. A Spearman's rank-order correlations test was used to correlate COMT enzyme activity correlated with COMT protein levels in control and COMT-Val-tg mice. In the attentional set-shifting test, a two-way ANOVA with genotype (control vs COMT-Val-tg) as a between-subjects factor and the different stages (SD, CD, CDRe, IDS, IDSRe, IDS2, IDSRe2, EDS, and EDSRe) as a within-subject factor was used to examine the number of trials to reach the criteria and timing needed to complete each stage. Two-way ANOVA with genotype (control or COMT-Val-tg) as a between-subjects factor and within-session 5 min intervals as a repeated-measure within-subject factor was used to analyze the total distance performed in the empty open-field arena during the object recognition test. Two-way ANOVA with genotype and treatment (vehicle or amphetamine) as independent variables was used to examine the exploration time during the acquisition session of the second new object recognition task. Two-way ANOVA with genotype and treatment (vehicle or amphetamine) as independent variables was used to examine the new object exploration during the retention session of the second new object recognition task. Two-tailed Fisher's exact analyses were used to compare genotypes for the number of mice reaching the learning criteria of the T-maze tasks. Two-way ANOVA with genotype (control vs COMT-Val-tg or COMT+/+, COMT+/−, and COMT−/−) as a between-subjects factor and the days of habituation (days 1 and 2) as a within-subject factor was used to examine the latency to retrieve the first hidden food pellet from the end of the T-maze alleys. Two-way ANOVA with genotype (control vs COMT-Val-tg) as a between-subjects factor and days of testing as a repeated-measure within-subject factor was used to analyze the correct choices made during the reversal phase of the T-maze task. Comparison of the COMT+/+, COMT+/−, and COMT−/− mice used one-way ANOVA to examine the days needed to reach the criteria in the T-maze task, the three parameters measured during the object exploration task (exploration time, the time spent avoiding the objects and the stretching attempts), the results on the CaMK family components and other genes in the PFC, the elevated plus maze parameters, the latency to reaction in the hot plate test, and the latency to tail flick. A two-way ANOVA with genotype (control vs COMT-Val-tg or COMT+/+, COMT+/−, and COMT−/−) and different intratrial delay (4, 30, 60, or 240 s) as independent variables was used to examine the percentage of correct choices made during the T-maze. Two-way ANOVAs with genotype (control or COMT-Val-tg; COMT+/+, COMT+/−, and COMT−/−) and acoustic stimulus (no stimulus or 120 dB stimulus for the startle amplitude; 74, 78, 82, 86, or 90 dB prepulse sound level for the PPI; 70, 82, 90, 100, 110, or 120 dB for the startle amplitude and threshold) as independent variables were used to examine the startle amplitude and PPI. A two-way ANOVA with genotype and body temperature before and after the stress (T1 and T2) as independent variables was used for SIH. Post hoc analyses for individual group comparisons used Newman–Keuls analyses. The accepted value for significance was p < 0.05.
Results
Gene divergence mutations in the first Kozak sequence favor MB-COMT
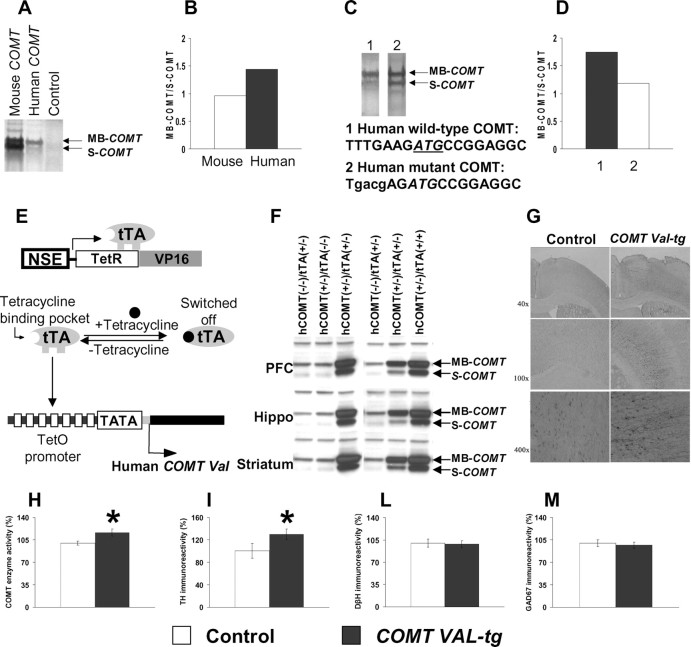
To elucidate the evolutionary consequence of COMT mutations, we performed in vitro mutagenesis. We confirmed that MB-COMT is generated preferentially from the human COMT mRNA but not from mouse COMT mRNA (Fig. 1 A,B). To test whether the first Kozak sequence of human COMT is more efficient than that of mouse COMT, we introduced the mouse Kozak sequence at the first codon into the human COMT. This experiment in reverse evolution showed that less MB-COMT was now generated from the mutant human COMT (Fig. 1 C,D), suggesting that gene divergence mutations make the first human Kozak sequence more efficient and expression of MB-COMT more abundant. MB-COMT has less enzyme capacity but much greater affinity for neurotransmitters such as DA (Lachman et al., 1996).
Figure 1.
Gene divergence Kozak mutation and generation of transgenic mice overexpressing the human COMT-Val gene. A, MB-COMT and S-COMT proteins were generated by in vitro protein synthesis using 35S-methionine as labeling substrate and mouse and human COMT cDNAs as templates. B, Ratio of the MB-COMT versus S-COMT proteins. C, MB-COMT and S-COMT proteins were generated as above and using human wild-type and Kozak mutant COMT cDNAs as templates. D, Mutation at the Kozak sequence of human COMT cDNA decreased the ratio of the MB-COMT versus S-COMT proteins. E, Schematic diagram of a tetracycline-regulated gene expression system. Neuron-specific expression of tTA containing the tetracycline binding domain from the tetracycline receptor (TetR) and transactivation domain from the viral transcription factor VP16 is driven by the NSE promoter. The tTA can bind to the tetracycline-responsive promoter, which contains TetO and minimal CMV promoter carrying only TATA box to activate the expression of the human COMT-Val transgene. Expression of the transgene can be switched off by tetracycline. F, Expression of human COMT-Val protein in the frontal cortex, hippocampus, and striatum of mice carrying the NSE-tTA alone [hCOMT(−/−)/tTA(+/−)], the human COMT-Val transgene alone [hCOMT(+/−)/tTA(−/−)], or both transgenes [hCOMT(+/−)/(tTA+/−) or (tTA+/+)]. G, Overexpression of the COMT gene was restricted to neurons and the major cell type expressing the human COMT-Val-tg was pyramidal neurons. Neurons expressing the COMT-Val-tg were detected by immunostaining with specific anti-human COMT antibody. H, COMT enzyme activity in the frontal cortex of the COMT-Val-tg mice and their controls. n = 17–21 per group. *p < 0.05 versus control mice. I–M, Immunoreactivity of (I) TH, (L) DβH and (M) GAD67 proteins in the frontal cortex of the COMT-Val-tg mice and their controls. n = 4–9 per group. Results are expressed as percentage over the average of the control group. Values represent mean ± SEM. *p < 0.05 versus control mice.
Generation of inducible transgenic mice overexpressing the human COMT-Val gene
To achieve neuron-specific human COMT-Val overexpression with temporal regulation, we generated double-transgenic mice expressing the tTA under the NSE promoter, and carrying the human COMT-Val gene under the tetracycline operator (TetO) construct (Fig. 1 E). Increased expression of human COMT-Val protein was detected in frontal cortex, hippocampus and striatum of only the bigenic mice carrying both tet-COMT-Val and NSE-tTA transgenes (COMT-Val-tg mice) indicating that the expression of the tet-COMT-Val transgene is tightly regulated by tTA (Fig. 1 F). The human COMT-Val gene was expressed primarily in pyramidal neurons (Fig. 1 G) and an estimation of the percentage of total cells expressing it was 14.0 ± 1.7 and 7.6 ± 2.7 in the PFC and striatum, respectively. Because the DA transporter has 1000-fold higher affinity for DA compared with COMT and is abundant in the striatum but not in the hippocampus and cortex, region-specific COMT overexpression may not be critical to produce a selective effect in the cortex, as confirmed by previous studies (Gogos et al., 1998; Tunbridge et al., 2004; Yavich et al., 2007).
To determine whether the human COMT-Val protein was functional in the brain, we measured enzyme activity in COMT-Val-tg and control mice. COMT enzyme activity in homogenate tissue from frontal cortex of the COMT-Val-tg mice was ∼16% higher than control mice (t test, p < 0.05; n = 17–21 per group) (Fig. 1 H), suggesting that the human COMT-Val was functional in the brain of the transgenic mice. Moreover, COMT enzyme activity correlated with COMT protein levels (Spearman's rho = 0.69, p = 0.055; n = 8).
TH, the rate-limiting enzyme in DA synthesis, is an index of DA terminal activity and presumably DA release. TH immunoreactivity in the frontal cortex was higher in COMT-Val-tg (t test, p < 0.05; n = 4 per group) (Fig. 1 I), consistent with a compensatory effect secondary to higher degradation of DA in COMT-Val-tg mice. In contrast, there was no genotype difference in the expression of DβH (p = 0.82; n = 9 per group) (Fig. 1 L), the rate limiting enzyme in the synthesis of NE, consistent with other evidence that COMT has a negligible role in cortical NE metabolism (Yavich et al., 2007). There also was no difference in the expression of GAD67 (p = 0.67; n = 4–5 per group) (Fig. 1 M), the biosynthetic enzyme for GABA.
Behavioral phenotyping
Altered COMT enzyme activity did not affect the general health or physical abilities of genetically modified mice (supplemental Tables S2, S3, available at www.jneurosci.org as supplemental material). We compared the two COMT genetically modified lines of mice to wild-type littermates carrying COMT-Leu as a control so as not to confound our results with potential effects of additional genetic manipulations (e.g., insertion of a transgene on a knock-out background) and to investigate whether an even small increase or decrease in COMT enzyme activity can produce clear behavioral effects.
Selective impairment in attentional set shifting in COMT-Val-tg mice
Human subjects with frontal lobe defects have attentional set-shifting deficits, as illustrated by the Wisconsin Card Sorting Task (WCST) (Owen et al., 1993) and the intradimensional/extradimensional shift (IEDS) subtest of the Cambridge Neuropsychological Test Automated Battery (CANTAB), a task that was specifically linked to PFC DA in the monkey (Roberts et al., 1994; Crofts et al., 2001). We tested 9 COMT-Val-tg and 10 control littermates in an attentional set-shifting “stuck-in-set” paradigm, adapted and improved from previous studies (Birrell and Brown, 2000; Garner et al., 2006) and modeled after the human WCST and the IEDS test of the CANTAB. Each mouse was trained to perform a series of discriminations, including reversals, two intradimensional shifts, and an extradimensional shift. Two control and two COMT-Val-tg mice were excluded because they were not reliably digging to retrieve the food reinforcement during the first or second day of habituation. All of the other mice readily learned to dig for food rewards in the two bowls and were focused on their activities during the task.
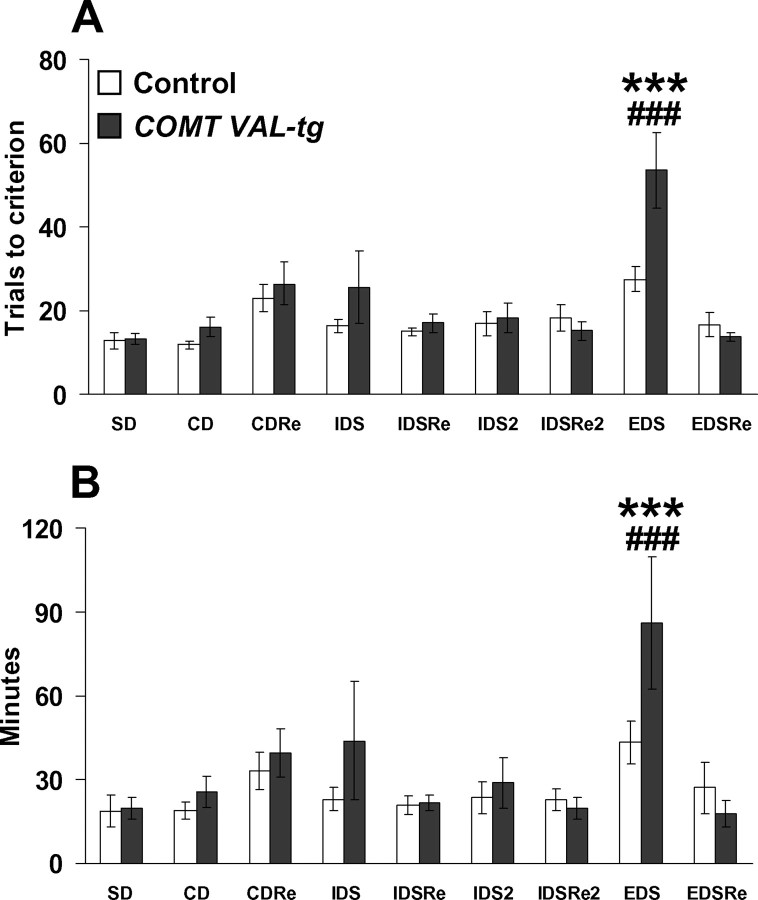
The analysis of the number of trials required to reach criteria revealed a significant genotype × discrimination interaction effect (F (8,104) = 3.07; p < 0.005), attributable entirely to impaired performance in COMT-Val-tg mice on the extradimensional shift (p < 0.0005) (Fig. 2 A). Analysis of time to complete each stage revealed a genotype × discrimination interaction effect (F (8,104) = 2.36; p < 0.05). COMT-Val-tg mice required more time to solve the EDS stage compared with all of the other discriminations and to the time required by controls (p < 0.0005) (Fig. 2 B). Consistent with studies in rats and in primates, control mice performed more poorly at the EDS stage compared with all of the other stages (supplemental text and Fig. S2, available at www.jneurosci.org as supplemental material).
Figure 2.
Selective impairment in shifting of attentional-set in COMT-Val-tg mice. A, B, Trials to criteria (A) and minutes to complete the different stages of the attentional set-shifting task (B) grouped by COMT-Val-tg and control littermates. Values represent mean ± SEM. n = 7–8 per group. ***p < 0.0005 versus the performance of control mice in all of the stages encountered; ### p < 0.0005 versus their own performance in all of the other stages. In our modified paradigm, the mice were able to acquire this demanding task in 3–7 d, a considerably shorter time than that reported in a previous study (Garner et al., 2006).
We also attempted to test the COMT knock-out mice in the attentional set-shifting task, but the mice failed to perform for reasons that were not attributable to cognitive performance. Soon after the beginning of the test (habituation day 1 and/or 2), both COMT+/− and COMT−/− mice were not reliably digging to retrieve the food reinforcement. Specifically, many of these mice attempted to jump out of the cage, spent time in biting the outer texture of the bowls or the digging media, and exhibited high levels of rearing, sniffing, and grooming. We believe these behaviors reflect the increased anxiety-like behavior manifest by these animals (see below).
Impaired object recognition memory in COMT-Val-tg mice and reversal by amphetamine treatment
Because the human COMT-Val/Met genotype also has been associated with tests involving recognition memory (Bertolino et al., 2006), we tested COMT-Val-tg mice and their littermate wild-type controls in a novel object recognition task. During the habituation session, control and COMT-Val-tg mice traveled similar distances in the empty open field (F (1,16) = 0.01; p = 0.90; n = 7–11) and showed similar locomotor habituation (F (1,176) = 18.59; p < 0.0001) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). During acquisition, control and COMT-Val-tg mice spent equal amounts of time exploring the objects (t = 0.09; df = 16; p = 0.93) (Fig. 3 A), suggesting no genotype differences in motivation, curiosity, motor, olfactory, tactile, or visual functions relevant to object recognition. However, during the retention trial, in contrast to controls, the COMT-Val-tg mice failed to spend more time exploring the novel object (t = 3.03; df = 16; p < 0.01) (Fig. 3 B), indicating impaired recognition memory.
Figure 3.
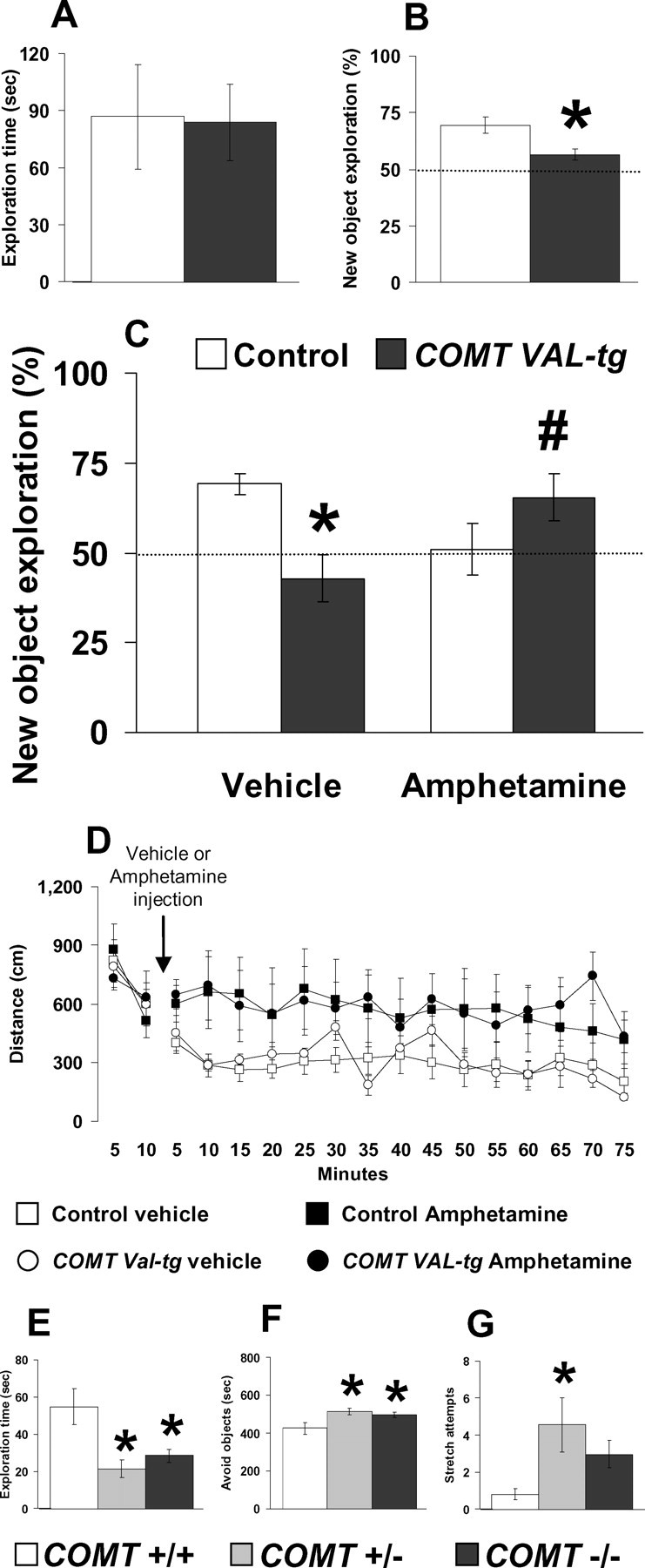
Impaired object recognition memory in COMT-Val-tg mice is reversed by amphetamine treatment. A, Time spent exploring two identical objects displayed by COMT-Val-tg and their control littermates during the 10 min acquisition session of the object recognition test. n = 8–11 per group. B, Percentage of the time spent exploring the new object over the total time spent in exploring both objects by COMT-Val-tg and control mice during the 5 min retention trial of the novel object recognition test, performed 1 h after the acquisition session. n = 7–11 per group. *p < 0.05 versus control mice. C, Percentage of the time spent exploring the new object over the total time spent in the exploration of the two objects during the 5 min retention trial with a 1 h delay, displayed by COMT-Val-tg and control mice treated with vehicle or amphetamine (1.5 mg/kg, i.p.) immediately after the acquisition session. The dotted lines correspond to chance levels (50%) of new object exploration. n = 7–12 per group. *p < 0.05 versus vehicle-treated control mice; # p < 0.05 versus vehicle-treated COMT-Val-tg mice. D, Ambulatory distance in 5 min bins displayed by control and COMT-Val-tg mice during the 10 min before and 1 h and 15 min after the vehicle or amphetamine (1.5 mg/kg, i.p.) injection. Note that these are the same open-field apparatus and timing used in the different phases of the novel object recognition memory test. n = 4–6 per group. E–G, Time spent exploring (E) and avoiding (F), and number of stretching attempts toward the two identical objects (G), displayed by COMT+/+, COMT+/−, and COMT−/− littermates during the 10 min acquisition session of the object recognition test. n = 5–7 per group. *p < 0.05 versus COMT+/+ mice. Values represent mean ± SEM.
To test whether lower DA levels in the COMT-Val-tg mice contributed to impaired recognition memory, control and COMT-Val-tg mice were treated with vehicle or a moderate dose of amphetamine (1.5 mg/kg, i.p.) immediately after the 10 min acquisition session. As found in the first cohort, in this second cohort of mice there were no differences in locomotor activity during the habituation session between the control and COMT-Val-tg mice (F (1,35) = 1.84; p = 0.20; n = 16–23 per group) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). Moreover, as found in the previous experiment, the analysis of the time spent exploring the two objects during the acquisition session revealed no genotype effect (F (1,34) = 1.20; p = 0.28), no assigned-treatment effect (F (1,34) = 0.01; p = 0.91), and no genotype × assigned-treatment interaction effect (F (1,34) = 0.01; p = 0.94; n = 7–12 per group) (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). Thus, these results strongly support the previous evidence that the COMT-Val-tg mice had no altered locomotion, exploration, curiosity, olfactory, tactile, or visual functions.
Analysis of the exploratory preference during the retention trial revealed no genotype effect (F (1,34) = 0.38; p = 0.54) nor treatment effect (F (1,34) = 0.43; p = 0.51), but an interesting genotype × amphetamine treatment interaction effect (F (1,34) = 12.33; p < 0.005). Post hoc analysis revealed that, as in our previous experiment, vehicle-treated COMT-Val-tg mice, in contrast to the vehicle-treated control mice, did not recognize and spent less time exploring the novel object (p < 0.05; n = 7–12 per group) (Fig. 3 C). However, whereas amphetamine treatment tended to reduce novel object exploration in control mice (p = 0.07), amphetamine increased novel object exploration in COMT-Val-tg (p < 0.05 vs vehicle-treated COMT-Val-tg mice) (Fig. 3 C). Amphetamine treatment did not affect sniffing behavior during the retention trial (supplemental text and Fig. S4, available at www.jneurosci.org as supplemental material). Thus, lower DA levels may be responsible for the memory deficits found in the COMT-Val-tg mice. The finding that mice with increased COMT enzyme activity and putatively reduced cortical DA benefit cognitively from amphetamine, whereas control mice are impaired by amphetamine, is consistent with the inverted U-shaped relationship between cortical DA and cognitive performance characterized in the monkey (Vijayraghavan et al., 2007) and is analogous to findings in humans that executive cognition is improved by amphetamine in individuals with the COMT-Val/Val genotype, but worsened in individuals with Met/Met genotypes (Mattay et al., 2003).
Amphetamine produced similar locomotor activation in COMT-Val-tg and control littermates
To test whether the amphetamine treatment differentially affects COMT genotypes on measures of locomotor activity, we tested a new cohort of 12 control and 9 COMT-Val-tg mice in conditions very similar to those used in the novel object recognition test. Again, there were no differences in the basal locomotor activity during the habituation session between the control and COMT-Val-tg mice (data not shown). On the following day, no locomotor activity differences were again present between groups of control and COMT-Val-tg mice during the first 10 min spent in the empty open field (equivalent to the 10 min acquisition session of the novel object recognition test; F (1,17) = 0.03; p = 0.87) (Fig. 3 D). After these 10 min, control and COMT-Val-tg mice were treated with vehicle or the same dose of amphetamine used in the novel object recognition test (1.5 mg/kg, i.p.) and put back into the empty open field for 1 h and 15 min (equivalent to the consolidation and retention phases of the novel object recognition test). Analysis of the distance traveled revealed no genotype effect (F (1,17) = 0.03; p = 0.87), a drug-treatment effect (F (1,17) = 5.72; p < 0.05), and no genotype × drug-treatment interaction effect (F (1,17) = 0.00; p = 0.98). Overall, amphetamine treatment slightly increased the locomotor activity in a genotype-independent way (p < 0.05; n = 4–6 per group) (Fig. 3 D). These results suggest that locomotor effects of amphetamine do not interact with the COMT genotype; thus, the changes in the recognition memory performance seen after amphetamine treatment were not dependent on aberrant locomotor activity in response to the psychostimulant drug.
Testing of COMT+/− and COMT−/− mice in the novel object recognition task reveals increased anxiety-like behavior
To determine the effect of genetically diminished COMT activity on recognition memory, we tested COMT−/−, COMT+/−, and COMT+/+ littermates in the same novel object recognition task. During the habituation session, COMT+/+, COMT+/−, and COMT−/− mice traveled similar distances and showed similar locomotor habituation in the empty open field (F (2,15) = 0.14; p = 0.87) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material), suggesting no genotype differences in general activity. However, after introduction of the novel objects during the acquisition session, COMT+/− and COMT−/− mice spent less time exploring the objects (F (2,15) = 7.16; p < 0.01; n = 5–7 per group) (Fig. 3 E). In fact, COMT+/− and COMT−/− mice spent more time avoiding the objects (F (2,14) = 4.64; p < 0.05) (Fig. 3 F) and exhibited more stretching attempts toward the objects without engaging in contact with them (F (2,15) = 4.36; p < 0.05) (Fig. 3 G). These results precluded an investigation of recognition memory in COMT knock-out mice.
Working memory impairment in COMT-Val-tg mice
Working memory deficits have been described as core cognitive features of schizophrenia and are related to PFC DA function (Vijayraghavan et al., 2007). Human subjects carrying the high-activity Val allele tend to perform more poorly on tasks involving working memory (Egan et al., 2001; Mattay et al., 2003; Tunbridge et al., 2006). We therefore tested the COMT transgenic and knock-out mice in a PFC-dependent working memory T-maze task (Aultman and Moghaddam, 2001; Kellendonk et al., 2006).
A similar percentage of the 8 COMT-Val-tg and 12 control mice tested acquired this T-maze task (75 and 83%, respectively; p = 0.88). However, COMT-Val-tg mice required significantly more days to reach the learning criteria (t = −2.33; df = 14; p < 0.05; n = 6–10 per group) (Fig. 4 A), indicating impaired acquisition of this working memory task. During the habituation phase of the task, COMT-Val-tg mice readily learn to run quickly through the maze to retrieve the food reward (F (1,18) = 41.32; p < 0.0001; n = 8–12 per group) (Fig. 4 B), a component of the T-maze task considered to depend on reference memory (Olton et al., 1979). Their habituation performance was not different from their control littermates (F (1,18) = 0.01; p = 0.92). Mice were further tested in the discrete paired-trial T-maze paradigm under more demanding conditions, consisting of four different intratrial delays, and decreasing the intertrial delay to 20 s, instead of 20 min. Analysis of the percentage of correct choices at the different intratrial delays revealed both a genotype effect (F (1,56) = 8.18; p < 0.01) and a delay effect (F (3,56) = 8.11; p < 0.0005). All groups displayed delay-dependent performance, but the COMT-Val-tg mice showed consistently worse performance compared with control littermates (p < 0.01; n = 6–10 per group) (Fig. 4 C). This profile indicates that even a relatively modest increase of COMT enzyme activity is detrimental to working memory performance.
Figure 4.
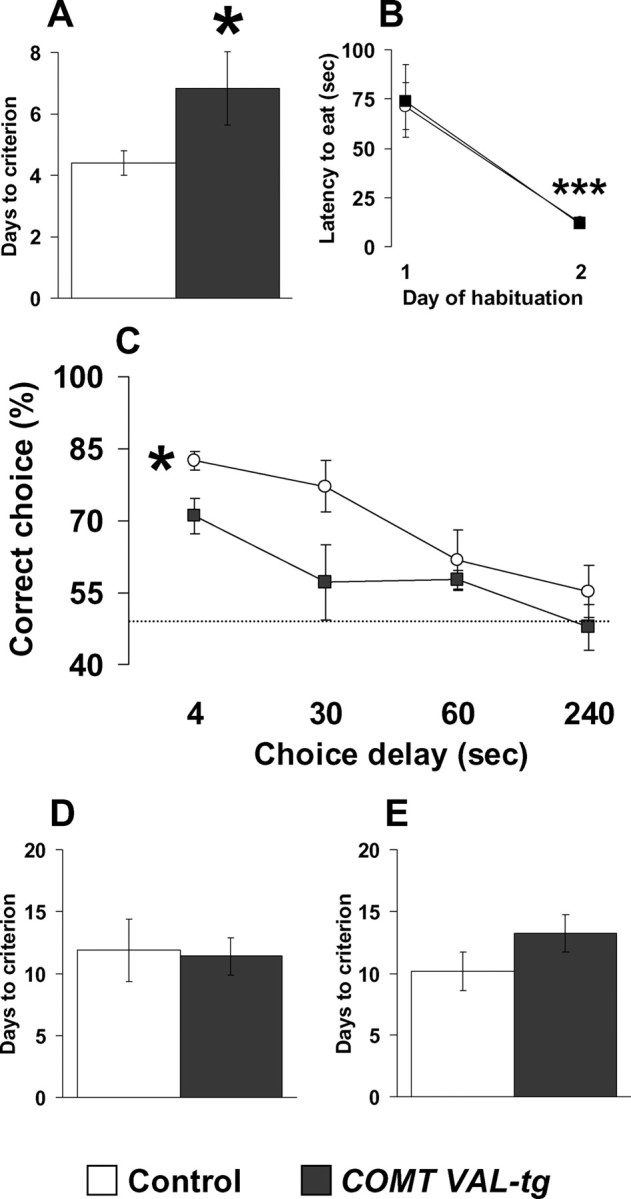
Specific working memory impairments in COMT-Val-tg mice. A, B, Days needed to reach the criteria (A) and latency to retrieve the hidden food pellet displayed by control and COMT-Val-tg mice during the discrete paired-trial T-maze task (B). n = 6–10 per group. *p < 0.05 versus control mice; ***p < 0.0001 versus day 1 of the habituation. C, Percentage of correct choices displayed by control and COMT-Val-tg mice during the discrete paired-trial variable-delay T-maze task with different intratrial delays randomly presented (4, 30, 60, and 240 s) and an intertrial delay of 20 s. The dotted line corresponds to chance levels (50%) of correct choices. n = 6–10 per group. *p < 0.01 versus control mice. D, E, Days needed to reach the criteria displayed by control and COMT-Val-tg mice during the continuous spatial alternation T-maze task (D; n = 8–15 per group) and during the reversal phase (E; n = 7–13 per group). Values represent mean ± SEM.
We also evaluated COMT-Val-tg in a continuous delayed alternation T-maze task involving a regularly repeated sequence of events rather than trial-specific experience. A similar percentage of the 19 COMT-Val-tg and 11 control mice tested acquired this T-maze task (79 and 73% respectively; p = 0.73); moreover, COMT-Val-tg and control mice required the same number of days to reach criterion (t = 0.17; df = 21; p = 0.86; n = 8–15 per group) (Fig. 4 D). To further rule out general cognitive deficits of the COMT-Val-tg mice, we tested these animals for acquisition of a simple spatial rule. Again, no genotype difference was present in the number of mice that attained the criteria (93 and 89% for COMT-Val-tg and control, respectively; p = 0.91) nor in the number of days to reach the criteria (t = −1.30; df = 18; p = 0.21; n = 7–13 per group) (Fig. 4 E) or in the learning curve through the day of testing (F (1,21) = 0.03; p = 0.85) (supplemental Fig. S5, available at www.jneurosci.org as supplemental material), indicating that the COMT-Val-tg mice manifest specific deficits in working memory functions that are not attributable to alterations in reference memory, spatial learning, motivation, food-reward associations, or nonspecific cognitive deficits.
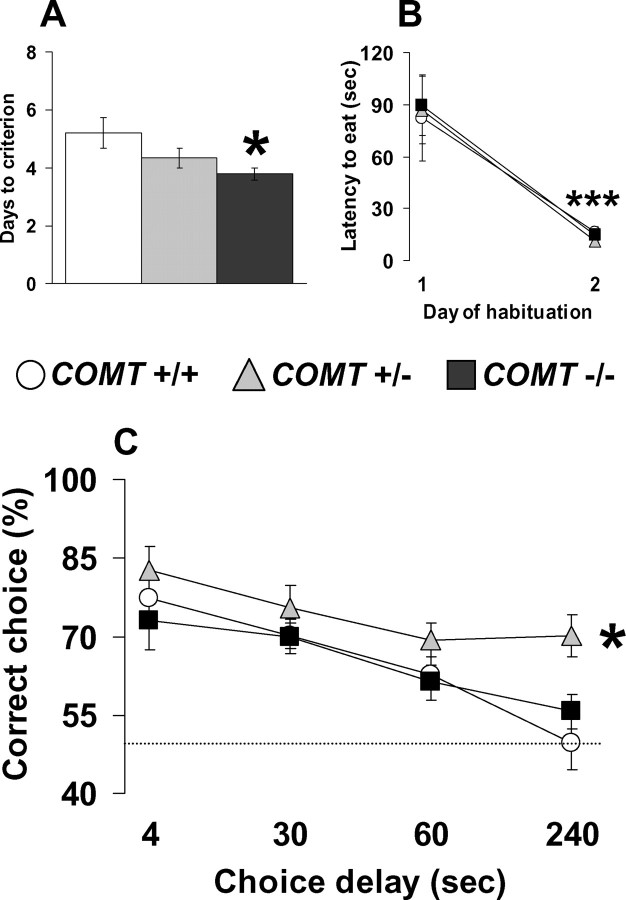
Improved working memory performance in COMT null mutant mice
Individuals carrying the Met allele, which confers reduced COMT activity and thereby relatively increased cortical synaptic DA (Slifstein et al., 2008), demonstrate improved working memory and executive function compared with Val allele carriers (Egan et al., 2001; Tunbridge et al., 2006). Thus, we investigated the working memory performance of COMT+/+, COMT+/−, and COMT−/− littermates in the same discrete paired-trial T-maze working memory task used in the COMT-Val-tg mice. COMT−/− littermates acquired this task faster than COMT+/− and COMT+/+ mice (p < 0.05; n = 10–14 per group) (Fig. 5 A). A similar number of the 14 COMT−/−, 13 COMT+/− and 10 COMT+/+ mice tested reached the criteria (100, 92, and 100%, respectively; p = 0.93). Moreover, COMT−/−, COMT+/−, and COMT+/+ mice readily learned to quickly retrieve the food reward during the habituation phase (F (1,33) = 39.76; p < 0.0001) (Fig. 5 B) and they did this similarly to the COMT-Val-tg mice. Thus, disruption of the COMT gene resulted in better working memory performance, opposite to the deficits in working memory seen in the COMT-Val-tg mice and in mice with medial PFC (mPFC) lesions (Kellendonk et al., 2006).
Figure 5.
Better working memory performance in COMT knock-out mice. A, B, Days needed to reach the criteria (A) and latency to retrieve the hidden food pellet (B) displayed by COMT+/+, COMT+/−, and COMT−/− mice during the discrete paired-trial T-maze task. n = 10–14 per group. *p < 0.05 versus COMT+/+ mice; ***p < 0.0001 versus day 1 of the habituation. C, Percentage of correct choices displayed by COMT+/+, COMT+/−, and COMT−/− during the discrete paired-trial variable-delay T-maze task with different intratrial delays randomly presented (4, 30, 60, and 240 s) and an intertrial delay of 20 s. The dotted line corresponds to chance levels (50%) of correct choices. n = 10–14 per group. *p < 0.01 versus COMT+/+ and COMT−/− mice. Values represent mean ± SEM.
Mice were further tested in the discrete paired-trial T-maze paradigm under more demanding conditions, consisting of four different intratrial delays, and decreasing the intertrial delay to 20 s, instead of 20 min. Analysis of the percentage of correct choices at the different intratrial delays revealed both a genotype effect (F (2,132) = 6.90; p < 0.005) and a delay effect (F (3,132) = 12.12; p < 0.0001). All groups displayed delay-dependent performance, but the COMT+/− mice showed consistent improvement in performance compared with COMT+/+ and COMT−/− (p < 0.01; n = 10–14 per group) (Fig. 5 C). This profile indicates that under more cognitively demanding conditions, partial decrease of the COMT enzyme activity (COMT+/−) is more beneficial to working memory performance than a complete absence or full presence of functional COMT.
COMT modulates expression of the Ca2+/calmodulin kinase superfamily in prefrontal cortex
To search for potential downstream molecular mechanisms underlying improved working memory performance in COMT−/− mice, we examined several components of the CaMK superfamily using both RT-qPCR and immunoblotting in the PFC. CaMK components, which are critical in the molecular biology of learning and memory, have been implicated also in working memory (Runyan et al., 2005). However, the specific role for the different components of the CaMK family in PFC-dependent cognitive abilities is still unclear.
PFC CaMKII protein immunoreactivity was decreased in COMT−/− mice (p < 0.05; n = 15–16 per group) (Fig. 6 A) and, conversely, increased in COMT-Val-tg mice (p < 0.05; n = 10 per group) (Fig. 6 B). The CaMKII protein is encoded by four genes (α-, β-, δ-, and γ-CaMKII); COMT−/− mice had decreased α-CaMKII mRNA expression in PFC (p < 0.05; n = 9–10 of group) (Fig. 6 C), but increased β-CaMKII (p < 0.05; n = 9–10 per group) (Fig. 6 D), δ-CaMKII (p < 0.05; n = 9–10 per group) (Fig. 6 E), and γ-CaMKII (p < 0.05; n = 9–10 per group) (Fig. 6 F).
Figure 6.
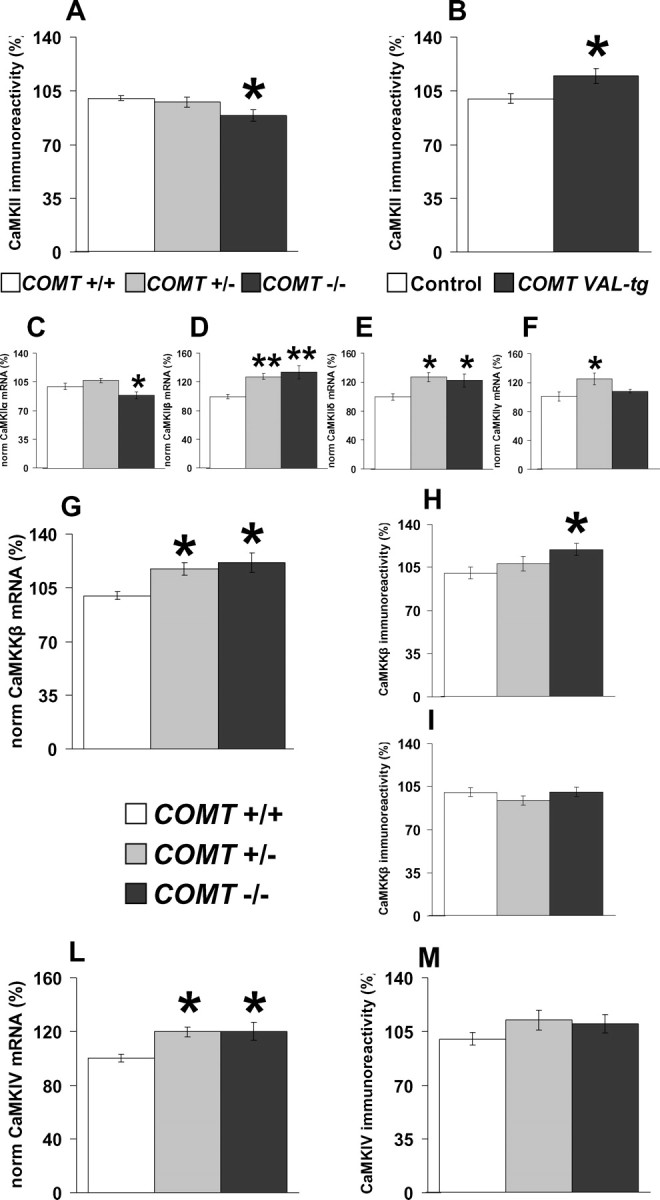
COMT specifically modulate PFC CaMK superfamily components. A, B, CaMKII protein immunoreactivity levels in the PFC of COMT+/+, COMT+/−, COMT−/− (A) and control and COMT-Val-tg mice (B) by immunoblot analysis. n = 10–16 per group. *p < 0.05 versus proper wild-type control group. C–F, Normalized expression levels of CaMKII mRNA α- (C), β- (D), δ- (E), and γ- (F) subunits in the PFC of COMT+/+, COMT+/− and COMT−/− mice. n = 9–10 per group. *p < 0.05; **p < 0.005 versus COMT+/+ mice. G–I, Normalized expression levels of CaMKKβ mRNA (G) and protein, heavier (H), and lighter (I) band by immunoblot analysis in the PFC of COMT+/+, COMT+/−, and COMT−/− mice. n = 9–10 per group. *p < 0.05 versus COMT+/+ mice. The CaMKKβ heavier band has been reported to be brain-specific (Anderson et al., 1998). L, M, Normalized expression levels of CaMKIV mRNA (L) and protein (M) in the PFC of COMT+/+, COMT+/−, and COMT−/− mice. n = 9–28 per group. *p < 0.05 versus COMT+/+ mice. Results are expressed as percentage of the COMT+/+ group for each experiment. Values represent mean ± SEM.
We also found in PFC that COMT−/− mice had increased CaMKKβ mRNA expression (p < 0.05; n = 9–10 per group) (Fig. 6 G) and protein levels of the brain-specific heavier band (p < 0.05; n = 9–10 per group) (Fig. 6 H), but not of the lighter nonspecific ubiquitous band (p = 0.31; n = 9–10 per group) (Fig. 6 I). COMT−/− and COMT+/− mice also manifest higher mRNA expression of CaMKIV (p < 0.05; n = 9–10 per group) (Fig. 6 L), a finding consistent with evidence that CaMKKβ regulates CaMKIV (Anderson et al., 1998). However, there was no difference between COMT−/− and COMT+/+ mice in CaMKIV protein immunoreactivity (p = 0.29; n = 25–28 per group) (Fig. 6 M).
COMT deficiency does not have a ubiquitous effect on PFC genes expression
To consider the specificity of the molecular effects of COMT genotype, we quantified several other genes in the PFC of COMT+/+, COMT+/− and COMT−/− mice. Although COMT deletion changed the expression of several components of the CaMK family in the PFC, we found no changes in the PFC protein levels of CaMKI (COMT+/+, 100.0 ± 2.0; COMT+/−, 103.5 ± 3.6; COMT−/−, 98.8 ± 4.5; F (2,27) = 0.47; p = 0.63; n = 10 per group) and CaMKKα (COMT+/+, 100.0 ± 5.3; COMT+/−, 104.5 ± 8.3; COMT−/−, 102.8 ± 8.6; F (2,25) = 0.09; p = 0.92; n = 9–10 per group). Moreover, in the PFC, COMT deletion did not affect several other genes, including adenylate cyclase 1 (COMT+/+, 100.1 ± 9.1; COMT+/−, 117.4 ± 8.5; COMT−/−, 115.5 ± 9.7; F (2,24) = 1.09; p = 0.35; n = 9 per group), cannabinoid receptor 1 (COMT+/+, 100.0 ± 7.5; COMT+/−, 102.2 ± 6.0; COMT−/−, 94.04 ± 6.1; F (2,29) = 0.45; p = 0.64; n = 9–12 per group), protein kinase cAMP dependent catalytic α (COMT+/+, 100.0 ± 4.4; COMT+/−, 97.1 ± 2.1; COMT−/−, 106.9 ± 2.1; F (2,25) = 2.85; p = 0.07; n = 9–10 per group), fasciculation and elongation protein ζ-1 (COMT+/+, 100.0 ± 3.9; COMT+/−, 106.2 ± 3.8; COMT−/−, 99.5 ± 2.8; F (2,28) = 1.21; p = 0.31; n = 8–12 per group), lissencephaly 1 (COMT+/+, 99.8 ± 3.6; COMT+/−, 100.6 ± 4.4; COMT−/−, 93.8 ± 1.8; F (2,29) = 1.17; p = 0.33; n = 9–12 per group), and two so-called housekeeping genes, actin (COMT+/+, 100.0 ± 6.6; COMT+/−, 100.0 ± 6.1; COMT−/−, 109.3 ± 3.6; F (2,29) = 0.96; p = 0.39; n = 9–12 per group) and β-glucuronidase (COMT+/+, 100.0 ± 4.7; COMT+/−, 93.3 ± 2.5; COMT−/−, 94.3 ± 5.8; F (2,24) = 0.57; p = 0.58; n = 9–10 per group).
Acoustic startle is decreased in COMT-Val-tg mice and increased in COMT−/− mice
The startle response elicited by sudden sensory stimuli is one of the most widely studied phenotypes that are highly conserved across mammalian species. Because the COMT-Val/Met genotype has been associated with stress related phenotypes in humans, we explored the startle response in our genetically altered mice. Compared with controls, COMT-Val-tg mice showed lower acoustic startle reactivity to the 120 dB stimulus (p < 0.0005), whereas levels of basal activity did not differ (p = 0.98; n = 11–18 per group) (Fig. 7 A). In contrast, COMT−/− mice showed higher startle reactivity to the 120 dB stimulus compared with COMT+/− and COMT+/+ littermates (p < 0.005), whereas levels of basal activity did not differ (p = 0.93; n = 11–12 per group) (Fig. 7 C). Analysis of PPI of a 120 dB acoustic startle stimulus showed no genotype effect in both the COMT transgenic and knock-out mice (F (1,27) = 1.98, p = 0.17, and F (2,31) = 0.63, p = 0.54, respectively). In both lines of mutant mice, PPI progressively increased with higher prepulse intensities (main effect of prepulse sound level, F (4,108) = 19.23, p < 0.0001, and F (4,124) = 45.89, p < 0.0001). No significant genotype × prepulse intensity interactions were detected (F (4,108) = 1.24, p = 0.30 and F (8,124) = 0.54, p = 0.82, for COMT transgenic and knock-out mice, respectively) (Fig. 7 B,D).
Figure 7.
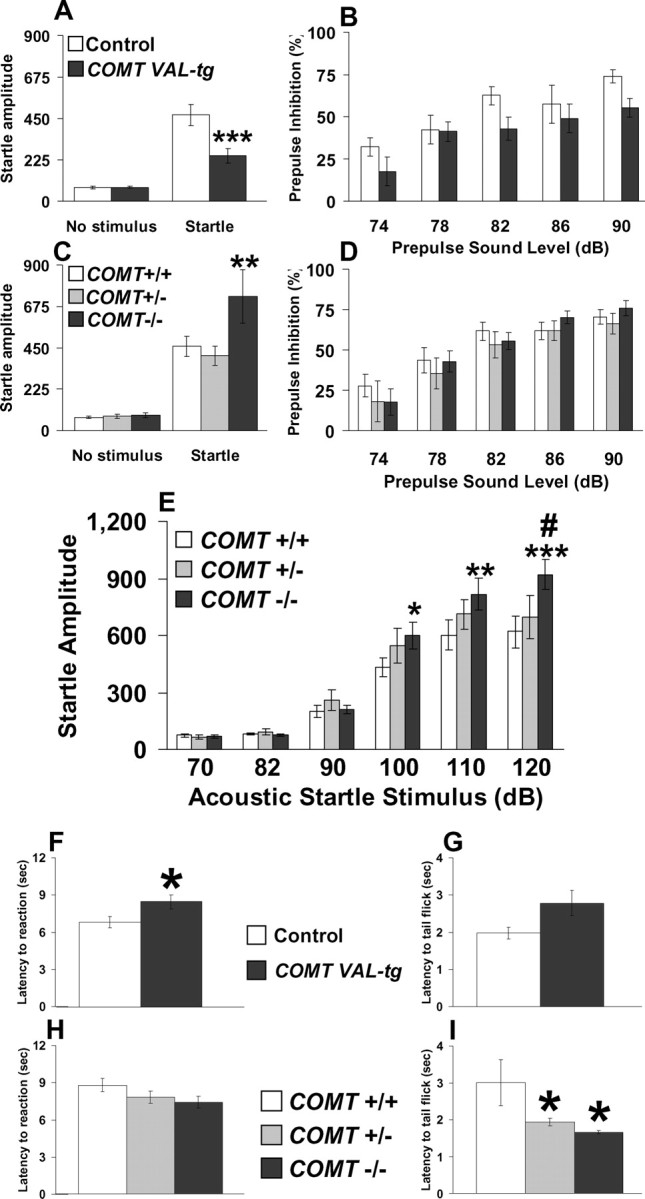
Acoustic startle and pain sensitivity are blunted in COMT-Val-tg mice and exaggerated in COMT−/− mice. A, C, Startle amplitude displayed by control and COMT-Val-tg mice (A) and COMT+/+, COMT+/−, and COMT−/− mice (C) after the presentation of no stimulus or a 120 dB stimulus (Startle). B, D, Percentage prepulse inhibition of the acoustic startle response displayed by the same COMT transgenic mice (B) and COMT knock-out mice (D) after the presentation of 74, 78, 82, 86, and 90 dB prepulse sound stimuli. n = 11–18 per group. ***p < 0.0005 versus control mice startle response; **p < 0.005 versus COMT+/+ and COMT+/− mice startle response. E, Startle reaction amplitude displayed by COMT+/+, COMT+/−, and COMT−/− mice after the presentation of 40 ms, 70, 82, 90, 100, 110, and 120 dB acoustic startle stimuli. n = 11–14 per group. *p < 0.05; **p < 0.001; ***p < 0.0001 versus COMT+/+ mice; # p < 0.01 versus COMT+/− mice. F, G, Comparison of COMT-Val-tg and control littermates on reaction latency in the hot plate test (F; n = 17–21 per group) and in the tail flick test (G; n = 24–31 per group). *p < 0.05 versus control mice. H, I, Comparison of COMT+/+, COMT+/−, and COMT−/− littermates on reaction latency in the hot plate test (H; n = 19–24 per group) and in the tail flick test (I; n = 13–15 per group). *p < 0.05 versus COMT+/+ mice. Values represent mean ± SEM.
To characterize further the modulation of the startle response by COMT genotype, we tested the acoustic startle amplitude and threshold to various sound levels in 13 COMT−/−, 11 COMT+/−, and 14 COMT+/+ naive littermates. The acoustic startle threshold was 100 dB for all genotypes; however, COMT−/− mice showed higher startle amplitude than COMT+/+ mice at 100 (p < 0.05) and 110 dB (p < 0.001), and higher startle amplitude than COMT+/+ (p < 0.0001) and COMT+/− (p < 0.01) mice at 120 dB (Fig. 7 E). These results indicate that genetic variations in COMT alter startle reactivity, whereas PPI processes are not affected under these conditions.
Pain sensitivity is decreased in COMT-Val-tg mice and increased in COMT−/− mice
Recent reports suggest that COMT genotypes are related to differences in human pain perception (Nackley et al., 2006). Thus, we tested our genetically modified mice with the hot plate and tail flick tests to assess the role of COMT in centrally mediated and spinal pain reflexes, respectively. COMT-Val-tg mice showed increased latency to reaction in the hot plate test (t = −2.2; df = 36; p < 0.05; n = 17–21 per group) (Fig. 7 F) and a tendency to higher latency to tail flick (t = −2.0; df = 53; p = 0.05; n = 24–31 per group) (Fig. 7 G) compared with controls. COMT−/− mice were not different from COMT+/− and COMT+/+ mice in the hot plate test (F (2,62) = 1.94; p = 0.15; n = 19–24 per group) (Fig. 7 H) but in the tail flick test a genotype effect was present (F (2,39) = 4.23; p < 0.05), wherein both COMT−/− and COMT+/− mice showed decreased tail flick latencies compared with COMT+/+ mice (p < 0.05; n = 13–15 per group) (Fig. 7 I). Thus, in line with recent human literature, increased COMT activity attenuated pain perception, whereas reduced COMT increased pain perception.
Stress-induced hyperthermia is blunted in COMT-Val-tg mice and exacerbated in COMT−/− mice
To further explore the role of COMT in stress reactivity and anxiety-related phenotypes, COMT mutant mice were tested in a stress-induced hyperthermia paradigm. A stress-induced rise in body temperature characterizes a large variety of species including humans and rodents, and is a well validated model for anxiety-related traits (Adriaan Bouwknecht et al., 2007). Control and COMT-Val-tg mice did not differ in their basal temperature (T1; p = 0.73). Although stress increased body temperature in both groups (T2; p < 0.0005), the response of COMT-Val-tg mice was blunted (p < 0.05; n = 13–16 per group) (Fig. 8 A).
Figure 8.
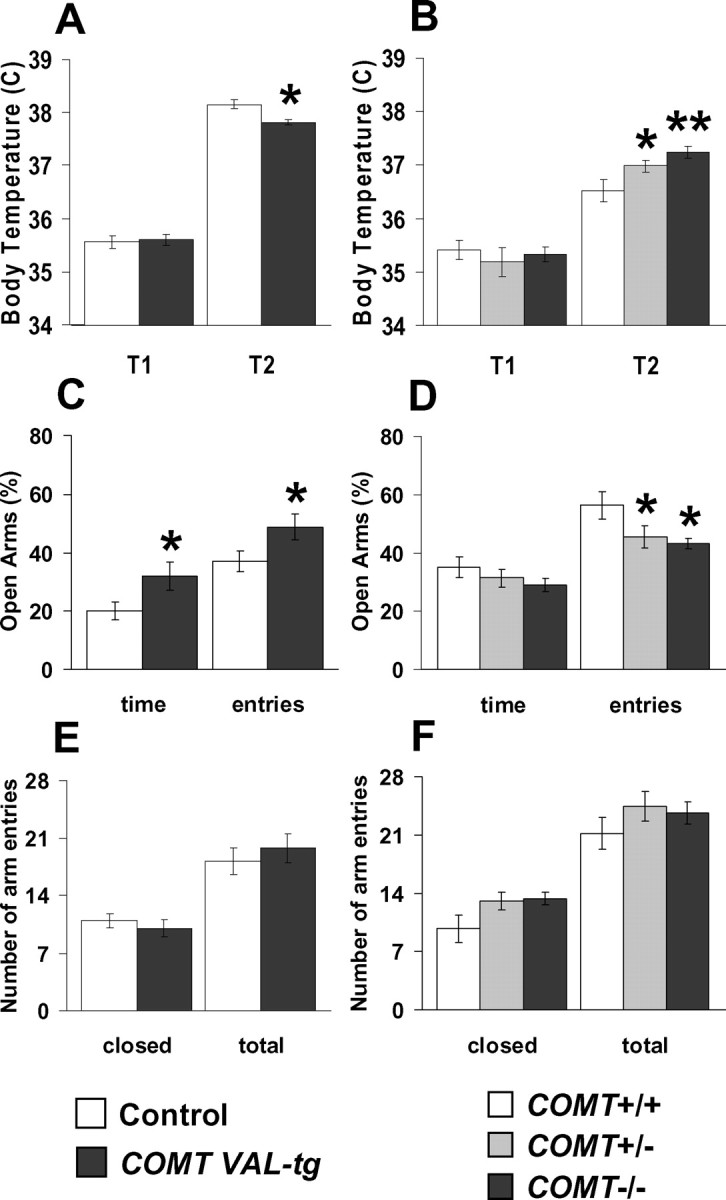
Anxiety-like traits are decreased in COMT-Val-tg mice and increased in COMT−/− mice. A, Body temperatures displayed by control and COMT-Val-tg mice before (T1) and 25 min after (T2) the manipulation of the mice, consisting of handling and insertion of the rectal probe, plus a supplemental stress, consisting of placing the animals in a clean new cage. n = 13–16 per group. *p < 0.05 versus T2 of control mice. B, Body temperatures displayed by COMT+/+, COMT+/−, and COMT−/− mice before (T1) and 10 min after (T2) handling and insertion of the rectal probe. n = 9–11 per group. *p < 0.05; **p < 0.005 versus T2 of COMT+/+ mice. C, D, In the elevated plus maze, the percentage of time spent in the open arms and number of visits to the open arms were (C) significantly higher for COMT-Val-tg mice and (D) less for COMT−/− and COMT+/− mice. n = 9–15 per group. *p < 0.05 versus proper wild-type control group. E, F, Locomotor activity in the elevated plus maze was not different between control and mutant mice as measured by closed arm entries and total arm entries. Values represent mean ± SEM.
COMT+/+, COMT+/−, and COMT−/− mice also did not differ in their basal temperature (T1; p = 0.53). Again, stress increased temperature in all three groups (p < 0.0005), but the effect was exaggerated in both COMT−/− and COMT+/− mice compared with COMT+/+ mice (p < 0.05; n = 9–11 per group) (Fig. 8 B). These results indicate that insertion of the human COMT-Val gene blunted, whereas deletion of the COMT gene exacerbated, autonomic reactivity to stress.
Anxiety-like traits are decreased in COMT-Val-tg mice and increased in COMT−/− mice
Finally, we tested the COMT genetically modified mice in the elevated plus maze paradigm, one of the most widely used tests of anxiety-related behavior in mice (Crawley, 2007). COMT-Val-tg mice spent more time in (t = −2.2; df = 23; p < 0.05) and entered more frequently (t = −2.1; df = 23; p < 0.05) the open arms of the plus-maze apparatus than did their control littermates (Fig. 8 C) (n = 10–15 per group). Conversely, COMT−/− and COMT+/− mice entered less frequently the open arms of the plus-maze apparatus than did their COMT+/+ littermates (p < 0.05; n = 9–13 per group) (Fig. 8 D). The decrease in anxiety-like behavior in COMT-Val-tg mice and increase in COMT−/− and COMT+/− mice was not a result of altered general exploration, as overall activity in closed-arm and total arm entries was not different between the different groups (for transgenic mice, p = 0.49 and p = 0.52; for knock-out mice, p = 0.08 and p = 0.42, respectively) (Fig. 8 E,F).
Discussion
This study demonstrates that genetic manipulation of COMT in mice affects specific cognitive and stress reactivity measures remarkably analogous to those reported in humans. Our inverse results from increased and decreased life-long changes in COMT activity elucidate the link of causality between functional polymorphisms in the COMT gene and diverse human behaviors. Moreover, COMT genotype effects on CaMK components reveal a potential molecular mechanism for the PFC-dependent cognitive behaviors linked with COMT variations.
It is tempting to speculate that the apparent trade-off between a genetic effect that improves memory but exaggerates emotional responsivity has potential implications for environmental selection and thus implicates evolutionary mechanisms. We demonstrate that, from mice to humans, the first codon Kozak sequence has changed to favor translation of the MB form of COMT in brain. Together with a progressive decrease in COMT activity associated with the transition from Leu to Val to Met (from mouse to monkey to human, respectively), these gene divergence changes are consistent with the increasing importance of COMT in central neurotransmission compared with its earlier primary role in peripheral detoxification.
Conversely to relative cognitive advantages but stress induced disadvantages of COMT−/− mice, relatively increased COMT activity produced specific cognitive disadvantages but a stress and pain sensitivity advantage. Thus, genetic variability in COMT represents an attractive biological mechanism by which a specific gene has pleotropic behavioral effects related to human cortical function, including cognition, emotion, and risk for mental illness. Indeed, we have demonstrated in our COMT mice all of the major behavioral and pharmacological associations that have been reported in humans. Because of the complexity of the human behavioral phenotypes and of genetic variation in human COMT, the evidence for a role of COMT in these various phenotypes has been controversial. The data in mice are robust and conclusive.
COMT and cortical DA function
In line with the functional importance of COMT in PFC DA signaling, we found increased TH expression in the PFC of the COMT-Val-tg mice. Similarly, postmortem human midbrains of COMT-Val homozygotes had higher TH mRNA levels than Val/Met heterozygotes (Akil et al., 2003). Early studies in rodents had suggested that COMT may be expressed also in glia (Kaplan et al., 1979); these findings, however, were based on two-dimensional immunohistochemistry and recent work has shown that COMT mRNA in mouse and human brain is expressed primarily if not exclusively in neurons (Matsumoto et al., 2003). In agreement with previous studies showing that COMT does not impact on cortical NE signaling (Gogos et al., 1998; Tunbridge et al., 2004; Yavich et al., 2007), we found no difference in DβH levels between COMT-Val-tg and control mice.
Contrary to previous claims, our results further support recent evidence that mice are able to develop an attentional-set (Garner et al., 2006), analogous to that described in rats, primates, and humans (Dias et al., 1997; Birrell and Brown, 2000). COMT-Val-tg mice displayed a selective impairment of the EDS, but no alteration in acquisition or reversal learning. This same double dissociation of EDS and reversal learning has been found in rats, monkeys, and humans with lesions of PFC and orbitofrontal cortex, respectively (Dias et al., 1997; Birrell and Brown, 2000; Robbins, 2007). Moreover, EDS appears to be modulated specifically by DA pathways in the PFC (Robbins, 2007). These mechanisms are consistent with our results, which closely mimic the human findings that poorer performance and greater PFC “inefficiency” in a comparable PFC-dependent test is associated with the high-activity COMT-Val allele (Egan et al., 2001). Additionally, it has been demonstrated that acute administration of a COMT inhibitor improves EDS performance in rats and increases mPFC DA (but not NE) under conditions of enhanced catecholamine efflux (Tunbridge et al., 2004).
COMT-Val-tg mice showed recognition memory deficits. Cognitive performance in this task involves prominently the perirhinal cortex and is thought to be hippocampal independent when contextual features are not changed (O'Brien et al., 2006). Acute treatment with amphetamine, which increases DA levels in the perirhinal, prefrontal, and entorhinal cortex (Pum et al., 2007), restored recognition memory performance of COMT-Val-tg mice but worsened performance of control. Amphetamine was injected immediately after the learning phase, and our locomotor activity data and microdialysis studies (Pum et al., 2007) suggest that amphetamine could be still active during the retention delay. Thus, amphetamine could have affected consolidation and/or retrieval but not encoding of objects memory. Analogously, in humans, amphetamine enhanced cognitive performance in subjects with the high-enzyme-activity COMT-Val/Val genotype, whereas in subjects with the low activity COMT-Met/Met genotype, who tend to have superior cognitive performance at baseline, the drug was deleterious (Mattay et al., 2003). These data further illustrate the inverted-U-shaped relationship between cognitive performance and cortical DA levels and its modulation by COMT.
COMT−/− mice acquired a clinically relevant discrete paired-trial alternation T-maze task faster than COMT+/+ and COMT+/− mice. In contrast, COMT-Val-tg mice required more days to acquire it than did their controls. This paradigm has been designed to recapitulate important elements of human and monkey working memory tasks in rodents (Aultman and Moghaddam, 2001). The working memory network across species is thought to be centered around the PFC (Chudasama and Robbins, 2006). In line with this, mice with mPFC lesions required more days to reach the learning criteria in the same test (Kellendonk et al., 2006), mimicking the COMT-Val-tg mice phenotype. Moreover, working memory impairments in this test have been linked to altered DA transmission and D1 receptor activation in rodent mPFC (Aultman and Moghaddam, 2001; Kellendonk et al., 2006). In an analogous way, disruption of DA dorsolateral PFC signaling, and specifically D1 receptor pathways, has been associated with profound working memory impairments in nonhuman primates (Vijayraghavan et al., 2007).
D1/D5 receptor pathways in rat PFC have been shown to modulate long-term potentiation of intrinsic excitability through the activation of CaMK pathways (Chen et al., 2007). Here, we add novel evidence for a role of CaMK pathways in PFC-dependent processes and its connections with COMT. In the PFC of COMT−/− mice, CaMKKβ mRNA and protein immunoreactivity were increased, as was mRNA of CaMKIV, which is regulated by CaMKKβ (Anderson et al., 1998). These results may explain the finding of enhanced CaMK activity found in rat mPFC after working memory training (Runyan et al., 2005). Moreover, disruption of CaMKKβ as well as CaMKIV has been shown to impair certain aspects of memory (Kang et al., 2001; Peters et al., 2003). In an opposite way, Runyan et al. (2005) found that intra-mPFC infusion of the CaMKII-preferring inhibitor KN-93 (N-[2-[N-(4-chlorocinnamyl)-N-methylaminomethyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulfonamide phosphate salt) produced an enhancement in working memory performance. In agreement, we found that COMT−/− mice, with improved working memory performance, manifest decreased PFC levels of CaMKII protein, whereas COMT-Val-tg mice, which have working memory deficits, had increased PFC CaMKII protein immunoreactivity. Reduced CaMKII protein levels in the PFC of COMT−/− mice may reflect decreased mRNA expression of the α-CaMKII subunit, because in adult mice the α- subunit is relatively more abundant in the forebrain (Greenstein et al., 2007). In contrast, the other subunits were found to be increased, in accordance with evidence that α- and β-CaMKII reciprocally regulate each others activity (Thiagarajan et al., 2002).
Despite the COMT genotype effects on working memory processes, COMT-Val-tg mice showed normal performance in discrimination learning, T-maze reference components, continuous alternation task, and reversal. These tasks involve a regularly repeated sequence of events rather than trial-specific experience, and they involve expectancy-based information processing rather than working memory mechanisms (Brito et al., 1987; Green and Stanton, 1989). Thus, our results highlight the COMT gene as a critical hot spot in the regulation of executive memory processes.
We found that in the discrete paired-trial variable-delay T-maze task, the COMT−/− mice were the best performers under low-stress conditions, whereas COMT+/− performed best under more challenging conditions. The apparent heterozygote advantage observed here might be related to the double dissociation of the COMT genotype extremes on cognition and stress-related phenotypes. Indeed, mild uncontrollable stressors impair PFC working memory functions in both humans and animals (Arnsten, 2000). Alternatively, the heterozygote advantage may be further evidence of the inverted-U-response between cortical DA levels and cognition.
COMT and emotionality
Our genetically altered mice showed dramatic effects of COMT on emotional and autonomic arousal. We found a gene-dosage effect in stress-induced hyperthermia, in acoustic startle reaction, in pain sensitivity and in anxiety-like traits, with COMT−/− mice overreacting and COMT-Val-tg mice showing blunted reactions.
COMT genotypes have been associated in humans with pain sensitivity. We confirmed and expanded these findings in our genetically modified mice. Moreover, our characterization of responses to diverse stressors suggests that COMT has a general role in stress reactivity and that pain sensitivity may be a proxy measure for this more general-behavior arousal response. In agreement with our findings, the COMT-Met allele has been linked with increased levels of anxiety, OCD, panic disorder, type-1 alcoholism, anger-related traits, reward dependence, and pain sensitivity (Drabant et al., 2006; Heinz and Smolka, 2006).
In conclusion, we demonstrate that increased COMT activity is a risk factor for cortically dependent cognitive dysfunctions but a protective factor in stressful situations, whereas COMT reduction enhances working memory processes but results in exaggerated stress reactivity. The COMT transgenic and knock-out mice recapitulate salient aspects of human behaviors associated with COMT polymorphisms and establish the biologic validity of these associations. Our COMT model reifies that a common genetic factor can be involved in diverse clinical disorders characterized by abnormal cognitive processing and stress reactivity and represents a promising new animal model for testing cognitive and stress related therapies. Finally, our results raise intriguing gene divergence implications of a functional trade-off between genetic variation that concurrently results in more efficient cognitive behaviors and less adaptive affective behaviors.
Footnotes
This work was supported by the Intramural Program of the National Institutes of Health–National Institute of Mental Health. We thank P. Patnaik and C. Bes for technical assistance with behavioral tests and G. Liu for mice genotyping. We thank Drs. M. Karayiorgou and J. A. Gogos (Columbia University, New York, NY) for generously donating the COMT−/− mouse breeders and Dr. A. Contarino for critical reading of this manuscript. We also thank Dr. J. Wu, and G. Ma, S. Le, S. Melhem, B. Stepp, H. Choxi, and R. Buerlein for technical assistance.
The authors declare that they have no financial conflict of interest.
References
- Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem. 1998;273:31880–31889. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 2001;153:353–364. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–705. [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, Caforio G, Petruzzella V, Kolachana B, Hariri A, Meyer-Lindenberg A, Nardini M, Weinberger DR, Scarabino T. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito LS, Yamasaki EN, Paumgartten FJ, Brito GN. Continuous and discrete T-maze alternation in rats: effects of intertrial and interrun intervals. Braz J Med Biol Res. 1987;20:125–135. [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bohanick JD, Nishihara M, Seamans JK, Yang CR. Dopamine D1/5 receptor-mediated long-term potentiation of intrinsic excitability in rat prefrontal cortical neurons: Ca2+-dependent intracellular signaling. J Neurophysiol. 2007;97:2448–2464. doi: 10.1152/jn.00317.2006. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Ed 2. Hoboken, NJ: Wiley; 2007. What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JP, Thogerson CM, Würbel H, Murray JD, Mench JA. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behav Brain Res. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Green RJ, Stanton ME. Differential ontogeny of working memory and reference memory in the rat. Behav Neurosci. 1989;103:98–105. doi: 10.1037//0735-7044.103.1.98. [DOI] [PubMed] [Google Scholar]
- Greenstein R, Novak G, Seeman P. Amphetamine sensitization elevates CaMKIIbeta mRNA. Synapse. 2007;61:827–834. doi: 10.1002/syn.20429. [DOI] [PubMed] [Google Scholar]
- Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci. 2006;17:359–367. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, O'Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol Psychiatry. 2005;10: 229:287–298. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- Kaplan GP, Hartman BK, Creveling CR. Immunohistochemical demonstration of catechol-o-methyltransferase in mammalian brain. Brain Res. 1979;167:241–250. doi: 10.1016/0006-8993(79)90819-9. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M, Kawachi I, Ascherio A, Colditz GA, Hunter DJ, De Vivo I. Association between catechol-O-methyltransferase and phobic anxiety. Am J Psychiatry. 2004;161:1703–1705. doi: 10.1176/appi.ajp.161.9.1703. [DOI] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- O'Brien N, Lehmann H, Lecluse V, Mumby DG. Enhanced context-dependency of object recognition in rats with hippocampal lesions. Behav Brain Res. 2006;170:156–162. doi: 10.1016/j.bbr.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelmann GE. Hippocampus, space, and memory. Behav Brain Sci. 1979;2:313–365. [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Peters M, Mizuno K, Ris L, Angelo M, Godaux E, Giese KP. Loss of Ca2+/calmodulin kinase kinase beta affects the formation of some, but not all, types of hippocampus-dependent long-term memory. J Neurosci. 2003;23:9752–9760. doi: 10.1523/JNEUROSCI.23-30-09752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley EC, Fineberg N, Harrison PJ. The met(158) allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: case-control study and meta-analysis. Mol Psychiatry. 2007;12:556–561. doi: 10.1038/sj.mp.4001951. [DOI] [PubMed] [Google Scholar]
- Pum M, Carey RJ, Huston JP, Muller CP. Dissociating effects of cocaine and d-amphetamine on dopamine and serotonin in the perirhinal, entorhinal, and prefrontal cortex of freely moving rats. Psychopharmacology (Berl) 2007;193:375–390. doi: 10.1007/s00213-007-0791-2. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn Mem. 2005;12:103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, Frankle WG, Weinberger DR, Laruelle M, Abi-Dargham A. COMT genotype predicts cortical-limbic D1 receptor availability measured with [(11)C]NNC112 and PET. Mol Psychiatry. 2008;13:821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Winterer G, Egan MF, Kolachana BS, Goldberg TE, Coppola R, Weinberger DR. Prefrontal electrophysiologic “noise” and catechol-O-methyltransferase genotype in schizophrenia. Biol Psychiatry. 2006;60:578–584. doi: 10.1016/j.biopsych.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Männistö PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]