Abstract
Background
Systemic steroids have been advocated in addition to antimicrobial therapy for severe Mycoplasma pneumoniae pneumonia. We evaluated the efficacy of clarithromycin, dexamethasone, or combination therapy for M. pneumoniae respiratory infection.
Methods
Mice infected with M. pneumoniae were treated with clarithromycin, dexamethasone, combined clarithromycin/dexamethasone, or placebo daily; mice were evaluated at baseline and after 1, 3, and 6 days of therapy. Outcome variables included M. pneumoniae culture; lung histopathologic score (HPS); bronchoalveolar lavage cytokine, chemokine and growth factor concentrations.
Results
Clarithromycin monotherapy resulted in the greatest reductions in M. pneumoniae concentrations. After 3 days of treatment, combination therapy significantly reduced lung HPS compared with placebo, clarithromycin, and dexamethasone alone; while after 6 days of therapy, clarithromycin alone and combination therapy significantly reduced lung HPS compared with placebo. IL-12 p40, RANTES, MCP-1, and KC were significantly lower in mice treated with clarithromycin alone and/or combination therapy compared with dexamethasone alone and/or placebo; combination therapy resulted in a significantly greater reduction than clarithromycin alone for IL-12 p40 and RANTES.
Conclusions
While monotherapy with clarithromycin had the greatest effect on reducing concentrations of M. pneumoniae, combination therapy had the greatest effect on decreasing cytokines and chemokines, as well as pulmonary histologic inflammation.
Keywords: Mycoplasma pneumoniae, asthma, pneumonia, clarithromycin, steroids, dexamethasone, cytokines, chemokines, macrolide, IL-12
Introduction
Mycoplasma pneumoniae is a common etiology of pediatric and adult community-acquired pneumonia, causing 10−40% of cases [1] [2] [3] [4]. Treating M. pneumoniae pneumonia with appropriate antibiotics, such as macrolides, has been found to significantly improve the course of disease in both animal models and human investigations [5] [6] [7] [8] [9] [10] [11] [12]. Observational data in both children and adults indicate that the addition of systemic steroids to antimicrobial therapy may improve the outcome of severe M. pneumoniae pneumonia. As a result of this clinical observation, systemic steroids have been advocated in addition to antibiotic therapy for severe M. pneumoniae pneumonia [13] [14] [15] [16]. Steroid therapy has been found to be of possible benefit for the treatment of inflammation related to some infectious diseases, such as certain types of bacterial meningitis [17] [18]. Alternatively, steroid therapy has been shown to be of no value for other infectious diseases, such as bronchiolitis, and may potentially be harmful [19] [20].
In addition, evidence of acute M. pneumoniae infection is found in up to 20% of acute asthma exacerbations in adolescents and adults [21] [22] [23] [24] [25]. For more severe asthma exacerbations, systemic steroids are given while antibiotics are not routinely administered, as the microbiological etiology of asthma exacerbations is not frequently determined in routine practice. Some evidence does suggest that appropriate antimicrobial therapy may be of value in the treatment of M. pneumoniae associated exacerbations of wheezing; however, more definitive data is needed [21] [26] [27]. Additionally, evidence suggests that macrolides may have anti-inflammatory properties independent of their antimicrobial effect [5].
The specific and comparative effects of treatment with macrolides, systemic steroids, or the combination of these on M. pneumoniae respiratory tract infection has not been fully investigated. The effect of systemic steroids on infection-induced airway inflammation and airway function is incompletely understood, in particular as related to infectious asthma. In the present study, we investigated the effect of clarithromycin, systemic dexamethasone, and combination clarithromycin/dexamethasone therapy on M. pneumoniae -induced airway inflammation in a murine model. In particular, we evaluated pulmonary histopathological inflammation, bronchoalveolar lavage (BAL) cytokine / chemokine / growth factor concentrations, markers of airway function, and M. pneumoniae quantification during the course of these therapies.
Materials and Methods
Organism and growth conditions
M. pneumoniae (ATCC 29342) was reconstituted in SP4 broth and subcultured after 24−48 hours in a flask containing 20 mL of SP4 media at 37°C. When the broth turned an orange hue (approximately 72 hours), the supernatant was decanted, and 2mL of fresh SP4 broth was added to the flask. A cell scraper was used to harvest the adherent mycoplasmas from the bottom of the flask. This achieved an M. pneumoniae concentration in the range of 108 colony forming units (CFU)/mL. Aliquots were stored at −80°C. All SP4 media contained nystatin (50 units/mL) and ampicillin (1.0 mg/mL) to inhibit growth of potential contaminants.
Animals and inoculation
Mice were obtained from commercial vendors (Jackson Labs), who confirmed their mycoplasma- and murine virus-free status. The Animal Resource Center at UT Southwestern Medical Center performed quarterly health surveillance on sentinel mice housed in the mouse storage room. Antibodies against mouse hepatitis virus, Sendai virus, pneumonia virus of mice, reo-3 virus, mouse encephalitis virus (GD-7), mouse rotavirus (EDIM), minute virus of mice, and Mycoplasma pulmonis were analyzed for in sentinel mice. Sentinel mice were also screened for pinworm and mites. The sentinel mice tested negative for these pathogens. Mice were housed in filter-top cages and allowed to acclimate to their new environment for 1 week. Isoflurane, an inhaled anesthetic, was used for inoculum sedation. Nine to 12 week-old female BALB/c mice were intranasally inoculated once with 107 CFU of M. pneumoniae in 50 μL of SP4 broth. All mice were housed in the same animal room and received identical daily care. Animal guidelines were followed in accordance with the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center at Dallas.
Treatment regimen
Treatment was initiated 1 day after M. pneumoniae inoculation. Clarithromycin (25 mg/kg) was administered subcutaneously (SQ) once daily [5]. Dexamethasone (0.5mg/kg) was administered intraperitoneally (IP) once daily ([28] [29] [30]. For the combined therapy, mice received clarithromycin (25 mg/kg) SQ and dexamethasone (0.5mg/kg) IP once daily. Clarithromycin and dexamethasone were reconstituted in sterile 5% dextrose water. Placebo groups received sterile 5% dextrose water administered SQ once daily.
Experimental Design and Sample Collection
Mice were evaluated after 1, 3, and 6 days of therapy. Samples were obtained from 7 to 10 mice per treatment group (4 groups: clarithromycin monotherapy, dexamethasone monotherapy, combined therapy, and placebo therapy) at each time point from repeated experiments. Mice were anesthetized with an intraperitoneal injection of 75 mg/kg ketamine and 5 mg/kg acepromazine before cardiac puncture. Blood was centrifuged at 3,500 × g for 10 min, and the serum was stored at −80°C. BAL specimens were obtained by instilling 500 μL of SP4 broth through a 25-gauge needle into the lungs, via the trachea, followed by aspiration of this fluid into a syringe. Lung specimens, including the trachea, were collected and fixed for histologic evaluation.
Culture
Twenty-five μL of undiluted BAL sample and serial 10-fold dilutions of BAL in SP4 broth (50 μL of undiluted BAL was used for the initial dilution) were immediately cultured on SP4 agar plates at 37°C, whereas the remaining undiluted BAL sample was stored at −80°C. Quantification was performed by counting colonies on plated specimens and expressed as log10 CFU/mL.
Histopathology
Histopathologic score (HPS) was determined by a single pathologist who was unaware of the treatment status of the animals from which specimens were taken. HPS was based on grading of peribronchiolar/ bronchial infiltrate, bronchiolar/bronchial luminal exudate, perivascular infiltrate, and parenchymal pneumonia (neutrophilic alveolar infiltrate). This HPS system assigned values from 0 to 26 (the greater the score the greater the inflammatory changes in the lung) [31]. In our experience, the extent of variation in HPS when the same slide is scored by the same pathologist on multiple times has been found to be 0 to 1.
Plethysmography
Whole-body, unrestrained, nonsedated plethysmography (Buxco, Troy, NY) was used to monitor the respiratory dynamics of mice in a quantitative manner at baseline (airway obstruction), and after methacholine exposure (airway hyperresponsiveness). Before methacholine exposure, mice were allowed to acclimate to the chamber and then plethysmography readings were recorded to establish baseline values. Next, mice were exposed once to aerosolized methacholine (25 mg/mouse); after exposure, plethysmography readings were recorded. Enhanced pause (Penh) is a dimensionless value that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration. Penh correlates with pulmonary airflow resistance or obstruction. Penh as measured by plethysmography has been previously validated in animal models of AHR [32] [33] [34] [35] [36].
BAL cytokines/chemokines
Concentrations of cytokines and chemokines in BAL specimens were assessed using Multiplex Bead Immunoassays (Bio-Rad Laboratories) in conjunction with the Luminex LabMAP system, following the manufacturer's instructions. Assay limits of detection per Bio-Rad Laboratories is as follows: IL-1β 0.8 pg/ml, IL-2 1.1 pg/ml, IL-4 0.5 pg/ml, IL-5 0.8 pg/ml, IL-6 1.1 pg/ml, IL-8 0.5 pg/ml, IL-9 0.7 pg/ml, IL-10 0.9 pg/ml, IL-12p70 0.5 pg/ml, IL-13 2.1 pg/ml, IL-17 0.2 pg/ml, EOTAXIN 14.6 pg/ml, G-CSF 1.1 pg/ml, GM-CSF 4.5 pg/ml, IFN-γ 19.3 pg/ml, MCP-1 6.7 pg/ml, MIP-1α 1.1 pg/ml, MIP-1β 1.1 pg/ml, PDGF 1.0 pg/ml, RANTES 1.2 pg/ml, TNFα 3.0 pg/ml, VEGF 0.5 pg/ml. For statistical analysis, samples with readings below the limit of the standard curve of the assay were assigned a value one-half that of the lowest detectable value.
Statistics
One Way ANOVA was used to compare treatment groups at each time point, if the data were normally distributed. In the instances where the data were not normally distributed, the Kruskal-Wallis test was used for comparisons. If a difference was found between groups, then a pairwise multiple comparison procedure was performed. A comparison was considered statistically significant if the p value was ≤ 0.05.
Results
Culture
Quantitative M. pneumoniae BAL cultures in mice treated with clarithromycin alone were significantly reduced compared with mice treated with placebo after 3 days of therapy; while after 6 days of therapy, M. pneumoniae cultures in mice treated with clarithromycin alone and combined therapy were both significantly reduced compared with mice treated with placebo or dexamethasone alone (Figure 1).
Figure 1.
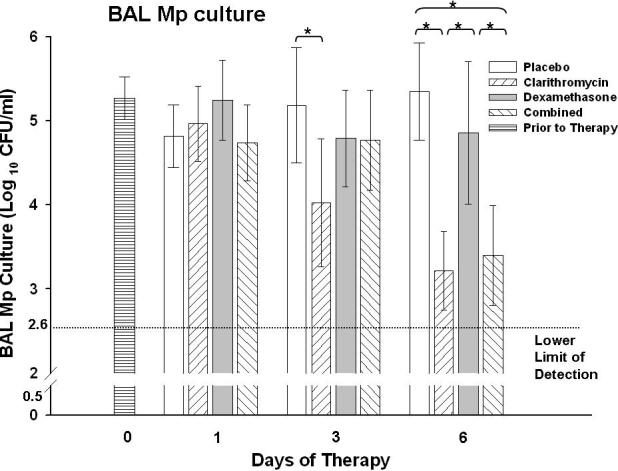
Quantitative M. pneumoniae (Mp) cultures of bronchoalveolar lavage (BAL) fluid samples from mice inoculated with Mp and treated with clarithromycin alone , dexamethasone alone, combined therapy, or placebo for 6 days (treatment began 1 day after inoculation). Bars represent results from seven to ten mice per treatment group at each time point from repeated experiments. Values shown are the means ± standard deviations (error bars). *, p < 0.05 between the two specified treatment groups at the time point by One Way ANOVA followed by pairwise multiple comparisons.
Lung histopathology
Lung HPS in mice treated with combined therapy was significantly reduced after 3 days compared with placebo, clarithromycin alone, and dexamethasone alone (Figure 2). After 6 days of therapy, HPS was significantly lower with clarithromycin monotherapy and with combined therapy compared with the placebo treated mice; in addition, combined therapy significantly reduced HPS compared with dexamethasone alone (Figure 2). Figure 3 demonstrates the histopathologic appearance of representative lungs after three days of therapy for all treatment groups.
Figure 2.
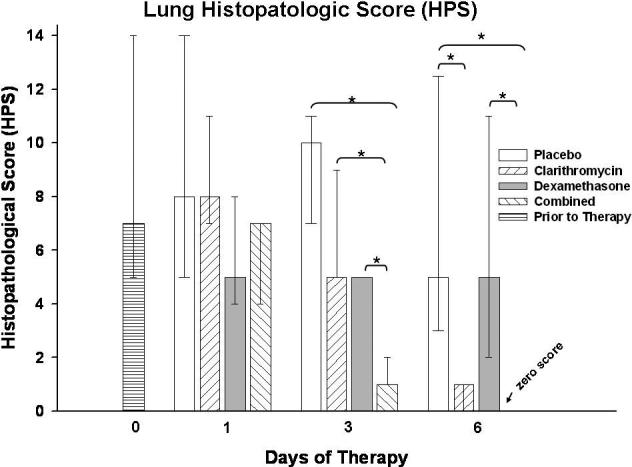
Lung histopathology score (HPS) from mice inoculated with M. pneumoniae (Mp) and treated with clarithromycin alone, dexamethasone alone, combined therapy, or placebo for 6 days (treatment began 1 day after inoculation). Bars represent results from seven to ten mice per treatment group at each time point from repeated experiments. Values shown are the medians and the 25th to 75th percentile (error bars). *, p < 0.05 between the two specified treatment groups at the time point by Kruskal-Wallis test followed by pairwise multiple comparisons.
Figure 3.
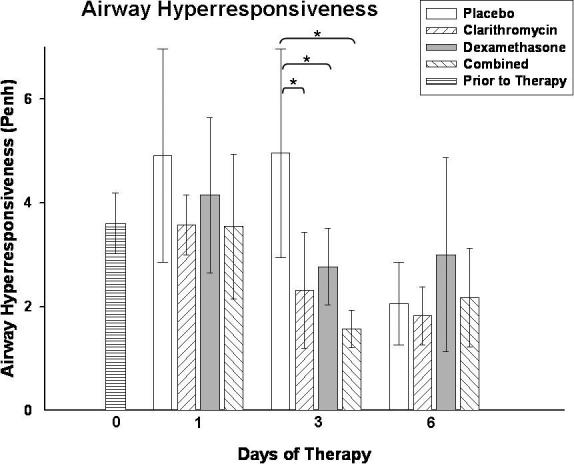
Comparative histopathological appearance of lungs from mice inoculated with M. pneumoniae and treated with clarithromycin alone (A, HPS = 5), dexamethasone alone (B, HPS = 5), combined therapy (C, HPS = 1), or placebo (D, HPS = 11) for 3 days (treatment began 1 day after inoculation). Magnification × 20.
Plethysmography
For airway obstruction, as measured by baseline plethysmography prior to methacholine exposure, there were no significant differences found between the treatment groups. Airway hyperresponsiveness, as measured post methacholine exposure, was significantly lower after 3 days of therapy in all treatment groups compared with placebo (Figure 4).
Figure 4.
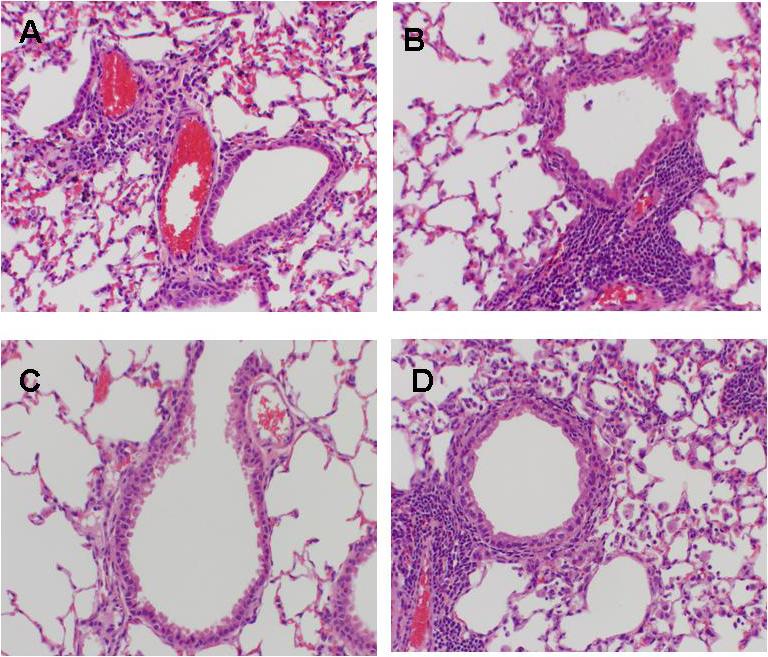
Airway hyperresponsiveness was assessed by whole-body plethysmography by measuring Penh after methacholine exposure in mice inoculated with M. pneumoniae (Mp) and treated with clarithromycin alone, dexamethasone alone, combined therapy, or placebo for 6 days (treatment began 1 day after inoculation). Bars represent results from seven to ten mice per treatment group at each time point from repeated experiments. Values shown are the means ± standard deviations (error bars). *, p < 0.05 between the two specified treatment groups at the time point by One Way ANOVA followed by pairwise multiple comparisons.
Cytokines / chemokines / growth factors
BAL concentrations of IL-12 p40, RANTES, MCP-1, and KC were significantly lower in mice treated with clarithromycin monotherapy and/or combined therapy compared with mice treated with dexamethasone alone and/or placebo, as depicted in Figure 5. No significant differences were found for the other 21 cytokines / chemokines / growth factors investigated.
Figure 5.
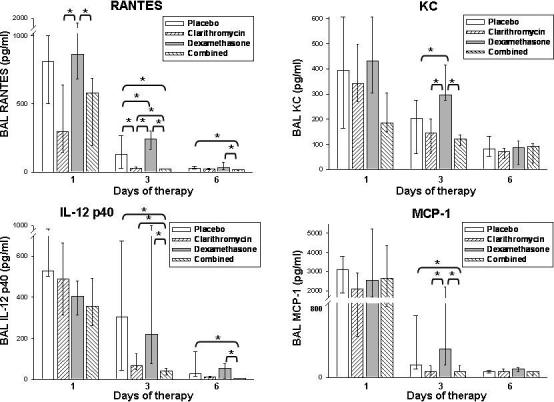
Cytokine and chemokine concentrations in bronchoalveolar lavage (BAL) fluid specimens in mice inoculated with M. pneumoniae (Mp) and treated with clarithromycin alone, dexamethasone alone, combined therapy, or placebo for 6 days (treatment began 1 day after inoculation). Bars represent results from seven to ten mice per treatment group at each time point from repeated experiments. Values shown are the medians and the 25th to 75th percentile (error bars). *, p < 0.05 between the two specified treatment groups at the time point by Kruskal-Wallis test followed by pairwise multiple comparisons.
Discussion
M. pneumoniae is generally associated with mild to moderate community-acquired pneumoniae that is self-limited and/or responds well to appropriate antimicrobial therapy. However, M. pneumoniae pneumonia may also be severe with accompanying acute respiratory failure that may not respond promptly to appropriate antimicrobial therapy [13] [14]. Severe pulmonary injury with M. pneumoniae pneumonia has been hypothesized to be due to an exuberant host immune response, rather than from direct microbial damage [37] [38] [39]. Immunopathogenic investigations in M. pneumoniae pneumonia animal models support this supposition [36] [39] [40] [41] [42]. The use of systemic steroids, in addition to antimicrobial therapy, to diminish the host response in severe M. pneumoniae pneumonia is supported by observational case series in both children and adults [13] [14] [15] [16]. While many observational to placebo-controlled, double blind, randomized investigations have demonstrated the beneficial role of antimicrobial therapy for M. pneumoniae respiratory tract infection in adults, the role of systemic steroids in the treatment of severe M. pneumoniae respiratory illness is not well defined [9] [10] [11]. Furthermore, systemic steroids are often proposed for the treatment of extrapulmonary manifestations of M. pneumoniae infection, particularly central nervous system manifestations, without clear data indicating the effect of steroid therapy on these manifestations.
In an experimental model of M. pneumoniae respiratory infection, we found that combination therapy consisting of clarithromycin with dexamethasone significantly reduced pulmonary histologic inflammation compared with placebo, as well as compared with clarithromycin alone and with dexamethasone alone after 3 days of therapy. After 6 days of the therapy the combination treatment group again had the lowest mean lung HPS; however, this was not significantly lower than that of clarithromycin alone. This may suggest that combined therapy is most beneficial in the early stages of inflammation or is most beneficial when lung inflammation is greatest, as the HPS for placebo peaked after 3 days of therapy (4 days after M. pneumoniae inoculation).
Of note, dexamethasone alone did not significantly reduce histologic pulmonary inflammation. In contrast to our steroid monotherapy results, Chu et al. found that the administration of daily inhaled fluticasone propionate for 5 days, beginning 2 days prior to M. pneumoniae inoculation, significantly decreased pulmonary histologic inflammation in a mouse model [43]. Bowden et al. found that in a Mycoplasma pulmonis chronic respiratory infection mouse model the administration of intraperitoneal dexamethasone for 2 weeks significantly reduced the thickness of tracheal mucosa, as a marker of tissue inflammation [28]. The differences in experimental methodology utilized in these investigations compared with the current investigation likely explain the differing results. Our steroid monotherapy results may be applicable to acute untreated M. pneumoniae infection.
Microbiologically, as expected, therapies that included clarithromycin significantly reduced quantitative M. pneumoniae cultures compared to therapies without clarithromycin. Dexamethasone monotherapy did not increase or decrease M. pneumoniae concentrations in the BAL. Bowden et al. compared treatment with dexamethasone to the antimicrobial agent oxytetracycline in the chronic M. pulmonis mouse model. Their group found that oxytetracycline significantly reduced quantitative mycoplasma cultures in lung tissue, while dexamethasone did not compared to placebo. In tracheal tissue, they found that both dexamethasone and oxytetracycline significantly reduced quantitative cultures [28]. Chu et al. found that inhaled fluticasone propionate appeared to significantly reduce lung concentrations of M. pneumoniae compared to placebo, while not reducing BAL M. pneumoniae concentrations [43]. As a whole, these results seem to indicate that antimicrobials with in vitro activity against M. pneumoniae are effective in reducing concentrations of M. pneumoniae in vivo, while steroid monotherapy does not increase concentrations of M. pneumoniae during active infection and may actually decrease concentrations of M. pneumoniae in some instances.
In contrast to the findings for M. pneumoniae culture and pulmonary histopathology, all three treatment regimens investigated significantly reduced methacholine airway hyperresponsiveness compared with placebo after 3 days of treatment, without significant differences found between the regimens. However, it must be noted that many authorities regard the measurement of the parameter enhanced pause (Penh), as performed in this investigation, as a limited screening of overall lung function rather than a rigorous evaluation of pulmonary mechanics. In addition, Penh may correlate with airflow in the whole airway, rather than solely with pulmonary airflow. Chu et al. noted that inhaled fluticasone propionate initiated prior to M. pneumoniae infection also significantly reduced methacholine airway hyperresponsiveness [43]. The pathogenic mechanisms involved in the reduction of airway hyperresponsiveness may be different for clarithromycin and dexamethasone therapy, as the effects on the other measured outcomes, especially cytokines and chemokines, did not parallel the airway hyperresponsiveness results. Dakhama et al. previously noted distinct differences in the in vitro adherence interactions of M. pneumoniae with cell culture after treatment with either erythromycin or dexamethasone [44]. It also appears that clarithromycin and dexamethasone therapy are not significantly additive or synergistic for decreasing airway hyperresponsiveness. Speculatively, these findings may clinically translate to indicate that macrolide therapy is as effective as steroid therapy for an asthma exacerbation due to M. pneumoniae infection in terms of airway hyperresponsiveness. Macrolides have been postulated to have host immunomodulating activity; however, past investigations in our laboratory indicate that the beneficial activity of macrolides in the treatment of M. pneumoniae respiratory infection is antimicrobial in nature, as opposed to a primary host immunomodulation mechanism [5] [6].
The significant differences detected for IL-12 p40, RANTES, MCP-1, and KC lend insight into the immunopathogenesis involved in M. pneumoniae respiratory disease and its treatment. Combination therapy resulted in the greatest reductions of these cytokines and chemokines; however, the absolute differences between combination therapy and clarithromycin alone were minor. Dexamethasone monotherapy significantly increased RANTES and KC concentrations compared to placebo. The significant differences noted for IL-12 p40 concentrations parallel the pulmonary histopathologic results found with the investigated regimens, in contrast to RANTES, MCP-1, and KC. IL-12 has been previously reported to play an important role in the immunopathogenesis of M. pneumoniae respiratory infection with less lung disease present in IL-12 knock out mice and more disease present with administration of exogenous IL-12 [36] [45] [46]. Conversely, the IL-12 p40, RANTES, MCP-1, and KC results did not parallel the airway hyperresponsiveness outcomes. This may mean that other unmeasured factors are more elemental in the pathogenesis of M. pneumoniae related airway hyperresponsiveness or that overlapping pathways are involved in airway hyperresponsiveness that clarithromycin and dexamethasone interact with through different mechanisms to achieve a similar outcome of reduced airway hyperresponsiveness. These chemokines and others have been found to be elevated and/or correlate with disease severity in mycoplasma infection [44] [6] [36] [47] [48] [49] [50].
In conclusion, combination therapy with clarithromycin and dexamethasone is more effective in reducing M. pneumoniae induced pulmonary inflammation than either clarithromycin alone or dexamethasone alone. This data lends support to the clinical observation that the addition of systemic steroids to antimicrobials may be of value in severe M. pneumoniae pneumonia. However, before final conclusions can be made on the role of adding steroids to antimicrobial therapy for the treatment of M. pneumoniae pneumonia, controlled clinical investigations in humans are necessary to determine the risks and benefits to patients, as this investigation has the inherent limitation of being done in a murine model. Currently, antimicrobials alone remain the primary therapy of M. pneumoniae pneumonia. Importantly, in our investigation dexamethasone monotherapy was not found to reduce pulmonary inflammation. Of the cytokines/chemokines evaluated, IL-12 concentrations appear to be the most closely linked with pulmonary histologic inflammation. The possibility of treating M. pneumoniae associated wheezing with clarithromycin without the addition of steroids should be further investigated.
Acknowledgements
This work was supported by the KO8 NIH grant of R.D.H. A.M. was supported in part by a grant from the NIH 1 UL1 RR024982-01. The authors have no conflicts of interest.
Funding statement: This work was supported by the KO8 NIH grant of R.D.H. A.M. was supported in part by a grant from the NIH 1 UL1 RR024982-01.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest.
Previously presented: Tagliabue C., Salvatore C.M., Techasaensiri C., Katz K., Gomez A.M., Esposito S., Principi N., Hardy R.D.. Comparison of clarithromycin, dexamethasone, or combination therapy for experimental Mycoplasma pneumoniae respiratory infection. Poster presentation (abstract B-91), 47th Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago, 17−20 September 2007.
References
- 1.Principi N, Esposito S, Blasi F, Allegra L. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis. 2001;32:1281–9. doi: 10.1086/319981. [DOI] [PubMed] [Google Scholar]
- 2.Clyde WA, Jr., Fernald GW. Mycoplasmas: the pathogens’ pathogens. Cell Immunol. 1983;82:88–97. doi: 10.1016/0008-8749(83)90143-0. [DOI] [PubMed] [Google Scholar]
- 3.Marston BJ, Plouffe JF, File TM, Jr., et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med. 1997;157:1709–18. [PubMed] [Google Scholar]
- 4.Gendrel D, Raymond J, Moulin F, et al. Etiology and response to antibiotic therapy of community-acquired pneumonia in French children. Eur J Clin Microbiol Infect Dis. 1997;16:388–91. doi: 10.1007/BF01726370. [DOI] [PubMed] [Google Scholar]
- 5.Hardy RD, Rios AM, Chavez-Bueno S, et al. Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae-induced pneumonia. Antimicrob Agents Chemother. 2003;47:1614–20. doi: 10.1128/AAC.47.5.1614-1620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonseca-Aten M, Salvatore CM, Mejias A, et al. Evaluation of LBM415 (NVP PDF-713), a novel peptide deformylase inhibitor, for treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2005;49:4128–36. doi: 10.1128/AAC.49.10.4128-4136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rios AM, Fonseca-Aten M, Mejias A, et al. Microbiologic and immunologic evaluation of a single high dose of azithromycin for treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2005;49:3970–3. doi: 10.1128/AAC.49.9.3970-3973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca-Aten M, Rios AM, Mejias A, et al. Treatment of experimental chronic pulmonary mycoplasmosis. Int J Antimicrob Agents. 2006;28:253–8. doi: 10.1016/j.ijantimicag.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 9.McCracken GH., Jr. Current status of antibiotic treatment for Mycoplasma pneumoniae infections. Pediatr Infect Dis. 1986;5:167–71. doi: 10.1097/00006454-198601000-00054. [DOI] [PubMed] [Google Scholar]
- 10.Kingston JR, Chanock RM, Mufson MA, et al. Eaton agent pneumonia. Jama. 1961;176:118–23. doi: 10.1001/jama.1961.03040150034009. [DOI] [PubMed] [Google Scholar]
- 11.Shames JM, George RB, Holliday WB, Rasch JR, Mogabgab WJ. Comparison of antibiotics in the treatment of mycoplasmal pneumonia. Arch Intern Med. 1970;125:680–4. [PubMed] [Google Scholar]
- 12.Esposito S, Bosis S, Cavagna R, et al. Characteristics of Streptococcus pneumoniae and atypical bacterial infections in children 2−5 years of age with community-acquired pneumonia. Clin Infect Dis. 2002;35:1345–52. doi: 10.1086/344191. [DOI] [PubMed] [Google Scholar]
- 13.Lee KY, Lee HS, Hong JH, et al. Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2006;41:263–8. doi: 10.1002/ppul.20374. [DOI] [PubMed] [Google Scholar]
- 14.Miyashita N, Obase Y, Ouchi K, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol. 2007;56:1625–9. doi: 10.1099/jmm.0.47119-0. [DOI] [PubMed] [Google Scholar]
- 15.Cimolai N. Corticosteroids and complicated Mycoplasma pneumoniae infection. Pediatr Pulmonol. 2006;41:1008–9. doi: 10.1002/ppul.20424. author reply 1010. [DOI] [PubMed] [Google Scholar]
- 16.Radisic M, Torn A, Gutierrez P, Defranchi HA, Pardo P. Severe acute lung injury caused by Mycoplasma pneumoniae: potential role for steroid pulses in treatment. Clin Infect Dis. 2000;31:1507–11. doi: 10.1086/317498. [DOI] [PubMed] [Google Scholar]
- 17.van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44–53. doi: 10.1056/NEJMra052116. [DOI] [PubMed] [Google Scholar]
- 18.Prasad K, Volmink J, Menon GR. Steroids for treating tuberculous meningitis. Cochrane Database Syst Rev. 2000:CD002244. doi: 10.1002/14651858.CD002244. [DOI] [PubMed] [Google Scholar]
- 19.Corneli HM, Zorc JJ, Majahan P, et al. A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N Engl J Med. 2007;357:331–9. doi: 10.1056/NEJMoa071255. [DOI] [PubMed] [Google Scholar]
- 20.Buckingham SC, Jafri HS, Bush AJ, et al. A randomized, double-blind, placebo-controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: effects on RSV quantity and clinical outcome. J Infect Dis. 2002;185:1222–8. doi: 10.1086/340024. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca-Aten M, Okada PJ, Bowlware KL, et al. Effect of clarithromycin on cytokines and chemokines in children with an acute exacerbation of recurrent wheezing: a double-blind, randomized, placebo-controlled trial. Ann Allergy Asthma Immunol. 2006;97:457–63. doi: 10.1016/S1081-1206(10)60935-0. [DOI] [PubMed] [Google Scholar]
- 22.Seggev JS, Lis I, Siman-Tov R, et al. Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults. Ann Allergy. 1986;57:263–5. [PubMed] [Google Scholar]
- 23.Gil JC, Cedillo RL, Mayagoitia BG, Paz MD. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann Allergy. 1993;70:23–5. [PubMed] [Google Scholar]
- 24.Lieberman D, Lieberman D, Printz S, et al. Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med. 2003;167:406–10. doi: 10.1164/rccm.200209-996OC. [DOI] [PubMed] [Google Scholar]
- 25.Biscardi S, Lorrot M, Marc E, et al. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–6. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 26.Esposito S, Blasi F, Arosio C, et al. Importance of acute Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with wheezing. Eur Respir J. 2000;16:1142–6. doi: 10.1034/j.1399-3003.2000.16f21.x. [DOI] [PubMed] [Google Scholar]
- 27.Marc E, Chaussain M, Moulin F, et al. Reduced lung diffusion capacity after Mycoplasma pneumoniae pneumonia. Pediatr Infect Dis J. 2000;19:706–10. doi: 10.1097/00006454-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Bowden JJ, Schoeb TR, Lindsey JR, McDonald DM. Dexamethasone and oxytetracycline reverse the potentiation of neurogenic inflammation in airways of rats with Mycoplasma pulmonis infection. Am J Respir Crit Care Med. 1994;150:1391–401. doi: 10.1164/ajrccm.150.5.7524980. [DOI] [PubMed] [Google Scholar]
- 29.Piedimonte G, McDonald DM, Nadel JA. Glucocorticoids inhibit neurogenic plasma extravasation and prevent virus-potentiated extravasation in the rat trachea. J Clin Invest. 1990;86:1409–15. doi: 10.1172/JCI114855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda RK, Nayar J, Cho JY, et al. Resolution of airway inflammation following ovalbumin inhalation: comparison of ISS DNA and corticosteroids. Am J Respir Cell Mol Biol. 2003;28:655–63. doi: 10.1165/rcmb.4853. [DOI] [PubMed] [Google Scholar]
- 31.Cimolai N, Taylor GP, Mah D, Morrison BJ. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol Immunol. 1992;36:465–78. doi: 10.1111/j.1348-0421.1992.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalo JA, Lloyd CM, Wen D, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–67. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamelmann E, Schwarze J, Takeda K, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–75. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 34.Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest. 1997;100:226–33. doi: 10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Schaik SM, Enhorning G, Vargas I, Welliver RC. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J Infect Dis. 1998;177:269–76. doi: 10.1086/514208. [DOI] [PubMed] [Google Scholar]
- 36.Fonseca-Aten M, Rios AM, Mejias A, et al. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am J Respir Cell Mol Biol. 2005;32:201–10. doi: 10.1165/rcmb.2004-0197OC. [DOI] [PubMed] [Google Scholar]
- 37.Foy HM. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin Infect Dis. 1993;17(Suppl 1):S37–46. doi: 10.1093/clinids/17.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 38.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka H, Narita M, Teramoto S, et al. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest. 2002;121:1493–7. doi: 10.1378/chest.121.5.1493. [DOI] [PubMed] [Google Scholar]
- 40.Cartner SC, Lindsey JR, Gibbs-Erwin J, Cassell GH, Simecka JW. Roles of innate and adaptive immunity in respiratory mycoplasmosis. Infect Immun. 1998;66:3485–91. doi: 10.1128/iai.66.8.3485-3491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denny FW, Taylor-Robinson D, Allison AC. The role of thymus-dependent immunity in Mycoplasma pulmonis infections of mice. J Med Microbiol. 1972;5:327–36. doi: 10.1099/00222615-5-3-327. [DOI] [PubMed] [Google Scholar]
- 42.Jones HP, Tabor L, Sun X, Woolard MD, Simecka JW. Depletion of CD8+ T cells exacerbates CD4+ Th cell-associated inflammatory lesions during murine mycoplasma respiratory disease. J Immunol. 2002;168:3493–501. doi: 10.4049/jimmunol.168.7.3493. [DOI] [PubMed] [Google Scholar]
- 43.Chu HW, Campbell JA, Rino JG, Harbeck RJ, Martin RJ. Inhaled fluticasone propionate reduces concentration of Mycoplasma pneumoniae, inflammation, and bronchial hyperresponsiveness in lungs of mice. J Infect Dis. 2004;189:1119–27. doi: 10.1086/382050. [DOI] [PubMed] [Google Scholar]
- 44.Dakhama A, Kraft M, Martin RJ, Gelfand EW. Induction of regulated upon activation, normal T cells expressed and secreted (RANTES) and transforming growth factor-beta 1 in airway epithelial cells by Mycoplasma pneumoniae. Am J Respir Cell Mol Biol. 2003;29:344–51. doi: 10.1165/rcmb.2002-0291OC. [DOI] [PubMed] [Google Scholar]
- 45.Salvatore CM, Fonseca-Aten M, Katz-Gaynor K, et al. Respiratory tract infection with Mycoplasma pneumoniae in interleukin-12 knockout mice results in improved bacterial clearance and reduced pulmonary inflammation. Infect Immun. 2007;75:236–42. doi: 10.1128/IAI.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salvatore CM, Fonseca-Aten M, Katz-Gaynor K, Gomez AM, Hardy RD. Intranasal IL-12 Therapy Inhibits Mycoplasma pneumoniae Clearance and Sustains Airway Obstruction in Murine Pneumonia. Infect Immun. 2007 doi: 10.1128/IAI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu T, Kida Y, Kuwano K. Mycoplasma pneumoniae-derived Lipopeptides Induce Acute Inflammatory Responses in the Lungs of Mice. Infect Immun. 2007 doi: 10.1128/IAI.00955-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun X, Jones HP, Hodge LM, Simecka JW. Cytokine and chemokine transcription profile during Mycoplasma pulmonis infection in susceptible and resistant strains of mice: macrophage inflammatory protein 1beta (CCL4) and monocyte chemoattractant protein 2 (CCL8) and accumulation of CCR5+ Th cells. Infect Immun. 2006;74:5943–54. doi: 10.1128/IAI.00082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu HW, Breed R, Rino JG, Harbeck RJ, Sills MR, Martin RJ. Repeated respiratory Mycoplasma pneumoniae infections in mice: effect of host genetic background. Microbes Infect. 2006;8:1764–72. doi: 10.1016/j.micinf.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Yang J, Hooper WC, Phillips DJ, Talkington DF. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev. 2004;15:157–68. doi: 10.1016/j.cytogfr.2004.01.001. [DOI] [PubMed] [Google Scholar]


