Abstract
Large-field visual motion elicits tracking eye movements at ultra-short latency, often termed Ocular Following Responses (OFRs). We recorded the initial OFRs of 3 human subjects when vertical sine-wave gratings were subject to horizontal motion in the form of successive ¼-wavelength steps. The gratings could occupy the full screen (45° wide, 30° high) or a number of horizontal strips, each 1° high and extending the full width of the display. These strips were always equally spaced vertically. In a first experiment, the gratings always had a contrast of 32%. Increasing the number of strips could reduce the response latency by up to 20 ms, so the magnitude of the initial OFRs was estimated from the change in eye position over the initial open-loop period measured with respect to response onset. A single (centered) strip (covering 3.3% of the screen) always elicited robust OFRs, and 3 strips (10% coverage) were sufficient to elicit the maximum OFR. Increasing the number of strips to 15 (50% coverage) had little impact, i.e., responses had asymptoted, and further increasing the coverage to 100% (full screen image) actually decreased the OFR so that it was now less than that elicited with only 1 strip. In a second experiment, the contrast of the gratings could be fixed at one of four levels ranging from 8% to 64% and the OFR showed essentially the same pattern of dependence on screen coverage except that the lower the contrast, the lower the level at which the response asymptoted. This indicated that the asymptote was not due simply to some upper limit on the magnitude of the eye movement or the underlying motion signals. We postulate that this asymptote is the result of normalization due to global divisive inhibition, which has often been described in visual-motion-selective neurons in the cortex. We further suggest that the decrease in the OFR when the image filled the screen was due to the increased continuity of the gratings which we postulate would favor the local inhibitory surround mechanisms over the central excitatory ones. This study indicates that robust OFRs can be elicited by much smaller motion stimuli than is commonly supposed and that introducing spatial discontinuities can increase the efficacy of the motion stimuli even while decreasing the area stimulated.
Keywords: Ocular Following Response (OFR), Response normalization, Surround inhibition
Introduction
Ocular following responses (OFRs) are the tracking eye movements elicited by large-field visual motion: see Miles (1998) for review. Two defining characteristics of the initial OFR are its ultra-short latency—less than 80 ms in humans (Gellman et al., 1990) and less than 60 ms in monkeys (Miles et al., 1986)—and its machine-like quality. Recent studies indicate that the OFRs to broadband motion stimuli depend critically on the Fourier composition of the images (Chen et al., 2005; Sheliga et al., 2005), consistent with mediation by local spatio-temporal filters like those in the motion energy model that is commonly used to describe the responses of motion-selective complex cells in the striate cortex (Adelson and Bergen, 1985; Watson and Ahumada, 1985). Further, the fundamental visual properties of the OFR, such as its dependence on contrast and spatial frequency, show remarkably little inter-subject variation, most probably because these characteristics directly reflect the activity of the underlying low-level motion detectors (Sheliga et al., 2005). This has led to the suggestion that the OFR can be used to probe the early cortical processing of visual motion (Kodaka et al., 2007).
Previous studies used large-field stimuli to elicit OFRs and, partly because of this, it has been common to assume that large-field stimuli are necessary. Indeed, the finding that the initial OFRs elicited by patterns moving within stationary circular apertures show strong dependence on the size of the aperture—increasing steadily until the pattern spans 20° or more (e.g., Barthelemy et al., 2006)—seem to imply that OFRs are best with large-field stimuli. In the present study, the OFR stimuli were confined to elongated apertures aligned with the motion and indicated that the large-field motion stimuli often used to elicit the OFR are not optimal because they evoke at least two forms of response suppression in the neural networks processing the visual motion. We will argue that one of these suppressive mechanisms involves global inhibitory interactions between the motion-sensitive neurons, which result in divisive normalization, and the other involves local inhibitory surround mechanisms, which render the neurons less responsive to wide-field stimuli. One major consequence of these suppressive mechanisms for the OFR is that some motion stimuli of quite modest proportions are favored over the usual large-field ones.
Methods
Most of the techniques were very similar to those used previously in our laboratory (Sheliga et al., 2005; 2006). Three subjects participated and experimental protocols were approved by the NEI Institutional Review Board concerned with the use of human subjects. The horizontal and vertical positions of the right eye were recorded with an electromagnetic induction technique (Robinson, 1963) using a scleral search coil embedded in a silastin ring (Collewijn et al., 1975), as described by Yang, FitzGibbon and Miles (2003). Visual stimuli were presented on a computer monitor that subtended 45° horizontally and 30° vertically. The visual motion stimuli consisted of 1-D vertical gratings with sinusoidal luminance profiles (spatial frequency, 0.25 cycles/°) that underwent successive ¼-wavelength shifts every frame (i.e., every 10 ms). On any given trial, the grating could occupy the full screen or horizontal strips extending the full width of the display. There could be 1, 3, 7, or 15 strips, each 1° high, with one always at the screen center and any others vertically distributed with equal spacing: see Fig. 1A. The luminance in the spaces between the strips of grating matched the mean luminance of the grating (43 cd/m2). In Experiment 1, all gratings had the same contrast (32%). In Experiment 2, the gratings could have one of four contrasts (8%, 16%, 32%, 64%).
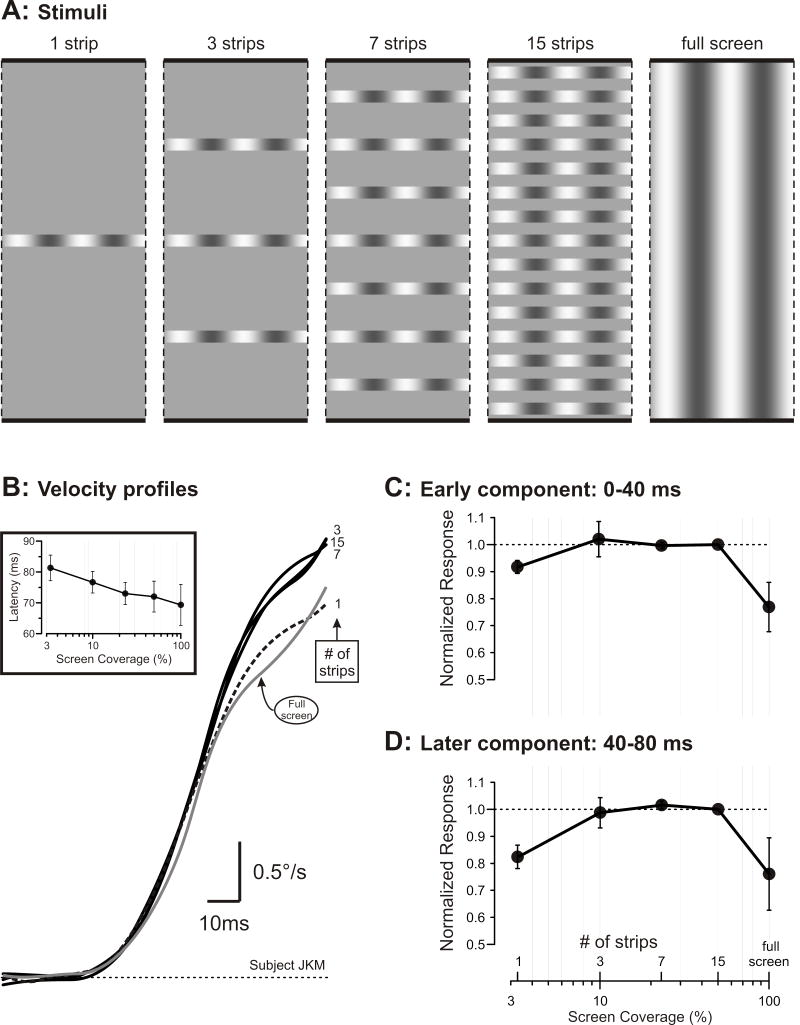
Figure 1. Dependence of the OFR on screen coverage (determined by the number of strips occupied by the grating).
A. Cartoons showing the vertical partitioning of the vertical gratings into horizontal strips. B. Mean horizontal R-L eye velocity profiles for one subject synchronized to the onset of the responses; inset shows dependence of latency on the screen coverage (means ±SD for three subjects); numbers at ends of traces indicate the number of strips making up the grating stimulus; grey trace, grating occupies full screen; dashed trace, grating occupies a single strip. C. Dependence of the “early component” of the OFR on screen coverage (mean normalized measures ±SD for three subjects). D. Dependence of the “later component” of the OFR on screen coverage (mean normalized measures ±SD for three subjects).
At the beginning of each trial, the grating pattern (randomly selected from a lookup table) appeared together with a central target spot that the subject was instructed to fixate. After the subject’s right eye had been positioned within 2° of the fixation target and no saccades had been detected for a randomized period of 800 to 1100 ms the fixation target disappeared and the apparent-motion stimulus began. The motion lasted for 200 ms (20 frames), at which point the screen became a uniform gray (39 cd/m2) marking the end of the trial. After an inter-trial interval of 500 ms a new grating pattern appeared together with a fixation target, commencing a new trial. The subjects were asked to refrain from blinking or making saccades except during the inter-trial intervals but were given no instructions relating to the motion stimuli.
The eye-position data were first smoothed with a 6-pole Butterworth filter (3 dB at 45 Hz) and then mean temporal profiles were computed for each subject for all the data obtained for each of the stimulus conditions. To improve the signal-to-noise, the mean horizontal response to each leftward motion stimulus was subtracted from the mean horizontal response to the corresponding rightward motion stimulus: the “mean R-L position responses”. By convention, rightward eye movements were positive so that these pooled responses were positive when OFRs were in the forward (i.e., stimulus) direction. Velocity responses were estimated at successive 1-ms intervals by computing the differences between the mean R-L position responses at intervals of 10 ms. The response onset was defined as the time when the mean R-L eye velocity first exceeded 0.2°/s, and the “initial OFRs” were quantified by measuring the changes in eye position over the 60-ms time periods starting with response onset. We also measured the changes in eye position over two 40-ms time periods, one starting with response onset (“early component”) and the other 40 ms after response onset (“later component”). For each subject, these various response measures were each normalized with respect to the measures obtained with the 15 strips and then mean response measures were calculated for each stimulus for the 3 subjects. The minimum latency of onset was ~70 ms so that the “initial OFR” and “early component” measures were restricted to the open-loop period (i.e., twice the reaction time) but the “later component” measures included some responses after the loop closed.
Results
Experiment 1
Figure 1B shows the mean R-L eye velocity response profiles of a sample subject when the grating stimulus occupied the full screen or was confined to 1, 3, 7 or 15 bands that occupied 3.3%, 10%, 23% or 50% of the area of the screen, respectively. Note that the latency tended to decrease linearly with the logarithm of the screen coverage, reducing by about 12 ms as the stimulus was increased from a single strip to full screen (see inset graph in Fig. 1B). To facilitate comparison, the traces in Fig. 1B have all been aligned on response onset, and it is immediately apparent that a 30-fold difference in the area of the stimulus—the difference between one strip and the full screen—had only a very modest impact on the initial OFRs. In fact, the profiles during the initial 30 or 40 ms of the responses all look very similar and then only later do those obtained with the single strip (dashed trace) and the full screen (grey trace) clearly begin to fall progressively below the other profiles. Because these eye velocity profiles seemed to suggest that the dependence on screen coverage changed ~40 ms into the response, we examined the OFR measures up to this point (“early component”) and beyond (“later component”) separately (see Methods). The mean normalized “early component” measures for the three subjects are plotted as a function of screen coverage in Fig. 1C and the mean normalized “later component” measures are similarly plotted in Fig. 1D. (Note the logarithmic abscissas in Fig. 1C, D.) It is now evident that, in fact, the “early” and “later” OFR measures showed qualitatively similar dependencies on the screen coverage and showed only relatively minor quantitative differences. Thus, as the number of strips increased from 1 to 3, the screen coverage increased from 3.3% to 10% and both the “early” and “later” response measures increased to a maximum; both measures then remained at this level until the number of strips reached 15, when the screen coverage was 50%, after which the measures showed a surprising decrease of ~24% as the grating filled the screen. Importantly, with just a single strip—only 1° wide and occupying only 3.3% of the screen—the “early” and “later” response measures were, on average, more than 90% and 80%, respectively, of the maxima and were actually greater than those elicited by the full screen stimulus by 19% and 8%, respectively.
Experiment 2
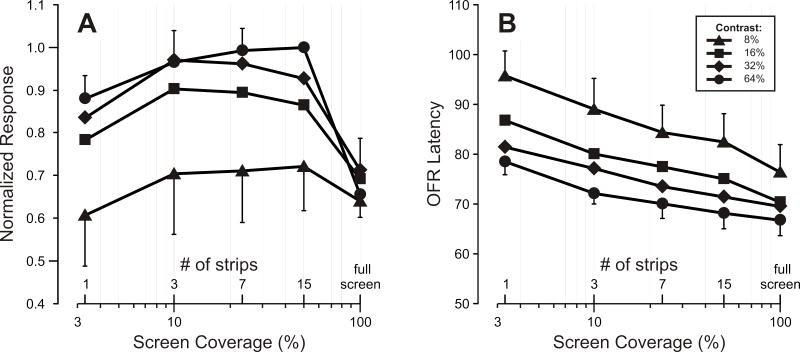
Figure 2 shows the dependence of the OFR on screen coverage when the gratings had contrasts of 8% (triangles), 16% (squares), 32% (diamonds) and 64% (circles). The mean normalized “initial OFR” measures are plotted in Fig. 2A and the data obtained at each contrast all show a pattern of dependence on screen coverage very similar to that seen in Fig. 1C, D, except that the lower the contrast, the lower the response level at which responses asymptoted (and the lower the response to the single band). The mean latencies of these responses are plotted in Fig. 2B and show the inverse dependence on screen coverage already apparent in the inset in Fig. 1B. It is now also apparent that the latency was inversely related to the contrast (cf., Miles et al., 1986).
Figure 2. Dependence of the OFR on screen coverage (determined by the number of strips occupied by the grating): Effect of altering the contrast of the gratings.
A. Dependence of the “initial OFR” measures on screen coverage (mean normalized measures ±SD for three subjects) at each of four contrast levels. B. Dependence of latency on the screen coverage (means ±SD for three subjects) at each of four contrast levels. Key, indicates the Michelson contrast.
Discussion
Our experiments indicate that robust OFRs can be elicited by much smaller motion stimuli than commonly supposed. Indeed, the initial OFR generally began to level off at its maximum when the grating occupied only 10% of the screen (3 strips). In addition, increasing the area of the grating from 50% of the screen to 100%, i.e., from 15 strips to full screen, actually decreased the OFR. The net result was that the initial OFRs to a single strip 1° wide that occupied only 3.3% of the screen were actually greater than those elicited by the full screen stimulus.
Our finding that the level at which the OFR asymptoted was contrast dependent indicated that this leveling off with screen coverage was not due simply to the passive attainment of some upper limit in the magnitude of the sensory motion signals or the motor response itself. Rather, we postulate an active process like the divisive normalization often described in visual-motion-sensitive neurons in the cortex and generally attributed to some global inhibitory process (Britten and Heuer, 1999; Carandini and Heeger, 1994; Carandini et al., 1997; Heeger, 1992; Heuer and Britten, 2002; Simoncelli and Heeger, 1998). Ideally, the responses of an ocular tracking mechanism to motion of a given speed and direction should be insensitive to the physical characteristics of the moving images and the current data indicate that, for a given contrast, the initial OFRs are independent of the size of the stimulus over a five-fold range (10-50% coverage). Over this range, there is clear vector averaging, exactly the sort of behavior one expects of a system subject to divisive normalization. We suggest that these effects are mediated by the same mechanism that is responsible for contrast gain control whereby the OFR saturates at relatively low contrast, ~30% (Masson and Castet, 2002; Sheliga et al., 2005).
We further postulate that the decrease in the OFR when the screen coverage increased from 50 to 100% was due to the increased continuity of the gratings as the image filled the screen and we invoke local inhibitory surround mechanisms to explain it (cf., Barthelemy et al., 2006). Direction-selective neurons with powerful inhibitory surrounds are commonplace in cortical area MT, which is a major source of the motion signals reaching MST, a region known to be critical for the genesis of the OFR (Takemura et al., 2007). Some MT neurons have antagonistic surrounds whose preferred direction of motion is the same as that at the center, rendering these neurons sensitive to local-motion contrast and insensitive to wide-field motion: see Born and Bradley (2005) for recent review. We postulate that it is because of such neurons that introducing spatial discontinuities increases the OFR—even while decreasing the area stimulated by motion—by reducing the activation of the antagonistic surrounds.
A crucial feature of the stimuli in the present study was that they were in effect seen through elongated apertures aligned with the axis of motion and hence were inherently broadband. Moving images confined to stationary circular apertures, as in the study of Barthélemy et al. (2006), become increasingly high-pass when the aperture is reduced in diameter, compromising the low spatial frequencies that are preferred by the OFR. Thus, the effects of the aperture here are less to do with its area than with its spatial-frequency bandwidth, which depends on the length of the aperture along the axis of motion. Many other studies have examined the so-called smooth pursuit tracking responses to single small moving spots that are obviously not confined to a stationary window. These pursuit responses have latencies that are generally at least twice that of the OFR (e.g., Heinen and Watamaniuk, 1998).
Acknowledgments
This research was supported by the intramural program of the National Eye Institute at the National Institutes of Health.
References
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A. 1985;2:284–99. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- Barthelemy FV, Vanzetta I, Masson GS. Behavioral receptive field for ocular following in humans: dynamics of spatial summation and center-surround interactions. J Neurophysiol. 2006;95:3712–26. doi: 10.1152/jn.00112.2006. [DOI] [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. Ann Rev Neurosci. 2005;28:157–89. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Britten KH, Heuer HW. Spatial summation in the receptive fields of MT neurons. J Neurosci. 1999;19:5074–84. doi: 10.1523/JNEUROSCI.19-12-05074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Summation and division by neurons in primate visual cortex. Science. 1994;264:1333–6. doi: 10.1126/science.8191289. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Movshon JA. Linearity and normalization in simple cells of the macaque primary visual cortex. J Neurosci. 1997;17:8621–44. doi: 10.1523/JNEUROSCI.17-21-08621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KJ, Sheliga BM, FitzGibbon EJ, Miles FA. Initial ocular following in humans depends critically on the fourier components of the motion stimulus. Ann N Y Acad Sci. 2005;1039:260–71. doi: 10.1196/annals.1325.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Van Der Mark F, Jansen TC. Precise recording of human eye movements. Vision Res. 1975;15:447–450. doi: 10.1016/0042-6989(75)90098-x. [DOI] [PubMed] [Google Scholar]
- Gellman RS, Carl JR, Miles FA. Short latency ocular-following responses in man. Vis Neurosci. 1990;5:107–122. doi: 10.1017/s0952523800000158. [DOI] [PubMed] [Google Scholar]
- Heeger DJ. Normalization of cell responses in cat striate cortex. Vis Neurosci. 1992;9:181–97. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Watamaniuk SN. Spatial integration in human smooth pursuit. Vision Res. 1998;38:3785–94. doi: 10.1016/s0042-6989(97)00422-7. [DOI] [PubMed] [Google Scholar]
- Heuer HW, Britten KH. Contrast dependence of response normalization in area MT of the rhesus macaque. J Neurophysiol. 2002;88:3398–408. doi: 10.1152/jn.00255.2002. [DOI] [PubMed] [Google Scholar]
- Kodaka Y, Sheliga BM, Fitzgibbon EJ, Miles FA. The vergence eye movements induced by radial optic flow: Some fundamental properties of the underlying local-motion detectors. Vision Res. 2007;47:2637–60. doi: 10.1016/j.visres.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson GS, Castet E. Parallel motion processing for the initiation of short-latency ocular following in humans. J Neurosci. 2002;22:5149–63. doi: 10.1523/JNEUROSCI.22-12-05149.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FA. The neural processing of 3-D visual information: evidence from eye movements. Eur J Neurosci. 1998;10:811–22. doi: 10.1046/j.1460-9568.1998.00112.x. [DOI] [PubMed] [Google Scholar]
- Miles FA, Kawano K, Optican LM. Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of visual input. J Neurophysiol. 1986;56:1321–54. doi: 10.1152/jn.1986.56.5.1321. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A Method of Measuring Eye Movement Using a Scleral Search Coil in a Magnetic Field. IEEE Trans Biomed Eng. 1963;10:137–45. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Chen KJ, FitzGibbon EJ, Miles FA. Initial ocular following in humans: a response to first-order motion energy. Vision Res. 2005;45:3307–21. doi: 10.1016/j.visres.2005.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Chen KJ, FitzGibbon EJ, Miles FA. The initial ocular following responses elicited by apparent-motion stimuli: Reversal by inter-stimulus intervals. Vision Res. 2006;46:979–92. doi: 10.1016/j.visres.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncelli EP, Heeger DJ. A model of neuronal responses in visual area MT. Vision Res. 1998;38:743–61. doi: 10.1016/s0042-6989(97)00183-1. [DOI] [PubMed] [Google Scholar]
- Takemura A, Murata Y, Kawano K, Miles FA. Deficits in short-latency tracking eye movements after chemical lesions in monkey cortical areas MT and MST. J Neurosci. 2007;27:529–41. doi: 10.1523/JNEUROSCI.3455-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AB, Ahumada AJ., Jr Model of human visual-motion sensing. J Opt Soc Am A. 1985;2:322–41. doi: 10.1364/josaa.2.000322. [DOI] [PubMed] [Google Scholar]
- Yang DS, FitzGibbon EJ, Miles FA. Short-latency disparity-vergence eye movements in humans: sensitivity to simulated orthogonal tropias. Vision Res. 2003;43:431–43. doi: 10.1016/s0042-6989(02)00572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]