Abstract
Preclinical studies of chemoprevention drugs given in combination at low doses show remarkable efficacy in preventing adenomas with little additional toxicities, suggesting a strategy to improve risk to benefit ratios for preventing recurrent adenomas. Three hundred seventy-five patients with history of resected (≥3 mm) adenomas were randomly assigned to receive oral difluoromethylornithine (DFMO) 500 mg and sulindac 150 mg once daily or matched placebos for 36 months, stratified by use of low-dose aspirin (81 mg) at baseline and clinical site. Follow-up colonoscopy was done 3 years after randomization or off-study. Colorectal adenoma recurrence was compared among the groups with log-binomial regression. Comparing the outcome in patients receiving placebos to those receiving active intervention, (a) the recurrence of one or more adenomas was 41.1% and 12.3% (risk ratio, 0.30; 95% confidence interval, 0.18–0.49; P < 0.001); (b) 8.5% had one or more advanced adenomas, compared with 0.7% of patients (risk ratio, 0.085; 95% confidence interval, 0.011–0.65; P < 0.001); and (c) 17 (13.2%) patients had multiple adenomas (>1) at the final colonoscopy, compared with 1 (0.7%; risk ratio, 0.055; 0.0074–0.41; P < 0.001). Serious adverse events (grade ≥3) occurred in 8.2% of patients in the placebo group, compared with 11% in the active intervention group (P = 0.35). There was no significant difference in the proportion of patients reporting hearing changes from baseline. Recurrent adenomatous polyps can be markedly reduced by a combination of low oral doses of DFMO and sulindac and with few side effects.
More than 50,000 people in the United States will die in 2007 from colorectal cancer. In the United States, cancer is the leading cause of death in people under age 74 years (1), and colorectal cancer is the second most common cause of cancer deaths after lung cancer (2). Colorectal cancer may be prevented by removal of precursor adenomas found during screening sigmoidoscopy or colonoscopy (3), although rates are variable and range from 30% to 90% depending highly on reimbursement policies (4, 5).
Diet and inflammation have been associated with risk of colorectal cancer (6), and a series of clinical trials have been conducted to test the efficacy of individual dietary supplements or anti-inflammatory agents to prevent the incidence or recurrence of colon polyps (7–14). Unfortunately, these trials have not translated into significant changes in medical practice for prevention or management of colon cancer for a variety of reasons, including lack of efficacy, unacceptable toxicities, and the availability of competing strategies for risk reduction (15).
Studies in rodent models have shown that combination chemoprevention strategies are often more effective than those using individual agents (16, 17). Difluoromethylornithine (DFMO) has been identified as a potent inhibitor of intestinal and colon carcinogenesis in animal models, especially in combination with nonsteroidal anti-inflammatory drugs (18–20). DFMO and the nonsteroidal anti-inflammatory drug sulindac also interact additively to prevent the growth and viability of human colon cancer cells (21). The results of a phase III clinical chemoprevention trial evaluating the combination of DFMO and sulindac for the prevention of colon polyp recurrence are reported here.
Materials and Methods
Study design
This study was a randomized, double-blind placebo-controlled trial to test whether the combination of a low dose of DFMO plus a low dose of sulindac reduces the recurrence of colorectal adenomas detected by standard colonoscopy. The trial involved seven clinical sites in the United States. The human subjects committee at each site approved the study protocol and written informed consent was provided by all patients before enrollment. Quality control to promote uniform practice and protocol compliance included meetings before enrollment and site inspections during and after the trial. An independent Data and Safety Monitoring Board reviewed safety and efficacy data twice yearly.
Recruitment and study population
Eligibility required patients of ages 40 to 80 years with a history of ≥1 resected adenoma of at least 3 mm within 5 y before study entry. A screening colonoscopy within 6 mo of study entry was done and all polyps removed and pathologically examined. A 1-mo placebo run-in period was used to assess compliance. Before randomization to the agents, pre-randomization screening was done and included baseline history, physical examination, pure-tone audiometry, and laboratory evaluations for baseline hematologic, renal, and hepatic status. Three years after randomization, colonoscopies were done. Gastroenterologists associated with the trial performed all study colonoscopies.
Subjects were ineligible if they had a history of familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, inflammatory bowel disease, or invasive cancer within 5 y before enrollment. Also ineligible were subjects with renal, hepatic, or bleeding disorders; subjects hypersensitive to selective inhibitors of cyclooxygenase-2, nonsteroidal anti-inflammatory drugs, salicylates, or sulfonamides; and subjects who had undergone large-bowel resection of >10 cm (excluding appendectomy). Participants with >20 dB uncorrectable hearing loss above age-adjusted norms (assessed by pure-tone audiometry) at any frequency in the normal hearing range were ineligible. To be randomized, participants had to show 80% adherence to the 1-mo run-in medication.
Safety evaluations during the study included physical examinations and laboratory evaluations at return visits after the run-in and 3, 6, 9, 12, and every 6 mo through the end of the study. Pure-tone audiograms were done at 18 and 36 mo or off-study, and repeated 6 mo later. Compliance with the protocol, including in-person and telephone visits, study medication, and blood draws, was monitored throughout the duration of the study.
Study treatment
DFMO was given orally at a dose of 500 mg and sulindac at a dose of 150 mg/d. The randomization used a blocked design and was stratified by clinical site and on the basis of the use (defined as ≤81 mg daily or ≤325 mg twice weekly) or nonuse of low-dose aspirin at study entry.
Assessment of end points, adverse events, and follow-up
The reports for all polyps removed during colonoscopies were submitted for central pathology review and the diagnosis of adenomas was confirmed using standard diagnostic criteria. Secondary efficacy end points included the number and size of colorectal adenomas and the total adenoma burden over the 3-y period. An additional a priori secondary end point was the detection of an advanced adenoma with any of the following characteristics: size of at least 1.0 cm by endoscopic measurements, villous or tubulovillous histologic features, high-grade dysplasia, and intramucosal or invasive carcinoma. Safety analyses were based on investigator-reported adverse events, serious adverse events, laboratory measurements, and physical examinations. Adverse events were coded according to the Coding Symbols for Thesaurus of Adverse Reaction Terms (COSTART) Body System.
Statistical analysis
Based on the two-sample test of binomial proportions, the trial was designed with a statistical power of 90% to detect a 50% decrease in the rate of recurrent adenomas experienced by the DFMO plus sulindac group, assuming a 35% cumulative incidence rate of adenomas in the placebo group, and 0.025 one-sided level of significance. Based on these assumptions, 292 subjects were required for end point evaluation. To account for a dropout rate of as much as 25%, a total of 375 subjects were randomized. The trial included a prespecified stopping rule allowing for early stopping in favor of efficacy or futility. Interim analyses were planned when ~60% and 80% of the maximal planned information for the trial were available. The stopping rule was chosen to maintain an overall one-sided type I error rate of 2.5%, using a one-sided O’Brien-Fleming (22) efficacy bound with a futility bound parameterized via the unified family of group sequential designs with P = 0.9 (23). Adjustments to the stopping rule to account for shifts in the actual timing of analyses while maintaining the desired type I error rate were done using the constrained boundaries method (24). Based on results observed at the second interim analysis, the Data Safety and Monitoring Board of the study recommended early termination in favor of efficacy. Here we present the results of data on the final intention-to-treat cohort of 267 evaluable patients. For the primary efficacy analysis, bias-adjusted estimates of the difference in recurrence rates and corresponding repeated confidence intervals were computed to account for the stopping rule (25).
In patients treated with DFMO plus sulindac compared with those treated with placebo, the relative risks of recurrent adenomas and of the development of at least one advanced adenoma were assessed by log-binomial regression. Treatment groups were compared with regard to the estimated censoring distribution due to early treatment termination using the Kaplan-Meier method. Investigator-reported adverse events were analyzed in total and according to prespecified categories to describe gastrointestinal, hematologic, and cardiovascular disorders; ototoxocity; and hospitalizations. The analyses included all events occurring after the first dose of study medication. The relative risk of hearing loss of at least 15 dB was assessed by log-binomial regression.
Results
Baseline characteristics
Baseline variables were similar across the two treatment groups (Table 1). Patient characteristics included age, gender, race/ethnicity, body mass index, colorectal cancer risk factors, prior use of low-dose aspirin, history of diabetes, hypertension, cardiovascular events, current smoking status, and colorectal cancer risk factors (i.e., number and characteristics of prestudy polyps).
Table 1.
Baseline characteristics of the patients were evenly distributed between arms
| Characteristic | Placebo (n = 184) | DFMO/sulindac (n = 191) |
|---|---|---|
| Age, y | ||
| Median | 60 | 60 |
| Mean ± SD | 61 ± 8.2 | 60 ± 8.6 |
| Range | 42–78 | 41–79 |
| Male sex, n (%) | 138 (75.0) | 147 (77.0) |
| Race or ethnic group, n (%) | ||
| White | 158 (85.9) | 155 (81.2) |
| Black | 6 (3.3) | 10 (5.2) |
| Hispanic | 12 (6.5) | 14 (7.3) |
| Asian or Pacific Islander | 4 (2.2) | 9 (4.7) |
| Other | 4 (2.2) | 3 (1.6) |
| Body mass index (mean ± SD) | ||
| Men | 28.4 ± 4.5 | 29.2 ± 5.5 |
| Women | 29.4 ± 7.5 | 27.7 ± 5.8 |
| No. reported adenomas* | 2.51 ± 2.3 | 2.49 ± 2.2 |
| Largest adenoma ≥ 1 cm, n (%)† | 40 (21.7) | 38 (19.9) |
| Use of low-dose aspirin, n (%) | 69 (37.5) | 77 (40.3) |
| History of cardiovascular disease, n (%) | 67/155 (43.2) | 73/158 (46.2) |
| History of high blood pressure or hypertension, n (%)‡ | 47/155 (30.3) | 48/158 (30.4) |
| History of diabetes, n (%)‡ | 21/151 (13.9) | 25/152 (16.4) |
| Current or prior cigarette smoker, n (%)‡,§ | 41/99 (41.4) | 42/100 (42.0) |
Placebo, n = 183; DFMO/sulindac, n = 189. Number of polyps reported as “multiple” for three patients.
Adenoma is defined as tubular, tubulovillous, villous, cancer in situ, adenoma, or tubular adenoma with high-grade dysplasia.
The denominator is the number of subjects for whom information was recorded. Missing values are not included.
Self-reported information.
Subject disposition
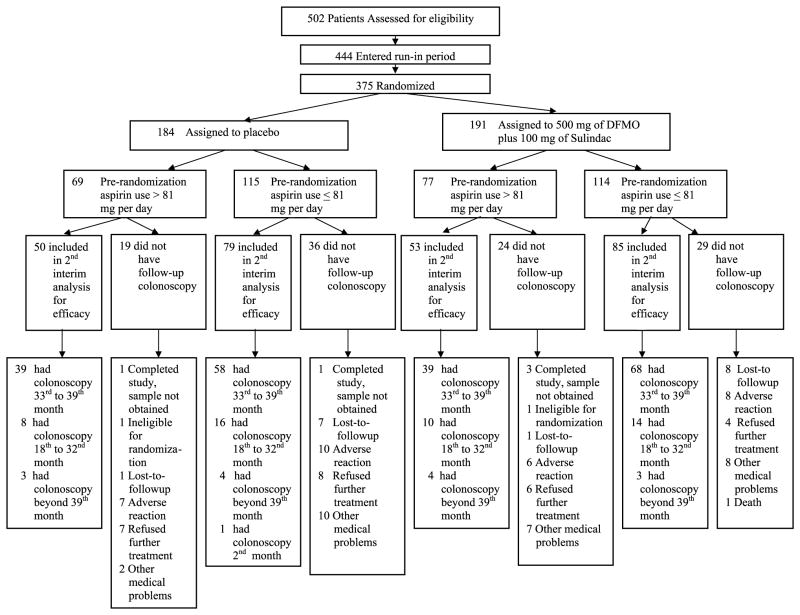
The study schema, presented in Fig. 1, indicates the number of patients randomized to treatment groups, the number of patients with data included in the final analyses, and the number of patients who had colonoscopies at time points other than between the 33- and 39-month window and timing of follow-up colonoscopies. Adherence to the treatment regimen was adequate. Seventy percent of patients in both groups adhered to the active intervention from 50% to 90% of the time. Twenty-five percent of patients adhered to the active intervention at least 90% of the time compared with 24% of patients receiving placebos.
Fig. 1.
Study schema.
After the first interim analysis (60% of patients completed the 3-y colonoscopy follow up), the Data and Safety Monitoring Board endorsed an accelerated analysis, following which the trial was stopped with appropriate follow-up of the remaining patients as the prescribed efficacy end points had been met and because long-term follow-up of the remaining patients on trial was unlikely to change the conclusions about efficacy.
Based on power calculations, 292 subjects would provide maximal information for end point evaluation; the rate of colonoscopy was 91% (267 of 292) in this cohort and the final analysis was based on data from these 267 evaluable patients, comprising 71% of the randomized cohort. The primary analysis for efficacy measured adenomas at any time after randomization (Table 2). In the placebo group, 53 patients had at least one adenoma, compared with 17 patients in the treatment group. The estimated cumulative recurrence was 41.1% in the placebo group and 12.3% in the treatment group, corresponding to a risk ratio of 0.30 [95% confidence interval (95% CI), 0.18–0.49; P < 0.001] or a reduction of 70%. Accounting for the interim analyses, the bias-adjusted point estimate of the difference in polyp recurrence between the treatment and placebo groups was −0.2793 with a corrected 95% CI of −0.3933 to −0.1718. Aspirin (≤81 mg) use did not seem to affect the number of total adenomas in either group. A sensitivity analysis in which adenomas were imputed at the observed placebo rate of recurrence for all patients without an end-of-study colonoscopy (Table 2) gave a risk ratio of 0.49 (95% CI, 0.36–0.69).
Table 2.
Risk of adenomas; evidence of substantial effect in active arm
| Follow-up colonoscopy2 to 39 mo after beginning treatment (n = 267)
|
Follow-up colonoscopy33 to 36 mo after beginning treatment (n = 204)
|
|||
|---|---|---|---|---|
| Placebo (n = 129) | DFMO/sulindac (n = 138) | Placebo (n = 97) | DFMO/sulindac (n = 107) | |
| Detection of any adenoma | ||||
| Cumulative incidence of adenomas detected at end of the treatment (%) | 53 (41.1) | 17 (12.3) | 42 (43.3) | 12 (11.2) |
| Risk ratio* (95% CI) | 0.30 (0.18–0.49) | 0.26 (0.15–0.46) | ||
| P | <0.001 | <0.001 | ||
| Detection of advanced adenomas† | ||||
| Cumulative incidence of advance adenomas detected at end of the treatment (%) | 11 (8.5) | 1 (0.7) | 9 (9.3) | 1 (0.9) |
| Risk ratio* (95% CI) | 0.085 (0.011–0.65) | 0.10 (0.013–0.78) | ||
| P | 0.001 | 0.004 | ||
| Detection of advanced adenomas with size ≥ 1 cm | ||||
| Cumulative incidence of advanced adenomas with size ≥ 1 cm detected at end of the treatment (%) | 9 (7.0) | 1 (0.7) | 7 (7.2) | 1 (0.9) |
| Risk ratio* (95% CI) | 0.10 (0.013–0.81) | 0.13 (0.016–1.03) | ||
| P | 0.004 | 0.02 | ||
| Detection of multiple adenomas (>1) | ||||
| Patients with >1 adenoma, incidence (%) | 17 (13.2) | 1 (0.7) | 15 (15.5) | 1 (0.9) |
| Risk ratio* (95% CI) | 0.055 (0.0074–0.41) | 0.060 (0.0081–0.45) | ||
| P | <0.001 | <0.001 | ||
| Sensitivity analysis imputing adenoma for patients without an end-point determination‡ | ||||
| Cumulative incidence of adenomas detected at end of the treatment (%) | 76/184 (41.3) | 39/191 (20.4) | ||
| Risk ratio* (95% CI) | 0.49 (0.36–0.69) | |||
| P | <0.001 | |||
Relative risk estimation by log-binomial regression. Likelihood ratio test P values are reported.
Advanced adenomas in the placebo group included tubulovillous (3), intramucosal carcinoma (2), size ≥1 cm (6), and one serrated adenoma with high-grade dysplasia; the one advanced adenoma in the treatment group was an adenoma >1 cm.
Sensitivity analysis imputing adenoma for all patients without an end-of-study colonoscopy at the placebo rate of recurrence.
In the placebo group, 11 patients had advanced adenomas, whereas in the DFMO plus sulindac group only one advanced adenoma was detected. This corresponds to a risk ratio of 0.085 (0.011–0.65; P < 0.001) or a reduction of 92%. In the placebo group, 17 patients had more than one adenoma, compared with one patient randomized to active intervention, indicating a reduction of 95% (risk ratio, 0.055; 95% CI, 0.0074–0.41; P < 0.001). Similar results were obtained in a smaller group of patients who had colonoscopies between 36 and 39 months on treatment (Table 2).
Safety
Adverse events were carefully monitored throughout the study (Tables 3 and 4). At least one serious adverse event requiring an overnight hospitalization was reported in 31 of 184 (16.9%) patients in the placebo group and in 42 of 191 (22.0%) patients in the DFMO plus sulindac group (risk ratio, 1.31; 95% CI, 0.86–1.98; P = 0.21). There was also no significant difference between the two arms in those patients experiencing a serious adverse event of grade ≥3 (Table 3). No drug-associated changes in serum levels of creatinine, alanine aminotransferase, or hemoglobin were measured. Reported renal, hypertensive, gastrointestinal, and cardiovascular disorders were analyzed separately, and no differences were detected between the two groups. Serious cardiovascular side effects (Table 4) occurred in 16 (8.4%) patients in the treatment arm and in 9 (4.9%) patients in the placebo arm (risk ratio, 1.71; 95% CI, 0.78–3.78; P = 0.17).
Table 3.
Incidence of adverse events after randomization categorized by COSTART
| Placebo (n = 184) | DFMO/sulindac (n = 191) | |
|---|---|---|
| Serious adverse events, no. patients with adverse event (%)* | ||
| Adverse events requiring overnight hospitalizations | 31 (16.9) | 42 (22.0) |
| Risk ratio (95% CI) | 1.31 (0.86–1.98) | |
| P | 0.21 | |
| All adverse events with a grade ≥3 | 15 (8.2) | 21 (11.0) |
| Risk ratio (95% CI) | 1.35 (0.72–2.53) | |
| P | 0.35 | |
| Deaths† | 1 (0.5) | 2 (1.1) |
| Risk ratio (95%) | NA | |
| Any adverse event, no. patients with adverse events (%) | ||
| All patients | 153 (83.2) | 171 (89.5) |
| Risk ratio (95% CI) | 1.08 (1.00–1.17) | |
| P | 0.07 | |
| Cardiovascular (95% CI) | 22 (12.0) | 28 (14.7) |
| Risk ratio (95% CI) | 1.23 (0.73–2.06) | |
| P | 0.44 | |
| Gastrointestinal events, no. patients (%) | 14 (7.6) | 24 (12.6) |
| Risk ratio (95% CI) | 1.65 (0.88–3.09) | |
| P | 0.11 | |
| Audiometric evaluation, no. patients with adverse event/no. patients in cohort (%)* | ||
| Self-reported hearing complaint reported as an adverse event | 53 (28.8) | 67 (35.1) |
| Risk ratio (95% CI) | 1.22 (0.90–1.64) | |
| P | 0.19 | |
| Self-reported study-related hearing complaint reported as adverse event | 30 (16.3) | 36 (18.8) |
| Risk ratio (95% CI) | 1.16 (0.74–1.80) | |
| P | 0.52 | |
Relative risk estimation by log-binomial regression. Likelihood ratio test P values are reported.
Of the three deaths, one subject died during the trial due to traffic accident and the other subjects died 1 and 3 y, respectively, after study completion from causes judged by the investigator as unrelated to the intervention.
Table 4.
Detailed assessment of adverse events
| Placebo (n = 184)
|
DFMO/sulindac (n = 191)
|
|||
|---|---|---|---|---|
| Aspirin (n = 69) | Non-aspirin (n = 115) | Aspirin (n = 77) | Non-aspirin (n = 114) | |
| Cardiovascular | ||||
| CAD, prior Hx* | 0 | 1 | 3 | 0 |
| CAD, new | 0 | 1 | 1 | 0 |
| MI, prior Hx | 1 | 0 | 1 | 0 |
| MI, new | 0 | 0 | 3 | 1 |
| CVA, prior Hx | 0 | 0 | 0 | 0 |
| CVA, new | 0 | 1 | 1 | 1 |
| CHF, prior Hx | 1 | 0 | 1 | 0 |
| Chest pain, new | 0 | 4 | 0 | 4 |
| Subtotal, prior Hx | 2 | 1 | 5 | 0 |
| Subtotal, new | 0 | 6 | 5 | 6 |
| Total | 9 (4.9) | 16 (8.4) | ||
| Risk ratio (95% CI)† | — | 1.71 (0.78–3.78) | ||
| P | — | 0.17 | ||
| Hearing | ||||
| Audiometric evaluations at end of study, no. patients with adverse events/no. patients in cohort (%)‡ | ||||
| Hearing loss at least 15 dB at ≥2 frequencies | 24/123 (19.5) | 35/136 (25.7) | ||
| Risk ratio (95% CI)† | 1.32 (0.83–2.09) | |||
| P | 0.23 | |||
| Hearing loss at least 15 dB at ≥2 consecutive frequencies | 12/123 (9.8) | 25/136 (18.4) | ||
| Risk ratio (95% CI)† | 1.88 (0.99–3.59) | |||
| P | 0.05 | |||
| Hearing loss at least 15 dB at ≥2 consecutive frequencies in the normal range (500–3,000 Hz) | 7/123 (5.7) | 14/136 (10.3) | ||
| Risk ratio (95% CI)† | 1.81 (0.75–4.33) | |||
| P | 0.17 | |||
| Bilateral audiometric changes at 36 mo or at least 6 mo off-study | ||||
| End of study, no. with bilateral changes (%)§ | 2/9 (22.2) | 10/21 (47.6) | ||
| Risk ratio (95% CI)† | — | 2.14 (0.58–7.88) | ||
| P | — | 0.18 | ||
| At 6 mo posttreatment3 | ||||
| Improved | 1/2 (50.0) | 3/10 (30.0) | ||
| Persistent | 0/2 (0.0) | 1/10 (10.0) | ||
| Unilateral hearing loss with development of bilateral hearing loss | 1/2 (50.0) | 2/10 (20.0) | ||
| Pending | 0/2 (0.0) | 4/10 (40.0) | ||
Prior Hx, subjects had prior history.
Relative risk estimation by log-binomial regression. Likelihood ratio test P values are reported.
All self-reported hearing complaints were recorded. Hearing thresholds were assessed for 259 patients with follow-up audiograms measured from 18 to 36 mo after beginning of therapy.
Unilateral or bilateral change from baseline at 36 mo (n = 30; placebo, n = 9; DFMO/sulindac, n = 21).
Bilateral changes at 36 mo or at least 6 mo off-study (n = 12; placebo, n = 2; DFMO/sulindac, n = 10).
There was no significant difference between groups in the proportions of patients with self-reported hearing loss (Table 3; risk ratio, 1.22; 95% CI, 0.90–1.64; P = 0.19), and in the normal speech range (500–3,000 Hz) no significant difference was noted between arms in the 259 tested participants (P = 0.17). For these models, after adjustment for age and gender, differences between groups remained nonsignificant (P = 0.19 and P = 0.15, respectively).
Of 262 participants who had air conduction audiograms at baseline and repeated audiograms from 18 to 36 months after beginning treatment, there were 25 of 136 (18.4%) in the DFMO plus sulindac group and 12 of 123 (9.8%) in the placebo group (P = 0.05) who experienced at least a 15-dB hearing reduction from baseline in ≥2 consecutive frequencies across the entire range tested (230–8,000 Hz; Table 4). Based on end-of-study audiograms, 30 patients had unilateral or bilateral hearing reductions of ≥15 dB from baseline in ≥2 consecutive frequencies across the entire range of frequencies tested. Of these, 12 had bilateral audiometric hearing reductions, 10 of 21 (48%) in the treatment arm and 2 of 9 (22%) in the placebo arm (P = 0.18). Follow-up audiograms obtained in these 12 patients showed that improvement occurred in 1 of 2 (50%) patients in the placebo arm and in 3 of 10 (30%) in the treatment group.
Discussion
The results from this randomized trial (Table 2) indicate that a low dose of the polyamine synthesis inhibitor DFMO plus the nonspecific nonsteroidal anti-inflammatory drug sulindac at a dose one half the usual therapeutic dose markedly reduced the recurrence of all adenomas (70% decrease), advanced adenomas (92% decrease), and recurrence of more than one adenoma (95% decrease) in a population of individuals at moderately high risk for sporadic adenomas (41% of patients receiving placebos developed recurrent adenomas). Although this study was not structured to determine whether colorectal cancer occurrence itself was affected, two participants in the placebo group, but none in the active treatment group, were diagnosed with colon cancer by the end of the study.
A major feature of this trial was the selection of a low dose of DFMO based on two sequential biomarker studies, the first 1 month in length and the second 12 months in length, in which a dose deescalation strategy was used (26–28). These studies allowed selection of the lowest dose of DFMO that produced a decrease in polyamine levels in the target of interest, colorectal mucosa. In the 12-month study, no evidence of a difference in toxicities between placebo and the low dose selected for the current trial was evident. Nevertheless, patients were carefully monitored for possible toxicities in the current trial.
At the time the current study was designed in 1997, there was no known concern about the cardiovascular toxicity of nonsteroidal anti-inflammatory drugs, including celecoxib and sulindac, with almost the entire focus on the decreased occurrence of gastrointestinal toxicity with cyclooxygenase-2 inhibitors (29, 30). At the time the current study was started (1999), selection of sulindac was based on its long-term prior broad use with an excellent clinical profile including gastrointestinal toxicity in the low range for nonsteroidal anti-inflammatory drugs and evidence of activity in familial adenomatous polyposis (31). Although the clinical cardiovascular (and cerebrovascular) toxicity had not yet surfaced at that time, we were concerned with using a specific or selective cyclooxygenase-2 inhibitor based on mechanistic considerations (32). No statistically significant increase of gastrointestinal, hematologic, or cardiovascular or cerebrovascular toxicity was found in our trial; however, the study was not designed to have adequate power to identify differences in toxicity rates between treatment groups.
Self-reported hearing changes were also not significantly different between the two groups. Although no evidence of a decrement in the normal speech range was documented, serial audiograms suggested a possible effect across a broader range of frequencies tested that was reversible in some cases. The complex details of the audiologic studies and analyses will be reported elsewhere.
The results in this randomized trial confirm the value of deescalation studies before launching a full phase III trial in identifying a dose and regimen for the therapeutic prevention of adenomas that is both efficacious and with minimal toxicity. Larger and longer-term trials will be needed to determine the absolute risk of cardiovascular and cerebrovascular events and the clinical implications of audiologic changes from this regimen. Longer-term studies will also be needed to determine whether this combination regimen can reduce colorectal cancer incidence or improve/complement colorectal surveillance strategies in very high-risk individuals, in patients with low stage prior colorectal cancers, or in the post-adjuvant setting.
Acknowledgments
We thank the writing committee: Frank L. Meyskens, Jr., Christine E. McLaren, Eugene W. Gerner, and Ernest Hawk; the members of the Data Safety and Monitoring Board: John Baron (Chair; Dartmouth College), Scott Emerson (University of Washington), Frank Sinicrope (Mayo Clinic, Rochester, MN), Tom O’Brien (Lankenau Institute), Mikel Aickin (University of Arizona), and Gerald R. Popelka (Stanford University); and the staff involved in this long-term study: Sharon Maxwell, Wesley Lagerberg, Rhonda Gage, Tashia Orr, Ellen Richmond, Angela Garcia, Rachel Gonzalez, Wen-Pin Chen, Kuo-Tung Li, Lu Wu, and Ann Booth.
Grant support: National Cancer Institute contract no. NO1-CN75019 (F.L. Meyskens, Jr. and C.E. McLaren) and grants CA59024 (F.L. Meyskens, Jr.); CA88078 (F.L. Meyskens, Jr. and C.E. McLaren); CA47396, CA72008, and CA95060 (E.W. Gerner); and CA63640 (C.H. Hagedorn).
Footnotes
Disclosure of Potential Conflicts of Interest
MJ Lawson: Bristol-Myers Squibb Commercial Research Grant, Elan Pharmaceuticals Consultant. The other authors disclosed no potential conflicts of interest.
References
- 1.Minino AM, Heron MP, Murphy SL, Kochanek KD. Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics S. Deaths: final data for 2004 Natl Vital Stat Rep. 2007;55:1–119. [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127:1661–9. doi: 10.1053/j.gastro.2004.09.052. [see comment] [DOI] [PubMed] [Google Scholar]
- 5.Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296:2815–22. doi: 10.1001/jama.296.23.2815. [DOI] [PubMed] [Google Scholar]
- 6.Greenwald P. Lifestyle and medical approaches to cancer prevention. Recent Results. Cancer Res. 2005;166:1–15. doi: 10.1007/3-540-26980-0_1. [DOI] [PubMed] [Google Scholar]
- 7.Alberts DS, Martinez ME, Roe DJ, et al. Phoenix Colon Cancer Prevention Physicians’ Network. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. N Engl J Med. 2000;342:1156–62. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 8.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 9.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. N Engl J Med. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 10.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 11.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–82. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 12.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 13.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 14.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1070–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 15.Lippman SM. The dilemma and promise of cancer chemoprevention. Nat Clin Pract Oncol. 2006;3:523. doi: 10.1038/ncponc0609. [DOI] [PubMed] [Google Scholar]
- 16.Sporn MB. Combination chemoprevention of cancer. Nature. 1980;287:107–8. doi: 10.1038/287107a0. [DOI] [PubMed] [Google Scholar]
- 17.Torrance CJ, Jackson PE, Montgomery E, et al. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–8. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]
- 18.Nigro ND, Bull AW, Boyd ME. Inhibition of intestinal carcinogenesis in rats: effect of difluoromethylornithine with piroxicam or fish oil. J Natl Cancer Inst. 1986;77:1309–13. [PubMed] [Google Scholar]
- 19.Meyskens FL, Jr, Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5:945–51. [PubMed] [Google Scholar]
- 20.Gerner EW, Meyskens FL, Jr, Goldschmid S, Lance P, Pelot D. Rationale for, and design of, a clinical trial targeting polyamine metabolism for colon cancer chemoprevention. Amino Acids. 2007;33:189–95. doi: 10.1007/s00726-007-0515-2. Epub 2007 Mar 30. [DOI] [PubMed] [Google Scholar]
- 21.Lawson KR, Ignatenko NA, Piazza GA, Cui H, Gerner EW. Influence of K-ras activation on the survival responses of Caco-2 cells to the chemopreventive agents sulindac and difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 2000;9:1155–62. [PubMed] [Google Scholar]
- 22.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 23.Kittelson JM, Emerson SS. A unifying family of group sequential test designs. Biometrics. 1999;55:874–82. doi: 10.1111/j.0006-341x.1999.00874.x. [DOI] [PubMed] [Google Scholar]
- 24.Burington BE, Emerson SS. Flexible implementations of group sequential stopping rules using constrained boundaries. Biometrics. 2003;59:770–7. doi: 10.1111/j.0006-341x.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 25.Jennison C, Turnbull BW. Group sequential methods with applications to clinical trials 1999. New York (NY): Chapman and Hall, CRC Press; [Google Scholar]
- 26.Meyskens FL, Jr, Emerson SS, Pelot D, et al. Dose de-escalation chemoprevention trial of α-difluoromethylornithine in patients with colon polyps. J Natl Cancer Inst. 1994;86:1122–30. doi: 10.1093/jnci/86.15.1122. [DOI] [PubMed] [Google Scholar]
- 27.Meyskens FL, Jr, Gerner E, Emerson S, et al. Effect of α-difluoromethylornithine on rectal mucosal levels for polyamines in a randomized, double-blinded trial for colon cancer prevention. J Natl Cancer Inst. 1998;90:1212–8. doi: 10.1093/jnci/90.16.1212. [DOI] [PubMed] [Google Scholar]
- 28.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–92. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy D. Nonsteroidal anti-inflammatory drug-related gastrointestinal toxicity: definitions and epidemiology. Am J Med. 1998;105:3–9S. doi: 10.1016/s0002-9343(98)00274-5. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy DM. Comparative toxicity of nonsteroidal anti-inflammatory drugs. Am J Med. 1999;107:37–46S. doi: 10.1016/s0002-9343(99)00366-6. discussion 46–7S [review] [DOI] [PubMed] [Google Scholar]
- 31.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–9. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]