Abstract
Objective and Design:
Women aged 50-79 years in Iowa, Wisconsin, and Oregon with intakes of lutein and zeaxanthin (L+Z) above the 78th (high) and below the 28th (low) percentiles in the Women's Health Initiative Observational Study (1994-98) were recruited four to seven years later (2001-04) into the Carotenoids in Age-Related Eye Disease Study (CAREDS) to evaluate associations between nuclear cataract (determined from slit-lamp photographs) and L+Z in the diet and serum in 1994-98 and macula in 2001-04 (N=1,802).
Results:
Women in the high dietary L+Z group had a 23% lower prevalence of nuclear cataract (age-adjusted odds ratio [OR]: 0.77, 95% confidence interval [CI]: 0.62-0.96) compared to those in the low group. Multivariable adjustment slightly attenuated the association (OR (95% CI): 0.81 (0.65-1.01)). Women in the highest vs. lowest quintile categories of diet or serum L+Z were 32% less likely to have nuclear cataract (multivariable-adjusted OR (95% CI): 0.68 (0.48-0.97), p trend=0.04 and 0.68 (0.47-0.98), p trend=0.01). Cross-sectional associations with macular pigment density were inverse but not statistically significant.
Conclusion:
Diets rich in lutein and zeaxanthin are moderately associated with decreased prevalence of nuclear cataract in older women. However, other protective aspects of such diets may, in part, explain these relationships.
INTRODUCTION
Age-related cataract is a common condition among older adults that has been gaining increased health policy importance. An estimated 20.5 million (17%) Americans older than 40 years have cataract in either eye, and 6.1 million (5%) have had cataracts extracted.1 The number of Americans affected by cataract and undergoing cataract surgery is expected to increase dramatically over the next 20 years as the US population ages.1 The large increase in cataract surgical procedures predicted for the US population as a whole is of significant health policy concern, as treatment for cataract accounts for approximately 60% of vision-related Medicare expenditures2. As more aging Americans need cataract surgery, there is concern about the ability of health care systems, particularly Medicare, to fund cataract surgeries.2 Therefore, identifying modifiable risk factors is of critical importance to improving health of older Americans and to the economic stability of the health care system.
Nuclear cataract is the most common type of cataract among older Americans3 and the most common type of cataract for which cataract surgery is performed 4. It is more common in women than men and among Caucasians.1 The pathogenesis of nuclear cataract is known to involve the accumulated stressors resulting from the inability to sufficiently defend against or repair the damage due to a variety of environmental stressors, including photochemical formation of free radicals. Accordingly, diets high in the antioxidant vitamins C and/or E have been inversely associated with nuclear cataract in many previous epidemiologic studies.5-11 Lutein and zeaxanthin, the most abundant lens carotenoids,12 scavenge superoxide and hydroxyl radicals13, protect against ultraviolet-B-induced lipid peroxidation in cultured lens epithelial cells,14 and may further play a role in membrane stability. 15 Diets high in lutein plus zeaxanthin have also been inversely associated with nuclear cataract or cataract extraction in one recent study 16 and several previous studies (reviewed by Moeller et al17 and Mares18).
The Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women's Health Initiative (WHI), was designed, in part, to evaluate the relationships of the carotenoids lutein and zeaxanthin to the prevalence of age-related nuclear cataract. This study provides data on these carotenoids in the diet, serum, and retina, as well as on numerous potential risk and protective factors. Women at high and low extremes of lutein plus zeaxanthin intake at WHI enrollment were recruited into CAREDS in order to maximize statistical power to evaluate these associations and associations with age-related macular degeneration (previously reported 19). The a priori hypothesis was that women with high intakes of lutein plus zeaxanthin in 1994-1998 have a lower prevalence of age-related nuclear cataract in 2001-2004 compared to women with low intakes of these carotenoids. We also report odds ratios for nuclear cataract across the full range of intakes and serum levels in this sample and on whether these relationships are altered after accounting for other dietary, lifestyle, and health attributes that may be correlated with high intakes or serum levels of lutein plus zeaxanthin and nuclear cataract.
The uptake of carotenoids into the intestine and eye is widely known to be highly variable but conditions that predict levels in the eye are largely unknown.20 Levels in the retina and lens may be reflected by the optical density of these carotenoids in the macula. Two previous studies have reported macular pigment optical density to be cross-sectionally related to lens optical density in 114 persons over age 5021 and in 39 people over age 55.22 The present study also reports cross-sectional associations of macular pigment optical density with nuclear cataract in the larger sample of women in CAREDS.
METHODS
The Women's Health Initiative (WHI) Observational Study was a prospective cohort study of the most common causes of mortality and morbidity among 93,676 postmenopausal women 50 to 79 years of age at enrollment, at 40 sites around the United States. The original cohort was recruited to the WHI at each of these sites through regional mass-mailings and mass-media strategies, from among women ineligible for or uninterested in participation in the WHI Clinical Trials. Participants were followed on average 7 years after enrollment. Women were excluded if they had medical conditions that predicted survival of less than three years, alcoholism, drug dependency, or mental illness.23, 24
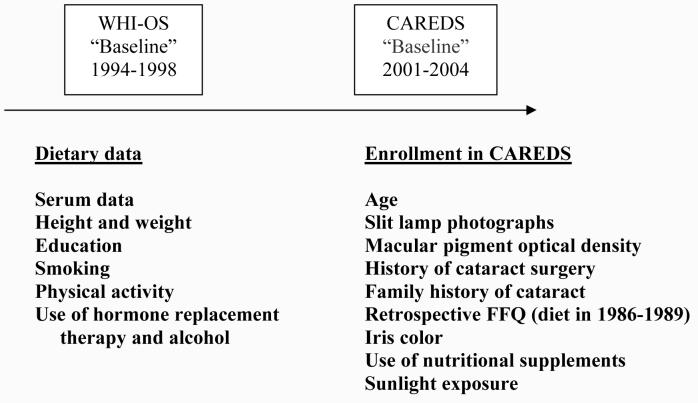
The CAREDS population consists of women who were enrolled in the observational study of the WHI at 3 of 40 sites: the University of Wisconsin (Madison), the University of Iowa (Iowa City), and the Kaiser Center for Health Research (Portland), who had intakes of lutein plus zeaxanthin that were above the 78th and below the 28th percentiles, as assessed at baseline enrollment into the WHI in 1994-1998 (Figure). This sampling strategy was used to maximize statistical power, as previously described. 25 Of the 3,143 women who fulfilled these criteria and formed the recruitment pool, 96 died or were lost to follow-up between sample selection in year 2000 and enrollment in the CAREDS in 2001-2004. Those remaining were sent letters inviting them to participate in the CAREDS. A total of 1,042 women declined participation and 2,005 were enrolled (64%). Of those enrolled, 1,894 participated in study visits.
Figure.
CAREDS Data Collection Timeline
Of those enrolled, one participant was later excluded because WHI found that her diet data was unreliable. We excluded 32 women reporting a history of trauma to both eyes, 1 woman reporting cataract extraction before the age of 40y, and 132 women with missing or ungradeable nuclear lens photographs. Thirty-seven participants were further excluded because of missing covariate data. Thus, 1,802 women comprised the analysis dataset for this investigation. All procedures conformed to the Declaration of Helsinki and were approved by the Institutional Review Board at each University.
To evaluate potential bias due to lack of participation in CAREDS, we compared characteristics of women in the recruitment dataset who did not enroll in CAREDS (N=1,138 declined, lost-to-follow-up, or died) to those who did (N=2,005). Women with high lutein intakes were more likely to join CAREDS than those with low intakes (69% vs. 60%, p<0.001). There was a slight but statistically non-significant (p=0.11) difference in the prevalence of self-reported cataract between the participants and non-participants (18% vs.21%).
We further compared women in the recruitment dataset who were included (N=1,802) in the final analysis dataset with those who were not (N=1,341). Women included in the final analysis dataset had, at WHI baseline, higher age-adjusted mean intakes of lutein plus zeaxanthin (1.8 mg/d vs. 1.6mg/d) and lower intakes of total fat (31% kcal vs. 33% kcal), saturated fat (10.8% vs. 11.2%), and polyunsaturated fat (6.4% vs. 6.7%); they were younger (median age 63 years vs. 65 years), had more formal education (78% with post high school education vs. 68%), and were more likely to be never-smokers (58% vs. 54%), to consume alcohol (73% vs. 68%), to use hormone replacement therapy (54% vs. 46%), to take dietary supplements (76% vs. 71%), to be physically active (≥ 21 kcals/week/kg in 27% vs. 20%), and to perceive their health as very good or excellent (63% vs. 55%). They were more likely to report a history of macular degeneration (5% vs. 4%) and less likely to have hypertension (32% vs. 36%) or a body mass index greater than 30 (24% vs. 33%) compared to women excluded from the analytic dataset (p<0.05 for all of the above). There was no statistically significant difference in the prevalence of self-reported cataract between those included and excluded from the analytic dataset (20% vs.19%, p=0.74).
Dietary Estimates
Primary dietary estimates were made from responses to a previously validated, semi-quantitative food frequency questionnaire (FFQ)26 at WHI baseline (1994-98). We also obtained estimates at CAREDS visits using the same questionnaire, querying intake 15-years prior to CAREDS (1986-1989) in order to estimate long-term diet stability. Retrospective FFQs have been found to be reliable indicators of past diet.27, 28 Nutrient and food group estimates in WHI and CAREDS were computed from responses to FFQs at the Fred Hutchinson Cancer Research Center. Data from the 2,004 participants with reliable FFQ data (≥600 kcal and ≤5000 kcal) at WHI baseline, 26 and the 1,995 participants with CAREDS 15-year past FFQ data collected retrospectively, were used to generate quintiles of nutrient intake, and tertiles of food and food group intake, for each time point.
Serum Analyses
Serum samples were obtained from participants at WHI baseline examinations (1994-1998) after a ≥ 10-hour fast and were stored at −80 degrees centigrade.29 Serum levels of lutein, zeaxanthin, and tocopherols were determined at Tufts University (2004-2005) by a reverse phase HPLC analysis.30 Levels of lutein and zeaxanthin represent the sum of the trans isomers. Blind duplicates were analyzed in each batch of serum analyses (in a total of 57 subjects). Mean coefficients of variation were 7.0% for all trans-lutein and 9.6% for all trans-zeaxanthin. Total triglycerides were determined by an automated chemistry analyzer based on an enzymatic method with a glycerol blank 31 and total cholesterol was determined enzymatically by the cholesterol oxidase method.32
Macular Pigment Optical Density
Measurements were made using a standardized protocol by the psychophysical method of heterochromatic flicker photometry (HFP) during CAREDS study visits conducted from 2001-04. This protocol, described in detail previously,20, 33 had high test-retest reliability (r=0.9) and participant responses at two wavelengths that are consistent with the absorption spectrum of lutein and zeaxanthin.33 Briefly, participants viewed a small test field superimposed on a blue background with the right eye. The test field alternates between a wavelength (blue or blue-green) that is absorbed by the macular pigment (MP) and a reference (green to yellow-green) wavelength that is outside the absorption band of MP. When the frequency of alternation is chosen correctly, the test field appears to flicker. When making measurements, the participant is instructed to adjust the energy of the bluish test light so that the flicker stops. The amount of bluish light that is required to produce this flicker null provides a measure of the optical density of MP (MPOD) at the retinal location of the test light. The subject was instructed to fixate at the center of the following targets: 0.25, 0.5, 1 and 1.75 degrees from the foveal center so that MPOD at different eccentricities was measured, in reference to a target at 7 degrees from the center. We used data from 0.5 degrees from the foveal center, the target with the lowest within to between person variability.33
Age-Related Nuclear Cataract
Lens photography and eye examinations were part of CAREDS study visits that took place between 2001-2004 using the standardized ETDRS protocol34 modified as in the AREDS study, for which grading reliability has been previously reported35. Briefly, both eyes were examined with slit lamp biomicroscopy. After pharmacological dilation of the pupils, a single nonstereoscopic photograph was taken of each eye with a modified Topcon slit lamp camera to grade nuclear sclerosis and nuclear color using the Age-Related Eye Disease Study (AREDS) protocol.36 Optical density of nuclear opacity was graded against a series of seven standard photographs producing continuous scores on a decimal scale that can range from 0 to 7.1. Severity of nuclear sclerosis was determined in eyes that had not previously undergone cataract extraction.
CAREDS questionnaires, completed before study visits, were reviewed with participants at the time of the CAREDS study visit. Eye history queries included dates of cataract surgeries in each eye, trauma to eyes, and physician diagnosed histories of cataract, glaucoma and macular degeneration and treatments and lifestyle changes that accompanied these conditions. Family history of cataract was also assessed, identifying all immediate family members who had physician-diagnosed cataracts prior to the age of 75 years.
The primary outcome was the presence of nuclear cataract, defined as a nuclear sclerosis severity score of 4 or greater in the worst eye and/or a history of cataract extraction in either eye. It was previously determined, in a similar population, that the incidence of cataract surgery was highest among people with photographically evident cataracts in the nuclear region of the lens4, suggesting that nuclear cataracts in women who had received cataract extractions were likely. All 1802 women in this dataset (described above) were eligible for this endpoint. A nuclear sclerosis severity score ≥ 4 was an additional outcome among women who had at least one natural lens for which lens photographs were gradable (1,577 women.).
Covariates
Age, family history of cataract (immediate family member under age 75 years when diagnosed), UV-B sunlight exposure (since age 18 years and in last 20 years based on outdoor activities during routine and vacation periods, living location, and use of protective gear [hats or sunglasses]), and use of nutritional supplements were queried in questionnaires submitted at the CAREDS study visit. Iris color was classified from photographs taken at the CAREDS eye exam. Additional demographic, lifestyle, and health history data were available from questionnaires completed at WHI study entry (education, smoking, physical activity, height, weight, hormone replacement therapy use, alcohol use, pulse pressure, and history of diabetes, hypertension, and cardiovascular disease).
Statistical Analyses
T-tests, ANCOVA, and Chi-Square tests were performed to assess the statistical significance of potential covariates in the high vs. low lutein plus zeaxanthin intake groups and by the presence or absence of nuclear cataract. Odds ratios and 95 percent confidence intervals for nuclear cataract and for nuclear sclerosis scores ≥ 4 (yes/no) were calculated comparing the high lutein plus zeaxanthin intake group to the low intake group using logistic regression (PROC LOGISTIC in SAS v.8.2, SAS Institute, Cary, NC).
A cataract risk factor model was determined for the CAREDS sample that included variables significantly related to nuclear cataract (p < 0.05) (Mares, et al, unpublished manuscript). After conducting age-adjusted analyses, this model was considered the starting point for further model building. The model included age (continuous), smoking status (never. past, current), iris color (blue, green, light brown, or dark brown/black), physical activity (kcals/week/kg), multivitamin use (>2 y vs. non-users), hormone replacement therapy use (never, past, current), pulse pressure (mm Hg), and body mass index (kg/m2).
Additional variables that were statistically significantly related (p≤0.10) to both nuclear cataract and lutein plus zeaxanthin intake group in CAREDS, or which were previously suspected to be biologically plausible confounders, were tested by adding them singly to the above risk factor model. These variables included intakes at WHI-Baseline of total energy (kcal), total fat (% kcal), saturated fat (% kcal), polyunsaturated fat (% kcal), fruits (servings/d), vegetables (servings/d), vitamin C (mg/d from food), vitamin E (mg/d from food), and gamma-tocopherol (mg/d); and use of high dose antioxidants at CAREDS Baseline (daily intake of at least 2 of the following 3 antioxidant supplements containing ≥120mg vitamin C, ≥60 IU (40mg) vitamin E, and ≥10,000 mcg beta-carotene). UV-B sunlight exposure (measured in Maryland sun years 37) was not found to be a significant confounder.
If the addition of the covariate changed the odds ratio by ≥ 10%, the covariate was selected for addition to a larger model. For both the cataract and nuclear sclerosis endpoints, the covariate that changed the odds ratio the greatest was added to the model first. The odds ratios comparing the high vs. low lutein plus zeaxanthin intake group were reassessed after the addition of each potential covariate. Only those covariates that still changed the odds ratio by ≥10% in the fuller model were retained in the final model. Dietary variables that met these criteria, but which were strongly correlated with dietary lutein plus zeaxanthin (r ≥ 0.5) were not included in the final model in order to limit issues of co linearity.
We tested for interactions to determine whether the associations between lutein plus zeaxanthin intake and nuclear cataract differed according to levels of other risk factors (age, smoking, and UV-B sunlight exposure). An alpha level of 0.10 was used to determine significance. If a significant interaction was found, analyses were conducted in both the overall sample and by strata.
In exploratory analyses, we examined the odds for nuclear cataract in women with high and low intakes of specific foods rich in lutein plus zeaxanthin, including spinach, dark green leafy vegetables, and fruits and vegetables in general. Tertiles of food intake were created using data for the 2,004 CAREDS participants with reliable WHI baseline FFQ data, both overall and within age-strata. Because of the low intake of cooked greens in our sample (spinach, mustard greens, turnip greens, and collards), comparisons were made in those eating < 1 versus ≥ 1 serving per month.
Serum lutein, zeaxanthin, and lutein plus zeaxanthin were divided into quintile categories. Odds ratios and 95% confidence intervals were calculated as described above, using the lowest quintile category as the referent group.
Exploratory analyses examined the cross-sectional relationship between levels of lutein and zeaxanthin in the macula (MPOD) and nuclear cataract. MPOD values were divided into quintile categories and odds ratios and 95% confidence intervals were calculated as described above.
RESULTS
Distribution of risk factors by level of lutein plus zeaxanthin in the diet
The median level of lutein plus zeaxanthin in the diet was approximately three times higher in women in the high lutein intake group compared to those in the low intake group (Table 1). Women in the high lutein intake group were more likely to be older, non-white, college educated, in a higher income bracket, more physically active, and have lower BMI's and serum triglycerides compared to those in the low lutein intake group. They also were more likely to rate their general health status as excellent or very good. Women with lower levels of dietary lutein plus zeaxanthin were more likely to live in Iowa and have blue eyes. Total energy intake was higher in the high lutein intake group, although the percentages of dietary fats in the diet were less than in the low lutein intake group. Intakes of dietary fiber, fruits, vegetables, many micronutrients and carotenoids, alcohol, and high dose antioxidant supplements were also higher in the high compared to the low lutein group, as were serum levels of lutein and zeaxanthin and alpha-tocopherol. Despite higher intakes of gamma-tocopherol, women in the high lutein intake group had lower serum levels of this tocopherol compared to the low lutein intake group. Results were similar when examining intakes as nutrient densities, except for omega-3 fatty acids (0.79 g/1000kcal/d in the low lutein group vs. 0.76 g/1000kcal/d in the high group, p<0.01) and alcohol (3.3 g/1000kcal/d in the low lutein group vs. 2.9 g/1000kcal/d in the high group, p=0.15).
TABLE 1.
Characteristics1 of CAREDS participants2 by level of lutein and zeaxanthin in the diet at WHI Baseline (N=1,802)
| Lutein plus Zeaxanthin Intake Group2 | ||
|---|---|---|
| Variable | Low | High |
| Number of participants in each group | 919 | 883 |
| Dietary lutein+zeaxanthin (μg/d) 3 | 791 | 28894, c |
| Median dietary lutein+zeaxanthin (mg/d) | 0.8 | 2.6 |
| Dietary lutein+zeaxanthin (mg/1000 kcal/d) 3 | 0.6 | 1.6 c |
| Median dietary lutein+zeaxanthin (mg/1000 kcal/d) | 0.6 | 1.4 |
| Demographics | ||
| Age Group (%) | ||
| ≤69 y | 52 | 47 a |
| 70-74 y | 24 | 24 |
| ≥75y | 24 | 29 a |
| Ethnicity (% Caucasian) | 98 | 97 a |
| Education (%) | ||
| High School | 30 | 14 c |
| College | 49 | 46 |
| Post-College Studies | 21 | 40 c |
| Income ≥ $ 75,000 per year (%) | 13 | 21 c |
| Study site (%) | ||
| Iowa | 37 | 30 b |
| Oregon | 33 | 33 |
| Wisconsin | 30 | 37 b |
| Lifestyle | ||
| Smoking | ||
| Never | 54 | 60 b |
| Past | 42 | 37 |
| Current | 5 | 3 b |
| Physical activity (kcal/week/kg body weight) | 12 | 18 c |
| Ocular Factors | ||
| Iris pigmentation (% blue) | 45 | 39 b |
| History of cataract diagnosis (%) | 48 | 47 |
| Family history of cataract (% yes) | 31 | 31 |
| Refractive error | −0.62 | −0.65 |
| Nuclear Cataract Outcomes | ||
|
Overall NuclearCataract (Either Nuclear Sclerosis Score ≥ 4 or Cataract Extraction (%)) |
43 | 38 a |
| Nuclear Sclerosis Score ≥ 4 (%) | 31 | 27 |
| Cataract extraction surgery (%) | 20 | 17 |
| Medical Factors | ||
| Body mass index (kg/m2) | 28 | 27 b |
| History of hypertension (% yes) | 29 | 26 |
| History of cardiovascular disease (% yes) | 25 | 24 |
| History of diabetes | 3 | 4 |
| Cholesterol lowering medications (%) | ||
| Never | 70 | 70 |
| Past | 4 | 5 |
| Current | 26 | 25 |
| Hormone Replacement Therapy (%) | ||
| Never | 34 | 33 |
| Past | 13 | 14 |
| Current | 53 | 53 |
| Systolic BP (mm Hg) | 127 | 127 |
| Pulse Pressure (mm Hg) | 53 | 53 |
| General Health (%) (N=1798) | ||
| Excellent/Very Good | 59 | 69 b |
| Good | 34 | 26 b |
| Fair/Poor | 7 | 4 b |
| Diet5 | ||
| Total energy (kcal) | 1316 | 1956 c |
| Total dietary fat (% kcal) | 34 | 28 c |
| Saturated fat (% kcal) | 12.0 | 9.6 c |
| Polyunsaturated fat (% kcal) | 6.9 | 6.0 c |
| Trans fat intake, mg/d | 3.4 | 4.0 c |
| Total omega-3 fatty acids, g/d | 1.0 | 1.5 c |
| Dietary fiber, g/d | 12 | 26 c |
| Alcohol intake (g/d) | 4.1 | 5.5 b |
| Fruit intake (servings/d) | 1.4 | 3.0 c |
| Vegetable intake (servings/d) | 1.2 | 3.9 c |
| Beta-carotene, μg/d | 2098 | 6994 c |
| Beta-cryptoxanthin, μg/d | 83 | 182 c |
| Vitamin C, mg/d | 74 | 165 c |
| Vitamin E, mg/d | 6.3 | 10.4 c |
| Gamma-tocopherol (mg/d) | 11.3 | 14.8 c |
| Zinc (mg/d) | 8.5 | 13.7 c |
| Supplement Use | ||
| Non-users of any supplement (% non-users) | 15 | 9 b |
| Among supplement users, % users of: | ||
| Multivitamins | 42 | 49 |
| Lutein or lutein + zeaxanthin | 4.8 | 4.7 |
| High dose antioxidants 6 | 28 | 35 b |
| High dose zinc 7 | 18 | 17 |
| Serum (N=1777-1778) | ||
| Total Cholesterol (mmol/L) | 5.8 | 5.8 |
| Triglycerides (mmol/L) | 1.9 | 1.7 b |
| Lutein (all-trans, μmol/L) | 0.21 | 0.31 c |
| Zeaxanthin (trans, μmol/L) | 0.054 | 0.064 c |
| Lutein+zeaxanthin (trans, μmol/L) | 0.26 | 0.37 c |
| Median serum lutein+zeaxanthin (trans, μmol/L) | 0.24 | 0.35 |
| Alpha-tocopherol (μmol/L) | 47 | 49 a |
| Gamma-tocopherol (μmol/L) | 6.2 | 4.8 c |
Data represent age-adjusted means or percentage of participants (directly standardized for age group (except for ‘Age-Group’): ≤69, 70-74, ≥75) unless otherwise indicated.
Participants were selected for intake into CAREDS if their intake of lutein + zeaxanthin was “low” (<28th percentile) or high (>78th percentile) relative to other women at each site in the larger WHI-Observational Study at WHI Baseline.
Means (not age-adjusted)
The difference between categories was statistically significant: a p ≤ 0.05; b p ≤ 0.01; c p ≤ 0.0001
Dietary intakes include food sources only
Daily intake of at least 2 of the following 3 antioxidants from supplements containing ≥ 120 mg vitamin C, ≥ 60 IU (40 mg) vitamin E, or ≥ 10,000 μg beta-carotene at CAREDS baseline
Daily intake of ≥ 15 mg supplemental zinc at CAREDS baseline
Association between dietary lutein plus zeaxanthin and nuclear cataract
The prevalence of nuclear cataract was lower among women in the high, compared to low, lutein intake groups after adjusting for age (Table 1). The prevalence of nuclear sclerosis scores ≥ 4 was marginally lower among women in the high lutein group vs. the low group, as was the prevalence of cataract extraction surgery. Women in the high lutein intake group were 23% less likely to have nuclear cataract after adjusting for age (Table 2). Further adjustment for other cataract risk factors did not substantially alter the odds ratio, although it was only marginally statistically significant (p=0.08). Odds ratios for nuclear sclerosis scores ≥ 4 were similar. In analyses in which both eyes were used as units of analyses, in generalized estimating equations, results were nearly identical (data not shown).
TABLE 2.
Odds ratios (95% confidence intervals) for overall nuclear cataract age-related nuclear sclerosis in women by levels of lutein plus zeaxanthin in the diet
| Lutein and Zeaxanthin Intake Group | ||||
|---|---|---|---|---|
| Low | --------------------------High-------------------------- | |||
| Median intake of LZ [range] (mg/d) | 0.8 [0.3-1.1] |
2.6 [2.0-10.7] |
||
| Referent | N (with outcome) | Age-adjusted model | Multivariable-adjusted model1 | |
|
Overall Nuclear Cataract (nuclear sclerosis score ≥ 4 and/or cataract |
1.00 | 1802 (733) | 0.77 (0.62-0.96) | 0.81 (0.65-1.01) |
| Nuclear Sclerosis Score ≥ 4 | 1.00 | 1571 (451) | 0.79 (0.62-1.00) | 0.80 (0.62-1.02) |
Odds ratios are adjusted for age (continuous), smoking status at WHI Baseline (never, past, current), iris pigmentation (dark brown, light brown, green, or blue), physical activity (METs), multivitamin use at CAREDS baseline (>2y vs. non-user), hormone replacement therapy use at WHI-Baseline (never, past, current), pulse pressure at WHI-Baseline (continuous), body mass index at WHI-Baseline (weight in kg/height in m2)
The associations were strengthened when women in this sample were further categorized by quintiles of lutein and zeaxanthin in the diet. Women in the highest vs. lowest quintile categories of diet lutein+zeaxanthin were 32% less likely to have nuclear cataract (multivariable-adjusted OR) (Table 3). Associations with nuclear sclerosis scores ≥ 4 were similar to those observed for nuclear cataract.
TABLE 3.
Odds ratios (95% confidence intervals) for overall nuclear cataract or nuclear sclerosis by levels of lutein plus zeaxanthin in the diet
| Quintile category of lutein + zeaxanthin | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P trend1 | |
| Diet Median Intakes per quintile2 | ||||||
| Lutein + zeaxanthin (mg/d) | 0.6 | 0.9 | 1.0 | 2.4 | 3.3 | |
|
Nuclear Cataract (nuclear sclerosis score ≥ 4 and/or cataract surgery) |
||||||
| Age-adjusted (95% CI)2 | 1.0 | 0.75 (0.54-1.04) | 0.83 (0.59-1.16) | 0.71 (0.51-1.00) | 0.64 (0.45-0.89) | 0.01 |
| Multivariable-adjusted (95% CI)3 | 1.00 | 0.76 (0.54-1.07) | 0.93 (0.66-1.32) | 0.77 (0.54-1.08) | 0.68 (0.48-0.97) | 0.04 |
| Nuclear Sclerosis Score ≥ 4 | ||||||
| Age-adjusted (95% CI)2 | 1.00 | 0.72 (0.50-1.05) | 0.83 (0.57-1.20) | 0.72 (0.50-1.05) | 0.63 (0.44-0.92) | 0.04 |
| Multivariable-adjusted (95% CI)3 | 1.00 | 0.72 (0.49-1.05) | 0.90 (0.62-1.33) | 0.74 (0.50-1.09) | 0.63 (0.43-0.94) | 0.04 |
| Median serum value [range], μmol/L | ||||||
| Lutein+zeaxanthin | 0.15 [0.04- 0.19] |
0.22 [0.19-0.25] | 0.29 [0.25-0.33] | 0.37 [0.33-0.42] | 0.50 [0.42-1.69] | |
|
Nuclear Cataract (nuclear sclerosis score ≥ 4 and/or cataract surgery) |
||||||
| Age-adjusted (95% CI) 2 | 1.00 | 0.84 (0.60-1.17) | 0.69 (0.50-0.97) | 0.76 (0.54-1.06) | 0.60 (0.43-0.84) | 0.001 |
| Multivariable-adjusted (95% CI) 4 | 1.00 | 0.90 (0.64-1.27) | 0.75 (0.53-1.07) | 0.83 (0.58-1.18) | 0.68 (0.47-0.98) | 0.01 |
| Nuclear Sclerosis Score ≥ 4 | ||||||
| Age-adjusted (95% CI) 2 | 1.00 | 0.77 (0.53-1.11) | 0.71 (0.49-1.03) | 0.77 (0.53-1.13) | 0.69 (0.47-0.99) | 0.04 |
| Multivariable-adjusted (95% CI) 4 | 1.00 | 0.85 (0.58-1.24) | 0.79 (0.54-1.16) | 0.87 (0.59-1.28) | 0.79 (0.53-1.18) | 0.16 |
P-trend analyses used lutein + zeaxanthin (μg/d in diet and μmol/L in serum) as a continuous variable in the logistic regression models
Odds ratios are adjusted for age only (continuous)
Multivariable-adjusted odds ratios are adjusted for age (continuous), smoking status at WHI Baseline (never, past, current), iris pigmentation (dark brown, light brown, green, or blue), physical activity (kcal/week/kg), multivitamin use at CAREDS baseline (>2y vs. non-user), hormone replacement therapy use at WHI-Baseline (never, past, current), pulse pressure at WHI-Baseline (continuous), body mass index at WHI-Baseline (weight in kg/height in m2)
24 participants missing serum data on lutein and zeaxanthin serum out of 1,802
We explored the possibility that age, smoking status, or UV-B exposure were modifying the relationship between dietary lutein plus zeaxanthin and nuclear cataract. No statistically significant interactions were observed for age (P for interaction = 0.67), smoking status (P for interaction = 0.64), or UV-B exposure (P for interaction = 0.60) with lutein intake group.
Association between serum lutein plus zeaxanthin and nuclear cataract
A 32% lower prevalence odds of nuclear cataract was observed for women in the highest quintile category of serum lutein+zeaxanthin compared to those in the lowest category (multivariable-adjusted OR) (Table 3). Associations with nuclear sclerosis scores ≥ 4 were attenuated compared to those observed for overall nuclear cataract, and were not statistically significant.
Confounding of the association between lutein plus zeaxanthin and nuclear cataract by dietary fat intake
Intake of total dietary fat (% kcal) significantly attenuated the associations between dietary lutein+zeaxanthin and nuclear cataract (Table 4). The associations with serum lutein + zeaxanthin were less strongly attenuated. The Pearson age-adjusted correlation coefficient between total dietary fat (% kcal, continuous) and lutein intake group was −0.35 (p < 0.0001).
TABLE 4.
The effect of total dietary fat on multivariable-adjusted odds ratios (95% confidence intervals) for nuclear cataract (nuclear sclerosis score ≥4 &/or cataract surgery) by levels of lutein plus zeaxanthin in the diet or serum
| Lutein and Zeaxanthin Intake Group (N=1,802) | ||||||
|---|---|---|---|---|---|---|
| Low Intake Group | High Intake Group | |||||
| Multivariable-adjusted (95% CI) | 1.00 | 0.81 (0.65-1.01) | ||||
| Multivariable-adjusted (95% CI) + total dietary fat |
1.00 | 0.90 (0.71-1.14) | ||||
| Quintile category of dietary lutein + zeaxanthin (N=1,802) | ||||||
| 1 | 2 | 3 | 4 | 5 | P-trend1 | |
| Multivariable-adjusted 2 | 1.00 | 0.76 (0.54-1.07) | 0.93 (0.66-1.32) | 0.77 (0.54-1.08) | 0.68 (0.48-0.97) | 0.04 |
| Multivariable-adjusted + total dietary fat3 |
1.00 |
0.78 (0.55-1.09) |
0.99 (0.70-1.41) |
0.86 (0.60-1.23) |
0.78 (0.54-1.12) |
0.27 |
| Quintile category of serum lutein and/or zeaxanthin (N=1,778) | ||||||
| 1 | 2 | 3 | 4 | 5 | P-trend1 | |
| Multivariable-adjusted 2 | 1.00 | 0.90 (0.64-1.27) | 0.75 (0.53-1.07) | 0.83 (0.58-1.18) | 0.68 (0.47-0.98) | 0.01 |
| Multivariable-adjusted + total dietary fat 3 |
1.00 |
0.91 (0.65-1.28) |
0.77 (0.55-1.09) |
0.87 (0.61-1.24) |
0.73 (0.50-1.05) |
0.04 |
P-trend analyses used dietary (μg/d) or serum (μmol/L) lutein + zeaxanthin as a continuous variable in the logistic regression models
Multivariable-adjusted odds ratios are adjusted for age (continuous), smoking status at WHI Baseline (never, past, current), iris pigmentation (dark brown, light brown, green, or blue), physical activity (kcal/week/kg), multivitamin use at CAREDS baseline (>2y vs. non-user), hormone replacement therapy use at WHI-Baseline (never, past, current), pulse pressure at WHI-Baseline (continuous), body mass index at WHI-Baseline (weight in kg/height in m2)
Odds ratios are adjusted for all of the variables listed above, plus intake of total dietary fat (% kcal/d)
Several other nutrients and food components attenuated the association between nuclear cataract and dietary lutein+zeaxanthin, including vitamin C, lycopene, beta-carotene, and fiber. These were not included in statistical models because of their high correlations with dietary lutein (Pearson age-adjusted correlation coefficients ranged from 0.61-0.77).
Association between lutein plus zeaxanthin and nuclear cataract after excluding those with dietary instability
Preliminary data suggested fluctuations in the level of lutein plus zeaxanthin in the diets of women in our sample prior to their enrollment in the WHI. Thus, we examined associations in women whose intake of lutein plus zeaxanthin did not change more than one quintile category between the CAREDS 15-year past FFQ and the WHI-Baseline FFQ (N=1,421). The association with nuclear cataract was similar to that observed in the overall dataset (age-adjusted OR [95% CI] comparing the highest versus lowest quintile category = 0.65 [0.44-0.95], p-trend = 0.07).
Association between food sources of lutein plus zeaxanthin and nuclear cataract
In exploratory analyses, we examined the odds for nuclear cataract in women with high and low intakes of fruits, vegetables, and specific foods rich in lutein and zeaxanthin. Women in the highest tertile categories of intake of fruits, vegetables, and fruits + vegetables were approximately 30% less likely to have nuclear cataract than women in the lowest tertiles of intake (Table 5). After multivariable adjustment, odds ratios were slightly attenuated and only the association with vegetable intake remained statistically significant. Associations with specific foods rich in lutein and zeaxanthin, such as cooked greens, dark green lettuces, and broccoli were in protective directions, but not statistically significant.
TABLE 5.
Odds ratios (95% confidence intervals) for nuclear cataract (nuclear sclerosis score ≥ 4 and/or cataract surgery) by levels of lutein-rich foods in the diet (n=1,802)
| Tertile Category of Intake of Specific Foods/Food Groups1 | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Fruit and vegetables | |||
| Median serv/week | 14 | 29 | 53 |
| Age-adjusted odds ratio (95% CI) 2 | 1.00 | 0.76 (0.58-0.98) | 0.70 (0.54-0.92) |
| Multivariable-adjusted odds ratio (95% CI) 3 | 1.00 | 0.80 (0.61-1.05) | 0.76 (0.58-1.00) |
| Fruit | |||
| Median serv/week | 5.6 | 14 | 25 |
| Age-adjusted odds ratio (95% CI) 2 | 1.00 | 0.92 (0.71-1.19) | 0.72 (0.56-0.94) |
| Multivariable-adjusted odds ratio (95% CI) 3 | 1.00 | 0.98 (0.75-1.28) | 0.78 (0.60-1.03) |
| Vegetables | |||
| Median serv/week | 7.0 | 15 | 29 |
| Age-adjusted odds ratio (95% CI) 2 | 1.00 | 0.75 (0.58-0.97) | 0.69 (0.53-0.90) |
| Multivariable-adjusted odds ratio (95% CI) 3 | 1.00 | 0.79 (0.61-1.03) | 0.74 (0.56-0.97) |
| Cooked greens 4 | |||
| Median serv/week | 0.0 | 0.8 | -- |
| Age-adjusted odds ratio (95% CI) 2 | 1.00 | 0.80 (0.64-1.01) | -- |
| Multivariable-adjusted odds ratio (95% CI) 3 | 1.00 | 0.82 (0.64-1.04) | -- |
| Mixed lettuce salad | |||
| Median serv/week | 0.5 | 2.0 | 5.1 |
| Age-adjusted odds ratio (95% CI) 2 | 1.00 | 0.76 (0.58-1.01) | 0.82 (0.64-1.04) |
| Multivariable-adjusted odds ratio (95% CI) 3 | 1.00 | 0.81 (0.61-1.07) | 0.86 (0.67-1.11) |
| Dark green leafy vegetables 5 | |||
| Median serv/week | 0.5 | 2.1 | 5.5 |
| Age-adjusted odds ratio (95% CI) 2 | 1.00 | 0.79 (0.61-1.02) | 0.77 (0.59-1.00) |
| Multivariable-adjusted odds ratio (95% CI) 3 | 1.00 | 0.85 (0.65-1.10) | 0.81 (0.62-1.07) |
| Broccoli | |||
| Median serv/week | 0.1 | 0.5 | 2.1 |
| Age-adjusted odds ratio (95% CI) 2 | 1.00 | 0.84 (0.65-1.09) | 0.83 (0.64-1.08) |
| Multivariable-adjusted odds ratio (95% CI) 3 | 1.00 | 0.87 (0.67-1.14) | 0.89 (0.68-1.17) |
Tertile categories are based on the number of medium-sized servings per week of each food or food group in all participants (n=2004)
Odds ratios are adjusted for age (continuous) only
Odds ratios are adjusted for age (continuous), smoking status at WHI Baseline (never, past, current), iris pigmentation (dark brown, light brown, green, or blue), physical activity (kcal/week/kg), family history of cataract (yes/no), multivitamin use at CAREDS baseline (>2y vs. non-user), hormone replacement therapy use at WHI-Baseline (never, past, current), pulse pressure at WHI-Baseline (continuous), body mass index at WHI-Baseline (weight in kg/height in m2)
Cooked greens include spinach, mustard greens, turnip greens, and collards. Due to the small number of individuals consuming cooked greens, intake was examined in those eating ≥ 1 serving/month vs. < 1 serving/month
Dark green leafy vegetables include mixed lettuce or spinach salad with vegetables, and cooked greens
Cross-sectional association between lutein plus zeaxanthin in the macula (MPOD) and nuclear cataract
Exploratory, cross-sectional analyses found a non-significant decrease in the prevalence odds of nuclear cataract for women in the highest quintile category of MPOD (median 0.63 OD units) compared to those in the lowest category (median 0.08 OD units)(age-adjusted OR (95% CI) = 0.80 (0.56-1.12), p-trend = 0.23) among the 1714 participants in these analyses that had MPOD values. The association was slightly attenuated after adjusting for other risk factors (smoking, iris pigmentation, physical activity, use of hormone replacement therapy, multivitamin use and pulse pressure) (OR 95% CI) = 0.84 (0.59-1.19) and further attenuated after adding to the model, variables that explain MPOD (lutein intake and BMI) 20 ( OR 95% CI = 0.88 (0.61-1.25). In analyses in which both eyes were used as units of analyses for the outcome, in generalized estimating equations, results were identical (data not shown).
COMMENT
We observed a moderate decrease in the prevalence of nuclear cataract among women with diets high in lutein plus zeaxanthin compared to those with diets lower in these carotenoids. This association was stronger when examining intake of lutein plus zeaxanthin across all levels of intake in this sample (by quintile categories) than when comparing the two groups of women sampled who had lutein and zeaxanthin intakes above the 78th and below the 28th quintiles. Associations were similarly strong for serum lutein plus zeaxanthin.
These results are consistent with a body of evidence that supports a protective relationship between lutein and zeaxanthin and nuclear cataract. Lutein and zeaxanthin are the predominant carotenoids in the lens12 and have been demonstrated to protect against photodamage in vitro. 14 Results of several previous epidemiological studies of samples with average intakes of lutein and zeaxanthin over wide ranges (0.8 to 3.3 mg/day) have suggested protective associations between high intakes of lutein and zeaxanthin and nuclear cataract, 16, 38-42 although the association, in one study, was no longer significant after adjusting for vitamin C 42 and the inverse association was not significant in another. 43 Serum levels of lutein and zeaxanthin, combined has been associated with nuclear cataract in longitudinal, 44 but not cross-sectional studies.45-47 , possibly reflecting error and bias related to recent diet change, since serum carotenoid levels reflect recent, not long-term, carotenoid intake.
There have been no significant associations reported between the intake of lutein and zeaxanthin and opacities in other regions of the lens. This may be due to the fact that opacities in other regions are less common and there is commonly low statistical power to assess these relationships. In one previous study16, the prevalence of cortical and posterior subcapsular opacities were lower among persons with intakes of lutein and zeaxanthin in the highest, compared to lowest, quintile, but not statistically significant. We did not obtain lens photographs in this study that would permit us to accurately determine the presence of lens opacities in these regions of the lens.
The associations observed between nuclear cataract and lutein and zeaxanthin in the diet and serum might reflect, in part, the overall benefits of diets high in fruits and vegetables or related diet patterns. Diets high in fruits and vegetables were associated with nuclear cataract in this study and were also related to decreased cataract extractions or cataracts of any type in two previous studies48, 49 Non-significant inverse associations between the intake of fruits and vegetables and nuclear cataract were observed, in a sub study of the Nurse's Health Study 50 and in the Beaver Dam Eye Study, in which the level of fruit and vegetable intake in the highest quintile was about half of that in the Nurses Health Study 38 39 . However, among specific fruits and vegetables, spinach, which is a concentrated source of lutein and zeaxanthin, has been the food item most consistently inversely associated with both nuclear opacities 38, 39, 51 and with opacities in any location.40, 41, 52, 53
We found that the association between nuclear cataract and dietary intakes of lutein and zeaxanthin were attenuated after adjusting for total dietary fat intake as a percentage of calories. To our knowledge, no other studies have reported dietary fat as a confounder of this relationship, or as a risk factor for nuclear cataract. Two studies of dietary fat and cataract reported relationships between nuclear cataract and linoleic and linolenic acids, and cataract and long-chain omega-3 fatty acids and cataract extraction, but not with total dietary fat intake.43, 54 Since the biologic plausibility of a direct association between dietary fat and cataract risk is weak at this time, we speculate that low dietary fat could be a marker for a diet pattern that has a high density of several micronutrients that could protect against cataract and may have contributed to the attenuation of the association by dietary fat (Table 4).
The observed protective associations between lutein and zeaxanthin the diet and serum to nuclear cataract in the observed study could reflect other aspects of diet or lifestyle that differ in women with diets high and low in fruits and vegetables that are unknown and unmeasured. We further evaluated cross-sectional associations of nuclear cataract with macular pigment that may reflect the status of these carotenoids in the eye, more specifically. Compared to the confidence intervals observed for the relationship of diet and serum lutein plus zeaxanthin to nuclear cataract, those for MPOD are not only weaker, but wider. This may be due to the cross-sectional nature of these analyses, as well as the variable retinal response to dietary carotenoids. The results are consistent, in direction, with two previous cross-sectional studies of lens optical density and MPOD.21, 22 The stronger odds ratios and tighter confidence intervals observed for the association of lutein plus zeaxanthin in the diet and serum may reflect the prospective nature of the analyses, the lack of confounding by recent diet change, or the fact that diets rich in lutein plus zeaxanthin reflect other unmeasured aspects of diet or lifestyle that protect against nuclear cataract. It might also reflect imperfect correlations between the levels of carotenoids in the macula and the lens. Prospective studies of MPOD to nuclear cataract are needed to clarify relationships with lutein and zeaxanthin status in the eye.
To our knowledge, this is the first study to examine the effect of an apparently poor serum response to intake of lutein plus zeaxanthin. However, excluding these apparently low responders did not change the observed associations between diet or serum levels of these carotenoids and nuclear cataract. Similarly, the exclusion of women whose reported intake of lutein plus zeaxanthin was relatively unstable did not change the observed associations for dietary lutein plus zeaxanthin and nuclear cataract.
In summary, we observed that diets rich in lutein and zeaxanthin were moderately associated with decreased prevalence of age-related nuclear cataract in women. Associations were similarly strong across quintiles of dietary and serum lutein plus zeaxanthin. High intakes of fruits and vegetables were also associated with reduced prevalence of nuclear cataract. These observations are consistent with those from previous observational studies. The possibility of a confounding influence of total dietary fat, which might reflect micronutrient-rich diet patterns, should be explored in further studies. Overall, our results suggest that diets rich in lutein and zeaxanthin protect against age-related nuclear cataract. However, other protective aspects of these diets may, in part, explain the observed relationships.
Acknowledgments
This research (supported by NIH grants EY13018 and DK 07665, and Research to Prevent Blindness) was part of the Carotenoids and Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women's Health Initiative. We thank the National Eye Institute for funding the CAREDS, and the National Heart, Lung and Blood Institute of the National Institutes of Health, U.S. Department of Health and Human Services, for funding the WHI program.
We are grateful to the Scientific Advisory Board (Natalie Kurinji, PhD, National Eye Institute; Sheila West, PhD (Chair); Neil Bressler, MD, Johns Hopkins University; Anne Lindblad, PhD, The EMMES Corporation; and Susan Mayne, PhD, Yale University) for their useful discussions and critical reading of the manuscript. We also thank the entire group of CAREDS Investigators: (Catherine Allen, PhD, Barbara Blodi, MD, Matthew Davis, MD, Larry Hubbard, MAT, Tara LaRowe, PhD, University of Wisconsin, Madison, WI; Karen Gehrs, MD, Robert Wallace, MD, University of Iowa Hospital & Clinics, Iowa City, IA; Elizabeth Johnson, PhD, Tufts University USDA Human Nutrition Research Center on Aging, Boston, MA; Michael Klein, MD, OHSU/Casey Eye Institute, Portland, OR; Cheryl Ritenbaugh, PhD, MPH, University of Arizona, Tucson, AZ; D Max Snodderly, PhD, Medical College of Georgia, Augusta, GA; Amy Millen, PhD, State University of New York-Buffalo, NY; Niyati Parekh, The Cancer Institute of New Jersey, New Brunswick, NY; Bill Wooten, PhD, Brown University, Providence, RI; and the CAREDS staff in Portland (Paula Smith, Susan K. Nolte, Debora Vahrenwald), Iowa City (Kelly O'Berry, Heather Stockman, Steven Wallace, Lindsey Fuhrmeister), Madison (Jane Armstrong, Michael Neider, Hugh Wabers, Janet Rowley, Tanya Judge, Lisa Oxton, Rickie Voland, Gail Ostrowski, Scott Burfield, Julie Ewing, Tracy Perkins) for their dedicated work.
We would also like to thank and acknowledge the Women's Health Initiative investigators, staff, and participants for their time and effort in obtaining the WHI data that were presented in this manuscript. Specifically, we would like to thank:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Barbara Alving, Jacques Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Jennifer Hays; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Evelyn Whitlock; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Howard Judd; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O'Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix.
REFERENCES
- 1.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004 Apr;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Ellwein LB, Urato CJ. Use of Eye Care and Associated Charges Among the Medicare Population: 1991-1998. Arch Ophthalmol. 2002 June 1;120(6):804–811. doi: 10.1001/archopht.120.6.804. 2002. [DOI] [PubMed] [Google Scholar]
- 3.Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004 Apr;122(4):487–494. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 4.Klein BE, Klein R, Moss SE. Incident cataract surgery: the Beaver Dam eye study. Ophthalmology. 1997 Apr;104(4):573–580. doi: 10.1016/s0161-6420(97)30267-x. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Chylack LT, Jr., Wu SY. The Lens Opacities Case-Control Study. Risk factors for cataract.[comment] Archives of Ophthalmology. 1991;109(2):244–251. doi: 10.1001/archopht.1991.01080020090051. [DOI] [PubMed] [Google Scholar]
- 6.Mares-Perlman JA, Brady WE, Klein BE, et al. Diet and nuclear lens opacities. Am J Epidemiol. 1995 Feb 15;141(4):322–334. doi: 10.1093/aje/141.4.322. [DOI] [PubMed] [Google Scholar]
- 7.Jacques PF, Felson DT, Tucker KL, et al. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997 Oct;66(4):929–936. doi: 10.1093/ajcn/66.4.929. [DOI] [PubMed] [Google Scholar]
- 8.Leske MC, Chylack LT, Jr., He Q, et al. Antioxidant vitamins and nuclear opacities: the longitudinal study of cataract. Ophthalmology. 1998;105(5):831–836. doi: 10.1016/s0161-6420(98)95021-7. [DOI] [PubMed] [Google Scholar]
- 9.Mares-Perlman JA, Lyle BJ, Klein R, et al. Vitamin supplement use and incident cataracts in a population-based study. Arch Ophthalmol. 2000 Nov;118(11):1556–1563. doi: 10.1001/archopht.118.11.1556. [DOI] [PubMed] [Google Scholar]
- 10.Vitale S, West S, Hallfrisch J, et al. Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiology. 1993 May;4(3):195–203. doi: 10.1097/00001648-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Chylack LT, Jr., Brown NP, Bron A, et al. The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiology. 2002;9(1):49–80. doi: 10.1076/opep.9.1.49.1717. [DOI] [PubMed] [Google Scholar]
- 12.Yeum KJ, Taylor A, Tang G, Russell RM. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Investigative Ophthalmology & Visual Science. 1995;36(13):2756–2761. [PubMed] [Google Scholar]
- 13.Trevithick-Sutton CC, Foote CS, Collins M, Trevithick JR. The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxyl radicals: a chemiluminescence and ESR study. Mol Vis. 2006;12:1127–1135. [PubMed] [Google Scholar]
- 14.Chitchumroonchokchai C, Bomser JA, Glamm JE, Failla ML. Xanthophylls and alpha-tocopherol decrease UVB-induced lipid peroxidation and stress signaling in human lens epithelial cells. J Nutr. 2004 Dec;134(12):3225–3232. doi: 10.1093/jn/134.12.3225. [DOI] [PubMed] [Google Scholar]
- 15.Gruseki WI. Carotenoid Orientation: Role in Membrane Stabilization. Marcel Dekker; 2004. [Google Scholar]
- 16.Vu HTV, Robman L, Hodge A, McCarty CA, Taylor HR. Lutein and Zeaxanthin and the Risk of Cataract: The Melbourne Visual Impairment Project. Invest. Ophthalmol. Vis. Sci. 2006 September 1;47(9):3783–3786. doi: 10.1167/iovs.05-0587. [DOI] [PubMed] [Google Scholar]
- 17.Moeller SM, Jacques PF, Blumberg JB. The potential role of dietary xanthophylls in cataract and age-related macular degeneration. Journal of the American College of Nutrition. 2000;19(5 Suppl):522S–527S. doi: 10.1080/07315724.2000.10718975. [DOI] [PubMed] [Google Scholar]
- 18.Mares JA. Carotenoids and Eye Disease: Epidemiologic Evidence. In: Krinsky NI, Mayne S, editors. Carotenoids in Health & Disease. Marcel Dekker, Inc.; New York, NY: 2003. Chapter 19. [Google Scholar]
- 19.Moeller SM, Parekh N, Tinker L, et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women's Health Initiative. Arch Ophthalmol. 2006 Aug;124(8):1151–1162. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- 20.Mares JA, LaRowe TL, Snodderly DM, et al. Predictors of macular pigment density in the Carotenoids in the Age-Related Eye Disease Study. American Journal of Clinical Nutrition. 2006 manuscript in press. [Google Scholar]
- 21.Berendschot TT, Broekmans WM, Klopping-Ketelaars IA, Kardinaal AF, Van Poppel G, Van Norren D. Lens aging in relation to nutritional determinants and possible risk factors for age-related cataract. Archives of Ophthalmology. 2002;120(12):1732–1737. doi: 10.1001/archopht.120.12.1732. [DOI] [PubMed] [Google Scholar]
- 22.Hammond BR, Jr., Wooten BR, Snodderly DM. Density of the human crystalline lens is related to the macular pigment carotenoids, lutein and zeaxanthin. Optometry & Vision Science. 1997;74(7):499–504. doi: 10.1097/00006324-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 23.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998 Feb;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 24.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003 Oct;13(9 Suppl):S107–121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 25.Mares JA, LaRowe TL, Snodderly DM, et al. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women's Health Initiative. Am J Clin Nutr. 2006 Nov;84(5):1107–1122. doi: 10.1093/ajcn/84.5.1107. [DOI] [PubMed] [Google Scholar]
- 26.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Annals of Epidemiology. 1999 Apr;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Ross LP, Walter CW, Peter G, et al. Nutrition and Physical Activity and Chronic Disease Prevention: Research Strategies and Recommendations. Journal of the National Cancer Institute. 2004;96(17):1276. doi: 10.1093/jnci/djh240. [DOI] [PubMed] [Google Scholar]
- 28.Wu ML, Whittemore AS, Jung DL. Errors in reported dietary intakes. II. Long-term recall. Am J Epidemiol. 1988 Nov;128(5):1137–1145. doi: 10.1093/oxfordjournals.aje.a115056. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003 Oct;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 30.Yeum KJ, Booth SL, Sadowski JA, et al. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. American Journal of Clinical Nutrition. 1996;64(4):594–602. doi: 10.1093/ajcn/64.4.594. [DOI] [PubMed] [Google Scholar]
- 31.Wahlfeld AW. Triglycerides: determination after enzymatic hydrolysis. In: Bergmeyer HU, editor. Methods in enzymatic analysis. 2nd English ed. Academic Press, Inc.; New York: 1974. pp. 1831–1835. [Google Scholar]
- 32.Allain CC, Poon LS, Chan SSG. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–477. [PubMed] [Google Scholar]
- 33.Snodderly DM, Mares JA, Wooten BR, et al. Macular pigment measurement by heterochromatic flicker photometry in older subjects: the carotenoids and age-related eye disease study. Investigative Ophthalmology & Visual Science. 2004 Feb.45(2):531–8. doi: 10.1167/iovs.03-0762. 2004. [DOI] [PubMed] [Google Scholar]
- 34.Early Treatment Diabetic Retinopathy Study. Manual of Operations. 1980 [Google Scholar]
- 35.Group A-REDSR A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8.[comment] Archives of Ophthalmology. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Age-Related Eye Disease Study Research G The age-related eye disease study (AREDS) system for classifying cataracts from photographs: AREDS report no. 4. American Journal of Ophthalmology. 2001 Feb.131(2):167–75. doi: 10.1016/s0002-9394(00)00732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarty CA, Lee SE, Livingston PM, Bissinella M, Taylor HR. Ocular exposure to UV-B in sunlight: the Melbourne visual impairment project model. Bull World Health Organ. 1996;74(4):353–360. [PMC free article] [PubMed] [Google Scholar]
- 38.Mares-Perlman JA, Brady WE, Klein BE, et al. Diet and nuclear lens opacities. Am J Epidemiol. 1995;141(4):322–334. doi: 10.1093/aje/141.4.322. [DOI] [PubMed] [Google Scholar]
- 39.Lyle BJ, Mares-Perlman JA, Klein BE, Klein R, Greger JL. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol. 1999;149(9):801–809. doi: 10.1093/oxfordjournals.aje.a009895. [DOI] [PubMed] [Google Scholar]
- 40.Chasan-Taber L, Willett WC, Seddon JM, et al. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am J Clin Nutr. 1999;70(4):509–516. doi: 10.1093/ajcn/70.4.509. [DOI] [PubMed] [Google Scholar]
- 41.Brown L, Rimm EB, Seddon JM, et al. A prospective study of carotenoid intake and risk of cataract extraction in US men.[comment] American Journal of Clinical Nutrition. 1999;70(4):517–524. doi: 10.1093/ajcn/70.4.517. [DOI] [PubMed] [Google Scholar]
- 42.Jacques PF, Chylack LT, Jr., Hankinson SE, et al. Long-term nutrient intake and early age-related nuclear lens opacities. Archives of Ophthalmology. 2001;119(7):1009–1019. doi: 10.1001/archopht.119.7.1009. [DOI] [PubMed] [Google Scholar]
- 43.Lu M, Taylor A, Chylack LT, Jr., et al. Dietary fat intake and early age-related lens opacities. Am J Clin Nutr. 2005 Apr;81(4):773–779. doi: 10.1093/ajcn/81.4.773. [DOI] [PubMed] [Google Scholar]
- 44.Lyle BJ, Mares-Perlman JA, Klein BE, et al. Serum carotenoids and tocopherols and incidence of age-related nuclear cataract. Am J Clin Nutr. 1999;69(2):272–277. doi: 10.1093/ajcn/69.2.272. [DOI] [PubMed] [Google Scholar]
- 45.Mares-Perlman JA, Brady WE, Klein BE, et al. Serum carotenoids and tocopherols and severity of nuclear and cortical opacities. Invest Ophthalmol Vis Sci. 1995;36(2):276–288. [PubMed] [Google Scholar]
- 46.Gale CR, Hall NF, Phillips DI, Martyn CN. Plasma antioxidant vitamins and carotenoids and age-related cataract. Ophthalmology. 2001 Nov;108(11):1992–1998. doi: 10.1016/s0161-6420(01)00833-8. [DOI] [PubMed] [Google Scholar]
- 47.Delcourt C, Carriere I, Delage M, Barberger-Gateau P, Schalch W, the PSG Plasma Lutein and Zeaxanthin and Other Carotenoids as Modifiable Risk Factors for Age-Related Maculopathy and Cataract: The POLA Study. Invest. Ophthalmol. Vis. Sci. 2006 June 1;47(6):2329–2335. doi: 10.1167/iovs.05-1235. 2006. [DOI] [PubMed] [Google Scholar]
- 48.Christen WG, Liu S, Schaumberg DA, Buring JE. Fruit and vegetable intake and the risk of cataract in women. Am J Clin Nutr. 2005 June 1;81(6):1417–1422. doi: 10.1093/ajcn/81.6.1417. 2005. [DOI] [PubMed] [Google Scholar]
- 49.Jacques PF, Chylack LT., Jr. Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am J Clin Nutr. 1991 Jan;53(1 Suppl):352S–355S. doi: 10.1093/ajcn/53.1.352S. [DOI] [PubMed] [Google Scholar]
- 50.Moeller SM, Taylor A, Tucker KL, et al. Overall Adherence to the Dietary Guidelines for Americans Is Associated with Reduced Prevalence of Early Age-Related Nuclear Lens Opacities in Women. J. Nutr. 2004 July 1;134(7):1812–1819. doi: 10.1093/jn/134.7.1812. 2004. [DOI] [PubMed] [Google Scholar]
- 51.Cumming RG, Mitchell P, Smith W. Diet and cataract: the Blue Mountains Eye Study. Ophthalmology. 2000;107(3):450–456. doi: 10.1016/s0161-6420(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 52.Tavani A, Negri E, La Vecchia C. Food and nutrient intake and risk of cataract. Ann Epidemiol. 1996 Jan;6(1):41–46. doi: 10.1016/1047-2797(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 53.Hankinson SE, Stampfer MJ, Seddon JM, et al. Nutrient intake and cataract extraction in women: a prospective study. Bmj. 1992 Aug 8;305(6849):335–339. doi: 10.1136/bmj.305.6849.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu M, Cho E, Taylor A, Hankinson SE, Willett WC, Jacques PF. Prospective Study of Dietary Fat and Risk of Cataract Extraction among US Women. Am. J. Epidemiol. 2005 May 15;161(10):948–959. doi: 10.1093/aje/kwi118. 2005. [DOI] [PubMed] [Google Scholar]