Abstract
Schizophrenia is widely thought to involve deficits of attention. However, the term attention can be defined so broadly that impaired performance on virtually any task could be construed as evidence for a deficit in attention, and this has slowed cumulative progress in understanding attention deficits in schizophrenia. To address this problem, we divide the general concept of attention into two distinct constructs: input selection, the selection of task-relevant inputs for further processing; and rule selection, the selective activation of task-appropriate rules. These constructs are closely tied to working memory, because input selection mechanisms are used to control the transfer of information into working memory and because working memory stores the rules used by rule selection mechanisms. These constructs are also closely tied to executive function, because executive systems are used to guide input selection and because rule selection is itself at key aspect of executive function. Within the domain of input selection, it is important to distinguish between the control of selection—the processes that guide attention to task-relevant inputs—and the implementation of selection—the processes that enhance the processing of the relevant inputs and suppress the irrelevant inputs. Current evidence suggests that schizophrenia involves a significant impairment in the control of selection but little or no impairment in the implementation of selection. Consequently, the CNTRICS participants agreed by consensus that attentional control should be a priority target for measurement and treatment research in schizophrenia.
Theorists have suggested that schizophrenia involves disorders of attention beginning with the original clinical descriptions of the illness (1, 2). However, the term attention has many meanings, and impairment in almost any task could be construed as a consequence of a deficit in attention. The situation is further complicated by the fact that—even when narrowly defined—attention serves to modulate and enhance the functioning of other cognitive systems (e.g., perception, memory, response selection) and interacts extensively with working memory and executive control systems, making it difficult to isolate the role of impairments in attention from impairments in these other systems.
The goal of this article is to present a framework for conceptualizing attention that derives from recent advances from the fields of cognitive psychology and cognitive neuroscience. This framework can be used to organize both the types of experimental paradigms that are used to measure attention and the pattern of impairments found when these paradigms are applied to patients with schizophrenia. It divides attention tasks according to whether they involve selection among competing inputs or selection among competing rules, and it makes a key distinction between the control of selection (i.e., the process of determining which inputs will be selected) and the implementation of selection (i.e., the process of enhancing these inputs and suppressing the other inputs). This framework makes it possible to organize the large and confusing literature on attention in schizophrenia, leading to the hypothesis that schizophrenia involves an impairment in control but not implementation.
Input Selection versus Rule Selection
Attention as Input Selection
In the most famous definition of attention, William James wrote:
Everyone knows what attention is. It is the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneously possible objects or trains of thought. Focalization, concentration, of consciousness are of its essence. It implies withdrawal from some things in order to deal effectively with others… (reference 3, pp. 381–382)
This remains the canonical definition of attention among cognitive psychologists and cognitive neuroscientists who primarily identify themselves as attention researchers (4). Here we will call this variety of attention input selection to emphasize that it involves giving a subset of inputs preferential access to a given cognitive process (i.e., “taking possession by the mind…one out of…several…possible objects or trains of thought”). The two most common experimental paradigms for examining input selection in basic science are the spatial cuing paradigm and the visual search paradigm, as illustrated in Figure 1. In both paradigms, observers select some sources of input for preferential processing at the expense of others (“…withdrawal from some things in order to deal effectively with others…”).
Figure 1.
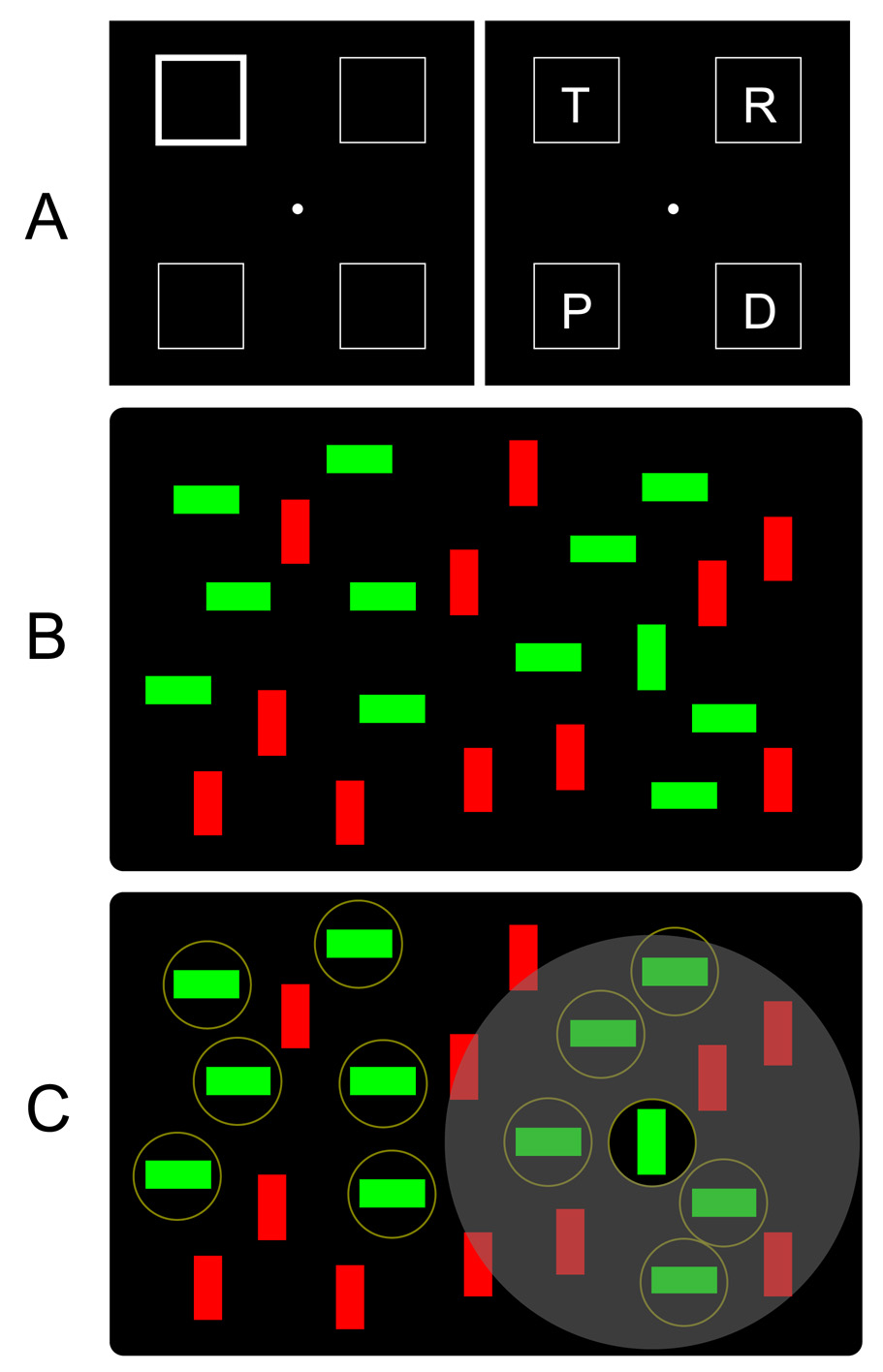
(A) Example of the spatial cuing task. In this version, a cue (a thickening of one of four location marker boxes) is used to direct attention to one location. After a brief delay, a target array appears, and the subject indicates whether it contains the letter T. In most experiments, the target is more likely to appear at the cued location (valid trials) than in an uncued location (invalid trials). This is largely an input selection task because the cue indicates which input source is relevant. (B) Example of the visual search task. In this version, the target is a green vertical bar, and the subjects makes a presence/absence response. This task can be performed by focusing attention to one item at a time, in random order, until the target is found. It can also be performed by limiting search just to the green items, which is much more efficient. In either case, this is largely an input selection task because—at any given moment—attention is used to select a subset of the objects for further processing. (C) Distinction between the control of selection (i.e., limiting attention to the circled items) and the implementation of selection (i.e., creating an annulus of suppression around the currently selected item).
The key to understanding input selection is to recognize that it operates primarily when multiple potential inputs compete for access to a process; otherwise, there would be no need to select only one potential input. This idea was popularized in the biased competition theory of Desimone and Duncan (5). According to this theory, inputs to a process compete with each other for further processing, and attention provides a bias signal that can allow a given input to win this competition, beating out other inputs that might be even more salient. For example, low-level sensory inputs in primary visual cortex provide the inputs to higher-level object recognition processes, and a faint object that is attended can out-compete an unattended bright object for object recognition (see Figure 2). However, when only one input is present, or when a highly salient input is the relevant input, no bias signal is needed to allow the relevant input to win the competition (e.g., a faint object presented alone will be fed into the object recognition machinery even without any bias from attention). Thus, input selection is most important when bottom-up salience is not sufficient to allow relevant inputs to win the competition for processing. In the spatial cuing paradigm, for example, the effects of cue validity are typically much stronger when the target must be selected from an array of distractors than when the target is presented in isolation (in which case the only source of competition is noise from other locations) (6). Similarly, the effects of attention at the single neuron level are stronger when a target and a distractor are presented simultaneously with a given neuron’s receptive field (7, 8) and when the target needs to overcome the greater salience of the distractor (9). Note that the key factor is the degree of competition between inputs, not the total amount of information being processed. When the inputs do not interfere (e.g., because they are spatially separated), they may be processed in parallel (10, 11).
Figure 2.
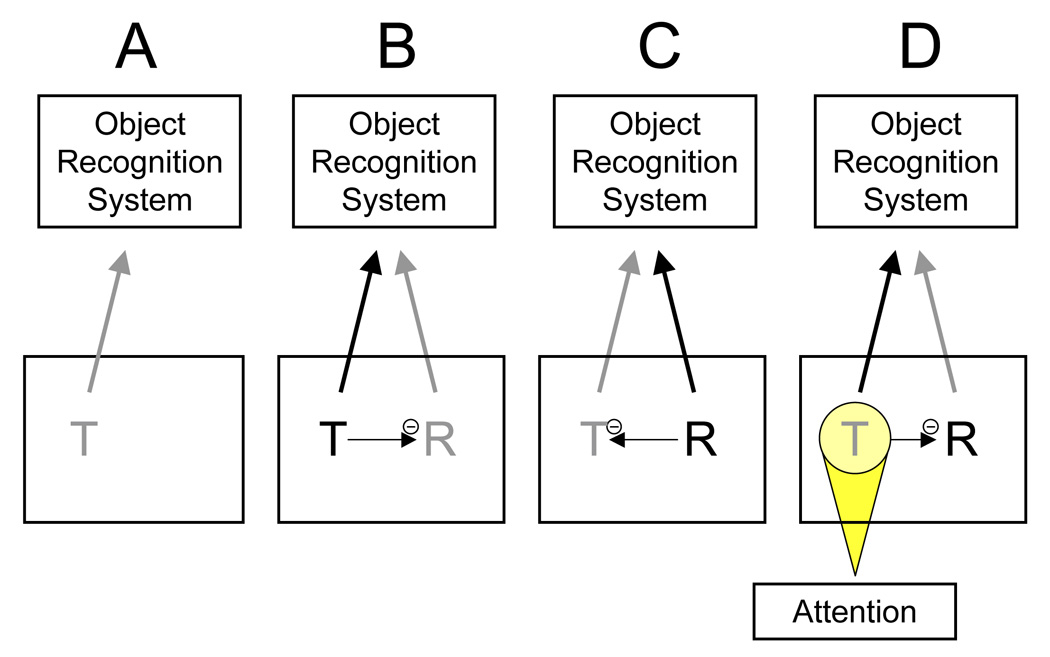
Example of the relationship between competition and attention. When a target object (the letter T) has no competition (A), it will be processed without any intervention by attention mechanisms even if it is weakly represented. When multiple objects are present but the target object is more salient than the nontarget objects (B), the target will automatically inhibit the other objects and can win the competition even without any help from attention. If, however, the target is weaker than the nontargets (C), but does not receive any help from attention, it will be inhibited by the stronger nontargets and lose the competition for representation. Attention can overcome the low salience of the target object by boosting its signal, allowing it to inhibit the stronger objects, and thus win the competition (D). According to this view, attention is necessary only when the relevant object is weaker than, or equal in strength to, the irrelevant objects.
It is important to note that input selection operates within different neurocognitive systems depending on the nature of the competition (4). When competition arises at the level of perception (e.g., in a crowded array of visual objects), attention influences which items are perceived (12). When competition arises at the level of working memory (e.g., when there is enough time to perceive all objects but the number of objects exceeds working memory capacity), attention influences which perceptual representations are stored in working memory (13). When competition arises at the stimulus-response translation stage (e.g., when multiple responses must be made in a short period of time), attention influences the prioritization of information at the stage of response selection (14).
Attention as Rule Selection
The term attention is also used to refer to a different type of process that we call rule selection, in which the observer must select among different rules that might govern the operation of ongoing processing rather than selecting among different inputs to the process. These rules might be either explicit task instructions or prepotent stimulus-response pairings that arise from extensive experience. The Stroop task (Figure 3A) is a prototypical example of a rule-selection task. When an observer is asked to name the ink color and ignore the word form, the name-the-ink-color rule must compete with the highly overlearned read-the-word rule, and it is the resolution of this competition between rules that is fundamentally responsible for the slowing of responses when the ink color and word form are incompatible.
Figure 3.
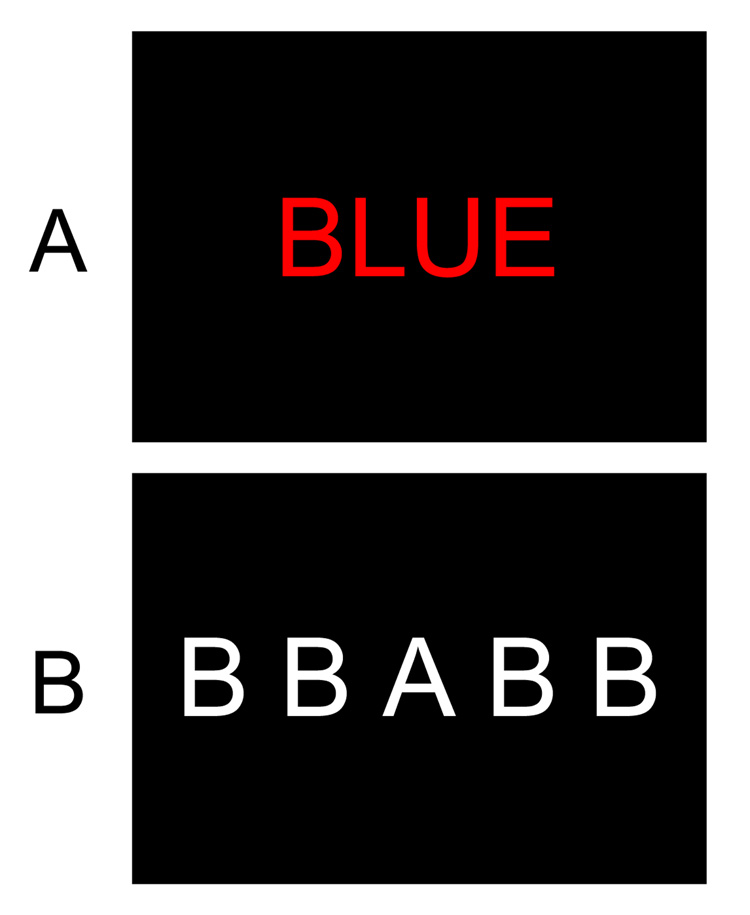
(A) Example of the Stroop task. Subjects are asked to name the ink color in one condition and to read the word in another condition. This is primarily a rule selection task, because the rules for the two tasks interfere with each other rather than the inputs. (B) Example of the flankers task. In this version, subjects press a left-hand button if the central letter is an A, and they press a right-hand button if the center letter is a B. On the trial shown here, the task-irrelevant flanking letters are incompatible with the central letter, which leads to slowed responses if the flanking letters are not adequately filtered. Although the flankers task is quite similar to the Stroop task, the flankers task is primarily an input selection because it stresses the need to apply a single rule to one input and preventing other inputs from being given access to this rule.
As in input selection, competition is key to rule selection. In the Stroop task, for example, interference in the name-the-ink-color condition is a consequence of interference from the highly overlearned read-the-word rule. However, the competition is between rules rather than inputs, because ink color and word form are separable dimensions of the same stimulus and cannot compete with each other (i.e., a color cannot compete with a shape; interference arises when the color and shape are translated into incompatible motor responses). In addition, incompatibility between the ink color and the word form in the Stroop task leads to slowed responses only when the observers are asked to name the ink color and not when they are asked to read the word; this sort of asymmetry would not ordinarily be expected when two inputs are competing with each other. A similar but more obvious case of rule selection is found in the task switching literature, in which many experiments present digits and require the subjects to switch between making a parity discrimination (odd or even) and making a magnitude discrimination (less than or greater than 5 – see, e.g., reference 15). In this case, different rules are applied to exactly the same information.
It might be tempting to argue that the Stroop task is really an input selection task in which attention selects either the ink color or the word name as the input to the verbal response system. However, different rules are necessary to transform an ink color versus a visual word form into a verbal response, so merely selecting one of these dimensions as the input is not enough to perform the task. The flankers task (Figure 3B), in which subjects respond to a central letter and attempt to ignore flanking letters (16), provides a useful contrasting example. In this task, responses are slowed when the flankers are associated with a different response than the target; a single rule is used, but a failure of input selection causes this rule to be applied to the wrong inputs. Thus the flankers task can be considered an example of an input selection task rather than a rule selection task. This task is complicated, however, by the fact that attention may operate either at a perceptual level (blocking the perceptual encoding of the flanking letters) or at a response level (blocking the perceived flanking letters from activating motor responses), depending on the extent of competition at the perceptual level (17).
It should be noted that, to a first order of approximation, rule selection is accomplished by prefrontal cortex whereas input selection is accomplished by posterior cortical regions, mapping onto the anterior and posterior attention systems described by Posner and Peterson (18).
Distinguishing Among Attention, Executive Control, and Working Memory
There is a great deal of overlap between conceptions of, and research on, the broad constructs of attention, executive control, working memory. This partly reflects the extensive interactions between these broad constructs in most cognitive tasks, and it partly reflects imprecision and inconsistency in the usage of these terms. However, these terms refer to constructs that can and should be distinguished.
Attention and Executive Control
A particularly clear definition of executive control was provided by Logan and Gordon (19), who defined executive processes as those processes whose outputs are the parameters that control the operation of other processes. For example, a categorization system could be set to classify an input of “4” as being an even number, as being less than “5”, as being a rhyme of “door”, as being a vertically oriented shape, etc. Control parameters are necessary to determine which of these outputs will be provided by the categorization system. In this case, the input to the executive control system might be the instruction to make a magnitude judgment, and the output of the executive control system would be the parameters that cause the categorization system to make a magnitude judgment rather than a parity judgment, rhyme judgment, curvature judgment, etc.
By this definition, rule selection would be a case of executive control. For example, when a subject performs the name-the-ink condition of the Stroop task, the role of attention is to send control parameters to the categorization and motor systems, causing them to implement the name-the-ink rule and inhibit the read-the-word rule. Consequently, Stroop interference is often viewed as a consequence of a failure of executive control, and the Stroop task is commonly used by researchers who are interested in executive control (and much less often by mainstream attention researchers). Moreover, when Baddeley first articulated the role of the central executive in his influential model of working memory (20), he indicated that the primary role of the executive is to override prepotent stimulus-response pairings, which is tantamount to rule selection. Thus, rule selection can be considered one of the fundamental executive control processes, and rule selection tasks can be considered examples of executive control tasks. This is entirely consistent with research practice in cognitive psychology and cognitive neuroscience.
Input selection processes, in contrast, are not executive control processes. When a letter presented at a cued location is identified and a letter presented concurrently at an uncued location is not identified, for example, attention is operating to bias the competition between the two inputs and not between two rules. This sort of selection will occur even if the letter presented at the uncued location has never been associated with a specific response. In contrast, rule selection is important primarily when it is necessary to suppress a response that has previously been associated with a particular stimulus. However, although input selection is not a form of executive control, it usually depends on executive control. That is, executive control systems need to set the parameters of the input selection system so that it will select the task-appropriate information (e.g., the letter at the location indicated by the cue). However, the challenging part of an input selection task is the selection of the to-be-attended input and the suppression of the to-be-ignored inputs, not the activation of the correct rule (which may be highly prepotent, as in the case of peripheral cues). As we will discuss later, it is possible to study executive control in the context of input selection tasks by placing different selection rules in competition with each other.
Attention and Working Memory
Attention and working memory are bidirectionally related. In one direction, input selection processes can be used to determine what information is stored in working memory. For example, a subject can be presented with 5 red letters and 5 blue letters and can be told that memory for the red letters will probably be tested after a few seconds. In this situation, the subject may identify all 10 letters but may store only the red letters in working memory (21, 22). In the opposite direction, the contents of working memory may provide a bias signal that influences input selection (5). For example, when the to-be-detected target changes from trial to trial in visual search, subjects store the identity of the target in working memory, which presumably biases selection in favor of this object (23, 24).
In addition to representing stimuli, working memory can store task rules (25), and these rules can be used to control the operation of attention (whether input selection or rule selection). For example, an individual might be asked to find a red apple in a fruit bowl and put it in the largest of three boxes, and this might be stored as two rules in working memory (e.g., “select a red apple” and “put the selected object in the large box.” Limits on working memory capacity would influence the degree of task complexity that could be handled by a given individual.
Control and Implementation of Selection
As discussed above, executive processes send control parameters to the input selection system that determine what types of inputs should be selected. These parameters will cause attention to be focused onto a given input, which in turn causes a facilitation of processing for the attended input and an inhibition of processing for the unattended inputs. One set of processes is used to identify the input that should be selected, and another set of processes is used to produce differential processing of the selected and unselected inputs. We call the first set of processes the control of selection and the second set of processes the implementation of selection. In the context of the spotlight metaphor of attention, the control of selection is analogous to pointing the beam in the correct direction, and the implementation of selection is analogous to the strength of the beam. The control of selection typically involves prefrontal and parietal cortices, and the implementation of selection typically occurs within the areas that process the inputs (e.g., within visual cortex).
Consider, for example, the visual search task shown in Figure 1B, in which the observer must indicate whether the array contains a green vertical bar. In healthy individuals, attentional control parameters can be set to favor the color green, allowing these individuals to limit search to a subset of the items (26). Figure 1C indicates this with circles around the green items, which indicate that attentional control mechanisms will flag these items for preferential treatment. However, flagging these items is only half of the problem; selective processing of the flagged items must somehow be implemented. That is, an additional mechanism must be present that allows the features of the flagged items to be identified without interference from surrounding items. This example of the implementation of selection is illustrated by a ring of suppression around the target item, which allows the features of the target item to be apprehended more clearly.
This distinction can also be seen in the example shown in Figure 2. Mechanisms of control determine which of the two letters is flagged, whereas mechanisms of implementation allow the flagged item to suppress the unflagged items and gain control over processing.
Unfortunately, it can be extremely difficult to separately measure the control and implementation of selection by means of behavioral measures. If responses are slow or inaccurate for a given target item, this could be due to a failure to direct attention to this item or a failure to effectively process this item and filter out distractors once attention has been directed to the item. However, control is easier to isolate than implementation because there are several techniques that can be used to determine whether attention has been misdirected. For example, if overt shifts of gaze are measured rather than shifts of covert attention, it is trivial to determine whether attention was directed to the right item or the wrong item (27, 28). Similarly, one can determine whether a salient distractor can attract attention to itself, thus slowing the allocation of attention to the target (29–31). Many of the best studies of the implementation of selection involve electrophysiological recordings, which can provide independent measures of the processing of attended and ignored stimuli (7, 8, 32).
Selective Disruption of Attentional Control in Schizophrenia
Although control and implementation can be difficult to isolate experimentally, the available evidence suggests that schizophrenia involves a deficit in the control of selection but not in the implementation of selection. The evidence for this proposal comes from tasks that stress one of these factors while making the other factor trivially easy. We will begin by discussing studies that assess the implementation of selection by making control very easy, and then we will turn to studies that assess the control of selection by making implementation very easy.
The Implementation of Selection in Schizophrenia
Consider, for example, the spatial cuing paradigm. In most experiments, attention is directed on the basis of a central or peripheral cue that is clearly visible, with no competition for attentional control. Because control is trivial in these paradigms, the observed attention effects presumably reflect the implementation of selection. We have identified 14 studies of schizophrenia between 1992 and 2002 using variations on this basic task, and in every case the patients showed a clear reaction time advantage for validly cued targets compared to invalidly cued targets, indicating that they were able to effectively enhance processing at the cued location compared to the uncued location (33–46). Although the precise pattern of results varied to some degree across studies, and deficits were observed under certain conditions in some of the studies, the overall pattern suggests that the implementation of selection is largely intact in schizophrenia patients.
A recent series of experiments used an analogous cuing approach to examine the ability of patients to selectively store relevant information in working memory (47). No matter what kind of cue was used, both schizophrenia patients and control subjects selectively stored the cued items in memory and kept the uncued items out of memory. Again, this suggests that the implementation of selection is not meaningfully impaired in schizophrenia.
The implementation of selection was recently assessed using event-related potentials (ERPs) in the context of a visual search task (48). Subjects searched for either a red target or a green target in an array of gray distractor objects, and the attention-related N2pc component (49, 50) was used to measure the allocation of attention to the target. The red and green colors were highly salient features, making attentional control very easy. However, the implementation of attention was stressed by requiring subjects to report the shape of the target and by surrounding the target with distractor objects that were very similar in shape. The N2pc component was found to be just as fast and just as large in schizophrenia patients as in control subjects. Thus, the implementation of selection appears to be intact in schizophrenia patients, although it remains possible that deficits would be observed under conditions that provide a greater challenge to the selection process (e.g., selecting a low-contrast target among high-contrast distractors).
The Control of Selection in Schizophrenia
Some of the best known examples of attentional dysfunction in schizophrenia are from rule selection tasks, such as the AX-CPT and Stroop tasks (51–53). As discussed above, impairments in such tasks can be well described as impairments in control. Because input selection tasks involve both control and implementation, schizophrenia patients may also show impaired performance in these tasks when they stress the control of selection. For example, Maruff et al. (54) used a variant of the spatial cuing paradigm in which a cue appearing in a peripheral location indicated that the target would appear in the opposite visual field. This task is analogous to an antisaccade task, because it requires the observer to shift covert attention away from rather than toward the cue. The cue automatically activates a shift of attention to its location, and this can be considered an example of a prepotent rule (i.e., there appears to be an automatic shift-attention-to-a-sudden-onset rule in visual perception tasks, which is analogous to the automatic read-the word rule in the Stroop task). This rule must be overridden in a top-down manner to produce a shift of attention away from the cue. Thus, this paradigm leads to the need to inhibit a prepotent rule to activate a task-appropriate rule, much as the Stroop task requires observers to inhibit the prepotent read-the-word rule and activate the task-appropriate name-the-ink-color rule. Maruff et al. (54) found that schizophrenia patients were slowed in this task compared to a task in which the target appeared at the location of the cue, demonstrating an inability to inhibit the prepotent rule of shifting attention toward the cue. This is very different from the pattern described above for the standard spatial cuing paradigm, in which subjects are required to shift attention toward the cued location. Thus, schizophrenia can lead to deficits in input selection tasks when they involve competition between the rules that govern the control of selection.
Schizophrenia may also lead to deficits in input selection tasks that require precise control over attention, even if there is no competition with an incompatible, prepotent rule. For example, Fuller et al. (55) tested schizophrenia patients and control subjects in four visual search tasks that required different degrees of attentional control. In one task, the target was defined by a single feature, but this feature was not very salient, and good control was presumably necessary to direct attention directly to the target without searching any of the distractors (see Figure 4A). Patients were strongly impaired in this task. They were also strongly impaired in a task that required the observers to shift attention back and forth between specific items on the left and right sides of the display, which also required precise attentional control (see Figure 4D). In contrast, patients were much less impaired in two tasks for which a random search process was highly effective, thus minimizing the need for precise control (Figures 4B and 4C). These different levels of impairment were not associated with the overall difficulty of the tasks, but were instead associated with the difficulty of the attentional control requirements of the tasks (see reference 56 for additional evidence of impaired attentional control in visual search).
Figure 4.
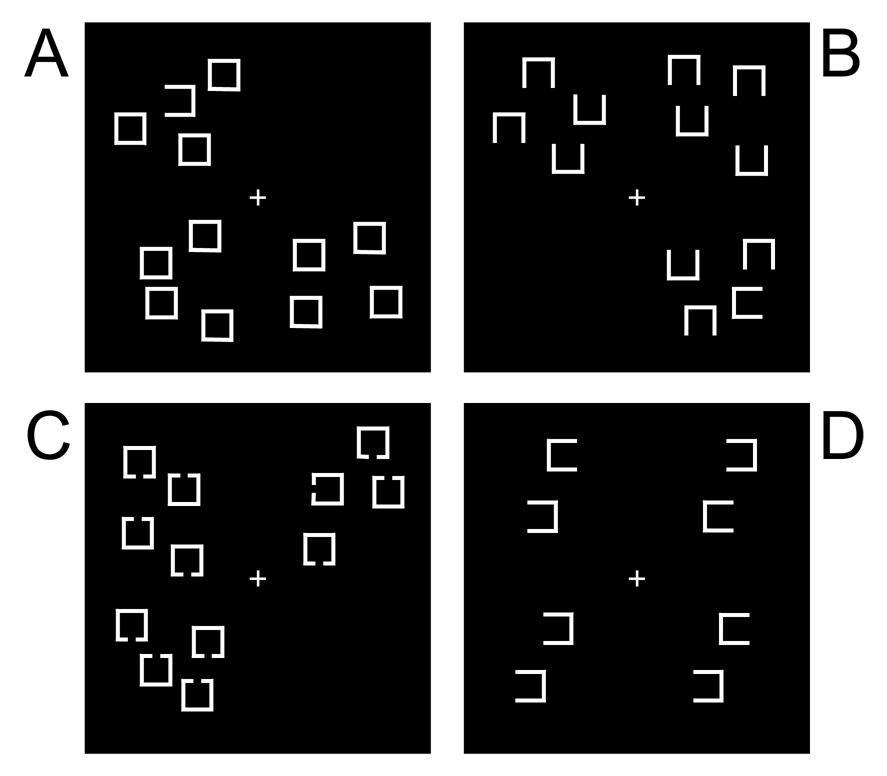
Visual search tasks used by Fuller et al. (55). The number of items in each stimulus array varied across trials, and performance was quantified as the slope of the function relating reaction time to the number of items in the stimulus array. The tasks are shown in order of ascending difficulty. In tasks A, B, and C, the target was a square with a gap on the left or right side, and subjects were required to press one of two buttons to indicate the side of this gap. In task A, the target was the only item with a gap, and performance could be optimized by controlling the search so that attention was directed immediately to this item rather than shifting randomly from item to item. In Task B, each item had a gap, making it impossible to use feature information to guide attention directly to the target. Thus, attention shifts randomly in this condition even in healthy individuals, and the need for precise attentional control is minimized. Task C was identical to Task B except that the gaps were smaller, making the task more difficulty without increasing the need for precise attentional control. In task C, the stimulus arrays consisted of pairs of items in the left and right visual fields, and subjects were required to find the one pair of identical items and report the direction of the gap in this pair. This task requires precise attentional control because an item in one visual field must be compared with a specific item in the opposite field, require a precise shift of attention to this item. Schizophrenia patients were most impaired in tasks A and C, the two tasks that stressed the control of selection.
Conclusions
Almost any task can be considered an attention task, and making sense of the pattern of impairment across tasks can be challenging. We have suggested two main ways of subdividing the broad construct of attention that may be helpful in understanding patient performance. First, we have noted that attention can be used to select between different inputs or to select between different rules. Second, we have made a distinction between the control of selection and the implementation of selection. The evidence reviewed here suggests that rule selection and the control of selection are impaired in schizophrenia whereas input selection and the implementation of selection are relatively unimpaired (especially when contributions from rule selection and attentional control are minimized). Consequently, the CNTRICS participants agreed by consensus that attentional control should be a priority target for measurement and treatment research in schizophrenia. The distinctions described in this article also help clarify the relationships among attention, executive control, and working memory. By subdividing the broad construct of attention in this manner, we hope that researchers will be able to develop tasks that more precisely assess the specific neurocognitive deficits that characterize schizophrenia so that new treatments can be developed and tested.
Acknowledgments
Preparation of this article was made possible by grants R01 MH065034 and MH06850 from the National Institute of Mental Health and by University of Maryland General Clinical Research Center Grant Number M01- RR-16500, National Institute of Health. We would like to thank Becky Fuller, Ben Robinson, and Elsie Braun for helping to shape the ideas and research discussed in this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Dr. Luck has served as a consultant to Merck. Dr. Gold receives royalty payments from the Brief Assessment of Cognition in Schizophrenia and has served as a consultant for Glaxo Smith Kline, Pfizer, and Solvay.
Contributor Information
Steven J. Luck, University of California, Davis
James M. Gold, Maryland Psychiatric Research Center
References
- 1.Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York: International Universities Press; 1911. [Google Scholar]
- 2.Kraepelin E. Dementia Praecox and Paraphrenia. 8th ed. Edinburgh: Livingstone; 1919. [Google Scholar]
- 3.James W. The Principles of Psychology. New York: Holt; 1890. [Google Scholar]
- 4.Luck SJ, Vecera SP. Attention. In: Yantis S, editor. Stevens' Handbook of Experimental Psychology: Vol 1: Sensation and Perception. 3rd ed. New York: Wiley; 2002. [Google Scholar]
- 5.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 6.Luck SJ, Hillyard SA, Mouloua M, Hawkins HL. Mechanisms of visual-spatial attention: Resource allocation or uncertainty reduction? Journal of Experimental Psychology Human Perception and Performance. 1996;22:725–737. doi: 10.1037//0096-1523.22.3.725. [DOI] [PubMed] [Google Scholar]
- 7.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 8.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen A, Ivry RB. Density effects in conjunction search: Evidence for a coarse location mechanism of feature integration. J Exp Psychol Hum Percept Perform. 1991;17:891–901. doi: 10.1037//0096-1523.17.4.891. [DOI] [PubMed] [Google Scholar]
- 11.VanRullen R, Reddy L, Fei-Fei L. Binding is a local problem for natural objects and scenes. Vision Res. 2005;45:3133–3144. doi: 10.1016/j.visres.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Treisman A. The binding problem. Curr Opin Neurobiol. 1996;6:171–178. doi: 10.1016/s0959-4388(96)80070-5. [DOI] [PubMed] [Google Scholar]
- 13.Vogel EK, Luck SJ, Shapiro KL. Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. J Exp Psychol Hum Percept Perform. 1998;24:1656–1674. doi: 10.1037//0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- 14.Pashler H. Dual-task interference in simple tasks: Data and theory. Psychol Bull. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- 15.Arrington CM, Logan GD. The cost of a voluntary task switch. Psychological Science. 2004;15:610–615. doi: 10.1111/j.0956-7976.2004.00728.x. [DOI] [PubMed] [Google Scholar]
- 16.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 17.Lavie N. Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- 18.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 19.Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- 20.Baddeley AD. Working Memory. Oxford: Clarendon; 1986. [Google Scholar]
- 21.Averbach E, Coriel AS. Short-term memory in vision. Bell System Technical Journal. 1961;40:309–328. [Google Scholar]
- 22.Vogel EK, Woodman GF, Luck SJ. Pushing around the locus of selection: Evidence for the flexible-selection hypothesis. J Cognit Neurosci. 2005;17:1907–1922. doi: 10.1162/089892905775008599. [DOI] [PubMed] [Google Scholar]
- 23.Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. J Neurophysiol. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- 24.Woodman GF, Luck SJ, Schall JD. The role of working memory representations in the control of attention. Cereb Cortex. 2007;17:i118–i124. doi: 10.1093/cercor/bhm065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logan GD. Working memory, task switching, and exectuive control in the task span procedure. J Exp Psychol Gen. 2004;133:218–236. doi: 10.1037/0096-3445.133.2.218. [DOI] [PubMed] [Google Scholar]
- 26.Egeth HE, Virzi RA, Garbart H. Searching for conjunctively defined targets. J Exp Psychol Hum Percept Perform. 1984;10:32–39. doi: 10.1037//0096-1523.10.1.32. [DOI] [PubMed] [Google Scholar]
- 27.Peterson MS, Kramer AF, Wang RF, Irwin DE, McCarley JS. Visual search has memory. Psychological Science. 2001;12:287–292. doi: 10.1111/1467-9280.00353. [DOI] [PubMed] [Google Scholar]
- 28.Williams CC, Pollatsek A. Searching for an O in an array of Cs: Eye movements track moment-to-moment processing in visual search. Percept Psychophys. 2007;69:372–381. doi: 10.3758/bf03193758. [DOI] [PubMed] [Google Scholar]
- 29.Theeuwes J. Stimulus-driven capture and attentional set: Selective search for color and visual abrupt onsets. J Exp Psychol Hum Percept Perform. 1994;20:799–806. doi: 10.1037//0096-1523.20.4.799. [DOI] [PubMed] [Google Scholar]
- 30.Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 1992;18:1030–1044. [PubMed] [Google Scholar]
- 31.Egeth HE, Yantis S. Visual attention: Control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- 32.Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32:4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- 33.Strauss ME, Alphs L, Boekamp J. Disengagement of attention in chronic schizophrenia. Psychiatry Res. 1992;43:87–92. doi: 10.1016/0165-1781(92)90144-r. [DOI] [PubMed] [Google Scholar]
- 34.Posner MI, Early TS, Reiman E, Pardo PJ, Dhawan M. Asymmetries in hemisphereic control of attention in schizophrenia. Arch Gen Psychiatry. 1988;45:814–821. doi: 10.1001/archpsyc.1988.01800330038004. [DOI] [PubMed] [Google Scholar]
- 35.Bustillo JR, Thaker G, Buchanan RW, Moran M, Kirkpatrack B, Carpenter WT. Visual information-processing impairments in deficit and nondeficit schizophrenia. Am J Psychiatry. 1997;154:647–654. doi: 10.1176/ajp.154.5.647. [DOI] [PubMed] [Google Scholar]
- 36.Maruff P, Hay D, Malone V, Currie J. Asymmetries in the convert orienting of visual spatial attention in schizophrenia. Neuropsychologia. 1995;33:1205–1223. doi: 10.1016/0028-3932(95)00037-4. [DOI] [PubMed] [Google Scholar]
- 37.Wigal SB, Swanson JM, Potkin SG. Lateralized attentional deficits in drug-free and medicated schizophrenic patients. Neuropsychologia. 1997;35:1519–1525. doi: 10.1016/s0028-3932(97)00087-0. [DOI] [PubMed] [Google Scholar]
- 38.Liotti M, Dazzi S, Umilta C. Deficits of the automatic orienting of attention in schizophrenic patients. J Psychiatr Res. 1993;27:119–130. doi: 10.1016/0022-3956(93)90056-8. [DOI] [PubMed] [Google Scholar]
- 39.Gold JM, Randolph C, Coppola R, Carpenter CJ, Goldberg TE, Weinberger DR. Visual orienting in schizophrenia. Schizophr Res. 1992;7:203–209. doi: 10.1016/0920-9964(92)90013-u. [DOI] [PubMed] [Google Scholar]
- 40.Nestor PG, Faux SF, McCarley RW, Penhune V, Shenton ME, Pollack SD, et al. Attentional cues in chronic schizophrenia: Abnormal disengagement of attention. J Abnorm Psychol. 1992;101:682–689. doi: 10.1037//0021-843x.101.4.682. [DOI] [PubMed] [Google Scholar]
- 41.Sereno AB, Holzman PS. Schizophr Res. Vol. 20. Netherlands: Elsevier Science Publishers BV; 1996. Spatial selective attention in schizophrenic, affective disorder, and normal subjects. [DOI] [PubMed] [Google Scholar]
- 42.Carter CS, Robertson LC, Chaderjian MR, Celaya LJ, Nordahl TE. Attentional asymmetry in schizophrenia: controlled and automatic processes. Biol Psychiatry. 1992;31:909–918. doi: 10.1016/0006-3223(92)90117-i. [DOI] [PubMed] [Google Scholar]
- 43.Larrison-Faucher A, Briand KA, Sereno AB. Delayed onset of inhibition of return in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:505–512. doi: 10.1016/s0278-5846(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 44.Fuentes LJ, Boucart M, Alvarez R, Vivas AB, Zimmerman MA. Inhibitory processing in visuospatial attention in healthy adults and schizophrenic patients. Schizophr Res. 1999;40:75–80. doi: 10.1016/s0920-9964(99)00044-4. [DOI] [PubMed] [Google Scholar]
- 45.Oie M, Rund BR, Sundet K. Covert visual attention in patients with early-onset schizophrenia. Schizophr Res. 1998;34:195–205. doi: 10.1016/s0920-9964(98)00092-9. [DOI] [PubMed] [Google Scholar]
- 46.Maruff P, Danckert J, Pantelis C, Currie J. Saccadic and attentional abnormalities in patients with schizophrenia. Psychol Med. 1998;28:1091–1100. doi: 10.1017/s0033291798007132. [DOI] [PubMed] [Google Scholar]
- 47.Gold JM, Fuller RL, Robinson B, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- 48.Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: Electrophysiological and behavioral evidence. Schizophr Res. 2006;85:174–195. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 49.Luck SJ, Hillyard SA. Spatial filtering during visual search: Evidence from human electrophysiology. J Exp Psychol Hum Percept Perform. 1994;20:1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- 50.Luck SJ, Girelli M, McDermott MT, Ford MA. Bridging the gap between monkey neurophysiology and human perception: An ambiguity resolution theory of visual selective attention. Cognit Psychol. 1997;33:64–87. doi: 10.1006/cogp.1997.0660. [DOI] [PubMed] [Google Scholar]
- 51.Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- 52.Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 53.Barch DM, Carter CS, Hachten PC, Usher M, Cohen JD. The "benefits" of distractibility: mechanisms underlying increased Stroop effects in schizophrenia. Schizophr Bull. 1999;25:749–762. doi: 10.1093/oxfordjournals.schbul.a033416. [DOI] [PubMed] [Google Scholar]
- 54.Maruff P, Pantelis C, Danckert J, Smith D, Currie J. Deficits in the endogenous redirection of covert visual attention in chronic schizophrenia. Neuropsychologia. 1996;34:1079–1084. doi: 10.1016/0028-3932(96)00035-8. [DOI] [PubMed] [Google Scholar]
- 55.Fuller RL, Luck SJ, Braun EL, Robinson B, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. J Abnorm Psychol. doi: 10.1037/0021-843X.115.2.266. (in press) [DOI] [PubMed] [Google Scholar]
- 56.Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophr Res. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


