Abstract
In a simple bipedal walking model, an impulsive push along the trailing limb (similar to ankle plantar flexion) or a torque at the hip can power level walking. This suggests a tradeoff between ankle and hip muscle requirements during human gait. People with anterior hip pain may benefit from walking with increased ankle pushoff if it reduces hip muscle forces. The purpose of our study was to determine if simple instructions to alter ankle pushoff can modify gait dynamics and if resulting changes in ankle pushoff have an effect on hip muscle requirements during gait. We hypothesized that changes in ankle kinetics would be inversely related to hip muscle kinetics. Ten healthy subjects walked on a custom split-belt force-measuring treadmill at 1.25 m/s. We recorded ground reaction forces and lower extremity kinematic data to calculate joint angles and internal muscle moments, powers and angular impulses. Subjects walked under three conditions: Natural Pushoff, Decreased Pushoff and Increased Pushoff. For the Decreased Pushoff condition, subjects were instructed to push less with their feet as they walked. Conversely, for the Increased Pushoff condition, subjects were instructed to push more with their feet. As predicted, walking with increased ankle pushoff resulted in lower peak hip flexion moment, power, and angular impulse as well as lower peak hip extension moment and angular impulse (p<0.05). Our results emphasize the interchange between hip and ankle kinetics in human walking and suggest that increased ankle pushoff during gait may help compensate for hip muscle weakness or injury and reduce hip joint forces.
Keywords: Gait, Hip Joint, Orthopaedics, Locomotion
1. Introduction
Insight into human gait can be gained by simple mechanical representations of walking. The simple walking model, as described by Garcia et al. (Garcia et al., 1998), consists of two rigid massless limbs connected at the “hip” by a hinge joint. The model has a point mass at the hips representing the body and a small mass at the end of each limb representing each foot. This bipedal model can be modified for different methods of actuation (McGeer, 1990; Kuo, 2002). One way to actuate the model is to apply an impulsive push along the trailing limb as it leaves the ground. This push redirects the center of mass forward and upward. A second method is to apply a torque between the limbs using a torsional spring. The torque generated by the spring pulls the swing limb forward. Comparison of these methods of powering gait indicates that there is a direct tradeoff between the impulsive push from the trailing limb and the rotational torque between the limbs. Using the hip torque alone to power gait is four times more energetically expensive than using the impulse alone (Kuo, 2002). Thus, a trailing limb impulsive pushoff is a much more economical way to generate walking in the simple bipedal model.
Although there is a clear tradeoff between a trailing limb push and a swing leg hip pull in the simple walking model, the interplay between ankle pushoff and hip torque generation may be less clear when applied to human gait. The impulsive push of the trailing limb can be analogous to ankle plantar flexion or “pushoff” in late stance. Clinicians refer to gait powered by ankle pushoff as using an “ankle strategy”, which is thought to be the preferred walking strategy for healthy young adults (Kerrigan et al., 1998; Mueller et al., 1994b). In the ankle strategy for walking, ankle plantar flexion power propels the leg into swing and accelerates body mass forward. The pushoff into swing is slightly different than in the simple walking model because the impulsive push in the model redirects the center of mass and swing occurs passively. In human gait, leg swing is not a completely passive process. Muscle activation is thought to contribute to leg acceleration and deceleration, thus increasing the energetic cost of walking (Doke et al., 2005; Gottschall and Kram, 2005).
A limitation of the simple walking model is that it does not accurately reproduce hip extension torque. The model’s torsional spring pulls the swing limb forward but has no effect on the stance limb or the movement of the center of mass. This type of actuation is similar to a “hip strategy” for walking where the hip flexor muscles of the swing leg concentrically contract to pull the leg forward (Mueller et al., 1994b). In human walking, however, both the hip flexor muscles and the hip extensor muscles are active during the gait cycle. McGibbon (McGibbon, 2003) proposes a second hip strategy in which the hip extensor muscles of the stance leg concentrically contract to posteriorly rotate the pelvis and assist with forward progression of the contralateral leg into swing. Individuals with diabetes mellitus demonstrate a higher hip flexion moment than ankle plantar flexion moment, opposite the pattern found in age-matched healthy individuals (Mueller et al., 1994a). This suggests there is a definitive tradeoff between a hip flexor strategy and an ankle strategy during human walking. Healthy elderly individuals demonstrate a greater hip extension angular impulse and a lower peak ankle plantar flexion angular impulse compared to healthy young adult subjects (DeVita and Hortobagyi, 2000). Correspondingly, this suggests a tradeoff between a hip extensor strategy and an ankle strategy.
Alteration of walking strategy has been recommended for some clinical populations. Mueller et al. (Mueller et al., 1994b) showed that instructing subjects to use an exaggerated hip strategy when walking could lead to a beneficial decrease in forefoot peak plantar pressures. Conversely, if individuals can decrease hip muscle forces by walking with increased ankle pushoff, people with hip pain may benefit from walking with an exaggerated ankle strategy. This alteration may be beneficial for patients with acetabular labral tears or idiopathic hip osteoarthritis. Patients with anterior hip pain may benefit from decreasing the anterior hip forces which may over time increase pain and lead to a tear of the acetabular labrum (Lewis and Sahrmann, 2006). Patients with idiopathic hip osteoarthritis may also benefit from decreased muscle forces as increased joint force is associated with earlier progress of disease (Recnik et al., 2007; Mavcic et al., 2004). In addition, it would also suggest that plantar flexor muscle weakness may be a cause of increased hip muscle forces during walking, leading to overuse injury of hip musculature or increased hip joint forces and subsequent joint injury.
The purpose of this study was to test whether directly altering ankle pushoff in late stance has an effect on hip muscle moments during walking in healthy young adults. We hypothesized that as subjects intentionally altered ankle pushoff, there would be an inverse relationship between peak ankle plantar flexion moment and peak hip flexion and extension moments.
2. Materials and Methods
2.1 Subjects
Ten healthy subjects (3 male, 7 female, age 27.0±7.2 years (mean±SD), height 1.69±0.09 m, and mass 63.2±11.3 kg) participated in the study. Written informed consent as approved by the University of Michigan Medical School Institutional Review Board was obtained from all subjects prior to any testing.
2.2 Instrumentation
We used a motion capture system (120 Hz) and reflective markers to record the kinematics of the ankle, knee, and hip joints (low pass Butterworth filtered at 6 Hz with zero phase lag to remove movement artifact). Subjects walked at 1.25 m/s on a custom built split-belt force-measuring treadmill (FIGURE 1) so we could measure vertical, horizontal and lateral ground reaction forces (1200 Hz). Each belt had a separate force platform mounted as its base to measure ground reaction forces from each leg separately. Details of the force treadmill measurement capabilities are included in Collins et al. (Collins et al., 2008).
FIGURE 1. Custom built force-measuring treadmill.
The treadmill has dual belts that are each mounted with a separate force platform as its base to measure ground reaction forces from each leg separately. The belts are 24 inches wide and mounted flush with the floor. The distance between the two belts is 0.75 inches. Each belt is connected to a separate motor (2.5 kW). Average belt speed variation when adult subjects walk on the treadmill at 1.25 m/s is 1.8%. Details of the measuring capabilities of the force treadmill are included in Collins et al. (Collins et al., 2008).
2.3 Conditions
Subjects walked under three conditions. For the Natural Pushoff condition, subjects were instructed to walk as they normally preferred. For the Increased Pushoff condition, subjects were instructed to “push more with your foot when you walk”. Conversely for the Decreased Pushoff condition, subjects were instructed to “push less with your foot when you walk”. We used only short simple instructions because we wanted to focus on relatively fast adaptations by the subjects. Subjects practiced walking under each condition for at least a minute prior to data collection. The natural condition was always tested first. The order of the two altered conditions was randomized.
2.4 Data acquisition and processing
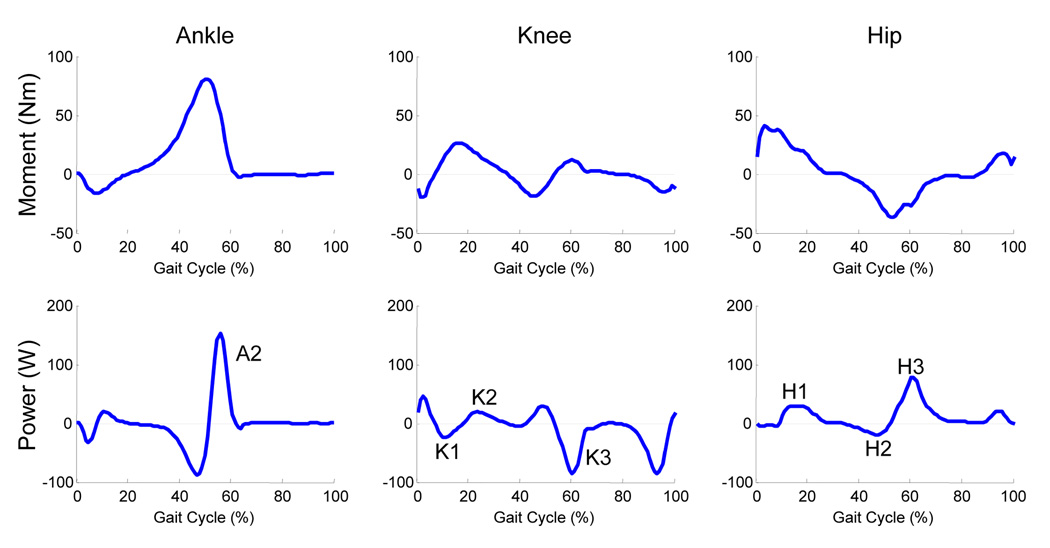
We recorded kinematic and kinetic data for 90 seconds of walking during each condition. We used commercially available software (Visual3D, C-Motion, Inc, Rockville, MD) to calculate internal muscle moments about the lower limb joints based on kinematic marker and force data. Lower limb inertial properties were estimated based on anthropometric measurements of the subjects (Zatsiorsky, 2002). Stride cycles were normalized from left heel strike (0%) to left heel strike (100%). Average maximum joint angles, peak ankle and hip net muscle moments and powers, and ankle and hip angular impulses of the left leg were calculated for each subject and condition. Angular impulses were calculated as the area under the corresponding positive or negative moment curve. We calculated angular impulse over the first 70% of the gait cycle (stance to early swing) to capture the activity of the hip flexor muscles during early swing (Winter et al., 1995). Peak moments and powers were calculated for specific phases of the gait cycle (FIGURE 2) keeping with previous convention (Winter, 1983).
FIGURE 2. Moment and power data for the left leg of a single representative subject.
Data are normalized from left heel strike to left heel strike. All major moment and power peaks were calculated for prescribed phases of the gait cycle keeping with previous convention (Winter, 1983). Ankle plantar flexion, knee extension and hip extension moments are positive. A2 represents ankle plantar flexion power during late stance. K1, K2, and K3 represent knee extension power, both absorption (negative) and generation (positive), during early stance, midstance and terminal stance respectively. H1 represents hip extension power while H2 and H3 represent hip flexion power.
2.5 Data analysis
We used a separate repeated measures analysis of variance (ANOVAs) for each variable . When the main effect of condition in the ANOVA was significant, post-hoc paired t-tests with Bonferonni correction were used to detect differences between the natural condition and the altered pushoff conditions. All statistical analyses were performed in SYSTAT 11 (SYSTAT Software, Inc, Point Richmond, CA). A p-value of less than 0.05 was considered significant.
3. Results
The simple instructions to and short practice by the subjects led to marked changes for the increased ankle pushoff condition but the instructions were not successful in producing substantial changes in the decreased pushoff condition (TABLE 1). Repeated measures ANOVAs revealed significant differences among the three conditions for all variables except maximum hip flexion and extension angles, ankle dorsiflexion angular impulse, ankle plantar flexion peak power, knee extension peak power during midstance and speed. Therefore, the rest of the results highlight the significant differences comparing the altered pushoff conditions to the natural condition using Bonferonni corrected paired t-tests.
TABLE 1. Mean and standard deviations of the kinematic, kinetic and temporal data from all subjects (N = 10).
Statistical analysis includes results of Bonferroni corrected post-hoc paired t-tests between natural and altered conditions. When repeated measures ANOVAs testing the main effect of condition were not significant (p>0.05), post-hoc tests were not conducted (NS). Bold text indicates significant differences (p<0.05).
| Condition | Statistical Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Decreased Pushoff | Natural Pushoff | Increased Pushoff | Natural vs. Decreased Pushoff | Natural vs. Increased Pushoff | |||
| KINEMATICS | t | p | t | p | |||
| Maximum Ankle DF (°) | −11.5 ±4.0 | −10.7 ±3.4 | −7.7 ±2.9 | −0.93 | 0.758 | 2.80 | 0.042 |
| Maximum Ankle PF (°) | 13.5 ±11.8 | 17.8 ±6.4 | 27.7 ±6.7 | −1.55 | 0.310 | 6.37 | <0.001 |
| Maximum Knee Extension (°) | 0 ±5.0 | 2.1 ±6.8 | 1.6 ±5.4 | 2.38 | 0.082 | 0.57 | 1.000 |
| Maximum Knee Flexion (°) | −65.4 ±6.5 | −68.6 ±7.4 | −64.6 ±6.6 | −3.26 | 0.020 | −3.66 | 0.010 |
| Maximum Hip Flexion (°) | −27.2 ±4.7 | −26.4 ±4.0 | −26.5 ±3.1 | NS | |||
| Maximum Hip Extension (°) | 12.9 ±3.2 | 13.5 ±3.6 | 14 ±5.3 | NS | |||
| KINETICS | |||||||
| Peak Ankle DF Moment (Nm) | −15.5 ±3.5 | −13.7 ±3.4 | −16.6 ±3.3 | −3.36 | 0.016 | −4.03 | 0.006 |
| Peak Ankle PF Moment (Nm) | 92.8 ±22.8 | 97.2 ±21.3 | 93.6 ±26.5 | −2.08 | 0.136 | −1.67 | 0.258 |
| Ankle DF Angular Impulse (Nms) | −1.6 ±0.7 | −1.3 ±0.6 | −1.4 ±0.4 | NS | |||
| Ankle PF Angular Impulse (Nms) | 23.6 ±9.5 | 24.3 ±8.3 | 30.9 ±9.9 | −0.71 | 0.992 | 7.52 | 0.001 |
| Peak Hip Flexion Moment (Nm) | −41.2 ±11.4 | −43.9 ±14.4 | −33.7 ±11.8 | 1.61 | 0.284 | 6.82 | <0.001 |
| Peak Hip Extension Moment (Nm) | 49.4 ±9.9 | 45 ±7.2 | 38.2 ±5.4 | 1.50 | 0.338 | −2.89 | 0.036 |
| Hip Flexion Angular Impulse (Nms) | −8.2 ±2.8 | −8.3 ±3.2 | −5.9 ±2.5 | 0.39 | 1.000 | 5.19 | 0.002 |
| Hip Extension Angular Impulse (Nms) | 8.1 ±3.6 | 7.1 ±2.7 | 6.1 ±2.9 | 1.87 | 0.190 | −2.88 | 0.036 |
| POWER | |||||||
| Ankle Plantar Flexion A2 (W) | 175.7 ±59.5 | 204 ±52.6 | 208.5 ±92.1 | NS | |||
| Knee Extension K1 (W) | −40.5 ±25.7 | −34.2 ±27.9 | −61.5 ±37.2 | 0.93 | 0.752 | 6.45 | |
| Knee Extension K2 (W) | 37.9 ±18.0 | 43.7 ±21.7 | 46.4 ±28.7 | NS | |||
| Knee Extension K3 (W) | −68.3 ±25.7 | −64.9 ±24.5 | −42.6 ±21.8 | 0.85 | 0.834 | −5.03 | 0.002 |
| Hip Extension H1 (W) | 46.9 ±26.4 | 37.3 ±19.6 | 33.8 ±18.3 | −1.65 | 0.266 | 1.74 | 0.234 |
| Hip Flexion H2 (W) | −30.1 ±13.2 | −27.9 ±13.1 | −22.4 ±12.8 | 1.72 | 0.238 | −3.40 | 0.016 |
| Hip Flexion H3 (W) | 72.2 ±20.8 | 70.1 ±16.4 | 45.1 ±12.2 | −0.55 | 1.000 | 7.39 | <0.001 |
| TEMPORAL | |||||||
| Step length (m) | 0.65 ±0.05 | 0.66 ±0.05 | 0.7 ±0.07 | 1.16 | 0.276 | −3.14 | 0.024 |
| Speed (m/s) | 1.25 ±0.00 | 1.25 ±0.00 | 1.25 ±0.00 | NS | |||
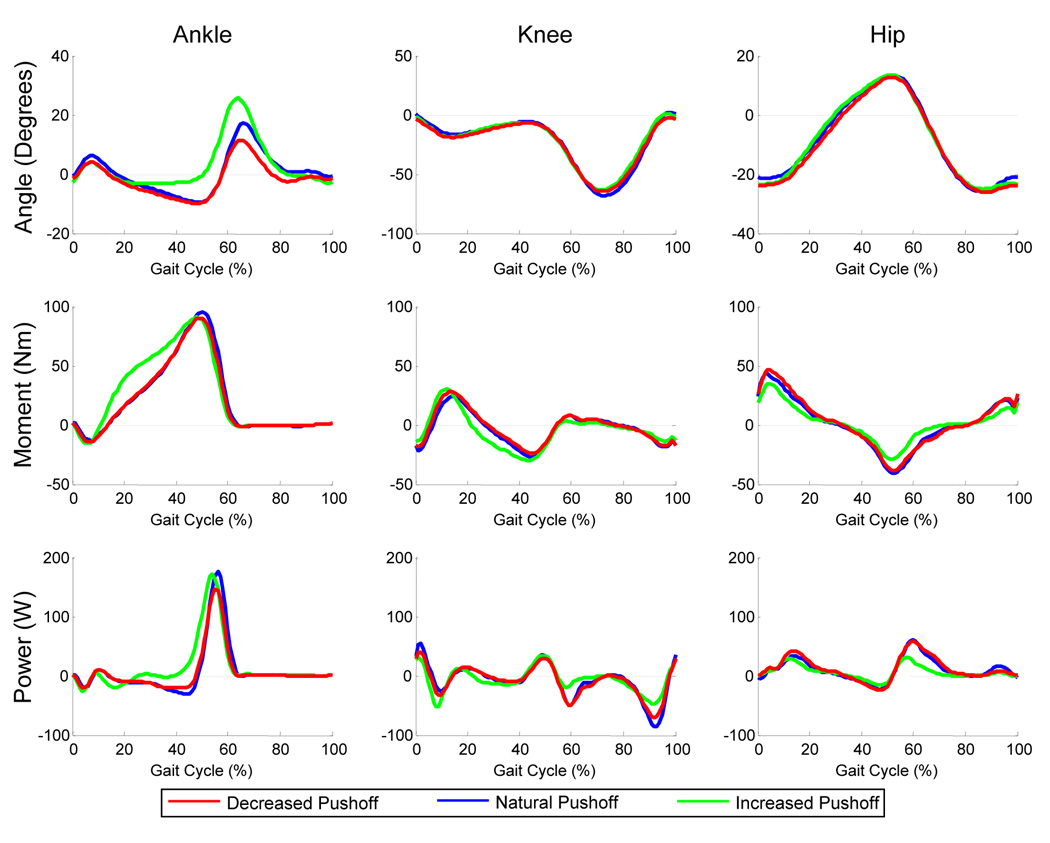
For the Increased Pushoff condition, subjects amplified ankle plantar flexion angular impulse by 27% (p<0.05) compared to the Natural Pushoff condition (FIGURE 3; TABLE 1). The greater plantar flexion angular impulse was due to a larger plantar flexion moment throughout midstance despite there being no change in the peak plantar flexion moment or power (FIGURE 3). Along with the change in ankle plantar flexion angular impulse, there were concurrent decreases in the hip flexion peak moment (23%), peak powers (20% H2, 36% H3), and angular impulse (29%) compared to natural walking (p<0.05). Hip extension peak moment and angular impulse were also reduced by 15% and 13% respectively (p<0.05). There were no significant changes in peak hip flexion or extension angles but step length was 4 cm longer in the Increased Pushoff condition when compared to the Natural Pushoff Condition (p<0.05).
FIGURE 3. Ankle, knee and hip joint kinematic and kinetic data.
Data represents the mean for all subjects (N=10) walking in each of the three conditions. Data are normalized from left heel strike to left heel strike. Ankle plantar flexion, knee extension and hip extension are positive. Ankle plantar flexion angular impulse was higher in the Increased Pushoff condition compared to the Natural Pushoff condition. There were concurrent decreases in hip flexion peak moment, peak powers, and angular impulse, and hip extension peak moment and angular impulse compared to the Natural Pushoff condition (p<0.05).
When instructed to walk with decreased pushoff, there were few differences compared to the Natural Pushoff condition. Plantar flexion angular impulse, peak power, and peak moment were not significantly different between the decreased ankle pushoff and natural conditions (p>0.07). The only kinetic difference was an increase in the dorsiflexion peak moment (p<0.05). It was 13% higher in the Decreased Pushoff condition than in the Natural Pushoff condition.
4. Discussion
The results of this study support the hypothesis that changes in ankle pushoff are inversely related to changes in the internal net hip muscle moments. When the ankle plantar flexion angular impulse was higher in the Increased Pushoff condition, both hip flexion and hip extension muscle moments and angular impulses were lower. In addition, hip flexion peak powers were also significantly lower when the plantar flexion angular impulse was increased. These findings agree with the prediction from the simple walking model that there is a tradeoff between the ankle and the hip during walking.
Our subjects also walked with a longer step length in the Increased Pushoff condition than in the Natural Pushoff condition. This observation is consistent with the literature comparing young adult and elderly gait. Young adult subjects walk with more pushoff and longer step lengths than elderly subjects (Kerrigan et al., 1998; DeVita and Hortobagyi, 2000; Judge et al., 1996). This finding may be interpreted to indicate that pushoff contributes to leg swing and the increased leg swing produces a longer step length (Judge et al., 1996). It may also be a potential limitation of this study. As we only controlled walking speed, and not cadence, we were unable to determine if the kinematic and kinetic differences we noted were solely due to the altered pushoff or also due to the change in step length. We chose to control walking speed instead of step length as joint moments are known to be affected by walking speed (Kirtley et al., 1985).
Previous studies comparing the gait of young adult subjects and elderly subjects have demonstrated that hip muscles may compensate for reductions in ankle plantar flexion moment or power. DeVita and Hortobagyi (DeVita and Hortobagyi, 2000) found that elderly subjects concurrently have lower ankle plantar flexion and higher hip extension angular impulses than young adult subjects when walking at the same speed. Kerrigan et al. (Kerrigan et al., 1998) also showed that when walking at a comfortable speed, elderly subjects walk with less pre-swing ankle power generation and a lower peak internal hip extension moment compared to young subjects. However, when asked to walk at a faster speed, elderly subjects increased the pre-swing ankle power generation but not to the level of the young subjects. Instead, the elderly subjects increased the internal hip extension moment to a level similar to the young subjects. Thus at the faster speed, the elderly subjects were gaining more of the walking energy from the hip than from the ankle when compared to young adult subjects. This illustrates the interaction between the hip extensor muscles and the ankle plantar flexor muscles during human gait.
A similar interaction between hip flexor muscles and ankle plantar flexor muscles has been noted in elderly subjects and subjects with diabetes mellitus. In a study focusing on the role of ankle plantar flexor muscles in step length, elderly subjects generated less ankle plantar flexion power and more hip flexion power during late stance than young adult subjects (Judge et al., 1996). Mueller et al. also presented data that subjects with diabetes mellitus walk with a smaller ankle plantar flexion moment and larger hip flexion moment compared to age-matched controls (Mueller et al., 1994a); statistical comparisons were not reported.
Although decreased ankle pushoff as evidenced by lower plantar flexion peak moment, power, or angular impulse have been observed naturally in elderly subjects and subjects with diabetes mellitus, we observed only minimal changes when our subjects were asked to “push less with your foot when you walk”. Healthy subjects may have difficulty reducing pushoff in the absence of ankle plantar flexor muscle weakness whereas this change may be a natural adaptation in the elderly (Kerrigan et al., 1998) and in people with diabetes mellitus (Mueller et al., 1994a). It may be that healthy subjects indeed can learn to walk with decreased ankle pushoff when given more feedback or time to train, but extensive training was not the focus of this study. Our purpose was to determine if there was an inverse relationship between hip and ankle kinetics demonstrable with simple instructions and gait modifications.
Mueller and colleagues propose that people with diabetes mellitus and peripheral neuropathy should be instructed to walk with a hip strategy (Mueller et al., 1994b). They found that walking with this strategy reduces the forefoot peak plantar pressures which are thought to lead to neuropathic foot ulcers (Mueller et al., 1994b). When walking with an exaggerated hip strategy, subjects in their study also decreased step length and hip extension range of motion, and increased dorsiflexion and hip flexion range of motion. We, however, did not see these changes in hip kinematics or step length in our Decrease Pushoff condition. This difference between the studies could be attributed to differences in subject instructions. In our study, subjects were simply instructed to push less with their foot. In addition, Mueller and colleagues told their subjects to “pull their leg forward from the hip”. As we were interested in the natural interplay between the ankle and hip, we did not want to artificially impose a change at the hip. The simple cue to decrease pushoff may have been insufficient or subjects may have required more training time to walk at the same speed with less pushoff.
In contrast to the studies illustrating decreased ankle pushoff, our study demonstrates the effects of increasing pushoff. The primary finding is that walking with an exaggerated ankle strategy results in decreases in both the hip extension and hip flexion peak moments and angular impulse. Using the simple walking model as a guide for understanding human gait, ankle pushoff would propel the center of mass forward and upward. This pushing of the center of mass would decrease the need for a hip extension moment acting on the stance leg to pull the center of mass forward. Unlike the hip extensor strategy described by McGibbon (McGibbon, 2003), the hip extension moment would act more on the center of mass through the stance leg than on the contralateral swing leg. The decreases in the hip flexion moment and power with increased pushoff supports the idea that pushoff contributes to leg swing thus decreasing the need for ispilateral hip flexion. As our subjects were instructed to alter the pushoff of both feet, it is difficult to determine if the changes in the hip extension and flexion moments occur ipsilateral or contralateral to the pushoff foot. Further study is required to parse out this difference.
The results of this study demonstrate that cues to increase ankle pushoff decrease the force requirements of the hip musculature. Walking with an exaggerated ankle strategy may have therapeutic applications. For people with anterior hip pain or an acetabular labral tear, walking with increased ankle pushoff has been recommended (Lewis and Sahrmann, 2006) and may be beneficial. Lewis and colleagues (Lewis et al., 2007) using a musculoskeletal model demonstrated that contraction of the hip flexor muscles while in a position of hip extension, as occurs at the end of stance phase, produces an anteriorly directed force from the femoral head onto the acetabulum. This anterior force, over time, may result in an acetabular labral tear (McCarthy et al., 2001; Mason, 2001), the majority of which are in the anterior region of the hip (McCarthy et al., 2001; Farjo et al., 1999; O'Leary et al., 2001; Santori and Villar, 2000; Klaue et al., 1991).
Walking with an exaggerated ankle strategy may also be beneficial for patients with hip osteoarthritis. Higher peak contact hip stress is associated with faster development and progression of idiopathic osteoarthritis (Recnik et al., 2007; Mavcic et al., 2004). Hip joint contact forces can be reduced by reducing the muscle forces at the hip (Seireg and Arvikar, 1975). Walking with increased ankle pushoff, which decreases the hip muscle force requirements, therefore may slow the progression of hip disease. The ability of subjects to consistently walk with an exaggerated ankle strategy and the therapeutic effect of this gait have yet to be tested.
Our main conclusion was that simple instructions to increase ankle pushoff can substantially decrease hip muscle moments and powers during human walking. The effect was large (~13-36%) given the amount of time the subject practiced (~1 minute) and suggests there may be clinical benefit from this type of intervention. Based on these results, a logical next step would be to determine if long-term gait modifications of increased pushoff can be maintained in clinical populations at risk of hip pain and/or injury.
Acknowledgements
The authors would like to thank the members of the Human Neuromechanics Laboratory for their assistance. This work was supported by the Medical Rehabilitation Research Training Program at University of Michigan which was funded by the National Institutes of Health (NIH), the National Institute of Child Health and Human Development (NICHD), the National Center for Medical Rehabilitation Research (NCMRR) Grants 5-T32-HD007422-17, and by F32-HD055010 and R01-NS45486.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors affirm that they have no financial affiliation or involvement with any commercial organization that has direct financial interest in any matter included in this manuscript.
Reference List
- Collins SH, Adamczyk PG, Ferris DP, Kuo AD. A simple method for calibrating force plates and force treadmills using an instrumented pole. Gait.Posture. 2008 doi: 10.1016/j.gaitpost.2008.06.010. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J.Appl.Physiol. 2000;88:1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Doke J, Donelan JM, Kuo AD. Mechanics and energetics of swinging the human leg. J.Exp.Biol. 2005;208:439–445. doi: 10.1242/jeb.01408. [DOI] [PubMed] [Google Scholar]
- Farjo LA, Glick JM, Sampson TG. Hip arthroscopy for acetabular labral tears. Arthroscopy. 1999;15:132–137. doi: 10.1053/ar.1999.v15.015013. [DOI] [PubMed] [Google Scholar]
- Garcia M, Chatterjee A, Ruina A, Coleman M. The simplest walking model: stability, complexity, and scaling. J.Biomech.Eng. 1998;120:281–288. doi: 10.1115/1.2798313. [DOI] [PubMed] [Google Scholar]
- Gottschall JS, Kram R. Energy cost and muscular activity required for leg swing during walking. J.Appl.Physiol. 2005;99:23–30. doi: 10.1152/japplphysiol.01190.2004. [DOI] [PubMed] [Google Scholar]
- Judge JO, Davis RB, III, Ounpuu S. Step length reductions in advanced age: the role of ankle and hip kinetics. J.Gerontol.A Biol.Sci.Med.Sci. 1996;51:M303–M312. doi: 10.1093/gerona/51a.6.m303. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Todd MK, Della CU, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch.Phys.Med.Rehabil. 1998;79:317–322. doi: 10.1016/s0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirtley C, Whittle MW, Jefferson RJ. Influence of walking speed on gait parameters. J.Biomed.Eng. 1985;7:282–288. doi: 10.1016/0141-5425(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J.Bone Joint Surg.Br. 1991;73:423–429. doi: 10.1302/0301-620X.73B3.1670443. [DOI] [PubMed] [Google Scholar]
- Kuo AD. Energetics of actively powered locomotion using the simplest walking model. J.Biomech.Eng. 2002;124:113–120. doi: 10.1115/1.1427703. [DOI] [PubMed] [Google Scholar]
- Lewis CL, Sahrmann SA. Acetabular labral tears. Phys.Ther. 2006;86:110–121. doi: 10.1093/ptj/86.1.110. [DOI] [PubMed] [Google Scholar]
- Lewis CL, Sahrmann SA, Moran DW. Anterior hip joint force increases with hip extension, decreased gluteal force, or decreased iliopsoas force. J.Biomech. 2007 doi: 10.1016/j.jbiomech.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB. Acetabular labral tears in the athlete. Clin.Sports Med. 2001;20:779–790. doi: 10.1016/s0278-5919(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Mavcic B, Slivnik T, Antolic V, Iglic A, Kralj-Iglic V. High contact hip stress is related to the development of hip pathology with increasing age. Clin.Biomech.(Bristol., Avon.) 2004;19:939–943. doi: 10.1016/j.clinbiomech.2004.06.003. [DOI] [PubMed] [Google Scholar]
- McCarthy JC, Noble PC, Schuck MR, Wright J, Lee J. The Otto E. Aufranc Award: The role of labral lesions to development of early degenerative hip disease. Clin.Orthop. 2001:25–37. doi: 10.1097/00003086-200112000-00004. [DOI] [PubMed] [Google Scholar]
- McGeer T. Passive Dynamic Walking. The International Journal of Robotics Research. 1990;9:62–82. [Google Scholar]
- McGibbon CA. Toward a better understanding of gait changes with age and disablement: neuromuscular adaptation. Exerc.Sport Sci.Rev. 2003;31:102–108. doi: 10.1097/00003677-200304000-00009. [DOI] [PubMed] [Google Scholar]
- Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys.Ther. 1994a;74:299–308. doi: 10.1093/ptj/74.4.299. [DOI] [PubMed] [Google Scholar]
- Mueller MJ, Sinacore DR, Hoogstrate S, Daly L. Hip and ankle walking strategies: effect on peak plantar pressures and implications for neuropathic ulceration. Arch.Phys.Med.Rehabil. 1994b;75:1196–1200. doi: 10.1016/0003-9993(94)90004-3. [DOI] [PubMed] [Google Scholar]
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181–188. doi: 10.1053/jars.2001.21481. [DOI] [PubMed] [Google Scholar]
- Recnik G, Kralj-Iglic V, Iglic A, Antolic V, Kramberger S, Vengust R. Higher peak contact hip stress predetermines the side of hip involved in idiopathic osteoarthritis. Clin.Biomech.(Bristol., Avon.) 2007;22:1119–1124. doi: 10.1016/j.clinbiomech.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Santori N, Villar RN. Acetabular labral tears: result of arthroscopic partial limbectomy. Arthroscopy. 2000;16:11–15. doi: 10.1016/s0749-8063(00)90121-x. [DOI] [PubMed] [Google Scholar]
- Seireg A, Arvikar The prediction of muscular load sharing and joint forces in the lower extremities during walking. J.Biomech. 1975;8:89–102. doi: 10.1016/0021-9290(75)90089-5. [DOI] [PubMed] [Google Scholar]
- Winter DA. Energy generation and absorption at the ankle and knee during fast, natural, and slow cadences. Clin.Orthop.Relat Res. 1983:147–154. [PubMed] [Google Scholar]
- Winter DA, Eng JJ, Ishac MG. A review of kinematic parameters in human walking. In: Craik RLB, Otais CA, editors. Gait analysis: theory and application. Mosby Pusblisher; 1995. pp. 252–270. [Google Scholar]
- Zatsiorsky VM. Kinetics of human motion. Champaign, IL: Human Kinetics; 2002. [Google Scholar]