Abstract
In this study, we have used the double-step paradigm to test saccadic gain adaptation during monocular viewing in one normal monkey, two monkeys with exotropia, and one monkey with esotropia. In this paradigm, the target for the saccade is displaced during the saccade, resulting in a consistent visual error. Studies in normal humans and monkeys have shown that the brain responds to this consistent visual error by gradually changing saccade gain. Using this technique, we were able to elicit adaptation in both the viewing eye and the nonviewing eye in the normal monkey and in monkeys with strabismus. The rate of adaptation was not significantly different in the viewing and nonviewing eyes in the normal and strabismic monkeys. The magnitude of adaptation as calculated by a percentage change in gain was also not significantly different in the viewing and the nonviewing eyes in the normal and strabismic monkeys. Our data show that animals with strabismus retain the ability to elicit a conjugate adaptation of saccades using this mechanism. We also suggest that the double-step paradigm elicits a conjugate adaptation of saccades whether the animal is viewing monocularly (our studies) or binocularly (data published in literature).
INTRODUCTION
Saccades are rapid eye movements that serve to redirect gaze or line of sight onto objects of interest. The rapidity of saccadic eye movements precludes any on-line correction of saccade amplitude in the face of an unexpected positional error. However, saccade gain in normal subjects is remarkably consistent, suggesting that calibration mechanisms exist to correct for consistent errors that may occur due to muscle or tissue changes or central problems. For example, in patients with abducens nerve palsies, monocular patching of the normal eye for a few days resulted in accurate saccades made with the paretic eye but larger than normal saccades made with the normal eye (Kommerell et al. 1976). This suggested that the saccadic system had adapted to increase the gain to both eyes such that the paretic (viewing) eye accurately acquired the target. This result has been replicated in monkeys where an eye was first made paretic by weakening an extraocular muscle (Optican and Robinson 1980; Viirre et al. 1988).
Laboratory evaluation of saccade adaptation mechanisms has used a second approach in normal humans and monkeys. In the double-step paradigm pioneered by Mclaughlin (1967), a consistent positional error induced by changing the position of the target during a saccadic eye movement resulted in a gradual change of saccadic gain in both humans and monkeys (Albano and King 1989; Deubel et al. 1986; Mclaughlin 1967; Straube et al. 1997). The stimulus for adaptation is the retinal error at the end of the initial saccade and not the motor error due to corrective saccades (Noto and Robinson 2001; Wallman and Fuchs 1998). Adaptation is direction- and amplitude-specific (Albano 1996; Frens and van Opstal 1994; Watanabe et al. 2000), i.e., adaptation to one direction and amplitude does not readily transfer to other directions or amplitudes. Adaptation can be tied to contextual cues such as eye position or head tilt (Shelhamer and Clendaniel 2002). Scudder et al. (1998) compared rates of adaptation following muscle paresis, and using the double-step paradigm, concluded that these two types of adaptation are similar when examined under controlled conditions. In perhaps the only adaptation study that measured movements of both eyes in normal human subjects, it has been shown that the double-step paradigm results in conjugate adaptation (Albano and Marrero 1995).
In our work with animals reared with sensory forms of strabismus, we have described horizontal saccade disconjugacy in monkeys with large horizontal misalignment (Fu et al. 2001; Tusa et al. 2002). The goals of this study were to determine whether the double-step paradigm could induce adaptation in animals with large horizontal misalignment and to compare the adaptation induced in the two eyes. Some of the results have been published in preliminary form (Das et al. 2002, 2003).
METHODS
Behavioral data were collected from three strabismic (S1, S2, S3) and one normal juvenile rhesus monkey (N1; Macaca mulatta) weighing 3-7 kg. Monkeys with strabismus were reared at the Yerkes National Primate Research Center using visual sensory deprivation methods for the first 4-6 mo of life, which were designed to induce ocular misalignment but not affect visual acuity (Tusa et al. 2002). For this study, we chose animals with large angles of exotropia or esotropia, no measurable latent nystagmus, and no amblyopia. They also had no noticeable eye preference and no fine stereo function (Fu et al. 2001; Tusa et al. 2002). S1 and S3 were exotropic (strabismus angle of 29° and 16° when measured during binocular viewing at primary gaze) and S2 was esotropic (strabismus angle of 21° when measured during binocular viewing at primary gaze).
Sterile surgical procedures carried out under aseptic conditions using isoflurane anesthesia (1.25-2.5%) were used to stereotaxically implant a head stabilization post and binocular scleral search coils when the animals were about 2 yr old (other details, including details of equipment and experimental setup, are provided in Das et al. 2001). All procedures were performed in strict compliance with National Institutes of Health guidelines and the protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Emory University.
The saccade adaptation testing began after the animal was trained to be able to make a large number of saccades (∼500-1,000; monocular or binocular viewing), in one experimental session. The animal was rewarded with a drop of juice for fixating the target with the viewing eye during monocular viewing or either eye during binocular viewing. The goal of the backward adaptation paradigm was to adaptively reduce saccade gain. In this paradigm, a trial began with the animal monocularly fixating a stationary target (within a ±3° window for a duration of 1.5 s) at primary position (i.e., straight ahead). The target then randomly jumped to a location either 10° or 15° to the right of primary gaze. A saccade to this new target location was detected by the computer (based on an ∼50°/s eye velocity criterion), and this triggered a backward jump equal to 30% of initial target movement (see Fig. 1 for example). Sometimes the second target jump occurred just after saccade offset (always within 30 ms), which is equally effective in driving adaptation (Shafer et al. 2000; also see Fig. 2). The trial ended when the monkey re-fixated the final target location for ∼1.5 s. The target was reset and a new trial was begun if the animal did not make a saccade to the target within 1.5 s. Trials were presented repeatedly until it appeared that the animal was consistently making saccades to the final target location rather than the initial target location. The four animals were each tested once with either the left or right eye viewing (separate days), resulting in a total of eight adaptation sessions.
FIG. 1.
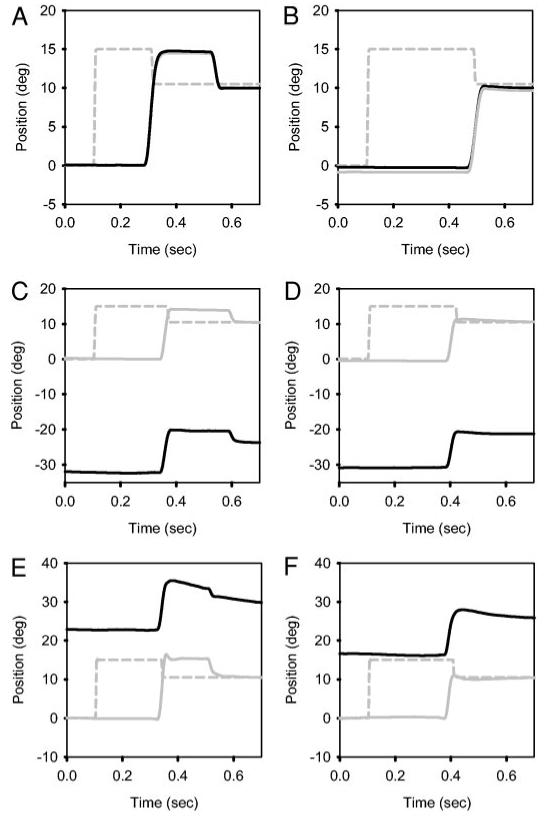
Single saccade trials showing saccade adaptation in both eyes during monocular viewing in a normal monkey (A and B), in monkey S3 with exotropia (C and D), and monkey S2 with esotropia (E and F). During initial trials (A, C, and E), the animals make a saccade to the initial target location and then make a corrective backward saccade. After repeated trials (B, D, and F), the animals make a saccade directly to the final target location. Gray solid line, right eye; black solid line, left eye; gray dashed line, target. Gain adaptation in the strabismic monkeys (C-F) appears qualitatively similar to adaptation in the normal monkey (A and B).
FIG. 2.
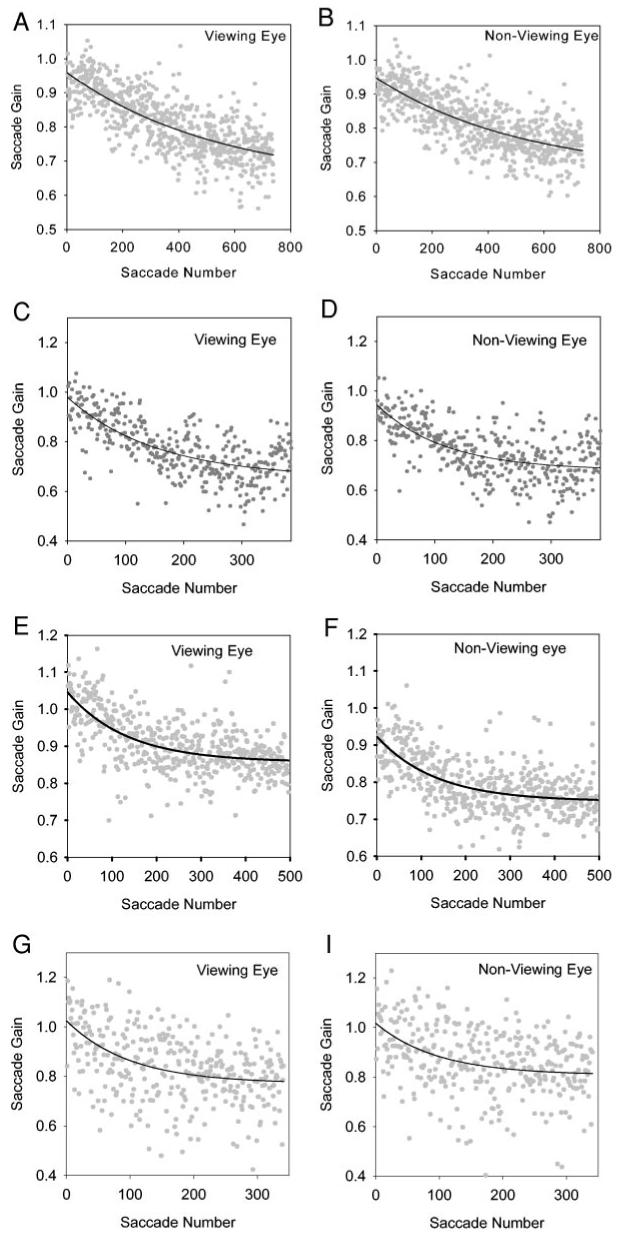
Gradual decrease of saccade gain elicited by the backward gain adaptation paradigm in normal monkey N1 (right eye viewing; A and B), exotropic monkey S1 (right eye viewing; C and D), exotropic monkey S3 (right eye viewing; E and F), and esotropic monkey S2 (right eye viewing; G and H). Each data point represents the gain of a single saccade plotted against the trial number. Panels show that saccade gain reduces exponentially and reaches asymptotic values. Adaptation occurs in both the viewing (A, C, E, and G) and nonviewing eye (B, D, F, and H), and the time constant of adaptation is similar in the 2 eyes. Figure also illustrates that saccade adaptation is similar in the normal monkey and monkeys with strabismus.
Data analysis of adaptation trials was partially automated using custom software built in MATLAB (Mathworks, Natick, MA). The computer displayed target position, eye position, eye velocity, and eye acceleration traces of a single trial on the screen. Velocity and acceleration signals were generated by digital differentiation of the position signal using a central difference algorithm. Position, velocity, and acceleration signals were filtered (“filtfilt” function in MATLAB signal processing toolbox) using software FIR filters (80 points; 0- to 80-Hz passband) also designed in MATLAB. The investigator viewed the traces and decided whether the trial was to be accepted or rejected. Trials that were rejected were usually those in which the initial saccade in the viewing eye did not appear to be directed toward the target. Also, if the initial saccade did not occur within 600 ms of the target step, the trial was discarded. Once a decision to accept the trial was made, mean and SD of control eye acceleration prior to the saccade were calculated over a 100-ms fixation period selected by the user. Saccade onset was automatically determined by the software as the first time point at which eye acceleration was >3 SD away from the control eye acceleration, and saccade offset was determined as the last time point at which eye deceleration was <3 SD away from the same mean eye acceleration. Although detection of saccade onset and offset was automated, the investigator visually examined the acceleration traces of every saccade and had the option of either accepting or changing the computer selection. Typically <10% of the computer’s marks were changed by the investigator. Saccade gain was calculated as the ratio of eye amplitude (difference between eye position at saccade offset and eye position at saccade onset) to the initial target amplitude. Throughout our analysis, we computed saccadic gain with respect to the first saccade and the initial target step.
We characterized saccade adaptation by fitting an exponential function to the saccadic gain and order number of the saccades (Straube et al. 1997). The equation of the fit used for backward adaptation was
| (1) |
In the equation above, λ is the time constant of decay, (G0 + A) is the initial gain, G0 is the asymptotic gain (i.e., saccadic gain if the experiment were continued until adaptation was complete), and n is the saccade order number. Therefore the change in gain due to adaptation is given by the parameter A. For each adaptation session, we compared the time constant (λ) of the exponential fit functions in the viewing and nonviewing eyes (t-test). We calculated a percentage change in gain in the viewing and nonviewing eyes from the initial and final gains estimated via the fit function and also from the actual data. To estimate the initial and final gain from the data, we took the means of the first and last 25 saccades in each adaptation session. Statistical comparison of the percentage change in gain in the viewing and nonviewing eyes were performed using a paired t-test (significance value of 0.05) on the entire group (8 viewing conditions as shown in the Tables) after verifying that the data were normally distributed. In addition to analysis of saccade gain, we also analyzed saccade kinematics by comparing saccade duration, latency, and peak velocity in the viewing and nonviewing eyes.
RESULTS
Figure 1, A and B, shows typical adaptation trials collected during monocular viewing in the normal monkey. Figure 1A shows that, during initial trials, the animal makes a saccade to the initial target location and then makes a corrective saccade to the final target location. Following adaptation, the animal makes a saccade directly to the final target location (Fig. 1B). Both the viewing and nonviewing eyes move conjugately and are adapted. Monkeys with strabismus show similar behaviorto normals. Figure 1, C and D, shows data from a monkey with exotropia during monocular viewing (S3). The viewing eye (right eye) is on target, while the nonviewing eye (left eye) is exotropic, with a strabismus angle of approximately 30°. Similar to normals, in initial trials, this strabismic monkey makes a saccade to the initial target location and then makes a corrective saccade. After adaptation, the animal makes a saccade directly to the final target location, and no corrective saccade is initiated. Both the viewing and nonviewing eyes follow the same pattern. Note, however, that the amplitude of the saccade in the viewing and the nonviewing eye is different. The nonviewing eye generally makes a smaller saccade both before and after adaptation. For the saccades illustrated, the gain of the viewing eye before adaptation is 0.95, and the gain of the viewing eye after adaptation is 0.80; the gain of the nonviewing eye before adaptation is 0.81, and the gain of the nonviewing after adaptation is 0.67. However, the ratio of gains in the viewing and nonviewing eyes before adaptation was 1.17, and ratio of gains after adaptation was 1.19. The percentage change in gain in the viewing eye was 15.8%, and the percentage change in gain in the nonviewing eye was 17.3%, suggesting that the effect of saccade adaptation in this paradigm is the same in the two eyes. Data from the esotropic monkey S2 are shown in Fig. 1, E (prior to adaptation) and F (after adaptation), and show that the esotropic animal also adapts saccadic gain in the double-step paradigm.
Figure 2 shows summary data collected during an adaptation session in N1 (normal monkey; Fig. 2, A and B), S1 (monkey with exotropia; Fig. 2, C and D), S3 (monkey with exotropia; Fig. 2, E and F), and S2 (monkey with esotropia; Fig. 2, G and H). Data from both the viewing and the nonviewing eyes are illustrated. Panels in Fig. 2 show that both the normal and strabismic animals adapt their saccade gain during monocular viewing. Furthermore, animals with strabismus adapt saccadic gain in both their viewing and their nonviewing eyes.
Comparison of adaptation in viewing and nonviewing eyes
Figure 2 also shows examples of the exponential fits to the viewing and nonviewing eye in N1, S1, S3, and S2. The parameters of the fit and the goodness of fit (estimated by the r2 value) are shown in Table 1 for all the conditions tested. The goodness of fit is variable with monkey S2 (Fig. 2, G and H), showing the greatest scatter of data. However, excluding this animal, the goodness of fit that we obtained (average r2 for viewing eye = 0.39 ± 0.15; average r2 for nonviewing eye = 0.37 ± 0.15) is comparable to those reported by Straube et al. (1997), who examined a number of normal animals over multiple sessions. Statistical analysis showed no significant differences between the time constant of adaptation in the viewing and the nonviewing eye (in 8/8 conditions in the strabismic and normal animals shown in Table 1; P > 0.05), suggesting that progression of saccade adaptation was similar in the two eyes.
TABLE 1. Exponential fit parameters.
| Subject_Viewing Condition | No. of Saccades Analyzed |
G0 |
A |
λ |
Goodness of Fit (r2) |
||||
|---|---|---|---|---|---|---|---|---|---|
| VE | NVE | VE | NVE | VE | NVE | VE | NVE | ||
| S1_LEV | 473 | 0.64 ± 0.06 | 0.67 ± 0.09 | 0.23 ± 0.06 | 0.23 ± 0.09 | 425 ± 184 | 481 ± 280 | 0.40 | 0.28 |
| S1_REV | 379 | 0.66 ± 0.02 | 0.68 ± 0.01 | 0.32 ± 0.02 | 0.26 ± 0.02 | 153 ± 21 | 116 ± 19 | 0.50 | 0.40 |
| S2_LEV | 335 | 0.83 ± 0.02 | 0.70 ± 0.02 | 0.10 ± 0.02 | 0.17 ± 0.03 | 56 ± 54 | 60 ± 32 | 0.06 | 0.10 |
| S2_REV | 340 | 0.77 ± 0.03 | 0.81 ± 0.03 | 0.25 ± 0.03 | 0.21 ± 0.03 | 99 ± 35 | 97 ± 45 | 0.18 | 0.12 |
| S3_LEV | 315 | 0.60 ± 0.01 | 0.56 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 137 ± 39 | 121 ± 33 | 0.13 | 0.14 |
| S3_REV | 492 | 0.86 ± 0.01 | 0.75 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 | 134 ± 17 | 133 ± 17 | 0.40 | 0.36 |
| N1_LEV | 269 | 0.65 ± 0.15 | 0.67 ± 0.08 | 0.31 ± 0.14 | 0.36 ± 0.07 | 272 ± 185 | 217 ± 90 | 0.34 | 0.48 |
| N1_REV | 737 | 0.63 ± 0.03 | 0.67 ± 0.03 | 0.33 ± 0.02 | 0.28 ± 0.02 | 543 ± 80 | 524 ± 96 | 0.58 | 0.56 |
Values in the table indicate the parameter estimate ± SE of the estimate. VE, viewing eye; NVE, non-viewing eye; LEV, left eye viewing; REV, right eye viewing; S1, S3, animals with exotropia; S2, animal with esotropia; N1, normal animal; G0, asymptotic gain estimated byfit; A, change in gain estimated by fit; λ, time constant of adaptation.
We also calculated the percentage change in gain in the viewing and nonviewing eyes (simply comparing the change in gain parameter, A, between the viewing and the nonviewing eye is susceptible to the pre-existing saccade disconjugacy). These data are shown in Fig. 3. Figure 3A shows the percentage change in gain in the viewing and nonviewing eyes calculated from the exponential fits. The asymptotic percentage change in gain ranged from -11.12 to -33.92% (mean: -24.32 ± 8.35%) in the viewing eye and from -14.97 to -35.15% (mean: -24.12 ± 6.25%) in the nonviewing eye. Statistical analysis showed that percentage change of saccadic gain in the viewing and nonviewing eye was not significantly different (paired t-test for the whole group; P = 0.88). Figure 3B shows the percentage change in gain calculated from the actual data (see METHODS). Using this method, the percentage change in gain ranged from -6.08 to -21.24% (mean -16.65 ± 5.20%) in the viewing eye and from -12.07 to -18.52% (mean: -15.30 ± 2.23%) in the nonviewing eye. Again, statistical analysis showed that percentage change of saccadic gain in the viewing and nonviewing eye was not significantly different (paired t-test for the whole group; P = 0.39).
FIG.3.
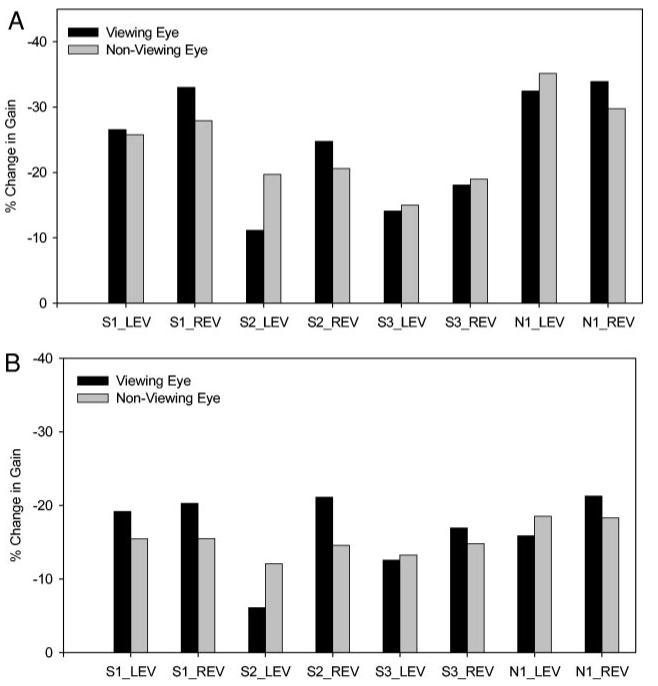
A: bar plot of percentage change in gain in viewing and nonviewing eyes estimated from the exponential fit functions (% change in gain = 100 × A/initial gain). Percentage change in gain in the viewing and nonviewing eyes is almost the same for all animals under all conditions tested. B: bar plot of percentage change in gain in viewing and nonviewing eyes estimated from actual data. Change in gain is calculated as the difference between the means of the last 25 saccades and 1st 25 saccades in each adaptation session. Again, percentage change in gain in viewing and nonviewing eyes are almost the same for all animals under all conditions tested. Percentage values in B are smaller than in A because experiments were not carried out until adaptation was complete. Abbreviations are as in Table 1. Note that percentage changes are negative values, indicating decrease in saccadic gain.
Saccade kinematics
We analyzed saccade kinematics by calculating peak velocity, latency, and duration of the initial saccade in the viewing and nonviewing eyes prior to (1st 25 saccades) and after (last 25 saccades) adaptation. The data are displayed in Table 2. The duration data show that there is very little difference in saccade durations in the viewing and the nonviewing eyes both before and after adaptation. Straube et al. (1997) have reported a postadaptation increase in latency in some but not all animals. We found a significant postadaptation increase in latency in the viewing eye in four of eight conditions tested (S1_LEV, S1_REV, S2_LEV, and N1_REV). In the other four conditions, latency changes in the viewing eye due to adaptation were not significant (P > 0.05). The same four testing conditions also yielded significant changes in postadaptation latency in the nonviewing eye. The peak velocity data showed considerable variability among the different animals and also between the viewing and nonviewing eyes (see Table 2). The peak velocity of postadaptation (last 25) saccades was consistently lower in both the viewing and the nonviewing eyes compared with preadaptation (1st 25) saccades. This might be a characteristic of the adaptation or could be partially due to fatigue (Straube et al. 1997). However, our analysis showed that postadaptation peak velocity reductions in the viewing and nonviewing eye were proportionate (<5% difference) in all but one condition tested (S2_LEV, % reduction of peak velocity in viewing eye = 6.75%; % reduction of peak velocity in nonviewing eye = 21.25%, resulting in a difference of 14.5%).
Table 2. Saccade kinematics.
| VE |
NVE |
|||
|---|---|---|---|---|
| Subject_Viewing Condition | First 25 Saccades | Last 25 Saccades | First 25 Saccades | Last 25 Saccades |
| S1_LEV | PV: 619 ± 81 | 397 ± 92 | 645 ± 86 | 408 ± 100 |
| Dur: 37 ± 4 | 51 ± 16 | 37 ± 4 | 53 ± 20 | |
| Lat: 167 ± 10 | 222 ± 48 | 167 ± 10 | 222 ± 45 | |
| S1_REV | 677 ± 121 | 535 ± 123 | 660 ± 86 | 542 ± 89 |
| 41 ± 6 | 38 ± 5 | 39 ± 6 | 38 ± 6 | |
| 180 ± 26 | 216 ± 70 | 180 ± 26 | 216 ± 70 | |
| S2_LEV | 683 ± 84 | 637 ± 130 | 857 ± 165 | 625 ± 189 |
| 34 ± 4 | 34 ± 6 | 28 ± 4 | 28 ± 6 | |
| 151 ± 12 | 178 ± 28 | 152 ± 12 | 178 ± 28 | |
| S2_REV | 716 ± 77 | 639 ± 169 | 577 ± 130 | 538 ± 113 |
| 38 ± 8 | 41 ± 6 | 43 ± 10 | 40 ± 7 | |
| 240 ± 108 | 191 ± 52 | 240 ± 108 | 193 ± 53 | |
| S3_LEV | 500 ± 62 | 357 ± 62 | 466 ± 72 | 313 ± 67 |
| 36 ± 5 | 42 ± 10 | 38 ± 5 | 43 ± 9 | |
| 315 ± 62 | 338 ± 66 | 315 ± 62 | 339 ± 67 | |
| S3_REV | 565 ± 121 | 502 ± 82 | 473 ± 88 | 425 ± 63 |
| 44 ± 5 | 44 ± 6 | 45 ± 5 | 46 ± 8 | |
| 243 ± 52 | 261 ± 35 | 243 ± 52 | 261 ± 35 | |
| N1_LEV | 505 ± 82 | 422 ± 76 | 561 ± 86 | 460 ± 82 |
| 43 ± 10 | 41 ± 10 | 43 ± 10 | 43 ± 9 | |
| 368 ± 111 | 363 ± 100 | 368 ± 111 | 363 ± 100 | |
| N1_REV | 523 ± 104 | 410 ± 73 | 508 ± 98 | 426 ± 65 |
| 42 ± 11 | 38 ± 6 | 40 ± 11 | 38 ± 6 | |
| 190 ± 28 | 254 ± 58 | 190 ± 28 | 253 ± 58 | |
Values in the table indicate mean ± SD. PV, peak velocity in deg/s; Dur, duration of saccade in ms; Lat, latency of saccade in ms. See Table 1 for other abbreviations.
In summary, our analysis of saccade kinematics (duration, latency, and peak velocity) showed that the effects of adaptation were very similar in both the viewing and the nonviewing eyes. Therefore the saccade kinematics data supported the saccade gain data in suggesting that the adaptation occurred in both the viewing and the nonviewing eyes.
DISCUSSION
Our goal was to examine the adaptive response to the double-step paradigm in a strabismic preparation during monocular viewing and determine whether animals with large strabismus and disconjugate saccades were able to adapt both eyes equally.
Our results show that, like normal monkeys, strabismic monkeys are able to adapt their saccade gain during the double-step paradigm. Bucci et al. (1997) suggested that loss of disconjugate adaptive control accompanied loss of binocular function in humans with large angles of strabismus. While we have not confirmed that the animals used in this study suffered from loss of disconjugate adaptive control, we know that these animals had impaired binocular function (Tusa et al. 2002). Our study indirectly suggests that a loss of disconjugate adaptive control may not generalize to other forms of adaptation.
We have also shown that adaptation occurs in both eyes during monocular viewing conditions in normal and strabismic monkeys. Our findings confirm the results of experiments conducted by Albano and Marrero (1995) in normal humans that showed transfer of adaptation occurs between the viewing and the nonviewing eye under monocular viewing conditions. Prior to conducting the experiments, one possible albeit extreme prediction would have been that, in animals with strabismus, adaptation would occur only in the viewing eye and that the nonviewing eye would remain unadapted. Such a result would have suggested that saccade adaptation functions via independent channels for each eye (similar to the idea proposed by Bucci et al. 1997 for disconjugate adaptation). However, our results suggest that even in an animal with a large misalignment, certain saccade adaptation mechanisms remain yoked.
Finally, we have shown that adaptation in the viewing and the nonviewing eye is equal (equal time constant and equal percentage change in gain) even in animals with large strabismus. While pre-existing unequal saccadic gains in the two eyes are likely to affect the magnitude of adaptation, it is unlikely to change the time course of adaptation in the two eyes as long as they are driven by the same adapting signal. Our results therefore suggest that a single central representation of positional retinal error (positional error in the viewing eye) drives adaptation for both eyes. The variability in saccade adaptation data, both ours (especially S2) and normal monkey data reported by Straube et al. 1997, makes it difficult to prove conclusively that the strategy used by strabismic animals to achieve adaptation is identical to that of normal monkeys. However, the sum total of all our analysis on viewing eye and nonviewing eye data (i.e., % change in gain, asymptotic % changes in gain, time constants, saccade latency effects, saccade velocity effects, and coupling of saccadic duration in viewing and nonviewing eyes) all seems to point to the fact that conjugate saccade adaptation mechanisms remain intact in animals with strabismus.
So what is the source of saccade disconjugacy in some animals and humans with large angles of strabismus? While our experiments cannot answer this question directly, they suggest that saccade disconjugacy is not due to a generalized failure of the adaptive process. The underlying cause of disconjugacy could be related to the specific inability to adapt disconjugately to asymmetries in the oculomotor plant (Bucci et al. 1997; Kapoula et al. 1997) or could be related to other motor aspects associated with strabismus (for example a miscalibrated neural integrator, aspects of torsional control, oculomotor muscle pulleys). It is believed that the rapid saccade adaptation elicited by the double step paradigm is due to cerebellar mechanisms possibly involving the vermis and the fastigial nucleus (Goldberg et al. 1993; Robinson et al. 2002; Scudder et al. 1998). Optican and Robinson (1980) showed that saccade adaptation induced after muscle paresis depends on the cerebellum. Some patients with cerebellar dysfunction also suffer from saccade disconjugacy, which could be interpreted as a lack of disconjugate adaptation (Versino et al. 1996). Possibly, the cerebellar flocculus/paraflocculus is important for this function. Taking these data into account, we suggest that the disconjugacy observed in animals and human with large angles of strabismus is probably due to the floccular complex in the cerebellum or motor mechanisms downstream from the cerebellum.
ACKNOWLEDGMENTS
We thank T. Brozyna for expert technical assistance.
GRANTS
This work was supported by National Institutes of Health Grants EY-06069, RR-00165, and NS-07480.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Albano JE. Adaptive changes in saccade amplitude: oculocentric or orbitocentric mapping? Vision Res. 1996;36:2087–2098. doi: 10.1016/0042-6989(96)89627-1. [DOI] [PubMed] [Google Scholar]
- Albano JE, King WM. Rapid adaptation of saccadic amplitude in humans and monkeys. Invest Ophthalmol Vis Sci. 1989;30:1883–1893. [PubMed] [Google Scholar]
- Albano JE, Marrero JA. Binocular interactions in rapid saccadic adaptation. Vision Res. 1995;35:3439–3450. doi: 10.1016/0042-6989(95)00079-t. [DOI] [PubMed] [Google Scholar]
- Bucci MP, Kapoula Z, Eggert T, Garraud L. Deficiency of adaptive control of the binocular coordination of saccades in strabismus. Vis Res. 1997;37:2767–2777. doi: 10.1016/s0042-6989(97)00093-x. [DOI] [PubMed] [Google Scholar]
- Das VE, Economides JR, Ono S, Mustari MJ. Information processing by parafoveal cells in the primate nucleus of the optic tract. Exp Brain Res. 2001;140:301–310. doi: 10.1007/s002210100816. [DOI] [PubMed] [Google Scholar]
- Das VE, Fu LN, Ono S, Tusa RJ, Mustari MJ. Saccade disconjugacy and adaptation in strabismic monkeys. Ann NY Acad Sci. doi: 10.1167/iovs.06-0955. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das VE, Ono S, Tusa RJ, Mustari MJ. Saccade gain adaptation in monkeys with strabismus. ARVO Abstr. 2002:2653. [Google Scholar]
- Deubel H, Wolf W, Hauske G. Adaptive gain control of saccadic eye movements. Human Neurobiol. 1986;5:245–253. [PubMed] [Google Scholar]
- Frens MA, Van Opstal AJ. Transfer of short-term adaptation in human saccadic eye movements. Exp Brain Res. 1994;100:293–306. doi: 10.1007/BF00227199. [DOI] [PubMed] [Google Scholar]
- Fu LN, Das VE, Mustari MJ, Tusa RJ. Saccadic disconjugacy in monkeys with strabismus. Soc Neurosci Abstr. 2001:71.42. [Google Scholar]
- Goldberg ME, Musil SY, Fitzgibbon EJ, Smith M, Olson CR. The role of the cerebellum in the control of saccadic eye movements. In: Mano N, Hamada I, Delong MR, editors. The Role of the Basal Ganglia and Cerebellum in Voluntary Movements. Elseiver; Amsterdam: 1993. pp. 203–211. [Google Scholar]
- Kapoula Z, Bucci MP, Eggert T, Garraud L. Impairment of the binocular coordination of saccades in strabismus. Vis Res. 1997;37:2757–2766. doi: 10.1016/s0042-6989(97)00064-3. [DOI] [PubMed] [Google Scholar]
- Kommerell G, Olivier D, Theopold H. Adaptive programming of phasic and tonic components in saccadic eye movements. Investigations of patients with abducens palsy. Invest Ophthalmol. 1976;15:657–660. [PubMed] [Google Scholar]
- Mclaughlin SC. Parametric adjustment in saccadic eye movements. Percept Psychophys. 1967;2:359–362. [Google Scholar]
- Noto CT, Robinson FR. Visual error is the stimulus for saccade gain adaptation. Cogn Brain Res. 2001;12:301–305. doi: 10.1016/s0926-6410(01)00062-3. [DOI] [PubMed] [Google Scholar]
- Optican LM, Robinson DA. Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol. 1980;44:1058–1076. doi: 10.1152/jn.1980.44.6.1058. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF, Noto CT. Cerebellar influences on saccade plasticity. Ann NY Acad Sci. 2002;956:155–163. doi: 10.1111/j.1749-6632.2002.tb02816.x. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Batourina EY, Tunder GS. Comparison of two methods of producing adaptation of saccade size and implications for the site of plasticity. J Neurophysiol. 1998;79:704–715. doi: 10.1152/jn.1998.79.2.704. [DOI] [PubMed] [Google Scholar]
- Shafer JL, Noto CT, Fuchs AF. Temporal characteristics of error signals driving saccadic gain adaptation in the macaque monkey. J Neurophysiol. 2000;84:88–95. doi: 10.1152/jn.2000.84.1.88. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Clendaniel RA. Context-specific adaptation of saccade gain. Exp Brain Res. 2002;146:441–450. doi: 10.1007/s00221-002-1199-1. [DOI] [PubMed] [Google Scholar]
- Straube A, Fuchs AF, Usher S, Robinson FR. Characteristics of saccadic gain adaptation in rhesus macaques. J Neurophysiol. 1997;77:874–895. doi: 10.1152/jn.1997.77.2.874. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Mustari MJ, Das VE, Boothe RG. Animal models for visual deprivation-induced strabismus and nystagmus. Ann NY Acad Sci. 2002;956:346–360. doi: 10.1111/j.1749-6632.2002.tb02833.x. [DOI] [PubMed] [Google Scholar]
- Versino M, Hurko O, Zee DS. Disorders of binocular control of eye movements in patients with cerebellar dysfunction. Brain. 1996;119:1933–1950. doi: 10.1093/brain/119.6.1933. [DOI] [PubMed] [Google Scholar]
- Viirre E, Cadera W, Vilis T. Monocular adaptation of the saccadic system and vestibulo-ocular reflex. Invest Ophthalmol Vis Sci. 1988;29:1339–1347. [PubMed] [Google Scholar]
- Wallman J, Fuchs AF. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol. 1998;80:2405–2416. doi: 10.1152/jn.1998.80.5.2405. [DOI] [PubMed] [Google Scholar]
- Watamabe S, Noto CT, Fuchs AF. Flexibility of saccade adaptation in the monkey: different gain states for saccades in the same direction. Exp Brain Res. 2000;130:169–176. doi: 10.1007/s002219900220. [DOI] [PubMed] [Google Scholar]


