Abstract
Triglyceride synthesis in mammalian tissues requires glycerol 3-phosphate as the source of triglyceride glycerol. In this study the relative contribution of glyceroneogenesis and glycolysis to triglyceride glycerol synthesis was quantified in vivo in adipose tissue, skeletal muscle, and liver of the rat in response to a chow diet (controls), 48-h fast, and lipogenic (high sucrose) diet. The rate of glyceroneogenesis was quantified using the tritium ([3H2]O) labeling of body water, and the contribution of glucose, via glycolysis, was determined using a [U-14C]glucose tracer. In epididymal and mesenteric adipose tissue of control rats, glyceroneogenesis accounted for ∼90% of triglyceride glycerol synthesis. Fasting for 48 h did not alter glyceroneogenesis in adipose tissue, whereas the contribution of glucose was negligible. In response to sucrose feeding, the synthesis of triglyceride glycerol via both glyceroneogenesis and glycolysis nearly doubled (versus controls); however, glyceroneogenesis remained quantitatively higher as compared with the contribution of glucose. Enhancement of triglyceride-fatty acid cycling by epinephrine infusion resulted in a higher rate of glyceroneogenesis in adipose tissue, as compared with controls, whereas the contribution of glucose via glycolysis was not measurable. Glyceroneogenesis provided the majority of triglyceride glycerol in the gastrocnemius and soleus. In the liver the fractional contribution of glyceroneogenesis remained constant (∼60%) under all conditions and was higher than that of glucose. Thus, glyceroneogenesis, in contrast to glucose, via glycolysis, is quantitatively the predominant source of triglyceride glycerol in adipose tissue, skeletal muscle, and liver of the rat during fasting and high sucrose feeding.
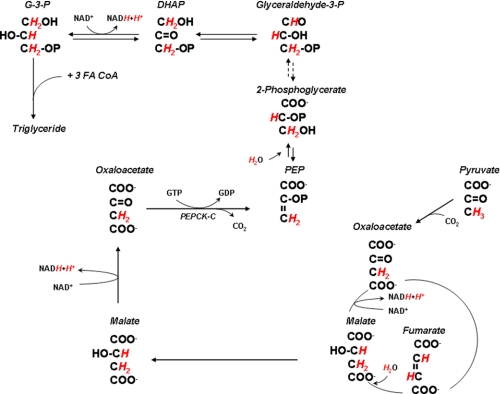
Triglyceride synthesis is critical for the accretion of fat and for the transport of lipids in the blood. In addition, triglyceride synthesis is an essential component of the triglyceride-fatty acid (TG-FA)3 cycle, in which fatty acids released from adipose tissue following lipolysis are re-esterified back to triglyceride (1, 2). Glycerol 3-phosphate (G-3-P) and fatty acyl-CoAs are the substrates for the synthesis of triglycerides. G-3-P can be formed by phosphorylation of glycerol via glycerol kinase or by the reduction of dihydroxyacetone phosphate via G-3-P dehydrogenase. Dihydroxyacetone phosphate can be derived from either glucose or pyruvate. Glycerol kinase, although highly active in the liver, is present at low activity in adipose tissue and skeletal muscle (3, 4). Glucose is generally considered to be the major carbon source for the synthesis of G-3-P in white and brown adipose tissue, skeletal muscle, and liver. However, the relative quantitative contribution of glucose and pyruvate (glyceroneogenesis) has not been examined systematically in vivo.
Glyceroneogenesis, the de novo synthesis of G-3-P from precursors other than glucose and glycerol (i.e. pyruvate, lactate, alanine, and citric acid cycle anions) has long been suggested as a potential pathway for triglyceride glycerol formation in adipose tissue (5, 6). The cytosolic form of phosphoenolpyruvate carboxykinase (GTP) (PEPCK-C) (EC 4.1.1.32), which catalyzes the GTP-dependent decarboxylation of oxaloacetate to form phosphoenolpyruvate, is a key regulatory enzyme in glyceroneogenesis. Ablation of PEPCK-C expression in adipose tissue resulted in mice that have a reduced fat mass, with some of the animals displaying lipodystrophy (7); conversely, mice overexpressing PEPCK-C in their adipose tissue became obese (8) and were markedly insulin-resistant when fed a high fat diet (9). Because fasting causes an increase in pyruvate incorporation into G-3-P as well as an increase in PEPCK-C activity, glyceroneogenesis has been implicated in the enhanced TG-FA cycling associated with fasting. Furthermore, a diet high in carbohydrate decreases the activity of PEPCK-C in white adipose tissue (10); these changes are largely due to alterations in gene transcription (11). We hypothesized that after 48 h fast PEPCK-C activity would be increased, resulting in enhanced glyceroneogenesis in the adipose tissue, whereas a high carbohydrate, lipogenic diet would decrease PEPCK-C activity and lower flux over this pathway.
Considerable triglyceride is present in skeletal muscle as lipid droplets deposited within the muscle fibers. Increases in the intramyocellular triglyceride pool have been observed in response to endurance training as well as in insulin-resistant states (12, 13). PEPCK-C is also expressed in low quantities in skeletal muscle, although its metabolic role in this tissue is not clear. Overexpression of PEPCK-C in the skeletal muscle of the mouse resulted in a marked increase in intramyocellular triglyceride levels (14). Guo and Jensen (15) reported that, whereas glucose and glycerol contributed equally to triglyceride glycerol synthesis in the gastrocnemius of 24-h-fasted rats, the majority of G-3-P was derived from what they termed the “indirect pathway,” which originated from reactions further down the glycolytic pathway.
In the liver the pathways of gluconeogenesis and glyceroneogenesis share a common set of reactions (16), so that these pathways cannot be functionally separated. Data from in vivo studies in humans fasted overnight (17) and from human subjects with type 2 diabetes (18) indicated that glyceroneogenesis and not glucose metabolism via glycolysis was the predominant source of the triglyceride glycerol in the circulation. However, the extent of hepatic glyceroneogenesis during an extended fast and in lipogenic conditions has not been examined in vivo.
In the present study we have quantified the relative contribution of glyceroneogenesis and glycolysis to the synthesis of triglyceride glycerol in vivo in white adipose tissue, skeletal muscle, and in the liver of rats over a range of physiological perturbations, i.e. controls, a 48-h fast, and in a lipogenic state induced by dietary sucrose supplementation. We have also examined glyceroneogenesis in adipose tissue in response to enhanced TG-FA cycling induced by the infusion of epinephrine.
EXPERIMENTAL PROCEDURES
Animals—Male Sprague-Dawley rats weighing ∼250–275 g with indwelling carotid artery and jugular vein catheters were obtained from Zivic-Miller Laboratories (Zelienople, PA).
3–4 days before transfer of animals to our institution, PE50 catheters were inserted in the right jugular vein and left carotid artery under sterile conditions and under anesthesia. The catheters were tunneled subcutaneously, and the distal end of each catheter was sutured to the dorsum of the neck. The catheters were filled with an anticoagulant solution, capped, and secured on the top of the neck. Post-operatively the animals were given analgesics for pain (0.2 ml of Ketosen, 10 mg/ml) and were placed on ad libitum rat chow and water.
The animals were housed in the Animal Resource Facility of Case Western Reserve University under controlled temperature (22 ± 1 °C) and light (on at 0600 h, off at 1800 h). All procedures involving the rats used in this study were reviewed and approved by the Institutional Animal Care and Use Committee and conformed to American Association for Accreditation of Laboratory Animal Care Guidelines. The animals were fed rat chow (Purina Mills Prolab RMH 1800; 21% protein, 14% fat, 65% carbohydrate) and water ad libitum and were provided with environmental enrichment (chew squares). After entry into the facility, the rats were allowed a minimum of 4 days to acclimate to the new surroundings before the study.
Glyceroneogenesis was quantified in four groups of animals: 1) controls (food was removed at 7 a.m. on the morning of the tracer isotope study; water was provided ad libitum), 2) fasting (food was removed 48 h before the tracer study; water was provided ad libitum), 3) high carbohydrate diet (in addition to rat chow, the animals were given sucrose water (20% w/v) for 5 days); food was removed at 7 a.m., sucrose water was discontinued, and animals received glucose intravenously (15 mg/kg/min, as a 10% solution) throughout the tracer study to maintain the lipogenic state), and 4) response to epinephrine infusion (food was removed at 7 a.m. on the day of the tracer study; water was provided ad libitum, and epinephrine (Hospira, Inc.; Lake Forest, IL) was infused (500 ng/kg/min) via the jugular vein catheter for 3 h).
Tracer Study Protocol (Fig. 1)—Rats were given an intraperitoneal injection of 0.5 mCi of [3H2]O (1.0 mCi/ml). This was followed immediately by [U-14C]glucose tracer in an isotonic saline solution as a prime constant rate infusion (prime = 9 μCi/kg, constant rate infusion = 9 μCi/kg/h) via the jugular vein catheter for 7 h. The animals were unrestrained, awake, and moved freely in the cage during the experiment. Blood samples (0.2 ml) were drawn from the carotid artery at 6, 6.5, and 7 h, and plasma was separated. At 7 h, the rats were given an anesthetic dose of sodium pentobarbital (Abbott Laboratories) via the carotid artery catheter, and the tissues of interest (epididymal and mesenteric fat, gastrocnemius, and soleus muscles) were harvested while the tracer infusion was continued. Plasma and tissues were stored at -80 °C until further analyses.
FIGURE 1.
Isotopic tracer study protocol. Rats were given 0. 5 mCi of [3H2]O at time 0 followed by a prime constant-rate infusion of [U-14C]glucose (prime = 9 μCi/kg; constant infusion = 9 μCi/kg/h) for 7 h. Animals moved freely about their cages during the study. Blood samples were collected during the final hour of the infusion, and tissues of interest were harvested while the tracer infusion was continued.
Triglyceride Extraction and the Isolation of Glycerol and Fatty Acids—Triglycerides were extracted from the tissues and plasma using a modification of the method of Folch et al. (19). Briefly, adipose tissue was homogenized in 2:1 (v/v) chloroform-methanol, whereas the muscle was homogenized in saline using an IKA-ULTRA-TURRAX T 25 basic tissue blender. Plasma samples and tissue homogenates were incubated in 2:1 (v/v) chloroform-methanol for 48 h at 4 °C, then 4 mm MgCl2 was added, and the solution was mixed thoroughly and centrifuged at 1000 × g for 1 h. The aqueous supernatant was removed, taken to dryness, and further processed as described below. The organic infranatant containing the triglycerides was dried and saponified by the addition of ethanolic KOH (0.5 n) followed by incubation at 70 °C for 1 h. To convert the carboxylate salts to the free acids, the samples were acidified by the addition of 6 n HCl. Fatty acids were extracted with hexane (Fluka) and dried. The aqueous layer containing glycerol was dried and reconstituted in a fixed volume, and the concentration of glycerol was determined fluorometrically (20).
Isolation of Plasma Metabolites—Plasma glucose, glycerol, and lactate were separated by ion-exchange chromatography (Bio-Rad AG 1-X8 hydrogen resin; AG 50W-X8 formate resin) (18). Glucose and glycerol, eluted in the neutral fraction, were further separated by liquid chromatography (Hewlett Packard Agilent 1100 Series HPLC with Agilent ChemStation software; Bio-Rad Aminex HPX-87P Carbohydrate Analysis Column, 300 × 7.8 mm; mobile phase 100% water, flow rate 0.6 ml/min, column temperature 80 °C). The eluates were dried and reconstituted in a fixed volume. The glucose concentration was determined enzymatically using the Beckman Glucose Analyzer by the glucose oxidase method. The concentrations of glycerol and lactate were determined enzymatically using a fluorometric assay (20, 21).
Measurements of Radioactivity—The radioactivity of 3Hina 20-μl aliquot of plasma was measured to determine the specific activity (SA) of body water. An aliquot of glucose, glycerol, and lactate, isolated from plasma samples (see above), was used to measure the total 3H and 14C radioactivity of each metabolite. The 3H and 14C radioactivity present in fatty acids and glycerol isolated (from triglycerides) in plasma and tissues was also measured.
Radioactivity of C-1 and (C-1 + C-3)—The 14C and 3H radioactivity of the C-1 and (C-1 + C-3) triglyceride glycerol carbons was determined after dimedon derivatization according to the method of Reeves (22). Briefly, glycerol samples were subjected to periodate oxidation, and the formaldehyde formed was precipitated as a dimedon derivative. Each glycerol sample was divided in half; one aliquot was taken directly through the dimedon derivatization procedure, and the other aliquot was phosphorylated using glycerol kinase, as described by Bederman et al. (23), to form G-3-P before the derivatization. Incubation of glycerol (or G-3-P) with periodic acid results in cleavage of vicinal diols. Phosphorylation of glycerol to form G-3-P prevents the cleavage of C-3 by periodate. Thus, radioactivity on (C-1 + C-3) and C-1-only can be determined from the same sample of triglyceride glycerol.
Activity of PEPCK-C—The activity of PEPCK-C was determined by the method of Ballard and Hanson (24) using adipose tissue isolated from 48-h fasted and sucrose-fed animals. The tissues were homogenized in 0.25 m sucrose containing 5 mm Tris-HCl at pH 7.4 and 1 mm dithiothreitol. The cytosolic fraction, prepared by centrifuging the homogenate at 30,000 × g for 30 min at 4 °C, was used to assay for PEPCK-C activity.
Calculations—The rate of appearance (Ra) of glucose in the blood was calculated during the isotopic steady state using the tracer dilution technique (25): Ra (μmol/kg/min) = I/SA, where I is the rate of infusion of [U-14C]glucose tracer (dpm/kg/min), and SA = specific activity of glucose (dpm/μmol).
The SA of pyruvate was calculated from that of plasma water assuming complete equilibrium between the hydrogens on plasma water and the hydrogens on C-3 of pyruvate (Fig. 2). Therefore, the SA of pyruvate = 3 × SA of body water, assuming all three hydrogens on C-3 of pyruvate are in complete equilibrium with body water (26, 27). SA of body water = 1.11 × SA of plasma. The measured SA of plasma was multiplied by 1.11 because water constitutes 90% of plasma.
FIGURE 2.
The incorporation of 3H into G-3-P from body water. Hydrogens of C-3 of pyruvate become labeled through exchange with body water during transamination with alanine and keto-enol tautomerization as well as during the equilibrium of malate with fumarate so that both hydrogens of C-3 of phosphoenolpyruvate, which forms the C-3 of glyceraldehyde-3-P, will have the same SA as that of body water. Additional labeling occurs at the level of the triose phosphate pool when C-1 and C-2 acquire 3H directly from the body water. Thus, the 3H of C-1 and C-2 of G-3-P were derived from the triose phosphate pool. The 3H of C-3 of G-3-P were derived entirely from pyruvate. Hydrogens labeled with 3H are highlighted in red.
The rate of gluconeogenesis was calculated by dividing the 3H SA of glucose with the estimated SA of the triose phosphate pool. The SA of the triose phosphate pool = 4 × SA of body water, as there are four hydrogens on each molecule of triose phosphate (dihydroxyacetone phosphate and glyceraldehyde-3-P) in equilibrium with body water (Fig. 2). It was assumed that all the hydrogens on the triose phosphate molecules are labeled to the same extent as body water (26, 27). The rate of glyceroneogenesis in the adipose tissue and skeletal muscle was calculated as follows: glyceroneogenesis (nmol/g/h) = (3H in triglyceride glycerol (dpm/g/h) × 2/5)/(pyruvate SA (dpm/μmol) × 2/3).
The 3H radioactivity of triglyceride glycerol was multiplied by 2/5 as only two hydrogens in the glycerol moiety are derived from pyruvate (Fig. 2). The SA of pyruvate was multiplied by 2/3 since only two hydrogens on C-3 of pyruvate are incorporated into triglyceride glycerol. The SA of 3H on C-1 and C-2 of triglyceride glycerol will be the same as that of body water because of complete equilibrium in the triose phosphate pool. In contrast, 3H on C-3 of triglyceride glycerol is derived from pyruvate.
The “total” (direct plus indirect via lactate) rate of the glycolytic contribution to triglyceride glycerol in adipose tissue and skeletal muscle was calculated as follows: total (direct plus indirect) incorporation of glucose into triglyceride glycerol (nmol/g/h) = 2 × (14C radioactivity in triglyceride glycerol (dpm/g/h))/(14C SA of glucose (dpm/μmol)).
The data are expressed as 3 carbon eq by multiplying the results by 2. The “direct” contribution of glucose carbon to triglyceride glycerol was calculated by subtracting the contribution of lactate (glyceroneogenesis) from the total glucose carbon incorporated into triglyceride glycerol. The contribution of lactate was calculated by multiplying the 14C SA of lactate with the estimated glyceroneogenic flux from pyruvate (corrected for the loss of 14C in the citric acid cycle; dilution factor 2.2) (28). Additional studies using four animals showed that the SA of the [14C]lactate pool reached steady state within 1 h of the start of the [14C]glucose infusion (data not shown).
Plasma triglyceride glycerol from glyceroneogenesis (%) = (3H SA plasma triglyceride glycerol (dpm/μmol) × 2/5)/(SA of pyruvate (dpm/μmol) × 2/3). Both the SA of triglyceride glycerol and pyruvate were adjusted, as only two hydrogens on C-3 of pyruvate are incorporated into the glycerol moiety. Plasma triglyceride glycerol from glucose (%) = 100 × (14C SA plasma triglyceride glycerol (dpm/μmol) × 2)/(14C SA glucose (dpm/μmol)). The SA of triglyceride glycerol was multiplied by 2 since two 3-carbon eq are formed from one molecule of glucose. The rate of lipogenesis was calculated as described by Katz and Rognstad (29).
Statistical Analysis—Values are expressed as the mean ± S.E. Analysis of variance was used to examine the differences between the three dietary groups. Two-tailed Student's t test (assuming unequal variance) was used to assess differences between two dietary groups for each tissue and within the same dietary group between two adipose tissue depots (or two types of skeletal muscle).
RESULTS
Glucose Kinetics, Gluconeogenesis, and the Source of Plasma Triglyceride Glycerol—The mean plasma glucose concentration of control and 48-h-fasted animals was 8.6 and 6.3 mm, respectively (Table 1). The mean plasma glucose concentration (9.8 mm) of the rats supplemented with sucrose was not significantly different when compared with controls. The glucose Ra was significantly lower in 48-h-fasted animals (17.7 μmol/kg/min) when compared with controls (35.6 μmol/kg/min) (Table 1). In the sucrose-supplemented group, intravenous glucose infusion resulted in complete suppression of endogenous glucose production. The fractional contribution of gluconeogenesis to glucose Ra, estimated from the incorporation of 3H of body water into glucose, was significantly higher in 48-h-fasted animals (58.6%) compared with the control group (28.5%) (Table 1). The contribution of gluconeogenesis to glucose Ra in the sucrose-supplemented group could not be calculated, because endogenous glucose production was completely suppressed.
TABLE 1.
Glucose kinetics, gluconeogenesis, and source of plasma triglyceride glycerol Values are the mean ± S.E. Conc., concentration in the plasma; GNG, contribution of gluconeogenesis to glucose Ra; GlyNG, contribution of glyceroneogenesis to plasma triglyceride; glycolysis, contribution of glucose to plasma triglyceride. Suc Sup + Glc Inf, sucrose-supplemented animals infused with glucose during the tracer study.
|
Glucose
|
Triglyceride
|
|||||
|---|---|---|---|---|---|---|
| Conc. | Ra | GNG | Conc. | GlyNG | Glycolysis | |
| mm | μmol/kg/min | % | mm | % | % | |
| Control (n = 5) | 8.6 ± 1.0 | 35.6 ± 4.3 | 28.5 ± 2.9 | 0.841 ± 0.07 | 57.6 ± 2.2 | 14.8 ± 1.9 |
| 48 h fast (n = 5) | 6.3 ± 0.6 | 17.7 ± 0.6a | 58.6 ± 1.6b | 0.844 ± 0.06 | 56.1 ± 6.7 | 10.6 ± 0.7 |
| Suc Sup + Glc Inf (n = 5) | 9.8 ± 0.9 | 0 | 0 | 0.803 ± 0.06 | 59.3 ± 2.7 | 27.8 ± 5.6 |
p < 0.05 vs. control
p < 0.01 vs. control
The plasma concentration of triglyceride were not different in the three groups (Table 1). The fraction of plasma triglyceride glycerol derived from glyceroneogenesis was ∼60% and not different among the three groups (Table 1). Approximately 15% of triglyceride glycerol was derived from glucose in the control group (Table 1). The contribution of glucose was lower in 48-h fasted rats (∼11%) and was higher in sucrose-supplemented animals (∼28%). Although sucrose supplementation resulted in a higher contribution of glucose to plasma triglyceride glycerol when compared with controls, the contribution of glucose was less than that of glyceroneogenesis (Table 1). However, the estimated contribution of glucose includes both the direct and indirect (via lactate) pathways. Therefore, the reported contribution of glucose to plasma triglyceride glycerol also includes the 14C incorporated into plasma triglycerides via glyceroneogenesis (from [14C]lactate).
Glyceroneogenesis and Glycolysis as a Source of Triglyceride Glycerol in Adipose Tissue—There was no significant (p = ns) difference in the triglyceride concentration in the adipose tissue of controls and 48-h-fasted animals. In the sucrose-supplemented animals, the concentration of triglyceride was significantly higher than controls (Table 2).
TABLE 2.
The contribution of glyceroneogenesis and glycolysis to triglyceride glycerol synthesis in adipose tissue Values are the mean ± S.E.; range is indicated in parentheses. GlyNG, glyceroneogenesis; Suc Sup + Glc Inf, sucrose-supplemented animals infused with glucose during tracer study. Glyceroneogenesis was quantified using tritium, [3H2]O, incorporation into triglyceride glycerol and is expressed as nmol of pyruvate/g/h. The contribution of glucose was quantified using [U-14C]glucose and is expressed as three carbon equivalents. Total, includes carbon coming directly from glucose plus indirectly via lactate. Direct, incorporation of glucose into triglycerides via triosephosphate.
|
Epididymal fat
|
Mesenteric fat
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
TG
|
GlyNG
|
Glycolysis
|
TG
|
GlyNG
|
Glycolysis
|
|||||
| Total | Direct | Total | Direct | |||||||
| μmol/g | nmol/g/h | nmol/g/h | μmol/g | nmol/g/h | nmol/g/h | |||||
| Control (n = 5) | 400 ± 21.6 | 604 ± 56.6 | 294 ± 37.9 | 82.3 ± 12.5 | 414 ± 17.7 | 789 ± 70.5 | 362 ± 45.0 | 86.3 ± 13.7 | ||
| 48-h fast (n = 5) | 352 ± 34.4 | 511 ± 65.0 | 66.3 ± 5.6a | (–24.5–11.5)b | 359 ± 45.9 | 816 ± 96.6 | 94.8 ± 6.63vb | (–42.0–10.5)a | ||
| Suc Sup + Glc Inf (n = 5) | 723 ± 19.2b | 1022 ± 57.1b | 671 ± 67.9a | 149 ± 19.3 | 695 ± 11.7a | 1410 ± 85.2b | 1111 ± 133a | 277 ± 34.1a | ||
p < 0.05 vs. control
p < 0.01 vs. control
Glyceroneogenesis was quantified using 3H incorporation into triglyceride glycerol and is expressed as incorporation of pyruvate equivalents. The rate of glyceroneogenesis in the control animals was ∼600 nmol/g/h in the epididymal adipose depot and ∼800 nmol/g/h in the mesenteric depot (Table 2). Glyceroneogenesis did not change in response to fasting for 48 h. In contrast, sucrose-supplementation resulted in a significant increase in this pathway in both adipose tissue depots. Glyceroneogenesis was higher in mesenteric adipose tissue as compared with epididymal adipose tissue in all groups; however, a statistically significant difference was only observed in the sucrose-supplemented group (Table 2).
Total and direct glucose carbon incorporated into triglyceride glycerol are displayed in Table 2. 48-h fast caused a significantly lower incorporation of total glucose carbon into triglyceride glycerol as compared with controls. In contrast, sucrose supplementation resulted in a higher total contribution of glucose carbon to triglyceride glycerol (Table 2). The direct contribution of glucose to triglyceride glycerol was ∼80 nmol/g/h in control animals in both adipose depots (Table 2). In the 48-h-fasted animals the direct contribution of glucose via glycolysis was negligible. In contrast, sucrose supplementation resulted in a doubling of the direct contribution of glucose to triglyceride glycerol in both adipose tissue depots.
We confirmed the predominance of glyceroneogenesis, as compared with glycolysis, by examining the 14C/3H ratio on C-1 and (C-1 + C-3) of triglyceride glycerol. C-1 and (C-1 + C-3) of glycerol were cleaved by periodate oxidation, and the radioactivity of the dimedon derivative was measured. We examined the mesenteric adipose tissue of sucrose supplemented rats because glyceroneogenesis was highest in the adipose tissue of this group. As shown in Table 3, the 14C/3H ratio was high on (C-1 + C-3) as compared with C-1 of triglyceride glycerol. A high 14C/3H ratio on C-3 (or C-1 + C-3) suggests a greater contribution of glyceroneogenesis relative to the direct contribution of glucose via glycolysis to triglyceride glycerol synthesis (Fig. 3).
TABLE 3.
14C and 3H radioactivity and 14C/3H ratio on C-1 and (C-1 + C-3) of triglyceride glycerol in the mesenteric adipose tissue The mean ± S.E. is reported for the 14C/3H ratio. C-1 and C-1 + C-3 (along with their corresponding hydrogens) were cleaved from triglyceride glycerol using periodate oxidation. The dimedon derivative of the formaldehyde formed was prepared as described under “Experimental Procedures.” 14C and 3H radioactivity was measured. Each row represents the values obtained from an individual rat of the sucrose supplemented group.
|
Animal
|
C-1 + C-3
|
C-1
|
||||
|---|---|---|---|---|---|---|
| 3H | 14C | 14C/3H | 3H | 14C | 14C/3Hb | |
| dpm | dpm | dpm | dpm | |||
| 1 | 1574 | 2948 | 1.87 | 1220 | 189 | 0.15 |
| 2 | 1893 | 2482 | 1.31 | 1255 | 133 | 0.11 |
| 3 | 1812 | 2593 | 1.43 | 1145 | 139 | 0.12 |
| 4 | 1186 | 1925 | 1.62 | 496 | 174 | 0.35 |
| 5 | 1099 | 1731 | 1.58 | 423 | 173 | 0.41 |
| Average | 1.56 ± 0.095a | 0.23 ± 0.063b | ||||
p < 0.05
p < 0.01 vs. (C-1 + C-3)
FIGURE 3.
The relative contribution of glyceroneogenesis and of glucose by the direct and indirect (via lactate) pathways and its impact on the 14C/3H ratio of C-1 and C-3 of triglyceride glycerol. The box represents the labeling pattern of G-3-P derived from [14C]glucose, [3H]pyruvate, and [14C]lactate. When G-3-P is formed from [14C]glucose, all the carbons of G-3-P will be equally labeled with 14C. In contrast, 3H, as a result of equilibrium in the triosephosphate pool (Fig. 2), will appear on C-1 and C-2 and not C-3. G-3-P formed from pyruvate will not have any 14C label, whereas the hydrogens on C-1, C-2, and C-3 will be completely labeled with 3H. On a stoichiometric equivalent basis, G-3-P formed from 1 molecule of glucose and 2 molecules of pyruvate will have a 14C/3H ratio on C-1 of 2 14C/8 3H = 0.25 and on C-3 of 2 14C/4 3H = 0.5. 14C of glucose can also be incorporated into G-3-P via [14C]lactate. However, as a result of randomization and the exchange of label in the TCA cycle, [14C]lactate entering the triose phosphate pool will have less label on C-1 relative to C-2 and C-3. Therefore, as the contribution of recycled glucose (via lactate) to G-3-P increases, there will be an increase in the 14C/3H ratio on C-3 and a decrease of the ratio on C-1. Thus, a high glyceroneogenic flux, relative to glycolytic flux, will result in a high 14C/3H ratio on C-3 (or C-1 + C-3) as compared with that on C-1. Carbons labeled with 14C are highlighted in blue, and hydrogens labeled with 3H are highlighted in red. PEP, phosphoenolpyruvate.
Fatty Acid Synthesis in Adipose Tissue—The incorporation of 14C of glucose into fatty acids was negligible in 48-h-fasted animals and high in controls in both the epididymal and mesenteric adipose tissue (Table 4). Furthermore, fatty acid synthesis in the sucrose-supplemented group was significantly higher as compared with control animals in both adipose tissue depots examined.
TABLE 4.
Incorporation of glucose into de novo synthesized fatty acids in adipose tissue Values are nmol/g/h, mean ± S.E. Fatty acids were obtained after hydrolysis of triglyceride isolated from epididymal and mesenteric adipose tissue depots as described under “Experimental Procedures.” The contribution of glucose carbon to the de novo synthesis of fatty acids was quantified during a prime constant-rate infusion of [U-14C]glucose. ND, non-detected; Suc Sup + Glc Inf, sucrose supplemented animals infused with glucose during tracer study.
| Epididymal fat | Mesenteric fat | |
|---|---|---|
| Control (n = 5) | 520 ± 160 | 360 ± 120 |
| 48-h fast (n = 5) | 0.84 ± 0.51 | ND |
| Suc Sup + Glc Inf (n = 5) | 5,984 ± 934a | 11,753 ± 1882a |
p < 0.05 vs. control
PEPCK-C Activity in the Adipose Tissue—Maximal PEPCK-C activity (0.33 units/g tissue) was noted in mesenteric fat of 48-h-fasted rats and was significantly higher than that of the sucrose-supplemented group (0.025 units/g of tissue) (Fig. 4). Similar qualitative changes were observed in the epididymal adipose depot (0.14 units/g tissue in 48-h-fasted rats and 0.020 units/g tissue in the sucrose supplemented group). PEPCK-C activity was significantly higher in mesenteric fat versus epididymal fat of 48-h-fasted animals.
FIGURE 4.
The activity of PEPCK-C in adipose tissue. Epididymal and mesenteric adipose tissue was collected from 48-h-fasted animals (gray bars) and from animals maintained on a sucrose supplemented diet and infused with glucose (black bars). The activity of PEPCK-C was determined in these tissues as described under “Experimental Procedures” and is expressed as the mean ± S.E. for three rats. A unit of activity is defined as 1 μmol of substrate converted to product/min/g tissue at 37 °C. *, p < 0.05; **, p < 0.01 versus 48 h fast.
Response to Enhanced TG-FA Cycling by Epinephrine—Epinephrine infusion elevated the concentration of glucose in the plasma and enhanced the rate of lipolysis, as indicated by the increase in the concentration of free glycerol in the plasma (data not shown). In the control rats infused with saline, there was no change in the plasma glucose and glycerol concentrations.
Infusion of epinephrine caused a significant increase in glyceroneogenesis in epididymal adipose tissue (Table 5). Similarly, glyceroneogenesis in mesenteric adipose tissue was significantly higher in the epinephrine-infused rats (978 nmol/g/h) as compared with the control animals (479 nmol/g/h). Total glucose carbon incorporated into adipose tissue triglyceride glycerol was higher in rats infused with epinephrine when compared with controls (Table 5). However, after accounting for the contribution via lactate to triglyceride glycerol, the direct contribution of glucose via glycolysis was negligible in both depots (Table 5).
TABLE 5.
The effect of epinephrine infusion on the contribution of glyceroneogenesis and glycolysis to triglyceride glycerol in the adipose tissue Values are the mean ± S.E.; range is indicated in parentheses. GlyNG, glyceroneogenesis, Epi Inf, epinephrine infusion. Glyceroneogenesis was quantified using tritium, [3H2]O, incorporation into triglyceride glycerol and is expressed as nmol of pyruvate/g/h. The contribution of glucose was quantified using [U-14C]glucose and is expressed as three carbon equivalents. Total, includes carbon coming directly from glucose plus indirectly via [14C]lactate. Direct, incorporation of glucose into triglycerides via triosephosphate.
|
Epididymal fat
|
Mesenteric fat
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
GlyNG
|
Glycolysis
|
GlyNG
|
Glycolysis
|
|||||
| Total | Direct | Total | Direct | |||||
| nmol/g/h | nmol/g/h | nmol/g/h | nmol/g/h | |||||
| Control (n = 4) | 367 ± 21.6 | 25.1 ± 1.6 | (–21.6–7.4) | 479 ± 38.2 | 31.7 ± 16.0 | (–27.2–3.1) | ||
| Epi Inf (500 ng/kg/min) (n = 4) | 672 ± 10.3a | 102 ± 17.7a | (–44.3–5.01) | 978 ± 182a | 144 ± 38.2 | (–103–9.3) | ||
p < 0.05 vs. control
The Relative Contribution of Glyceroneogenesis and Glucose to Triglyceride Glycerol Synthesis in Skeletal Muscle—The concentration of triglyceride in the gastrocnemius and soleus muscles were not different among the three groups (Table 6). Glyceroneogenesis was quantifiable in both gastrocnemius and soleus in vivo and was not different in the two muscle types. The rate of glyceroneogenesis in the control animals was ∼75 nmol/g/h (Table 6). Sucrose supplementation resulted in a significant increase in glyceroneogenesis in the gastrocnemius only.
TABLE 6.
The contribution of glyceroneogenesis and glucose to triglyceride glycerol synthesis in skeletal muscle Values are the mean ± S.E.; range is indicated in parentheses. GlyNG, glyceroneogenesis. Suc Sup + Glc Inf, sucrose-supplemented animals infused with glucose during tracer study. Glyceroneogenesis was quantified using tritium, [3H2]O, incorporation into triglyceride glycerol and is expressed as nmol of pyruvate/g/h. The contribution of glucose was quantified using [U-14C]glucose and is expressed as three carbon equivalents. Total, includes carbon coming directly from glucose plus indirectly via lactate. Direct, incorporation of glucose into triglycerides via triosephosphate.
|
Gastrocnemius
|
Soleus
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
TG
|
GlyNG
|
Glycolysis
|
TG
|
GlyNG
|
Glycolysis
|
|||
| Total | Direct | Total | Direct | |||||
| μmol/g | nmol/g/h | nmol/g/h | nmol/g/h | μmol/g | nmol/g/h | nmol/g/h | nmol/g/h | |
| Control (n = 5) | 5.7 ± 0.3 | 75.1 ± 9.3 | 19.5 ± 2.8 | (–3.5–2.0) | 6.4 ± 0.3 | 78.2 ± 16.3 | 18.3 ± 2.3 | (–3.5–4.0) |
| 48-h fast (n = 5) | 5.3 ± 0.6 | 118 ± 18.1 | 12.0 ± 0.5 | (–10.5–2.0) | 6.3 ± 0.4 | 127 ± 21.0 | 10.9 ± 1.8 | (–10.5–0.5) |
| Suc Sup + Glc Inf (n = 5) | 5.4 ± 0.2 | 109 ± 4.0a | 25.8 ± 1.5 | (–3.0–2.5) | 5.1 ± 0.3 | 104 ± 3.2 | 31.7 ± 3.1a | (–3.0–1.5) |
p < 0.05
Total glucose carbon incorporated into triglyceride glycerol was ∼20 nmol/g/h in the gastrocnemius and soleus of control rats (Table 6). Fasting for 48 h did not change the total glucose carbon incorporated into triglyceride glycerol in the skeletal muscle. In response to sucrose supplementation, a significant increase in total glucose incorporated into triglyceride glycerol was seen only in the soleus. After accounting for the contribution of lactate to triglyceride glycerol, the direct synthesis of triglyceride glycerol from glucose via glycolysis was negligible in both the gastrocnemius and soleus from all three groups of animals (Table 6).
DISCUSSION
In the present study we have examined the relative contribution of glyceroneogenesis and glucose via glycolysis to triglyceride glycerol synthesis in the rat. Our data show that glyceroneogenesis is quantitatively the predominant pathway for triglyceride glycerol synthesis in white adipose tissue, skeletal muscle, and liver during extended fasting as well as during periods of glucose availability. Surprisingly, the highest rates of glyceroneogenesis in adipose tissue were observed in sucrose-supplemented animals, when fatty acid synthesis and triglyceride deposition were high.
We used tritium ([3H2]O) labeling of body water in combination with [U-14C]glucose to quantify the relative contributions of glyceroneogenesis and glucose, respectively, to G-3-P synthesis in vivo. Botion et al. (30) used a similar method but estimated glyceroneogenic flux by subtracting the rate of triglyceride glycerol synthesis obtained with [U-14C]glucose from that obtained with [3H2]O. Because a significant amount of [14C]glucose carbon is incorporated into G-3-P via [14C]lactate, such an approach will underestimate glyceroneogenesis. For this reason, Botion et al. (30) described their results as the minimal contribution of glyceroneogenesis to triglyceride glycerol formation. In this study we have quantified the direct contribution of glucose to triglyceride glycerol synthesis by subtracting from total glucose incorporation in triglyceride glycerol the estimated contribution of 14C via lactate. Therefore, our data represent the “true” contribution of glucose to triglyceride glycerol. In addition, we confirmed the predominant role of glyceroneogenesis by examining the relative ratio of 14C/3H on C-1 and (C-1 + C-3) of triglyceride glycerol (Table 3). These data suggest that the major sources of carbon in triglyceride glycerol are pyruvate, lactate, and alanine (or the carbon skeletons of any of other gluconeogenic amino acid) via glyceroneogenesis. The negative values for the contribution of glucose to triglyceride glycerol synthesis could be due to the following. First, we determined the [14C]lactate SA after its isolation from the plasma. Although we are confident in the accuracy of our results, a small underestimation of the concentration of lactate could result in an overestimation of [14C]lactate SA. A higher [14C]lactate SA will result in an overestimation of the glyceroneogenic flux from [14C]lactate. Second, the dilution factor (2.2) for the loss of label in the citric acid cycle was based on literature values for the liver (28); we could not find a similar estimate for the adipose tissue. We may have underestimated the dilution in the adipose tissue, as glyceroneogenic flux relative to citric acid cycle flux in this tissue is far less than gluconeogenic flux relative to citric acid cycle flux in the liver. The smaller dilution factor will result in overestimation of glyceroneogenesis from lactate. Third, we estimated the 3H labeling of C-3 of glycerol from the total tritium labeling of triglyceride glycerol. This would result in an overestimation of label at C-3 and, therefore, an overestimation of glyceroneogenesis. For these reasons, we have elected to display both the total glucose incorporation into triglyceride glycerol and the estimated flux direct from glucose in Tables 2, 5, and 6. Because of the possible overestimation of glyceroneogenesis via lactate, the estimated contribution of glucose in the present study is “minimal.”
Glyceroneogenesis in Adipose Tissue—We have quantified glyceroneogenesis in adipose tissue in response to fasting, sucrose supplementation, and intravenous infusion of epinephrine. Our data show that during fasting there was no change in the rate of glyceroneogenesis, as compared with controls, and the direct contribution of glucose to triglyceride glycerol was negligible. Fasting is associated with a lower expression of GLUT 4 (31, 32) and a decreased uptake and phosphorylation of 2-deoxyglucose by adipocytes in vitro (33). There was a 50% lower rate of glucose uptake by adipose tissue, as measured by arterio-venous difference (34) in rats that were fasted for 48 h. Experimental deletion of GLUT 4 in the adipose tissue of mice, although reducing glucose uptake, did not impact on the triglyceride mass (35). Because PEPCK-C activity increases in the adipose tissue of fasted rats (36), we anticipated that glyceroneogenesis would be increased after 48 h of starvation to provide G-3-P for TG-FA cycling. Although not measured, the TG-FA cycling may already be high in control animals (∼8 h fast), so that starvation for 48 h did not cause a further increase in TG-FA cycling and, therefore, no increase in glyceroneogenesis.
Both high carbohydrate feeding and intravenous glucose administration have been shown to increase the concentration of plasma insulin, increase glucose uptake by the adipose tissue, and promote lipogenesis (37–40). In vivo data using 2H2O tracer showed that glucose was the predominant source of triglyceride glycerol in both visceral and epididymal adipose tissue in the mice fed a high carbohydrate diet for 75 days (41). Based upon these data, we expected glyceroneogenesis would be lower and the contribution of glucose higher in rats maintained on a sucrose-supplemented diet. The direct contribution of glucose to triglyceride glycerol synthesis was higher than controls in response to a sucrose supplemented diet (Table 2). Glyceroneogenesis also increased and remained the predominant pathway for triglyceride glycerol synthesis. In addition, a 90% increase in lipogenesis was observed in the adipose tissue. Wolfe and Peters (38) demonstrated that lipolysis continued to occur in humans when glucose was infused at 8 mg/kg/min and that the rate of appearance of fatty acids in plasma was significantly reduced due to enhanced intracellular TG-FA cycling. The observed increase in glyceroneogenesis in the sucrose supplemented animals in our study may support triglyceride glycerol synthesis for enhanced intracellular TG-FA cycling as well as the esterification of de novo synthesized fatty acids. Increased de novo lipogenesis in the adipose tissue was noted in the sucrose-fed rats in our study. It is possible that a fraction of the labeled fatty acids came from lipids that were synthesized in the liver as a consequence of sucrose feeding (39) and transported to the adipose tissue. The rate of lipogenesis has been shown to decrease markedly in the adipose tissue of rats with age (42, 43). Because we detected 14C radioactivity in fatty acids isolated from plasma triglyceride of sucrose-supplemented animals, such a possibility cannot be excluded.
Our finding of a high rate of glyceroneogenesis in sucrose-fed animals is at variance with that of Chen et al. (41), who showed a lower fractional contribution of glyceroneogenesis and a higher contribution of glucose in mice fed a high carbohydrate diet for 75 days. These differences may be related to the short duration of our study, the specific difference in dietary regimen (i.e. high carbohydrate and low fat diet versus sucrose supplementation followed by glucose infusion in our study), and the tracer method employed. Chen et al. (41) suggested that their method was optimal for calculating the fractional rate of synthesis of triglyceride glycerol. In contrast, our technique was optimized for measuring quantitative rates of glyceroneogenic flux over a short period. In addition, the experimental approach of Chen et al. (41), utilizing long term incorporation of label, represents a cumulative effect of feeding and fasting.
Response to Epinephrine Infusion—The effect of enhanced TG-FA cycling on glyceroneogenesis was examined by determining its response to epinephrine infusion. Epinephrine, a β-adrenergic agonist, increases TG-FA cycling in humans (44) and decreases glucose uptake by the adipose tissue (45). In addition, infusion of epinephrine causes an elevation in the concentration of lactate in the blood (46). Epinephrine infusion at 500 ng/kg/min for 3 h resulted in a high rate of glyceroneogenesis. There was no measurable contribution of glucose to triglyceride glycerol formation during epinephrine infusion. These data suggest that enhanced TG-FA cycling is associated with high rate of glyceroneogenesis.
As expected, PEPCK-C activity was higher in epididymal and mesenteric adipose depots from rats fasted for 48 h as compared with animals maintained on a high carbohydrate diet. However, the relative changes in the activity of PEPCK-C did not correlate with the measured rate of glyceroneogenesis. Glyceroneogenesis was highest in the adipose tissue of rats fed a sucrose supplemented diet, when PEPCK-C activity was at its lowest. Nevertheless, the PEPCK-C activity was always higher than the respective rate of glyceroneogenesis. High flux and low activity suggests that glyceroneogenic flux may be dependent on the provision of substrate (both pyruvate/lactate and fatty acids) or that PEPCK-C in adipose tissue is controlled by an allosteric regulator. The latter suggestion is interesting but unlikely, as no such compound has yet been identified that regulates this enzyme. It is possible that PEPCK-C plays additional roles other than supporting glyceroneogenesis in the adipose tissue and other tissues in which this pathway is active. For example, deletion of the gene for PEPCK-C in the liver resulted in a marked reduction of citric acid cycle flux (47), presumably due to an altered rate of cataplerosis in the tissue. This area clearly needs further study.
Glucose Uptake and Glyceroneogenesis by the Adipose Tissue— The key issues raised by our study are as follows. 1) Glucose uptake occurs in adipose tissue, but glucose carbon is not incorporated directly into G-3-P for triglyceride synthesis, and 2) high rate of glyceroneogenesis is evident in the presence of glucose uptake in this tissue. The simultaneous occurrence of glucose metabolism and glyceroneogenesis suggests either (a) intracellular compartmentation of G-3-P in two different, non-mixing pools, or (b) the existence of two different cell types in the adipose tissue, a predominantly glycolytic cell and a predominantly triglyceride storing cell in which glyceroneogenesis is dominant (Fig. 5).
FIGURE 5.
The two compartment (or two cell-type) hypothesis. Two different cell types exist within an adipose tissue depot; 1) undifferentiated adipocytes which do not synthesize triglyceride and are predominantly glycolytic (left side); 2) differentiated adipocytes which display the phenotype characteristic of mature adipocytes (namely a large triglyceride reservoir) and synthesize triglyceride (right side). The differentiated adipocytes may rely mostly on glyceroneogenesis for the deposition of triglyceride.
Currently there are no data in the literature that support the existence of two separate functional pools of G-3-P, i.e. one for glycolysis and the other for glyceroneogenesis, in the adipose tissue. However, there are multiple examples of apparently non-mixing intracellular pools of biochemical intermediates in several different metabolic pathways, e.g. acyl-CoA, diacylglycerol, leucine (48–50).
Evidence of multiple cell types in the adipose tissue is well documented. In addition to adipocytes, it contains endothelial cells and macrophages that primarily metabolize glucose to lactate (51, 52). Furthermore, undifferentiated preadipocytes do not deposit triglycerides and may be primarily glycolytic (53). Mature adipocytes are fully differentiated cells that exhibit an adipocyte-like phenotype, namely a large reservoir of triglyceride. In addition, PEPCK expression is a late event, occurring during terminal differentiation of the adipocyte (54). Smith (55) has proposed that mature adipocytes range from young to old and are insulin-sensitive to insulin-resistant, respectively. Our data are a composite of the contribution of glyceroneogenesis and glucose metabolism in all of the cells present in the adipose tissue. It is possible that the glycolytic cells metabolize glucose to lactate, which is then released into the interstitium where it is available as a glyceroneogenic substrate for the triglyceride-storing cells. Adipocytes express the gene for the monocarboxylate transporter-1, which may be responsible for both lactate release, as well as its uptake from the interstitium of adipose tissue (56). In vivo studies in healthy, lean humans using microdialysis techniques show that after an overnight fast the interstitial concentration of lactate is significantly higher in abdominal and femoral subcutaneous adipose tissue than in plasma, suggesting a local release of lactate (57). Furthermore, in response to an oral glucose load, both plasma and interstitial lactate levels increased in parallel; however, the interstitial lactate concentration was much higher than that in the plasma (57). Increased glucose metabolism, induced by overexpression of hepatic glucokinase in the adipose tissue of mice, resulted in higher glucose uptake and higher lactate production without an increase in triglyceride synthesis (58). Only future studies will confirm or refute these two hypotheses, i.e. functionally separate non-mixing pools of G-3-P or the two cell types of adipocytes.
Glyceroneogenesis in the Skeletal Muscle—Our data are the first demonstration of glyceroneogenesis in skeletal muscles. In response to fasting as well as sucrose feeding, glyceroneogenesis was the main contributor to triglyceride glycerol formation, whereas the direct contribution of glucose was not measurable. The low incorporation of glucose noted in this study may be due in part to the relatively small triglyceride pool in skeletal muscle; the intramyocellular triglyceride pool is typically a few μmol/g of muscle (59). During fasting, glyceroneogenesis would be expected to provide the G-3-P for triglyceride glycerol synthesis, as insulin-mediated GLUT 4 translocation and glucose uptake by the skeletal muscle is decreased (60). Furthermore, TG-FA cycling is active in the oxidative muscle of overnight-fasted rats (61). Although we anticipated that glyceroneogenesis would be a functional pathway, due to the presence of PEPCK-C activity in skeletal muscle (14), the dominance of this pathway was unexpected. Even more surprising was the lack of a direct contribution of glucose to G-3-P synthesis, given that in response to a glucose load, skeletal muscle is responsible for the majority (∼85%) of insulin-mediated glucose uptake (62). Our data demonstrating a marginal contribution of glucose to triglyceride glycerol in skeletal muscle are consistent with the report of Guo and Jensen (15), who, using [6-3H]glucose as a tracer, did not observe incorporation of 3H in triglyceride glycerol. In contrast, when [6-14C]glucose was employed, a significant amount (∼20%) of 14C incorporation in triglyceride glycerol was observed. Because 3H from glucose is lost to body water during the equilibration with pyruvate, the authors suggested that glucose carbon was incorporated into triglyceride glycerol via the indirect pathway (glyceroneogenesis).
Hepatic Glyceroneogenesis—The fractional contribution of gluconeogenesis to the glucose Ra changed as expected (about 30% lower in controls, which increased to ∼60% after a 48-h fast), whereas glyceroneogenesis remained constant at about ∼60% under all conditions studied. Furthermore, glyceroneogenesis, and not glucose metabolism via glycolysis, was the dominant pathway for hepatic triglyceride glycerol synthesis, even in sucrose-fed, glucose-infused animals. This was surprising, as exogenous glucose infusion would be expected to increase hepatic glucose uptake, and glucose should then become the major source of G-3-P. The levels of exogenous glucose infusion may not have been high enough to increase hepatic glucose uptake to a point where glucose, via glycolysis, became the major source of G-3-P. If the rate of infusion of glucose were increased, a corresponding increase in glucose uptake and its subsequent conversion to triglyceride glycerol might have been observed. However, Kalhan et al. (18) did not observe any effect of high glucose and insulin infusion during a hyperinsulinemic clamp on the relative contribution of pyruvate or glucose to triglyceride glycerol in type 2 diabetic subjects.
The present study clearly demonstrates that glyceroneogenesis occurs in adipose tissue, skeletal muscle, and liver of rat. This pathway may play a significant role in the control of triglyceride turnover and be a potential target for the treatment of obesity and diabetes (63), emphasizing the importance of PEPCK-C in both carbohydrate and lipid metabolism. However, several fundamental metabolic questions concerning glyceroneogenesis need further study. The carbon source for the G-3-P in adipose tissue and skeletal muscle is not well understood. Lactate is clearly a major precursor of triglyceride glycerol via glyceroneogenesis, but others, such as alanine and the carbon skeletons of gluconeogenic amino acids that enter the citric acid cycle are also potential candidates (16). The source of the NADH for the synthesis of G-3-P is also not well established. Glyceroneogenesis requires two molecules of NADH in the cytosol for every molecule of G-3-P synthesized; one is required at the glyceraldehyde-3-phosphate dehydrogenase step and the other at G-3-P dehydrogenase. One molecule of NADH is produced via cytosolic NAD-malate dehydrogenase when malate is oxidized to oxaloacetate. The source of the other molecule of NADH is not clear for any intermediate other than lactate, which generates an additional reducing equivalent in the cytosol when it is oxidized to pyruvate via lactate dehydrogenase. It is possible that the malate-aspartate shuttle produces net cytosolic NADH, essentially bringing reducing equivalents from the mitochondria to the cytosol to support glyceroneogenesis or that dihydroxyacetone phosphate is reduced to G-3-P via the flavoprotein dehydrogenase in the inner mitochondrial membrane (i.e. a reversal of the G-3-P shuttle). In the latter case the electrons for the synthesis of NADH would be generated in the mitochondria by fatty acid oxidation. Both of the problems outlined above require further study before the metabolic regulation of glyceroneogenesis will be understood.
Acknowledgments
We thank Visvanathan Chandramouli, Parvin Hakimi, and Clarita Duenas for technical assistance in this study.
This work was supported, in whole or in part, by National Institutes of Health Grants DK-58620 and DK-25541 (to R. W. H.) and HD-11089 and HD-042154 (to S. C. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TG, triglyceride; FA, fatty acid; Ra, rate of appearance; G-3-P, glycerol 3-phosphate; PEPCK, phosphoenolpyruvate carboxykinase; SA, specific activity.
References
- 1.Newsholme, E. A., and Crabtree, B. (1976) Biochem. Soc. Symp. 61-109 [PubMed]
- 2.Reshef, L., Olswang, Y., Cassuto, H., Blum, B., Croniger, C. M., Kalhan, S. C., Tilghman, S. M., and Hanson, R. W. (2003) J. Biol. Chem. 278 30413-30416 [DOI] [PubMed] [Google Scholar]
- 3.Robinson, J., and Newsholme, E. A. (1967) Biochem. J. 104 2-4 [Google Scholar]
- 4.Newsholme, E. A., and Taylor, K. (1969) Biochem. J. 112 465-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard, F. J., Hanson, R. W., and Leveille, G. A. (1967) J. Biol. Chem. 242 2746-2750 [PubMed] [Google Scholar]
- 6.Reshef, L., Niv, J., and Shapiro, B. (1967) J. Lipid Res. 8 688-691 [PubMed] [Google Scholar]
- 7.Olswang, Y., Cohen, H., Papo, O., Cassuto, H., Croniger, C. M., Hakimi, P., Tilghman, S. M., Hanson, R. W., and Reshef, L. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 625-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franckhauser, S., Munoz, S., Pujol, A., Casellas, A., Riu, E., Otaegui, P., Su, B., and Bosch, F. (2002) Diabetes 51 624-630 [DOI] [PubMed] [Google Scholar]
- 9.Franckhauser, S., Munoz, S., Elias, I., Ferre, T., and Bosch, F. (2006) Diabetes 55 273-280 [DOI] [PubMed] [Google Scholar]
- 10.Chaves, V. E., Frasson, D., Martins-Santos, M. E., Boschini, R. P., Garofalo, M. A., Festuccia, W. T., Kettelhut, I. C., and Migliorini, R. H. (2006) J. Nutr. 136 2475-2480 [DOI] [PubMed] [Google Scholar]
- 11.Hanson, R. W., and Reshef, L. (1997) Annu. Rev. Biochem. 66 581-611 [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster, B. H., He, J., Watkins, S., and Kelley, D. E. (2001) J. Clin. Endocrinol. Metab. 86 5755-5761 [DOI] [PubMed] [Google Scholar]
- 13.Krssak, M., Falk, P. K., Dresner, A., DiPietro, L., Vogel, S. M., Rothman, D. L., Roden, M., and Shulman, G. I. (1999) Diabetologia 42 113-116 [DOI] [PubMed] [Google Scholar]
- 14.Hakimi, P., Yang, J., Casadesus, G., Massillon, D., Tolentino-Silva, F., Nye, C. K., Cabrera, M. E., Hagen, D. R., Utter, C. B., Baghdy, Y., Johnson, D. H., Wilson, D. L., Kirwan, J. P., Kalhan, S. C., and Hanson, R. W. (2007) J. Biol. Chem. 282 32844-32855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, Z., and Jensen, M. D. (1999) J. Biol. Chem. 274 23702-23706 [DOI] [PubMed] [Google Scholar]
- 16.Owen, O. E., Kalhan, S. C., and Hanson, R. W. (2002) J. Biol. Chem. 277 30409-30412 [DOI] [PubMed] [Google Scholar]
- 17.Kalhan, S. C., Mahajan, S., Burkett, E., Reshef, L., and Hanson, R. W. (2001) J. Biol. Chem. 276 12928-12931 [DOI] [PubMed] [Google Scholar]
- 18.Kalhan, S. C., Bugianesi, E., McCullough, A. J., Hanson, R. W., and Kelley, D. E. (2008) Metabolism 57 305-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folch, J., Lees, M., and Sloane Stanley, G. H. (1957) J. Biol. Chem. 226 497-509 [PubMed] [Google Scholar]
- 20.Wieland, O. (1974) Methods of Enzymatic Analysis, Academic Press, Inc., London, 1404-1409
- 21.Gutmann, I., and Wahlefeld, A. W. (1974) Methods of Enzymatic Analysis, Academic Press, Inc., London, 1464-1472
- 22.Reeves, R. E. (1941) J. Am. Chem. Soc. 63 1476-1477 [Google Scholar]
- 23.Bederman, I. R., Dufner, D. A., Alexander, J. C., and Previs, S. F. (2006) Am. J. Physiol. Endocrinol. Metab. 290 E1048-E1056 [DOI] [PubMed] [Google Scholar]
- 24.Ballard, F. J., and Hanson, R. W. (1967) Biochem. J. 104 866-871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steele, R., Wall, J. S., De Bodo, R. C., and Altszuler, N. (1956) Am. J. Physiol. 187 15-24 [DOI] [PubMed] [Google Scholar]
- 26.Rognstad, R., Clark, D. G., and Katz, J. (1974) Eur. J Biochem. 47 383-388 [DOI] [PubMed] [Google Scholar]
- 27.Postle, A. D., and Bloxham, D. P. (1980) Biochem. J. 192 65-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hetenyi, G., Jr. (1982) Fed. Proc. 41 104-109 [PubMed] [Google Scholar]
- 29.Katz, J., and Rognstad, R. (1966) J. Biol. Chem. 241 3600-3610 [PubMed] [Google Scholar]
- 30.Botion, L. M., Brito, M. N., Brito, N. A., Brito, S. R., Kettelhut, I. C., and Migliorini, R. H. (1998) Metabolism 47 1217-1221 [DOI] [PubMed] [Google Scholar]
- 31.Berger, J., Biswas, C., Vicario, P. P., Strout, H. V., Saperstein, R., and Pilch, P. F. (1989) Nature 340 70-72 [DOI] [PubMed] [Google Scholar]
- 32.Ezaki, O. (1997) Biochem. Biophys. Res. Commun. 241 1-6 [DOI] [PubMed] [Google Scholar]
- 33.Bay, U., and Froesch, E. R. (1986) Mol. Cell. Endocrinol. 47 217-224 [DOI] [PubMed] [Google Scholar]
- 34.Kowalski, T. J., Wu, G., and Watford, M. (1997) Am. J. Physiol. 273 E613-E622 [DOI] [PubMed] [Google Scholar]
- 35.Abel, E. D., Peroni, O., Kim, J. K., Kim, Y. B., Boss, O., Hadro, E., Minnemann, T., Shulman, G. I., and Kahn, B. B. (2001) Nature 409 729-733 [DOI] [PubMed] [Google Scholar]
- 36.Reshef, L., Meyuhas, O., Boshwitz, C., Hanson, R. W., and Ballard, F. J. (1972) Isr. J. Med. Sci. 8 372-381 [PubMed] [Google Scholar]
- 37.Frayn, K. N., Shadid, S., Hamlani, R., Humphreys, S. M., Clark, M. L., Fielding, B. A., Boland, O., and Coppack, S. W. (1994) Am. J. Physiol. 266 E308-E317 [DOI] [PubMed] [Google Scholar]
- 38.Wolfe, R. R., and Peters, E. J. (1987) Am. J. Physiol. 252 E218-E223 [DOI] [PubMed] [Google Scholar]
- 39.Schwarz, J. M., Neese, R. A., Turner, S., Dare, D., and Hellerstein, M. K. (1995) J. Clin. Investig. 96 2735-2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotani, K., Peroni, O. D., Minokoshi, Y., Boss, O., and Kahn, B. B. (2004) J. Clin. Investig. 114 1666-1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen, J. L., Peacock, E., Samady, W., Turner, S. M., Neese, R. A., Hellerstein, M. K., and Murphy, E. J. (2005) J. Biol. Chem. 280 25396-25402 [DOI] [PubMed] [Google Scholar]
- 42.Karbowska, J., Kochan, Z., and Swierczynski, J. (2001) Metabolism 50 734-738 [DOI] [PubMed] [Google Scholar]
- 43.Shrago, E., Glennon, J. A., and Gordon, E. S. (1971) Metabolism 20 54-62 [DOI] [PubMed] [Google Scholar]
- 44.Wolfe, R. R., Peters, E. J., Klein, S., Holland, O. B., Rosenblatt, J., and Gary, H., Jr. (1987) Am. J. Physiol. 252 E189-E196 [DOI] [PubMed] [Google Scholar]
- 45.Kashiwagi, A., Huecksteadt, T. P., and Foley, J. E. (1983) J. Biol. Chem. 258 13685-13692 [PubMed] [Google Scholar]
- 46.Stainsby, W. N., Sumners, C., and Andrew, G. M. (1984) J. Appl. Physiol. 57 321-325 [DOI] [PubMed] [Google Scholar]
- 47.Burgess, S. C., Hausler, N., Merritt, M., Jeffrey, F. M., Storey, C., Milde, A., Koshy, S., Lindner, J., Magnuson, M. A., Malloy, C. R., and Sherry, A. D. (2004) J. Biol. Chem. 279 48941-48949 [DOI] [PubMed] [Google Scholar]
- 48.Carrasco, S., and Merida, I. (2004) Mol. Biol. Cell 15 2932-2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berge, R. K., Osmundsen, H., Aarsland, A., and Farstad, M. (1983) Int. J. Biochem. 15 205-209 [DOI] [PubMed] [Google Scholar]
- 50.Schneible, P. A., Airhart, J., and Low, R. B. (1981) J. Biol. Chem. 256 4888-4894 [PubMed] [Google Scholar]
- 51.Newsholme, P., Curi, R., Gordon, S., and Newsholme, E. A. (1986) Biochem. J. 239 121-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newsholme, P., Gordon, S., and Newsholme, E. A. (1987) Biochem. J. 242 631-636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tchkonia, T., Tchoukalova, Y. D., Giorgadze, N., Pirtskhalava, T., Karagiannides, I., Forse, R. A., Koo, A., Stevenson, M., Chinnappan, D., Cartwright, A., Jensen, M. D., and Kirkland, J. L. (2005) Am. J. Physiol. Endocrinol. Metab. 288 E267-E277 [DOI] [PubMed] [Google Scholar]
- 54.Tontonoz, P., Hu, E., Devine, J., Beale, E. G., and Spiegelman, B. M. (1995) Mol. Cell. Biol. 15 351-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith, S. A. (2003) Biochimie (Paris) 85 1219-1230 [DOI] [PubMed] [Google Scholar]
- 56.Bonen, A., Heynen, M., and Hatta, H. (2006) Appl. Physiol. Nutr. Metab. 31 31-39 [DOI] [PubMed] [Google Scholar]
- 57.Jansson, P. A., Larsson, A., Smith, U., and Lonnroth, P. (1994) J. Clin. Investig. 93 240-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franckhauser, S., Munoz, S., Hidalgo, A., Ferre, T., Mas, A., Elias, I., Cerdan, S., Pujol, A., and Bosch, F. (2007) Diabetes 56 Suppl. 1, A458 [Google Scholar]
- 59.Guo, Z., and Jensen, M. D. (1998) J. Appl. Physiol. 84 1674-1679 [DOI] [PubMed] [Google Scholar]
- 60.James, D. E., Brown, R., Navarro, J., and Pilch, P. F. (1988) Nature 333 183-185 [DOI] [PubMed] [Google Scholar]
- 61.Tagliaferro, A. R., Dobbin, S., Curi, R., Leighton, B., Meeker, L. D., and Newsholme, E. A. (1990) Int. J. Obes. 14 957-971 [PubMed] [Google Scholar]
- 62.DeFronzo, R. A., Jacot, E., Jequier, E., Maeder, E., Wahren, J., and Felber, J. P. (1981) Diabetes 30 1000-1007 [DOI] [PubMed] [Google Scholar]
- 63.Cadoudal, T., Fouque, F., Benelli, C., and Forest, C. (2008) Med. Sci. (Paris) 24 407-414 [DOI] [PubMed] [Google Scholar]