Abstract
The human colorectal epithelium is maintained by multipotent stem cells that give rise to absorptive, mucous, and endocrine lineages. Recent evidence suggests that human colorectal cancers are likewise maintained by a minority population of so-called cancer stem cells. We have previously established a human colorectal cancer cell line with multipotent characteristics (HRA-19) and developed a serum-free medium that induces endocrine, mucous and absorptive lineage commitment by HRA-19 cells in vitro. In this study, we investigate the role of the β1 integrin family of cell surface extracellular matrix receptors in multilineage differentiation by these multipotent human colorectal cancer cells. We show that endocrine and mucous lineage commitment is blocked in the presence of function-blocking antibodies to β1 integrin. Function-blocking antibodies to α2 integrin also blocked both HRA-19 endocrine lineage commitment and enterocytic differentiation by Caco-2 human colon cancer cells; both effects being abrogated by the MEK inhibitor, PD98059, suggesting a role for ERK signaling in α2-mediated regulation of colorectal cancer cell differentiation. To further explore the role of α2 integrin in multilineage differentiation, we established multipotent cells expressing high levels of wild-type α2 integrin or a non-signaling chimeric α2 integrin. Overexpression of wild-type α2 integrin in HRA-19 cells significantly enhanced endocrine and mucous lineage commitment, while cells expressing the non-signaling chimeric α2 integrin had negligible ability for either endocrine or mucous lineage commitment. This study indicates that the collagen receptor α2β1 integrin is a regulator of cell fate in human multipotent colorectal cancer cells.
A small population of multipotent epithelial stem cells maintains the integrity and function of the adult intestinal epithelium (1) Colorectal epithelial stem cells proliferate slowly giving rise to daughter cells that undergo a phase of rapid proliferation and then differentiate into absorptive, mucous, and endocrine cells. Homeostasis requires a precise balance between stem cell renewal and generation of lineage-committed cells; processes regulated by the Wnt, TGF-β, Hedgehog, and Notch pathways (2). Dysregulation of the Wnt signaling pathway, a critical regulator of normal stem cell renewal, is commonly present in colorectal cancer as the result of well described mutations in Wnt signaling components(3). This suggests that signaling cascades that promote normal colorectal epithelial stem cell renewal persist in colorectal cancer cells. Indeed there is growing support for the idea that human cancers, including colorectal cancer, are diseases of stem cells (4, 5). It has been shown that only a small minority of tumor cells, termed cancer stem cells, are able to initiate tumor growth. Furthermore, putative human colorectal cancer stem cells have been isolated on the basis of their expression of epithelial cell adhesion molecule and CD44 (6) or CD133 (7, 8). However the relationship between cancer stem cells and their normal counterparts remains to be elucidated. This will require a greater understanding of the mechanisms that balance self-renewal and differentiation in colorectal epithelial stem cells and colorectal cancer cells.
Maintenance of stem cells is thought to require a specialized tissue microenvironment known as a stem cell niche. The intestinal stem cell niche, like the intestinal stem cell, remains poorly defined, but it seems probable that intestinal stem cell behavior will be specified by the integration of signaling pathways triggered by soluble factors and stem cell adhesion to other cell types or extracellular matrix proteins (9, 10).
The extracellular matrix is a powerful regulator of stem cell function (11, 12). Cell-matrix interactions are mediated, to a large extent, by the integrin family of transmembrane receptors (13). Integrins mediate bi-directional signaling between the extracellular milieu and intracellular pathways. Integrins are heterodimers (one α and one β subunit (14)), without intrinsic catalytic activity, that signal by association with a diverse range of proteins including cytoskeletal proteins and kinases. Integrins can activate growth factor signaling pathways (15) and regulate many cell functions including proliferation, differentiation, and matrix assembly.
Elevated β1 integrin expression is a hallmark of skin (16), prostate (17), and neural stem cells (18); and β1 integrins regulate epidermal (19), neural (20), and embryonic (21) stem cell fate. β1 integrins are also candidate intestinal stem cell regulators as they are highly expressed in the stem cell region, and epithelial cells with high β1 expression show enhanced clonogenicity in vitro (22). Conditional deletion of β1 integrin in intestinal epithelium did not decrease adhesion as expected but instead increased proliferation, reduced differentiation, and increased expression of the putative stem cell marker Musashi-1 (23), suggesting that β1 integrins regulate intestinal stem cell fate. What is not clear is whether stem cell regulation is mediated entirely by the β1 integrin chain or in the context of a particular αβ heterodimer.
We previously established a multipotent human colorectal cancer cell line, HRA-19 (24) Clones of this cell line execute a multilineage differentiation program forming absorptive, mucous, and endocrine cells in xenografts (24) and in vitro upon transfer to serum-free medium (25). This study investigates the role of cell surface αβ1 integrin matrix receptors in lineage commitment in these multipotent human colon cancer cells in vitro. We report that α2 integrin regulates cell fate in human colorectal cancer cells.
EXPERIMENTAL PROCEDURES
Materials—Azide-free antibodies (Abs)2 to β1 (JB1A LM534), α1 (FB12), α2 (P1E6), α4 (P1H4), α5 (P1D6), αv (P3G8), and α6 (NKI-GoH3); mAb to α2β1 integrin (MAB1998), Ab against the α1 integrin cytoplasmic domain (AB1934), and mAb to human chromogranin (MAB5268) (Chemicon), α2 integrin mAb (611016) (BD Transduction), and mucous mAb, PR4D4 (kind gift from George Elia, CRUK). Integrin Abs used in this study have previously been shown to block integrin function: β1 (JB1A (26)), α1 (FB12 (27)), α2 (P1E6 (28)), α3 (P1B5 (29)), α3 (P1H4 (30)), α5 (P1D6 (29)), α6 (NKI-GoH3 (31)), and αv (P3G8 (32)).
Endocrine and Mucous Lineage Commitment Assay—Twice-cloned HRA-19a1.1 cells (24) were used in this study. Multiplex PCR analysis performed at the ECACC (Porton Down) confirmed that cells do have a unique profile. Lineage commitment experiments were performed as previously described (25) or with minor modifications. Briefly, cells were seeded into 8-chamber plastic slides (Nunc) at a dilution equivalent to a 1:5 split ratio (∼1.2 × 104cells/0.5 ml/chamber) (cells are transferred as a mixture of cell clumps and single cells; single cell suspension is not possible without major cell damage) in DMEM with 10% fetal calf serum. On Day 3, cells were transferred to serum-free medium (ITA): DMEM with 2 mm glutamine, ascorbic acid (10 μg/ml), insulin/transferrin/selenium (ITS-X: Invitrogen), and incubated at 37 °C. Monolayers were stained for endocrine cells (chromogranin) on Day 5 and mucous cells (PR4D4) on Day 6 (33) by immunocytochemistry. G418 was omitted during the lineage commitment assay as it inhibited differentiation.3 Values were normalized for cell number using the WST-1 reagent (Roche Applied Science) as described by the supplier.
Immunofluorescence—Immunofluorescence used ethanol-fixed cells with Abs to chromogranin (LK2H10: Chemicon) or α2 integrin (BD Transduction) followed by Alexa488-Rb anti-Ms immunoglobulins (Invitrogen).
Cell Adhesion Assay—Multiwell plates (Nunc Maxisorp) were coated with collagen I or IV (6 μg/ml) overnight at 4 °C, washed, and blocked with 1% bovine serum albumin in DPBS. 105 cells per well were incubated for 2 h at 37 °Cto allow attachment. The adherent cell number was measured using crystal violet staining, where the absorbance was read at 595 nm. Blank values from bovine serum albumin-coated wells (typically less than 5% of maximal adhesion) were subtracted from test values. Antibodies were incubated with cells for 15 min at 37 °C before adding to the matrix-coated wells.
Plasmid Constructs and Transfection—Integrin constructs in the pAWneo2 expression vector were a kind gift from Dr. J. Ivaska. Constructs were checked by sequencing and transfected into cells in 10-cm dishes using 10 μg of DNA and 37.5 μl of Fugene 6 (Roche Applied Science) prepared in Optimem medium (Invitrogen) and overnight incubation at 37 °C. Cells were transferred to DMEM with 10% fetal calf serum for 24 h, then G418 200 μg/ml (Invitrogen) was added. G418-resistant colonies were selected with cloning cylinders. Cells grew very slowly in G418, and selection took many months. Transfected cells were maintained in DMEM/10% fetal calf serum supplemented with 2 mm glutamine and 200 μg/ml G418.
Immunoblotting—Lysates were prepared with non-reducing SDS lysis buffer (Cell Signaling). Equal amounts of protein (RC-DC assay, Bio-Rad) were separated on 3–8% Tris-acetate gels (Invitrogen) and blotted onto nitrocellulose. Blots were blocked with 5% milk solution, rinsed in wash buffer (10 mm Tris-HCl, 0.1 m NaCl, 0.1% Tween 20), and incubated overnight with antibodies (β1 integrin; mAb 1965(Chemicon) or α2 integrin (611016)) in the blot wash. Blots were washed and incubated in HRPrabbit anti-mouse antibodies (Dako) in blot wash for 1 h at room temperature, washed, and developed using ECL-Plus (Amersham Biosciences).
Biotinylation and Immunoprecipitation—Cells were surface-biotinylated in 1 mg/ml Sulfo-NHS-Biotin (freshly prepared) (Pierce) in DPBS for 30 min at room temperature with gentle shaking. Cells were washed and lysed in 1% Triton X-100, 2 mm EDTA, 0.15 m NaCl, 50 mm Tris-HCl, 1 mm phenylmethylsulfonyl fluoride. For immunoprecipitation, lysates were precleared with protein-G-agarose (Roche Applied Science), then incubated with antibodies to β1 (1965), α2 (PIE6), or α2β1 (1998) for 4 h at 4 °C. Immune complexes were collected onto protein-G-agarose. Following electrophoresis and blotting, biotinylated proteins were detected with streptavidinHRP (Pierce). Immunoprecipitation of α2α1 integrin was performed with AB1934 to the cytoplasmic domain of α1 integrin and detected on blots with α2 integrin antibody.
Alkaline Phosphatase Activity in Caco-2 Cells—Subconfluent cells were transferred to serum-free medium (TSG) containing 0.2% bovine serum albumin, transferrin (5 μg/ml), sodium selenite (5 ng/ml), and 1 mm glutamine for 24 h, then harvested with 0.0125% trypsin/versene and added to an equal volume of soy bean trypsin inhibitor (0.5 mg/ml). Cells were plated at 0.6 × 104 cells/well in TSG medium into 96-well plates either untreated or coated with 10 μg/ml α2 integrin antibody (AK7) or MsIgG (Biolegend). After 72 h, the cell number was estimated with WST-1 reagent (Roche Applied Science). Alkaline phosphatase activity was measured using p-nitrophenyl phosphate (Chemicon); the reaction product p-nitrophenol was measured at 405 nm. Absorbance was normalized using the WST-1 values.
RESULTS
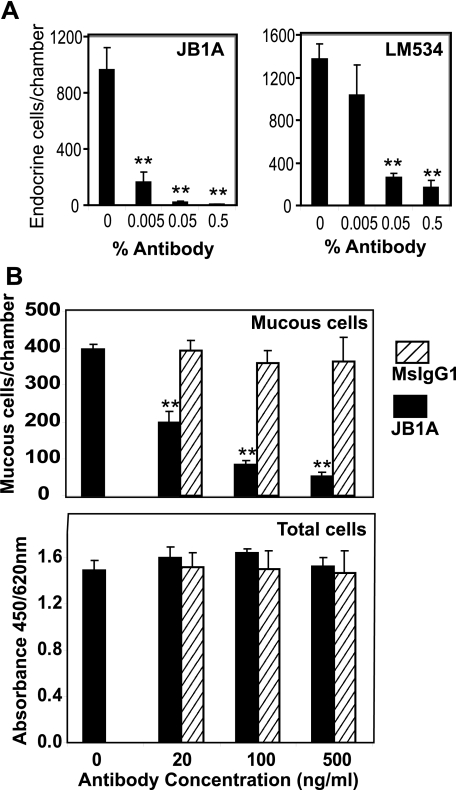
Endocrine Lineage Commitment Is Regulated by α2β1 Integrin—To determine whether β1 integrins were involved in lineage commitment, HRA-19 cells were transferred to serum-free medium to induce endocrine lineage commitment in the presence of a β1 antibody (JB1A), which blocks cell adhesion (26) and signaling (34). JB1A reduced endocrine lineage commitment to 2% of control values (Fig. 1A). Furthermore LM534, another β1 antibody that binds to the extracellular domain of β1 integrin, also reduced endocrine lineage commitment significantly (Fig. 1A). Function-blocking β1 integrin antibody, JB1A, also blocked mucous lineage commitment in HRA-19 cells (Fig. 1B) while addition of equivalent amounts of isotype control antibody did not affect mucous cell numbers. In addition, total cell number was unaffected by treatment with JB1A or isotype control, indicating that the antibodies had not affected attachment, proliferation, or survival (Fig. 1C). These results indicate a role for the β1 integrins in regulating endocrine and mucous lineage commitment in HRA-19 cells. However, integrins are heterodimers and modulation of β1 chain function could potentially be affecting all members of the β1 integrin family. Therefore, we sought to identify which β1 integrin heterodimer(s) was involved in blocking endocrine/mucous lineage commitment.
FIGURE 1.
β1 integrins regulate endocrine and mucous lineage commitment by HRA-19 cells. A, HRA-19 cells were seeded into 8-chamber plastic slides in serum-free medium with varying dilutions of β1 integrin mAbs JB1A or LM534. On Day 5, cells were fixed and stained for the endocrine lineage marker chromogranin using immunocytochemistry. Data shown are mean ± S.D. (n = 7). **, p < 0.001. Results are representative of three independent experiments. B, HRA-19 cells were grown in 8-chamber slides for 3 days and then transferred to serum-free medium. On Day 7, monolayers were stained with the colonic mucous antibody, PR4D4 using immunocytochemistry. Data shown are mean ± S.D. (n = 4) **, p < 0.001. The cell number was determined in replicate wells using the WST1 reagent (absorbance: 450/620 nm) (n = 4). Results are representative of three independent experiments.
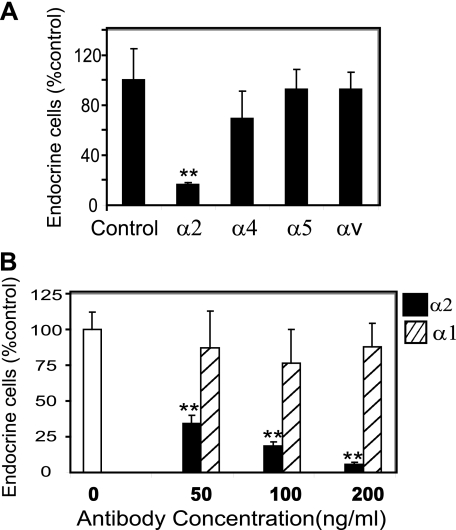
HRA-19 endocrine lineage commitment was induced in the presence of function-blocking antibodies to a range of α integrin chains known to form heterodimers with β1 integrin. Only antibodies to α2 integrin were shown to markedly reduce the ability of HRA-19 cells to generate endocrine cells while other α chain antibodies had no effect (Fig. 2A). The α2 chain antibody gave a dose-responsive inhibition of endocrine lineage commitment while an antibody recognizing the α1 chain of another collagen receptor, α1β1 integrin, had no effect at the same doses (Fig. 2B). Previous work has shown that α2 integrin only partners β1 integrin to form the α2β1 heterodimer (35); therefore these experiments suggest that α2β1 integrin regulates cell fate.
FIGURE 2.
α2 integrin regulates endocrine lineage commitment in HRA-19 cells. A, HRA-19 cells were seeded into 8-chamber slides in serum-free medium with and without antibodies to α2 (P1E6), α4 (P1H4), α5 (P1D6), and αv (P3G8) integrin, all at 500 ng/ml. Experiments were also attempted with α6 antibody (NKI-GoH3), but cell attachment was severely affected, and therefore data could not be collected. Data shown are mean ± S.D. (n = 3) **, p < 0.0001. Results are representative of a series of experiments performed with control and antibody-treated cells: α2 mAb P1E6 (five independent experiments), α4 mAb P1H4 (three independent experiments), α5 mAb P1D6 (two independent experiments), αv mAb P3G8 (three independent experiments). B, cells were seeded into 8-chamber slides in the presence of differing doses of antibodies to α1 integrin (FB12) or α2 integrin (PIE6). Data are the mean ± S.D. (n = 4) **, p < 0.0001. This experiment is representative of two independent experiments. Values are presented as % control for comparison.
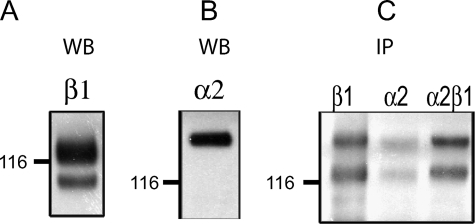
HRA-19 Cells Express α2β1 Integrin—Immunoblotting was used to analyze integrin expression in HRA-19 cells. Lysates contained two β1 integrin bands representing the immature (smaller band) and the mature glycosylated forms (Fig. 3A) (36). α2 integrin expression was also demonstrated (Fig. 3B). α2β1 integrin was demonstrated at the cell surface by biotinylation of live cells and immunoprecipitation with mAb to β1 integrin (Fig. 3C). Only the fully glycosylated β1 integrin band is seen at the cell surface along with a β1 integrin-associated protein which co-migrates with α2 integrin (Fig. 3C). Immunoprecipitation with antibodies to α2 integrin and α2β1 integrin complex also revealed two biotinylated protein bands corresponding in molecular weight to the α2 and β1 integrin (Fig. 3C).
FIGURE 3.
Integrin expression in HRA-19, human colorectal cancer cells. Western blot analysis of β1(A) and α2(B) integrin expression in lysates of HRA-19 cells. C, surface expression of α2β1 integrin in HRA-19 cells, demonstrated by biotinylation, lysis, and immunoprecipitation with antibodies to β1, α2, and α2β1 integrin. Biotinylated proteins were detected with streptavidinHRP.
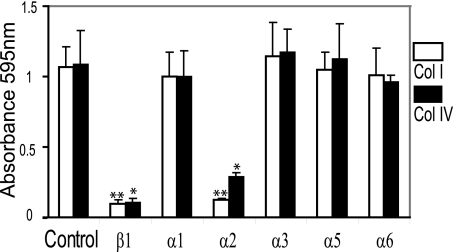
α2β1 Integrin Is a Collagen Receptor in HRA-19 Cells—α2β1 is a major collagen receptor (37) in many cell types. Cell adhesion experiments were used to establish whether α2β1-mediated collagen binding in HRA-19 cells. Attachment to collagen I and IV was blocked by antibodies to β1 and α2 integrin (Fig. 4), indicating that α2β1 integrin is a receptor for both collagen I and IV in HRA-19 cells. Antibodies recognizing other α chains did not significantly reduce cell binding to either collagen I or collagen IV (Fig. 4).
FIGURE 4.
α2β1 integrin is a collagen receptor in HRA-19, human colorectal cancer cells. Equal numbers of HRA-19 cells were seeded into wells coated with either human collagen I or IV and allowed to attach for 2 h at 37 °C. Attached cell number was measured using crystal violet staining and measurement at 595 nm: α1, 2 mg/ml; α2, 0.37 mg/ml; α3, 1.25 mg/ml; α5, 2 mg/ml; α6, 0.25 mg/ml; β1, 2253 5 mg/ml. Data are the mean ± S.D. (n = 3) **, p < 0.001; *, p < 0.005. Results are representative of a series of independent experiments performed on collagen I and collagen IV always including control wells and a range of antibodies; α1 (two experiments), α2 (four experiments), α3 (three experiments),α5 (three experiments), α6 (two experiments), β1 (five experiments).
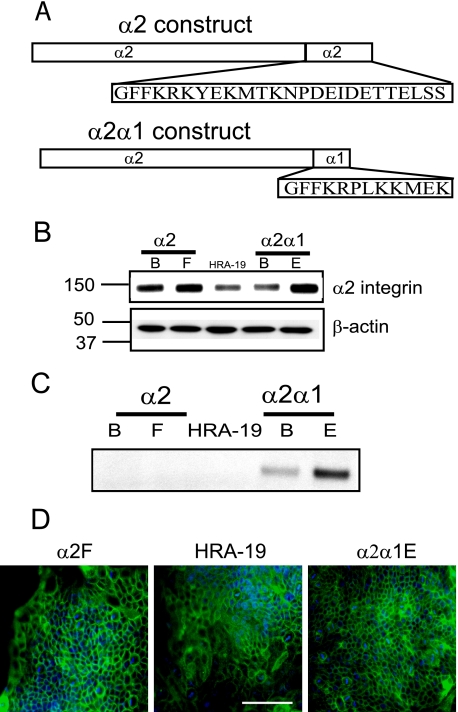
Integrin α2 Cytoplasmic Tail Is Required for Endocrine and Mucous Lineage Commitment—To support a role for the α2 integrin chain in cell fate regulation, we generated HRA-19 transfectants overexpressing either wild-type α2 integrin or a non-signaling chimeric protein composed of the extracellular and transmembrane domain of α2 integrin and the cytoplasmic domain of α1 integrin (Fig. 5A). Cell colonies were analyzed for their expression of α2 integrin (Fig. 5B) and α2α1 integrin (Fig. 5C). Two colonies, α2B and α2F, were chosen for further study as they showed markedly higher α2 integrin expression than the parent cell line (Fig. 5B). The chimeric protein was immunoprecipitated using an antibody to the cytoplasmic tail of α1 integrin and then detected on Western blots using an Ab to the extracellular region of the α2 chain (Fig. 5C). The α2 band was not observed in α1 immunoprecipitates of parent cells or α2 transfectants (α2B or α2F) but was present in chimeric transfectants α2α1B and α2α1E cells (Fig. 5C), which were selected for use in subsequent experiments. In the parent HRA-19 cells, α2 integrin is primarily localized at cell-cell contacts (Fig. 5D), as shown previously in the intestine (38), a localization retained by cells transfected with either wild-type α2 integrin ((α2F) or chimeric α2α1 integrin (α2α1E) (Fig. 5D).
FIGURE 5.
Expression ofα2 integrin constructs in HRA-19, human colorectal cancer cells. A, wild-type α2 and chimeric α2α1 integrin constructs transfected into HRA-19 cells. B, α2 integrin expression in α2 and α2α1 transfectants and HRA-19 cells. The experiment was performed twice. C, α2α1 integrin expression. α2α1 integrin was immunoprecipitated using an α1 cytoplasmic domain antibody, then detected using Western blot with an α2 extracellular domain antibody. The chimeric protein band was found only in α2α1-transfected colonies, α2α1β and α2α1E. The experiment was performed five times. D, α2 integrin localization was examined by immunofluorescence in α2F, HRA-19, and α2α1E cells. Bar, 100 μm.
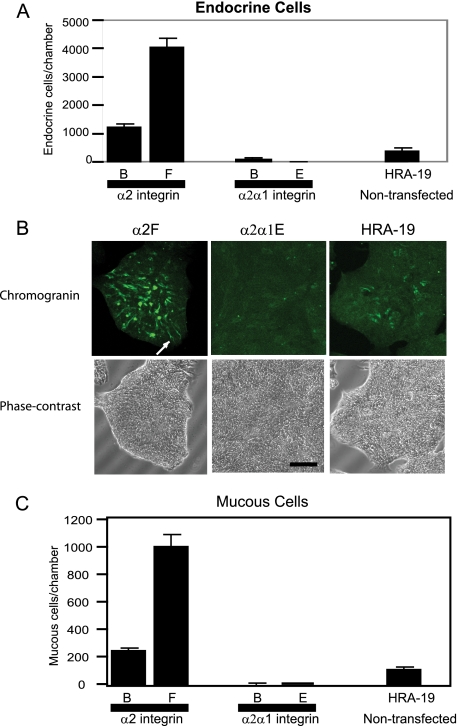
Parent cells, α2 and α2α1 transfectants were induced to undergo lineage commitment by growth in serum-free (ITA) medium. Endocrine cell numbers were much higher in the wild-type α2 transfectants, α2B and α2F, than in the chimeric transfectants α2α1B and α2α1E, which showed little endocrine lineage commitment (Fig. 6, A and B). α2F and α2α1E cells had the highest expression of α2 and α2α1 proteins, respectively (Fig. 5, B and C) and these colonies showed the most extreme phenotypes with α2F cells showing 10.5-fold higher endocrine lineage commitment than the parent cells while α2α1E cells show only 2% of parent endocrine cell lineage commitment. Immunofluorescence staining of HRA-19 monolayers for chromogranin shows differential endocrine lineage commitment (Fig. 6B) between parent cells and transfectants. Phase contrast images are included to show that cells are present in the α2α1E monolayers, but endocrine lineage commitment is negligible. α2F cells contain many typical chromogranin-positive endocrine cells with long processes (Fig. 6B, white arrow).
FIGURE 6.
α2 integrin regulates colorectal epithelial stem cell fate. A, endocrine lineage commitment (chromogranin expression) in cells after 48 h in serum-free medium. The mean ± S.D is shown. The experiment was performed three times. Endocrine and mucous cell numbers were normalized to an absorbance of 1 obtained with the WST-1 cell proliferation reagent to eliminate variation in cell number. Cells used were α2-transfected colonies α2B and α2F, chimeric α2α1-transfected colonies α2α1B and α2α1E and the parent non-transfected cell line HRA-19. B, chromogranin expression in α2F, α2α1E cells, and HRA-19 cells after 48 h in serum-free medium. Images obtained using a confocal microscope. The white arrow shows typical endocrine cell with a long process. Phase contrast images of the same fields. Bar, 100 μm. C, mucous lineage commitment in parent and transfected cell colonies detected with mucous antibody PR4D4 after 72 h in serum-free medium. The mean ± S.D. is shown. The experiment was performed three times.
To further investigate the lineage commitment program of the transfectants, we examined the ability of transfectants to generate mucous cells when transferred to serum-free medium (ITA). Again we found that α2F cell monolayers contained 9.8-fold parent cell mucous cell numbers while α2α1E cells contained only 4% of parent cell mucous numbers (Fig. 6C). These results strongly suggest that α2 integrin regulates colorectal epithelial cell fate by a mechanism requiring signaling via the α2 cytoplasmic tail.
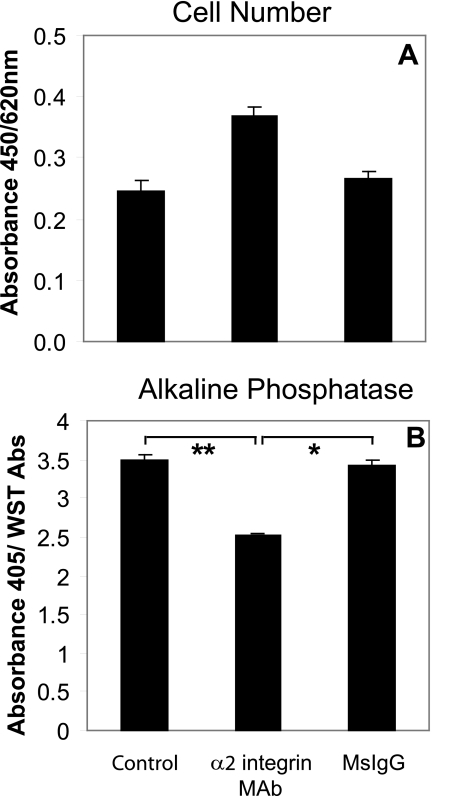
α2 Integrin Regulates Caco-2 Enterocytic Differentiation—To examine the wider significance of α2 integrin-mediated effects in human colon cancer cells, enterocytic differentiation was investigated in the well differentiated Caco-2 cell line. Caco-2 cells were shown to express the enterocytic differentiation marker, alkaline phosphatase when grown for several days in serum-free medium (TSG). The growth of cells on surfaces coated with an α2 integrin antibody increased cell proliferation (Fig. 7A) and reduced alkaline phosphatase expression (Fig. 7B). These results show that α2 integrin regulates differentiation in other colorectal carcinoma cells and can modulate enterocytic as well as endocrine and mucous lineage commitment.
FIGURE 7.
α2 integrin regulates enterocytic differentiation of Caco-2 cells. Caco-2 cells were seeded into control wells or wells coated with either α2 integrin Ab (AK7) or MsIgG (both 5 μg/ml) in TSG medium. A, cell number after 72 h in TSG medium measured with WST-1 reagent. B, alkaline phosphatase expression after 72 h in TSG medium normalized using WST-1 values; quadruplicate wells. Values shown are the mean ± S.D. **, p < 0.001; *, p < 0.005. The experiment was performed four times.
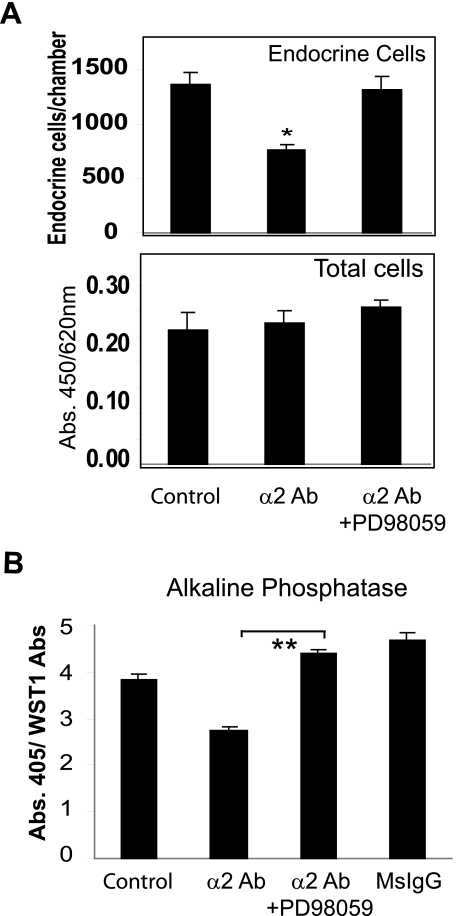
α2 Integrin Regulates Stem Cell Behavior via the ERK Signaling Pathway—The extracellular signal-regulated kinase (ERK MAPK) signaling pathway is important in intestinal epithelial differentiation (39, 40), and its dysregulation in colorectal cancer is thought to play a part in progression of this disease (41). The MEK inhibitor PD98059, which blocks ERK signaling, was used to determine whether α2 integrin regulation of lineage commitment was mediated via this signaling pathway. PD98059 abrogated the α2 integrin-mediated reduction in endocrine lineage commitment in HRA-19 cells (Fig. 8A) and enterocytic differentiation in Caco-2 cells (Fig. 8B) without a change in cell number (Fig. 8A).
FIGURE 8.
α2 integrin effects are mediated via the ERK signaling pathway. A, HRA-19 cells were transferred to ITA medium with or without the α2 integrin antibody P1E6 (25 ng/ml) and the MEK inhibitor PD98059 (10 μm) or DMSO control. Chromogranin-positive cells were detected by immunocytochemistry. The experiment was performed three times. Quadruplicate chambers were used for each condition. Mean ± S.D. is shown *, p < 0.005. The cell number was determined in replicate wells using the WST1 reagent (absorbance 450/620 nm). B, Caco-2 cells were plated in TSG medium onto surfaces coated with either α2 integrin mAb AK7 or MsIgG (10 μg/ml) with or without the MEK inhibitor PD98059 (10 μm). Alkaline phosphatase expression (abs: 405 nm) was normalized for cell number with the WST-1 reagent (abs 450 nm/abs 620 nm). Quadruplicate wells were used for each condition; mean ± S.D. The experiment was performed four times, **, p < 0.001.
DISCUSSION
The β1 integrin family of cell surface extracellular matrix receptors are known stem cell regulators, but their role in intestinal epithelial stem cell fate has yet to be established. To define the role of β1 integrins in cell fate decisions in multipotent human colorectal cancer cells, we induced lineage commitment in the presence of β1 integrin function-blocking antibodies. Endocrine and mucous lineage commitments were inhibited in the presence of β1 integrin Ab JB1A, which blocks β1 integrin-mediated adhesion and signaling (34). No change in morphology or cell adhesion was observed during antibody treatment, suggesting that the effects were on intracellular signaling rather than cell adhesion. Conditional knock-out of β1 integrin in adult mouse intestine results in enhanced proliferation and decreased differentiation suggesting perturbation of stem cell behavior (23). Somewhat surprisingly, β1 integrin knock-out did not appear to modulate intestinal cell adhesion, suggesting that a signaling, rather than an adhesive, function of β1 integrin was involved in specifying stem cell fate. Likewise, in this study, β1 integrin antibodies did not change cell morphology or perturb cell adhesion but markedly inhibited the ability of cells to undergo endocrine or mucous lineage commitment, suggesting that β1 integrin signaling is also involved in regulating the balance between cell renewal and lineage commitment in human colorectal cancer cells. These function-blocking experiments suggested a role for β1 integrin in regulating cell fate however β1 integrin partners with one of at least 12 α integrin chains to form matrix-specific heterodimers. Therefore, we sought to establish whether the observed effects of β1 integrin blockade were due to modulation of a specific αβ1 heterodimer(s). Endocrine lineage commitment was induced in HRA-19 cells in the presence of function-blocking antibodies to α integrin chains known to associate with β1 integrin. We show that a function-blocking antibody to the α2 integrin chain specifically and efficiently blocked endocrine lineage commitment by HRA-19 cells. As α2 integrin is only known to associate with β1 integrin, this finding suggests that a2β1 integrin is a regulator of stem cell fate. α2 integrin mAb and β1 integrin mAb gave similar blockade of endocrine lineage commitment suggesting that α2β1 integrin is the sole member of the β1 integrin family involved in cell fate determination. Our results support the lack of involvement of β1 integrins: α1β1, α4β1, α5β1, and αvβ1.
We next investigated α2β1 integrin expression in HRA-19 cells and showed α2 and β1 integrin expression by immunoblotting. Surface biotinylation following by immunoprecipitation demonstrated that α2β1 integrin is present on the HRA-19 cell surface and is the major β1 integrin heterodimer. Adhesion assays confirmed that α2β1 integrin was a collagen receptor mediating HRA-19 binding to collagen I and collagen IV.
To provide further evidence for a role of α2 integrin in specifying colorectal cancer stem cell fate and gain some mechanistic insight, multipotent colorectal cancer cells with permanent modifications to α2 integrin function were derived. Endocrine and mucous lineage commitment of colorectal cancer cells expressing highly elevated levels of wild-type α2 integrin were compared with parent cells and also cells expressing a non-signaling chimeric α2 integrin. This chimeric α2α1 integrin comprised the extracellular and transmembrane domain of the α2 chain but the cytoplasmic domain, crucial for α2-mediated cell signaling (42, 43), was replaced with that from the α1 chain. α1β1 integrin (another collagen receptor) did not appear to be endogenously expressed by HRA-19 cells as cell adhesion to collagen could not be blocked by antibodies to α1 integrin. Furthermore α1 integrin mAb did not modulate lineage commitment in these cells. HRA-19 cells expressing high levels of wild-type α2 integrin demonstrated a marked increase in both endocrine and mucous lineage commitment under serum-free conditions while cells expressing the chimeric protein showed a general failure to execute the colorectal lineage commitment program. These results suggest that α2β1 integrin regulates cell fate in human colorectal epithelial cells via a mechanism requiring the α2 cytoplasmic tail. Elevated α2β1 integrin expression is found on epidermal (16) and prostate stem cells (17). In the intestine, α2 integrin is expressed in the stem/progenitor cell zone and down-regulated during normal differentiation (22) suggesting a possible role for α2β1 in lineage commitment.
β1 integrin is a known stem cell regulator in a variety of stem cells; however, the question of which β1 integrin heterodimer(s) is involved has not yet been addressed. Our data raise the possibility that α2β1 integrin is the β1 heterodimer involved in regulating other stem cell types. Elevated α2β1 integrin expression is found on epidermal (16) and prostatic stem cells (17) while collagen, an α2β1 integrin ligand, blocks differentiation of mouse embryonic stem cells (44). Furthermore rare prostate cancer stem cells with self-renewal and differentiation potential have been isolated on the basis of a CD44+, α2β1 integrin+, CD133+ phenotype (45), suggesting shared characteristics between normal and neoplastic prostate epithelial stem cells.
To examine whether α2 integrin signaling was involved more widely in the differentiation of human colorectal cancer cells we investigated the well characterized cell line, Caco-2. Blockade of α2 integrin signaling in Caco-2 cells with function-blocking antibody was shown to promote proliferation and inhibit differentiation, again supporting a role for α2β1 integrin in balancing cell renewal and differentiation. Previous studies have linked α2 integrin function with the ERK signaling pathway (46) in human colon cancer cells. Furthermore normal intestinal stem cells express the MAPK family member ERK1 (47) while loss of ERK activation accompanies intestinal epithelial differentiation in vitro (40). This suggests a role for ERK signaling in maintaining self-renewal in intestinal epithelial stem cells. To determine whether α2β1 integrin-mediated effects required ERK signaling, we used the MEK signaling inhibitor PD98059, which abrogated the ability of α2 integrin antibodies to block endocrine lineage commitment in HRA-19 cells and enterocytic differentiation in Caco-2 cells. These preliminary results suggest that α2 integrin regulates ERK signaling, although further experiments will be required to confirm this possibility and identify other cell signaling pathways triggered by α2β1 integrin.
Several studies have suggested a link between α2β1 integrin and the development of human cancer. A functional association exists between α2β1 integrin and the EGF receptor (48, 49), a kinase whose aberrant signaling is associated with many cancer types including colorectal cancer where anti-EGFR reagents are under investigation as potential therapeutic agents (50). In addition, α2β1 integrin has been implicated as a promoter of malignant phenotype in pancreatic cancer cells (51) and metastasis to bone (52). Finally, it is intriguing that E-cadherin, a tumor suppressor, is found to be a ligand for α2β1 integrin (53). The functional significance of this finding remains uncertain but E-cadherin-α2β1 integrin interaction could be involved in the modulation of Wnt signaling as E-cadherin also binds β-catenin, a pivotal protein in this pathway.
Evidence is accumulating to support the idea that human colorectal cancer is a stem cell disease. Cancer stem cells are thought to initiate tumor growth and generate heterogeneity within tumor cell populations, which suggests that successful therapy will depend upon elimination of cancer stem cells. However many questions remain about the role that cancer stem cells play in cancer development (54) and much remains unknown about the molecular mechanisms, which balance self-renewal and lineage commitment in normal and neoplastic colorectal epithelial cells.
Our study indicates that α2 integrin regulates cell fate in cloned multipotent human colorectal cancer cells (HRA-19) probably via α2β1 integrin signaling. Previous studies support a role for β1 integrins as stem cell regulators in normal intestinal epithelium, suggesting that colorectal cancer cells retain elements of integrin-regulated cell fate decisions. Identification of the molecular mechanisms that regulate colorectal epithelial cell fate may explain the diminished differentiation that is the hallmark of colorectal cancer and suggest new therapeutic strategies.
Acknowledgments
We thank Carine de Bettignies for technical assistance. We thank Dr Johanna Ivaska, University of Turku, Finland for his generous gift of the integrin constructs.
This work was supported by Grant 060688 from The Wellcome Trust. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.

Author's Choice—Final version full access.
Footnotes
The abbreviations used are: Ab, antibody; mAb, monoclonal antibody; DMEM, Dulbecco's modified Eagle's medium; ERK, extracellular signal-regulated kinase.
S. C. Kirkland, unpublished observations.
References
- 1.Brittan, M., and Wright, N. A. (2004) Gut 53 899-910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sancho, E., Batlle, E., and Clevers, H. (2004) Annu. Rev. Cell Dev. Biol. 20 695-723 [DOI] [PubMed] [Google Scholar]
- 3.Reya, T., and Clevers, H. (2005) Nature 434 843-850 [DOI] [PubMed] [Google Scholar]
- 4.Dalerba, P., Cho, R. W., and Clarke, M. F. (2007) Annu. Rev. Med. 58 267-284 [DOI] [PubMed] [Google Scholar]
- 5.Jordan, C. T., Guzman, M. L., and Noble, M. (2006) N. Engl. J. Med. 355 1253-1261 [DOI] [PubMed] [Google Scholar]
- 6.Dalerba, P., Dylla, S. J., Park, I.-K., Liu, R., Wang, X., Cho, R. W., Hoey, T., Gurney, A., Huang, E. H., Simeone, D. M., Shelton, A. A., Parmiani, G., Castelli, C., and Clarke, M. F. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 10158-10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien, C. A., Pollett, A., Gallinger, S., and Dick, J. E. (2007) Nature 445 106-110 [DOI] [PubMed] [Google Scholar]
- 8.Ricci-Vitiani, L., Lombardi, D. G., Pilozzi, E., Biffoni, M., Todaro, M., Peschle, C., and De Maria, R. (2007) Nature 445 111-115 [DOI] [PubMed] [Google Scholar]
- 9.Ohlstein, B., Kai, T., Decotto, E., and Spradling, A. (2004) Curr. Opin. Cell Biol. 16 693-699 [DOI] [PubMed] [Google Scholar]
- 10.Moore, K. A., and Lemischka, I. R. (2006) Science 311 1880-1885 [DOI] [PubMed] [Google Scholar]
- 11.Matsubara, T., Tsutsumi, S., Pan, H., Hiraoka, H., Oda, R., Nishimura, M., Kawaguchi, H., Nakamura, K., and Kato, Y. (2004) Biochem. Biophys. Res. Commun. 313 503-508 [DOI] [PubMed] [Google Scholar]
- 12.Flaim, C. J., Chien, S., and Bhatia, S. N. (2005) Nat. Methods 2 119-125 [DOI] [PubMed] [Google Scholar]
- 13.Miranti, C., and Brugge, J. (2002) Nat. Cell Biol. 4 83-90 [DOI] [PubMed] [Google Scholar]
- 14.Hynes, R. (1992) Cell 69 11-25 [DOI] [PubMed] [Google Scholar]
- 15.Comoglio, P. M., Boccaccio, C., and Trusolino, L. (2003) Curr. Opin. Cell Biol. 15 565-571 [DOI] [PubMed] [Google Scholar]
- 16.Jones, P., Harper, S., and Watt, F. (1995) Cell 80 83-93 [DOI] [PubMed] [Google Scholar]
- 17.Collins, A. T., Habib, F. K., Maitland, N. J., and Neal, D. E. (2001) J. Cell Sci. 114 3865-3872 [DOI] [PubMed] [Google Scholar]
- 18.Campos, L. S., Leone, D. P., Relvas, J. B., Brakebusch, C., Fassler, R., Suter, U., and French-Constant, C. (2004) Development 131 3433-3444 [DOI] [PubMed] [Google Scholar]
- 19.Zhu, A. J., Haase, I., and Watt, F. M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 6728-6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leone, D. P., Relvas, J. B., Campos, L. S., Hemmi, S., Brakebusch, C., Fassler, R., ffrench-Constant, C., and Suter, U. (2005) J. Cell Sci. 118 2589-2599 [DOI] [PubMed] [Google Scholar]
- 21.Rohwedel, J., Guan, K., Zuschratter, W., Jin, S., Ahnert-Hilger, G., Furst, D., Fassler, R., and Wobus, A. M. (1998) Dev. Biol. 201 167-184 [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto, K., Beauchamp, R., and Whitehead, R. (2002) Gastroenterology 123 1941-1948 [DOI] [PubMed] [Google Scholar]
- 23.Jones, R. G., Li, X., Gray, P. D., Kuang, J., Clayton, F., Samowitz, W. S., Madison, B. B., Gumucio, D. L., and Kuwada, S. K. (2006) J. Cell Biol. 175 505-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkland, S. (1988) Cancer 61 1359-1363 [DOI] [PubMed] [Google Scholar]
- 25.Kirkland, S. C., and Henderson, K. (2001) J. Cell Sci. 114 2055-2064 [DOI] [PubMed] [Google Scholar]
- 26.Ni, H., and Wilkins, J. (1998) Cell Adh. Commun. 5 257-271 [DOI] [PubMed] [Google Scholar]
- 27.Fabbri, M., Castellani, P., Gotwals, P., Kotelianski, V., Zardi, L., and Zocchi, M. (1996) Tissue Antigens 48 47-51 [DOI] [PubMed] [Google Scholar]
- 28.Hers, I., Berlanga, O., Tiekstra, M. J., Kamiguti, A. S., Theakston, R. D. G., and Watson, S. P. (2000) Eur. J. Biochem. 267 2088-2097 [DOI] [PubMed] [Google Scholar]
- 29.Wayner, E., Carter, W., Piotrowicz, R., and Kunicki, T. (1988) J. Cell Biol. 107 1881-1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crook, M., Sothgate, K., and Newby, A. (2002) J. Vasc. Res. 39 221-229 [DOI] [PubMed] [Google Scholar]
- 31.Jiang, F., Georges-Labouesse, E., and Harrison, L. (2001) Mol. Med. 7 107-114 [PMC free article] [PubMed] [Google Scholar]
- 32.Wayner, E., Orlando, R., and Cheresh, D. (1991) J. Cell Biol. 113 919-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson, K., and Kirkland, S. C. (1996) Differentiation 60 259-268 [DOI] [PubMed] [Google Scholar]
- 34.Radel, C., and Rizzo, V. (2005) Am. J. Physiol. Heart Circ. Physiol. 288 H936-H945 [DOI] [PubMed] [Google Scholar]
- 35.Hynes, R. O. (2002) Cell 110 673-687 [DOI] [PubMed] [Google Scholar]
- 36.Heino, J., Ignotz, R., Hemler, M., Crouse, C., and Massague, J. (1989) J. Biol. Chem. 264 380-388 [PubMed] [Google Scholar]
- 37.Heino, J. (2000) Matrix Biol. 19 319-323 [DOI] [PubMed] [Google Scholar]
- 38.Lussier, C., Basora, N., Bouatrouss, Y., and Beaulieu, J. (2000) Microsc. Res. Technique 51 169-178 [DOI] [PubMed] [Google Scholar]
- 39.Ding, Q., Wang, Q., and Evers, B. (2001) Biochem. Biophys. Res. Commun. 284 282-288 [DOI] [PubMed] [Google Scholar]
- 40.Taupin, D., and Podolsky, D. (1999) Gastroenterology 116 1072-1080 [DOI] [PubMed] [Google Scholar]
- 41.Fang, J. Y., and Richardson, B. C. (2005) Lancet Oncol. 6 322-327 [DOI] [PubMed] [Google Scholar]
- 42.Ivaska, J., Reunanen, H., Westermarck, J., Koivisto, L., Kahari, V.-M., and Heino, J. (1999) J. Cell Biol. 147 401-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klekotka, P. A., Santoro, S. A., Wang, H., and Zutter, M. M. (2001) J. Biol. Chem. 276 32353-32361 [DOI] [PubMed] [Google Scholar]
- 44.Chen, S. S., Fitzgerald, W., Zimmerberg, J., Kleinman, H. K., and Margolis, L. (2007) Stem Cells 25 553-561 [DOI] [PubMed] [Google Scholar]
- 45.Collins, A. T., Berry, P. A., Hyde, C., Stower, M. J., and Maitland, N. J. (2005) Cancer Res. 65 10946-10951 [DOI] [PubMed] [Google Scholar]
- 46.Sawhney, R. S., Sharma, B., Humphrey, L. E., and Brattain, M. G. (2003) J. Biol. Chem. 278 19861-19869 [DOI] [PubMed] [Google Scholar]
- 47.Stappenbeck, T. S., Mills, J. C., and Gordon, J. I. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 1004-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, X., Miyamoto, S., and Mekada, E. (2000) J. Cell Sci. 113 2139-2147 [DOI] [PubMed] [Google Scholar]
- 49.Ning, Y., Buranda, T., and Hudson, L. G. (2007) J. Biol. Chem. 282 6380-6387 [DOI] [PubMed] [Google Scholar]
- 50.Scartozzi, M., Pierantoni, C., Berardi, R., Squadroni, M., and Cascinu, S. (2006) Exp. Opin. Therap. Targets 10 281-287 [DOI] [PubMed] [Google Scholar]
- 51.Grzesiak, J., and Bouvet, M. (2006) Br. J. Cancer 94 1311-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall, C. L., Dai, J., van Golen, K. L., Keller, E. T., and Long, M. W. (2006) Cancer Res. 66 8648-8654 [DOI] [PubMed] [Google Scholar]
- 53.Whittard, J. D., Craig, S. E., Mould, A. P., Koch, A., Pertz, O., Engel, J., and Humphries, M. J. (2002) Matrix Biol. 21 525-532 [DOI] [PubMed] [Google Scholar]
- 54.Hill, R. P. (2006) Cancer Res. 66 1891-1896 [DOI] [PubMed] [Google Scholar]