Abstract
Our current paradigm for obesity assumes that reduced thermogenic capacity increases susceptibility to obesity, whereas enhanced thermogenic capacity protects against obesity. Here we report that elimination of two major thermogenic pathways encoded by the mitochondrial uncoupling protein (Ucp1) and mitochondrial glycerol-3-phosphate dehydrogenase (Gdm) result in mice with increased resistance to diet-induced obesity when housed at 28 °C, provided prior adaptation occurred at 20 °C. Obesity resistant Gdm-/-·Ucp1-/- mice maintained at 28 °C have increased energy expenditure, in part through conversion of white to brown adipocytes in inguinal fat. Increased oxygen consumption in inguinal fat cell suspensions and the up-regulation of genes of mitochondrial function and fat metabolism indicated increased thermogenic activity, despite the absence of UCP1, whereas liver and skeletal muscle showed no changes in gene expression. Additionally, comparisons of energy expenditure in UCP1-deficient and wild type mice fed an obesogenic diet indicates that UCP1-based brown fat-based thermogenesis plays no role in so-called diet-induced thermogenesis. Accordingly, a new paradigm for obesity emerges in which the inactivation of major thermogenic pathways force the induction of alternative pathways that increase metabolic inefficiency.
Obesity is a disorder of energy homeostasis that results when energy intake exceeds energy expenditure. This simplistic relationship belies the complexity of the behavioral and physiological mechanisms underlying phenotypic variability among humans in both energy intake and energy expenditure (1–3). A homeostatic model has been developed based on neuroendocrine polypeptides, secreted by peripheral tissues, that act on centers in the hypothalamus to reciprocally regulate feeding behavior and energy expenditure. Although it has been proposed that these neuropeptides act simultaneously to regulate energy intake and expenditure, the thermogenic targets in peripheral tissues are largely unknown (4–6). Indeed, little attention has been given to centrally controlled thermogenic mechanisms that are independent of mitochondrial uncoupling proteins (UCPs),3 either UCP1, for which there is strong evidence for a role in rodent models of obesity (7), or the UCP homologues that continue to be proposed for mitochondrial uncoupling functions with little supporting evidence (8, 9). Given the paucity of evidence for UCP1 activity in adult tissues, there is a pressing need to identify novel mechanisms for activating thermogenesis that can be applied to the obesity problem.
The uncoupling of mitochondrial substrate oxidation from ATP synthesis by UCP1 in brown adipose tissue is widely recognized for its role in maintaining a normal body temperature during exposure to cold (10–13). There is also strong evidence that inducing UCP1-based thermogenesis through pharmacological or genetic manipulation reduces excessive adiposity (14). However, these experimentally induced phenotypes may not be indicative of a normal physiological mechanism for the homeostatic maintenance of energy balance. Indeed, the response of UCP1-deficient mice and wild type mice to a high fat diet is strongly affected by ambient temperature. Mutant and wild type mice maintained near thermoneutrality have the same level of adiposity when fed a high fat diet (15). However, as the ambient temperature declines, the resistance of UCP1-deficient mice to diet-induced obesity (DIO) suggests that alternative thermogenic mechanisms are activated/induced to generate sufficient heat to maintain body temperature. Unlike brown fat thermogenesis, these alternative biochemical and physiological mechanisms appear to be less efficient for the production and distribution of heat, requiring expenditure of more calories to maintain body temperature and thereby indirectly reducing adiposity.
Inguinal fat depots in UCP1-deficient mice, acclimated to cold, acquire the morphology of brown adipocytes, with gene expression data indicating an enhanced capacity for β-oxidation of fatty acids in mitochondria, and altered patterns of protein expression for phospholambans, regulators of the SERCA activity, suggested that a thermogenic mechanisms based upon Ca2+ cycling may have been induced (16). To enhance our ability to detect and evaluate alternative thermogenic mechanisms, we have achieved a further reduction in the endogenous thermogenic capacity of Ucp1 mutant mice by the targeted inactivation of the mitochondrial glycerol-3-phosphate dehydrogenase gene (Gdm) (17). The glycerol-3-phosphate shuttle has been proposed as a source of metabolic inefficiency, because of the generation of only two instead of three ATPs/mol of NADH generated by glycolysis (18). The protein levels of GDM and the cytoplasmic glycerol-3-phosphate dehydrogenase are much higher in interscapular brown adipose tissue than any other tissue (19, 20), suggesting that heat production from the glycerol phosphate shuttle is a parallel thermogenic pathway that could independently supplement UCP1 thermogenesis. Here we report that Gdm-/-·Ucp1-/- mice fed a high fat diet are more resistant to dietary obesity than mice lacking either UCP1 or GDM. The resistance of Gdm-/-·Ucp1-/- mice to DIO depends on a prior conditioning at a lower ambient temperature (20 °C). In addition, and most importantly, changes in the inducible thermogenic capacity that reduced DIO were sustained for a period of 10 weeks, even after switching to a higher ambient temperature (28 °C). We propose that in the absence of two recognized heat-generating systems, by being forced to utilize metabolically costly and less efficient alternative thermogenic mechanisms for maintaining body temperature, the Gdm-/-·Ucp1-/- mice become highly resistant to DIO and have increased insulin sensitivity.
EXPERIMENTAL PROCEDURES
Animals and Study Design—Gdm-/-·Ucp1-/- mice on a C57BL/6J (B6) genetic background were generated as follows: Gdm-/-·Ucp1+/+ mice were crossed with Gdm+/+·Ucp1-/- to generate the F1 offspring (Gdm+/-·Ucp1+/-) that were intercrossed to generate the F2 mice. The double knock-out (Gdm-Ucp1-/-) mice were fully fertile and maintained by full-sib mating. Only male mice were studied. In the studies conducted, 10–12-week-old mice were single-housed, fed rodent chow (PicoLab Rodent Diet 20; LabDiet, Richmond, IN) ad libitum, and reared at 28 °C ambient temperature. All of the animal experiments were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee in accordance with National Institutes of Health guidelines for care and use of laboratory animals.
Acute Cold Exposure Experiment—Mice previously maintained at 28 °C were exposed to an ambient temperature of 4 °C, and body temperature was measured every 60 min for 3 h with a rectal probe (TH-8; Physitemp Instruments Inc., Clifton, NJ).
Cold Adaptation Experiment—Mice maintained at 28 °C for at least 1 week, following a test for acute sensitivity to cold, were then subjected to a slow reduction (2 °C daily) of ambient temperature until the temperature reached 4 °C. The mice were kept at 4 °C for 24–48 h prior to sacrifice.
High Fat Diet Experiments—Three studies were conducted in which the mice were fed a high fat diet (D12230; Research Diets Inc., New Brunswick, NJ). In Experiment 1, the mice fed a high fat diet were reared at 20 °C ambient temperature for 10 weeks and then at 28 °C for another 10 weeks. In Experiment 2, the mice fed a high fat diet were reared at 28 °C ambient temperature for 8 weeks. In Experiment 3, the mice fed a high fat diet were reared at 20 °C ambient temperature for 4 weeks and then at 28 °C for another 4 weeks.
Phenotypes of Energy Balance—Body composition was analyzed by NMR (Bruker). Oxygen consumption, carbon dioxide production, and physical activity of individual mice were measured in 16-chamber Oxymax lab animal monitoring system, CLAMS (Columbus Instruments, Columbus, OH), housed in a temperature-controlled incubator.
Quantitative Reverse Transcription-PCR—Total RNA was prepared from tissues homogenized in TRI reagent (Molecular Research Center Inc., Cincinnati, OH). Genomic DNA was removed from total RNA by treating with RNase-free DNase (Qiagen). RNA was further purified using the RNeasy kit (Qiagen) and protected from RNase degradation by treatment with SUPERase-In (Ambion, Austin, TX). The quality and quantity of the isolated RNA were assessed using a NanoDrop spectrometer (NanoDrop Technologies, Wilmington, DE). Quantitative reverse transcription-PCR was performed using total RNA with specific primers and probes designed using Primer-Express™, version 2.0.0 (Applied Biosystems, Foster City, CA). TaqMan probes were used for quantification of some target genes using the TaqMan one-step reverse transcriptase PCR mastermix (Applied Biosystems). Other target genes were quantified using SYBR® Green PCR mastermix (Applied Biosystems). All of the gene expression data were normalized to the level of cyclophilin B. Primer and probe sequences are available upon request.
Metabolite and Hormone Determinations—Glucose tolerance and insulin tolerance tests were performed after overnight fasting using an intraperitoneal injection of 20% glucose solution (2 g/kg of body weight) or insulin (0.5 IU/kg of body weight; Humulin R, Eli Lilly and Company, Indianapolis, IN). Blood glucose levels were determined using a OneTouch Profile blood glucose meter (LifeScan Inc., Milpitas, CA). Blood triglyceride and ketone levels were measured using a Cardiochek PA Professional meter (HealthCheck Systems, Brooklyn, NY). Serum lactic acid levels were measured using a lactate assay kit (Trinity BioTech, Berkeley Heights, NJ). Tissue triglycerides were measured in chloroform/methanol extracts using the L-type TG H kit (Wako Chemicals, Richmond, VA). Total serum triiodothyronine (T3) and thyroxine (T4) levels were measured by enzyme immunoassay (Alpco Diagnostics, Salem, NH).
Histology of Inguinal Fat—Inguinal fat pads were fixed in Bouin's solution (Sigma-Aldrich), and paraffin-embedded sections were stained with hematoxylin-eosin and examined using a Zeiss Axioskop 40 microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Tissue Oxygen Consumption—Oxygen consumption in inguinal adipose tissue was measured with a Clark-type polarographic oxygen sensor (Oxygraph, Hansatech Instruments, Norfolk, UK). Freshly dissected inguinal adipose tissue was finely minced in a freshly oxygenated (95% O2, 5% CO2) Krebs-Ringer phosphate buffer (pH 7.4) with 1% fatty acid-free bovine serum albumin. All measurements were performed with minced tissue in bovine serum albumin-free Krebs-Ringer bicarbonate buffer (pH 7.4). Basal respiration was monitored for 1–2 min, after which succinate (5 mm) was added, and respiration was measured for 5–7 min. Tissue oxygen consumption was normalized to DNA content, which was quantified using the fluorescent dye bisbenzimide (Hoechst 33258; Sigma) (21).
Statistical Analysis—The data are expressed as the means ± S.E. Unpaired t test was used to compare differences between groups (Statview, version 5.0.1; SAS Institute Inc., Cary, NC). Analysis of variance with Bonferroni post hoc test was used when more than two groups were compared.
RESULTS
Gdm-/-·Ucp1-/- Mice Increase Energy Expenditure during Cold Adaptation—To determine whether Gdm-/-·Ucp1-/- mice are able to survive acute cold exposure, wild type and Gdm-/-·Ucp1-/- mice maintained at 28 °C were placed at 4 °C. Wild type mice were fully able to defend their body temperature, whereas 100% of the Gdm-/-·Ucp1-/- became hypothermic, with their body temperature dropping to less than 30 °C within 1–3 h of cold exposure (supplemental Table S1). Cold-sensitive mutant mice were immediately returned to a 28 °C ambient temperature and allowed to recover. However, when the ambient temperature of these recovered Gdm-/-·Ucp1-/- mice was gradually reduced at a rate of 2 °C per day, 80–90% of the mice were able to tolerate an ambient temperature of 4 °C (supplemental Table S1). This ability to adapt to cold ambient temperatures resembles our previous findings with the Ucp1-/- mice. Gdm-/- mice are cold tolerant (17), unless they are made hypothyroid (22), and the Gdm-/-·Ucp1-/- mice do not appear to have increased cold intolerance compared with Ucp1-/- mice.
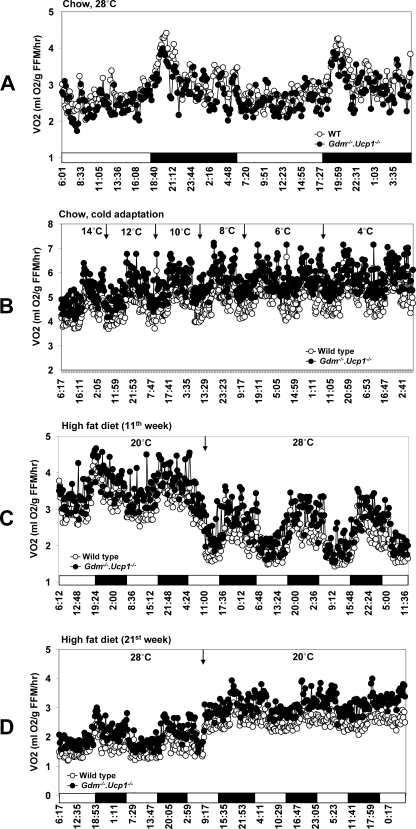
To determine the mechanism by which Gdm-/-·Ucp1-/- mice are able to survive cold adaptation, we measured energy expenditure using indirect calorimetry. At 28 °C, wild type mice had slightly higher oxygen consumption compared with Gdm-/-·Ucp1-/- mice (Fig. 1A); however, during acclimation to the cold environment, Gdm-/-·Ucp1-/- had higher VO2 than wild type mice (Fig. 1B). Because similar increases in VO2 consumption during cold adaptation were observed in our previous studies of cold-adapted Ucp1-/- mice and T3 or leptin-treated Lepob/ob·Ucp1-/- mice, an enhanced capacity to stimulate oxygen consumption over that normally found for wild type mice appears to be an essential requirement for adaptation to the cold. Gene expression analyses of tissues from wild type and Gdm-/-·Ucp1-/- mice showed that, similar to the adaptive response of Ucp1-/- mice (16), adaptation of Gdm-/-·Ucp1-/- mice to the cold (4 °C) was accompanied by induction of genes associated with fat oxidation, mitochondrial biogenesis, mitochondria transporters, and transcription factors known to regulate these genes. This induction in gene expression was found in inguinal fat and red and white gastrocnemius skeletal muscle but not in liver (Table 1 and supplemental Tables S2–S5).
FIGURE 1.
Effect of diet and ambient temperature on oxygen consumption (VO2) in WT and Gdm-/-·Ucp1-/- mice. A, mice were fed chow diet and kept at 28 °C. B, mice were acclimated to cold until ambient temperature reached 4 °C. C and D, mice were fed a high fat diet for 20 weeks and reared at 20 °C for 10 weeks and at 28 °C for 10 weeks. At the 11th week of the experiment, the temperature was switched from 20 to 28 °C (C). At the 21st week, temperature was switched from 28 to 20 °C (D).
TABLE 1.
Summary of gene expression in WT and Gdm–/–·Ucp1–/– mice during cold adaptation with chow diet and with high fat diet for 20 weeks (20 °C for 10 weeks and at 28 °C for 10 weeks) The values in bold type are significantly different at p < 0.05. The number of mice used was five/genotype in each study.
|
Gene
|
Ratio of
Gdm–/–·Ucp1–/–
to WT
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
Cold adaptation to 4 °C with chow diet
|
High fat diet at 20 °C for 10 weeks and at 28 °C for 10 weeks
|
|||||||
|
Liver
|
Gas
|
Inguinal fat
|
Liver
|
Gas
|
Inguinal fat
|
|||
| Red | White | Red | White | |||||
| Glucose metabolism | ||||||||
| Glut2 | 1.0 | 0.8 | ||||||
| Glut4 | 1.6 | 1.4 | 2.3 | 1.0 | 1.1 | 2.7 | ||
| Pdk4 | 0.8 | 1.8 | 6.7 | 0.5 | 1.9 | 6.7 | ||
| Pepck | 0.4 | 2.3 | 10.4 | 1.2 | 2.5 | 0.8 | 3.6 | 36.8 |
| Lipid metabolism | ||||||||
| Acox | 0.7 | 1.9 | 1.9 | 1.2 | 0.7 | 0.9 | 1.3 | 1.7 |
| Acsl2 | 0.8 | 1.1 | 1.6 | 0.9 | 1.3 | 0.9 | 1.0 | 1.6 |
| Hsl | 2.2 | 1.9 | 1.0 | 1.9 | 0.6 | 1.2 | 2.1 | |
| Cpt1a | 0.6 | 0.3 | ||||||
| Cpt1b | 1.4 | 1.2 | 6.4 | 0.7 | 0.9 | 9.3 | ||
| Scd1 | 0.8 | 1.7 | 1.6 | 1.3 | 0.4 | 0.7 | 0.9 | 2.1 |
| Mitochondrial function | ||||||||
| Slc25a25 | 0.7 | 1.8 | 3.7 | 0.4 | 0.8 | 0.7 | 1.1 | 2.8 |
| Mt-co2 | 0.9 | 1.6 | 1.4 | 1.3 | 0.7 | 0.7 | 0.9 | 2.6 |
| Ion transport | ||||||||
| Slc20a2 | 1.2 | 1.4 | 1.7 | 0.9 | 1.0 | 2.3 | ||
| Transcription | ||||||||
| Pgc1-α | 2.2 | 2.7 | 3.3 | 1.8 | 1.0 | 1.2 | 1.1 | 14 |
| PPARα | 0.8 | 2.9 | 3.1 | 3.2 | 1.3 | 1.1 | 1.5 | 10.8 |
| PPARδ | 1.0 | 1.7 | 1.8 | 1.3 | 0.4 | 1.1 | 1.0 | 1.8 |
| PPARγ2 | 1.4 | 1.4 | 0.5 | 0.3 | 1.3 | 1.9 | 2.5 | |
| Srebp1a | 0.4 | 0.8 | 1.1 | 1.1 | 0.7 | 0.8 | 0.9 | 1.5 |
| Srebp1c | 1.2 | 0.8 | 1.2 | 0.5 | 1.2 | 1.1 | 1.2 | 2.3 |
| Thyroid metabolism | ||||||||
| T4-Dio2 | 0.7 | 0.7 | 6.3 | 0.8 | 1.3 | 30.3 | ||
| Calcium cycling | ||||||||
| Serca2a | 1.3 | 1.0 | 1.2 | 1.3 | 0.6 | 1.0 | 1.5 | 1.7 |
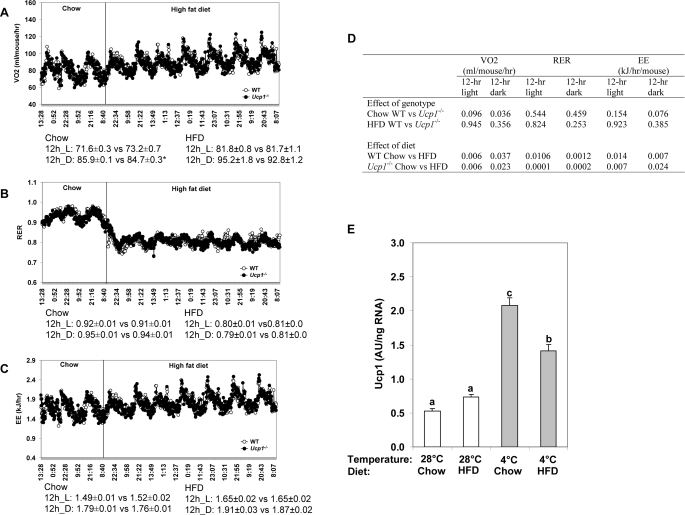
Gdm-/-·Ucp1-/- Mice Are Protected from Diet-induced Obesity—A central tenet of thermogenesis and obesity has been that UCP1 is the major thermogenic mechanism underlying diet-induced thermogenesis (4, 23). Accordingly, energy expenditure was expected to be lower in UCP1-deficient mice than in wild type mice fed a high fat-high sucrose diet. In addition, fat oxidation may also be suppressed in the UCP1-deficient mice. We tested this hypothesis and found, that there was no difference in oxygen consumption between wild type and UCP1-deficient mice when they were fed either a low fat chow diet or an obesogenic diet (Fig. 2, A and D). Upon switching to the obesogenic diet, oxygen consumption increased equally in both Ucp1-/- and wild type mice (Fig. 2, A and D). Furthermore, the RER showed the expected drop from ∼0.92 to 0.78 when the fat composition of the diet was increased, and similar to the oxygen consumption data, the values are indistinguishable between Ucp1-/- and wild type mice (Fig. 2, B and D). Accordingly, the experiment showed highly significant effects of diet on both energy expenditure and substrate utilization, but no significant genotype effects (Fig. 2, B–D). Consistent with this energy expenditure data, Ucp1 mRNA levels in the interscapular brown fat of wild type mice fed chow or obesogenic diets at both 28 and 4 °C were not significantly different from each other; if anything the HF diet suppressed Ucp1 mRNA induction when mice were exposed to an ambient temperature of 4 °C for 1 week (Fig. 2E). This evidence does not support the idea that UCP1 is part of a mechanism for diet-induced thermogenesis in mice fed an obesogenic/cafeteria diet; rather, the increase in oxygen consumption when animals switch from a diet high in carbohydrates to one rich in lipids is likely a consequence in the reduction in P/O ratio when fat is metabolized compared with carbohydrate (24).
FIGURE 2.
Analysis of diet-induced thermogenesis in Ucp1-/- and wild type mice. Effect of chow and high fat diet (HFD) on oxygen consumption (VO2) (A), RER (B), and energy expenditure (C) in WT (n = 8; body weight = 29.7 ± 0.01) and Ucp1-/- (n = 8; body weight = 28.4 ± 0.73) mice at 23 °C. The values presented under each graph correspond to the means ± S.E. in WT and Ucp1-/-, respectively. *, p < 0.05. D, statistical analysis (p values) for the effects of genotype and diet on VO2, RER, and energy expenditure. E, effect of ambient temperature (28 °C versus 4 °C) and diet (chow versus high fat diet) on Ucp1 gene expression in brown adipose tissue of WT mice (n = 9/group). The mice were fed chow or high fat diet (HFD) and kept at 4 or 28 °C ambient temperature for 1 week. a, b, and c indicate means that are statistically significant at p < 0.05.
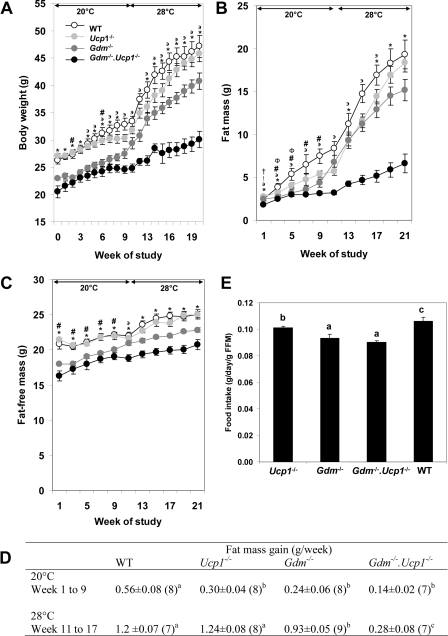
If it is calorically more costly to maintain body temperature by UCP1-independent thermogenesis, then an additional deletion of a thermogenic pathway should further reduce the development of adiposity. Accordingly, we evaluated the progressive loss of thermogenic mechanisms on the effects of ambient temperature on the development of DIO in wild type, Ucp1-/-, Gdm-/-, and Gdm-/-·Ucp1-/- mice, rationalizing that the deletion of a second thermogenic mechanism would further increase the demand for alternative thermogenesis and increase resistance to DIO. Ten-week-old mutant and wild type mice were fed a high fat diet at an ambient temperature of 20 °C for 10 weeks, and then the ambient temperature was raised to 28 °C for another 10 weeks (Fig. 3). The rate of body weight gain at 20 °C was slower for all genotypes than at 28 °C and more attenuated for the Ucp -/-, Gdm-/-, and Gdm-/-·Ucp1-/- than wild type mice (Fig. 3A). The effects of ambient temperature were clearly evident from the variation in the deposition of fat mass (Fig. 3, B and D). The most striking effects of ambient temperature were observed on the severely attenuated body weight and fat mass gain in the Gdm-/-·Ucp1-/- mice when the ambient temperature was raised to 28 °C. Estimates of the rate of increase in fat mass of the four genotypes fed a high fat diet at ambient temperatures of 20 and 28 °C show that at 20 °C wild type mice have the largest rate of increase, whereas Ucp1-/-, Gdm-/-, and Gdm-/-·Ucp1-/- mice were not significantly different from each other (Fig. 3D). However, when the ambient temperature is increased to 28 °C, the rate of fat mass accumulation in wild type mice and Ucp1-/- mice increased at a similar rate, Gdm-/- was slightly slower, whereas the Gdm-/-·Ucp1-/- mice showed only a modest increase in rate from that observed at 20 °C (Fig. 3D). Two-way analysis of variance on the fat mass gain during weeks 11–17 at 28 °C showed that both effects of genotype (p < 0.0001) and time (p < 0.0001) were significant; the interaction between genotype and time was likewise significant (p = 0.0135). When the ambient temperature is increased to 28 °C, the differences in fat mass accumulation during weeks 11–17 in wild type mice (8.4 ± 0.49) and Ucp1-/- mice (8.7 ± 0.46) are similar; Gdm-/- (6.5 ± 0.44) was slightly decreased, whereas the Gdm-/-· Ucp1-/- mice (1.9 ± 0.49) showed a significant decrease in fat mass. Inspection of the data suggests that the UCP1 and GDM phenotypes are synergistic, and they function as parallel thermogenic systems. Elimination of both Gdm and Ucp1 genes resulted in much lower fat mass accumulation than what would be predicted by the additive effect of the single mutants. These data indicate that there is a synergistic effect of the single gene deletions in causing reduced fat mass accumulation in the Gdm-/-·Ucp1-/- mice. Fat-free mass is significantly reduced in GDM-deficient mice and must be considered in calculations of energy expenditure (Fig. 3C). The variation in food intake on the adiposity phenotypes was minor (Fig. 3E), possibly accounting for some of the difference in adiposity between wild type and Ucp1-/- mice but not for the much greater reduction in fat mass of Gdm-/- and Gdm-/-·Ucp1-/- mice.
FIGURE 3.
Phenotypes of energy balance in wild type, Ucp1-/-,
Gdm-/-, and
Gdm-/-·Ucp1-/- mice. Shown
is the effect of a high fat diet on body weight (A), fat mass
(B), fat-free mass (C), rate of fat mass gain (D),
and food intake (E) in WT (n = 7–8),
Ucp1-/- (n = 8), Gdm-/-
(n = 8–9), and
Gdm-/-·Ucp1-/- (n = 7)
mice kept at 20 °C for 10 weeks and at 28 °C for 10 weeks. The data
represent means ± S.E. The following symbols indicate no significant
difference between groups: *, WT versus
Ucp1-/-; #, Gdm-/- versus
Gdm-/-·Ucp1-/-;  ,
Gdm-/- versus Ucp1-/-; †, WT
versus Gdm-/-; Φ,
Gdm-/-·Ucp1-/- versus
Ucp1-/-; !, WT versus
Gdm-/-·Ucp1-/-. a, b, and
c indicate means that are statistically significant at p
< 0.05 (D and E).
,
Gdm-/- versus Ucp1-/-; †, WT
versus Gdm-/-; Φ,
Gdm-/-·Ucp1-/- versus
Ucp1-/-; !, WT versus
Gdm-/-·Ucp1-/-. a, b, and
c indicate means that are statistically significant at p
< 0.05 (D and E).
Higher Energy Expenditure in Gdm-/-·Ucp1-/- Mice— The striking resistance of Gdm-/-·Ucp1-/- mice to DIO at 28 °C suggested that a difference in energy expenditure might be present even at an ambient temperature near thermoneutrality. Energy expenditure, monitored by indirect calorimetry at temperature transition points during the study (weeks 11 and 12, when ambient temperature was increased from 20 to 28 °C, and weeks 20 and 21, when ambient temperature was again reduced from 28 to 20 °C) showed that the Gdm-/-·Ucp1-/- mice had higher VO2 than wild type mice during both light and dark periods at both ambient temperatures (Fig. 1, C and D, and Table 2). By comparing A and B in Fig. 1, one can deduce that at 28 °C energy expenditure in wild type and Gdm-/-·Ucp1-/- mice is similar, but as the ambient temperature is reduced during the gradual cold adaptation, energy expenditure in Gdm-/-·Ucp1-/- becomes higher (Fig. 1B). Accordingly, when mice were fed a high fat diet and maintained at 20 °C during the first 10 weeks, a higher energy expenditure in Gdm-/-·Ucp1-/- mice was expected (Fig. 1C and Table 2). The higher level of energy expenditure of Gdm-/-·Ucp1-/- mice persisted even after the ambient temperature was elevated to 28 °C when assayed during the 11th week (Fig. 1C and Table 2). Unexpectedly, this elevated energy expenditure in Gdm-/-·Ucp1-/- continued to the 21st week when the mice were again placed in the metabolic chamber, indicating that significantly higher energy expenditure was sustained in Gdm-/-·Ucp1-/- mice during a period of 10 weeks at 28 °C while they were fed a high fat diet (Fig. 1D and Table 2). This elevated energy expenditure coincided with the severe suppression of DIO in the Gdm-/-·Ucp1-/- mice at 28 °C (Fig. 3A). To assess the continued response of the mice to changes in ambient temperature, the ambient temperature was again reduced to 20 °C during the 21st week, and the expected ambient temperature-dependent increase in energy expenditure was observed both in the wild type and mutant mice (Fig. 1D). This enhanced capacity for energy expenditure was dependent on prior conditioning at 20 °C, because Gdm-/-·Ucp1-/- mice fed a high fat diet at 28 °C and not exposed to 20 °C had comparable levels of obesity as wild type mice (adiposity indexes of 0.45 ± 0.04 versus 0.42 ± 0.07; Fig. 4D) and indistinguishable rates of oxygen consumption (12 h of light, 2.6 ± 0.05 versus 2.5 ± 0.17 ml O2/g fat free mass (FFM)/h, p = 0.290; 12 h of dark, 3.2 ± 0.10 versus 3.0 ± 0.25 ml O2/gm fat free mass (FFM)/h; p = 0.273).
TABLE 2.
Energy expenditure, RER, and physical activity in WT and Gdm–/–·Ucp1–/– mice fed a high fat diet and reared at 20 °C for 10 weeks and then at 28 °C for 10 weeks All of the values represent the means ± S.E. p values are the results of statistical analysis using a Student's t test.
| Temperature | Light conditions | WT (n = 6) | Gdm–/–·Ucp1–/– (n = 7) | |
|---|---|---|---|---|
| °C | ||||
| VO2 (ml O2/g of FFM/h) | ||||
| 11th week | 20 | 12 h of light | 2.9 ± 0.09 | 3.3 ± 0.02a |
| 20 | 12 h of dark | 3.5 ± 0.13 | 3.9 ± 0.05a | |
| 28 | 12 h of light | 1.8 ± 0.05 | 2.1 ± 0.01a | |
| 28 | 12 h of dark | 2.4 ± 0.01 | 2.9 ± 0.04a | |
| 21st week | 28 | 12 h of light | 1.5 ± 0.04 | 1.8 ± 0.05a |
| 28 | 12 h of dark | 1.8 ± 0.04 | 2.3 ± 0.08a | |
| 20 | 12 h of light | 2.3 ± 0.05 | 2.8 ± 0.09a | |
| 20 | 12 h of dark | 2.7 ± 0.11 | 3.3 ± 0.11a | |
| RER | ||||
| 11th week | 20 | 12 h of light | 0.87 ± 0.008 | 0.88 ± 0.002 |
| 20 | 12 h of dark | 0.86 ± 0.007 | 0.89 ± 0.007a | |
| 28 | 12 h of light | 0.93 ± 0.007 | 0.89 ± 0.007a | |
| 28 | 12 h of dark | 0.91 ± 0.009 | 0.91 ± 0.009 | |
| 21st week | 28 | 12 h of light | 0.85 ± 0.011 | 0.86 ± 0.008 |
| 28 | 12 h of dark | 0.84 ± 0.015 | 0.87 ± 0.004 | |
| 20 | 12 h of light | 0.84 ± 0.006 | 0.84 ± 0.005 | |
| 20 | 12 h of dark | 0.82 ± 0.001 | 0.84 ± 0.010 | |
| Ambulatory physical activity (total counts) | ||||
| 11th week | 20 | 12 h of light | 3138 ± 1014 | 2624 ± 105 |
| 20 | 12 h of dark | 13129 ± 3478 | 10512 ± 811 | |
| 28 | 12 h of light | 2626 ± 500 | 2831 ± 272 | |
| 28 | 12 h of dark | 10740 ± 2792 | 11259 ± 2429 | |
| 21st week | 28 | 12 h of light | 1757 ± 580 | 1859 ± 303 |
| 28 | 12 h of dark | 6465 ± 1660 | 7561 ± 1135 | |
| 20 | 12 h of light | 2357 ± 658 | 2685 ± 382 | |
| 20 | 12 h of dark | 9843 ± 2385 | 11076 ± 1149 |
p < 0.05
FIGURE 4.
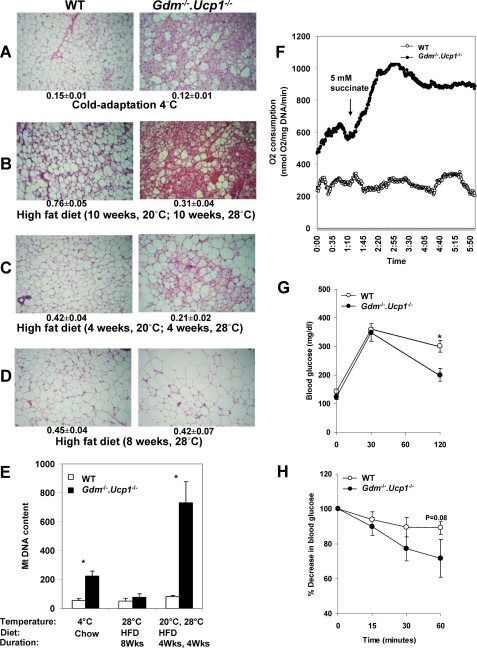
Evidence showing increased energy expenditure from the inguinal fat of Gdm-/-·Ucp1-/- mice and increased insulin sensitivity. A–D, histology of the inguinal fat. A representative section is shown for each experimental group. Adiposity index corresponding to the ratio between fat mass and fat-free mass (AI, mean ± S.E.) is shown below each panel. The number of mice used for each experiment (A, WT = 6 versus Gdm-/-·Ucp1-/- = 5; B, WT = 7 versus Gdm-/-·Ucp1-/- = 7; C, WT = 5 versus Gdm-/-·Ucp1-/- = 5; D, WT = 5 versus Gdm-/-·Ucp1-/- = 5). E, inguinal fat mitochondrial DNA content is the ratio between MT-encoded mt-co2 and nucleus-encoded Ucp2. The values represent the means ± S.E. *, p < 0.05. F, mean tissue O2 consumption in inguinal fat measured with a Clark-type electrode in WT (n = 4) and Gdm-/-·Ucp1-/- (n = 3) mice fed a high fat diet for 4 weeks at 20 °C and 4 weeks at 28 °C. G and H, blood glucose profile during intraperitoneal glucose tolerance test (G) and insulin tolerance test (H) in WT and Gdm-/-·Ucp1-/- mice (n = 6–7/group) after 20 weeks on high fat diet. The mice were kept at 20 °C for 10 weeks and 28 °C for 10 weeks. The values correspond to the means ± S.E.; *, p < 0.05.
Despite feeding a high fat diet, significant differences in RER were evident during the dark h at 20 °C and light hours at 28 °C (Table 2, 11th week). Gdm-/-·Ucp1-/- mice had higher RER during the dark hours at 20 °C and lower RER during the light hours at 28 °C (Table 2). Whether this 3.5% difference in RER is biologically significant is questionable. However, differences in RER between genotypes were not evident during the 21st week (Table 2). Ambulatory physical activity did not differ between genotypes during the 11th or 21st weeks (Table 2) and was much greater during the dark than light hours at both temperatures. Moreover, the mice were more active at 20 °C than at 28 °C at all measurement periods, but differences between genotypes were not observed.
Thermogenesis in Inguinal Fat Protects Gdm-/-·Ucp1-/- Mice from Diet-induced Obesity—Resistance to DIO in Gdm·Ucp1-/- mice depends upon a prior induction in the capacity for energy expenditure at a reduced temperature. This capacity is sustained for at least 10 weeks following a return to an ambient temperature of 28 °C. To identify the source of this capacity for energy expenditure, we have analyzed the morphology of white fat depots (Fig. 4, A–D) and analyzed gene expression in inguinal fat, skeletal muscle, and liver (Table 1). There was a dramatic induction of brown adipocyte-like cells in the inguinal fat in Gdm-/-·Ucp1-/- mice fed a high fat diet and housed at 20 °C that was sustained even after the mice were transferred from an ambient temperature of 20 to 28 °C for either 4 or 10 weeks (Fig. 4, B and C). The induction of brown adipocytes in inguinal fat of Gdm-/-·Ucp1-/- mice is even more robust than that found in mice adapted to an ambient temperature of 4 °C (Fig. 4A). No induction occurs in the inguinal fat of either wild type or Gdm-/-·Ucp1-/- mice fed a high fat diet and maintained at 28 °C for 8 weeks (Fig. 4D), indicating that ambient temperature and genotype, but not a high fat-high sucrose diet, are required to cause transformation of the white adipose tissue morphology. In Gdm-/- mice exposed to 20 °C for 10 weeks and then 28 °C for 10 weeks and fed a high fat diet, very little induction of brown adipocytes could be detected (supplemental Fig. S1).
This morphological evidence for brown adipocyte induction in white adipose tissue is supported by data showing almost a 10-fold induction in the level of mitochondrial DNA in inguinal fat of mice conditioned at 20 °C and then transferred to 28 °C while fed a high fat diet (Fig. 4E). Although not as extensive, brown adipocytes were also induced in retroperitoneal and epididymal fat depots (data not shown) in mice conditioned at 20 °C and then transferred to 28 °C. To assess whether this brown adipocyte morphology in inguinal fat, accompanied by a high mitochondrial content, but without either UCP1 or GDM, was capable of enhancing a thermogenic response that could suppress DIO, oxygen consumption was measured in cell suspensions of inguinal fat with a Clarke electrode. Measurements of basal oxygen consumption in inguinal fat of double mutant mice showed a 2-fold increase compared with wild type mice (278 ± 51 versus 582 ± 9 nmol O2/mg DNA/min, p < 0.05) (Fig. 4F). Under the conditions in which mice were fed a high fat diet and maintained at an ambient temperature of 20 °C for 4 weeks and then at 28 °C for another 4 weeks, the inguinal adipose tissue of the Gdm-/-, Ucp1-/- mice showed a striking increase (64%) in succinate-responsive oxygen consumption as compared with wild type mice (Fig. 4F).
Selective Induction of Gene Expression in Thermogenic Inguinal Fat—The remarkable changes in morphology and oxygen consumption were accompanied by changes in the gene expression profile in inguinal fat from Gdm-/-·Ucp1-/- mice pre-exposed to low ambient temperature, but no changes in gene expression were observed in skeletal muscle or liver (Table 1 and supplemental Tables S6–S9). Prominent increases in the expression of genes of lipid oxidation, including Acox, and CptI were observed. Acsl linked to activation of fatty acids essential for channeling acyl CoA toward oxidation was also up-regulated, as was hormone-sensitive lipase (Hsl), which catalyzes triglyceride hydrolysis in adipocytes. The elevated expression of the three PPAR isoforms, as well as Pgc1α, Srebp1a and 1c, genes involved in the transcriptional regulation of lipid metabolism and mitochondrial biogenesis, further supports enhanced activity in the inguinal fat. There was also induction of genes involved in lipid anabolism as evidenced by up-regulation of PPARγ, involved in transcriptional regulation of lipid storage and lipogenesis and Scd1 (stearoyl CoA desaturase 1), one of the key lipogenic enzymes. In addition to PPARγ, Srebp1a and Srebp1c were also up-regulated, providing further evidence for enhanced lipid biosynthesis.
PPARα, PPARδ, and PPARγ are all subject to transcriptional co-activation by Pgc1α (25); therefore, the 14-fold induction of Pgc1α in Gdm-/-·Ucp1-/- mice, compared with wild type mice, suggests a significant up-regulation of overall lipid metabolism, in addition to the pivotal role played by Pgc1α in mitochondrial biogenesis that is occurring in the inguinal fat (Fig. 4E). Another important factor that potentially contributes to thermogenesis in the inguinal fat is Dio2. Although there was no systemic increase in T3 and T4 levels in Gdm-/-·Ucp1-/- mice (supplemental Table S10), it is possible that the 30-fold induction in Dio2 mRNA levels contributes to a local increase in T3 to support increased metabolism in the inguinal fat, possibly through induction of SERCA activity.
Although enhanced lipid oxidation is necessary for heat production, in itself it is not sufficient. Previous comprehensive microarray gene expression analysis of skeletal muscle from cold-adapted Ucp1-/- mice highlighted increased expression in mitochondrial carriers and genes of Ca2+ cycling. These two solute carriers, Slc25a25 and Slc20a2, and the Ca2+ cycling component Serca2a also induced in the inguinal fat of Gdm-/-·Ucp1-/- mice compared with wild type and provide a potential thermogenic mechanism. It is important to reiterate that neither red or white gastrocnemius skeletal muscle nor liver showed any significant changes in gene expression (Table 1 and supplemental Tables S6–S9).
Insulin Sensitivity in Gdm-/-·Ucp1-/- Mice—To assess whether the major reduction in adiposity in Gdm·Ucp1-/- mice may be associated with a form of lipodystrophy, glucose and insulin tolerance tests were performed with wild type and mutant mice fed an obesogenic diet for 20 weeks. Following an intraperitoneal glucose tolerance test, the double knock-out animals displayed a much more efficient clearance of systemic glucose levels than wild type (Fig. 4G). In addition, Gdm-/-·Ucp1-/- mice were more sensitive to exogenous insulin during the insulin tolerance test compared with wild type mice (Fig. 4H). Hepatic and skeletal muscle triglyceride content in Gdm-/-·Ucp1-/- mice was 1.3-fold lower than in wild type mice (supplemental Table S10). The Gdm-/-·Ucp1-/- mice were more insulin-sensitive and had lower levels of nonadipose lipid accumulation than wild type mice, traits consistent with the increased energy expenditure that reduces overall adiposity in these mice and not with a form of lipodystrophy.
DISCUSSION
In this study we show that Gdm-/-·Ucp1-/- mice fed a high fat diet and initially exposed to an ambient temperature (20 °C), an environment that requires the activation of thermogenesis to maintain body temperature, will continue to express the induced thermogenic mechanisms in inguinal fat, but not skeletal muscle or liver, even after the mouse has been transferred to and maintained for 10 weeks at an ambient temperature of 28 °C. This inducible thermogenic mechanism in adipose tissue, although not required for the maintenance of body temperature at 28 °C, strongly suppresses the development of obesity and suggests the existence of novel thermogenic mechanisms, independent of muscle shivering, UCP1, or the glycerol phosphate cycle, that is able to reduce a positive energy balance.
In previous studies, changes in morphology and gene expression have been found in both skeletal muscle and adipose tissue of UCP1-deficient mice that could account for their ability to tolerate the cold. However, these earlier experiments have not been able to resolve the contribution of muscle shivering to either body temperature or body weight phenotypes, a thermogenic mechanism proposed to be the only alternative available to UCP1-deficient mice (26). Shivering certainly occurs when a UCP1-deficient mouse is placed in the cold (4 °C), as is obvious from the shaking of the mouse when it is exposed acutely, but the inability of this shivering to actually protect the mouse is equally obvious, because the body temperature will drop to below 30 °C in about 3 h. Accordingly, one can equally conclude that shivering is largely ineffective in contributing sufficiently to thermogenesis in the UCP1-deficient mouse to make a difference. To tolerate the cold a UCP1-deficient mouse must be gradually adapted by slowly reducing the ambient temperature (16). The changes occurring in the skeletal muscle affect the capacity for lipid oxidation, Ca2+ cycling, and mitochondrial content. These molecular changes could also be associated with a shivering-based mechanism; however, except that those changes occurring in adipose tissue must be independent of shivering.
Alternative thermogenic mechanisms based upon mitochondrial solute carriers, especially up-regulated in inguinal fat, are candidates that need to be evaluated. Of particular note is the ATP/Mg-Pi carrier, SLC25A25, which is induced in skeletal muscle from Lepob/ob·Ucp1-/- mice following leptin treatment when the ambient temperature went below 20 °C. This carrier was induced in both skeletal muscle and inguinal fat of Gdm-/-·Ucp1-/- mice adapted to 4 °C; however, no changes in its expression were detected in inguinal fat or skeletal muscle of cold-adapted Ucp1-/- mice (data not shown). The ATP/Mg-Pi carrier has been proposed to function in the regulation of matrix adenine nucleotide content (27, 28). Likewise, in our previous study in Ucp1-/- mice, adenine nucleotide transporter was up-regulated after cold adaptation (16). Finally, considering the evidence for enhanced expression of genes associated with both lipid synthesis and oxidation, a thermogenic mechanism based on lipid turnover needs to be kept in mind (29).
In addition to the above mentioned mitochondrial carriers, Serca, previously reported to be up-regulated in inguinal fat Ucp1-/- mice, was likewise induced in the inguinal fat of Gdm-/-·Ucp1-/- mice. We have previously shown the potential involvement of Ca2+-ATPase-based thermogenesis for cold adaptation in inguinal fat of Ucp1-/- mice (16) and in the skeletal muscle of Lepob/ob·Ucp1-/- mice (30). Calcium cycling has been proposed as a heat-generating mechanism in fish thermogenic organs (31), mammalian malignant hyperthermia (32), avian nonshivering thermogenesis (33), and recently as an additional source of heat production contributing to brown adipose tissue thermogenesis (34). The importance of this mechanism in thermogenesis in the inguinal fat of Ucp1-/- mice was shown by the significant changes in the phosphorylation of phospholamban, a Ca2+ cycling regulatory component of SERCA (16).
Inguinal fat is unique in the facile conversion of its white adipocyte population to brown adipocytes (35). Whereas the visceral fat depots in C57Bl/6J mice show very little conversion to brown adipocytes when stimulated adrenergically, the conversion is equally robust in the inguinal fat of B6 mice and other strains that are considered high inducers of the brown adipocyte phenotype (35). Consistent with this special sensitivity of the inguinal fat, we previously showed conversion to brown adipocytes in the inguinal fat of Ucp1-/- mice exposed to a 20 °C environment but not in the visceral fat depot (15). Now we show that in the Gdm-/-·Ucp1-/- mice, the brown adipocyte trait is preserved even after 10 weeks at 28 °C. Because mice fed a high fat diet at 28 °C do not show the brown adipocyte phenotype and have similar levels of adiposity as wild type mice (Fig. 4D), the results suggest that once induced by a lower ambient temperature (20 °C), the initial demand for fat oxidation and the generation of heat by the inguinal fat is preserved by the obesogenic diet, even after the temperature is increased to near thermoneutrality. The stimulus for the appearance of brown adipocytes in the inguinal fat of Gdm-/-·Ucp1-/- mice is highly dependent on adrenergic stimulation because double mutant mice reared at 28 °C during high fat feeding had scarcely any brown adipocytes. Although the mechanism for the enhanced ability of the inguinal fat depot to induce the brown adipocyte phenotype is unknown, it is likely to be found in the transient appearance of brown adipocytes found in white fat depots during post-natal development (36). Many studies have established that downstream of the adrenergic receptors, enhanced cAMP action on protein kinase A is essential and can be achieved by several pathways (37–40). The cAMP-dependent protein kinase pathway subsequently sets in motion an activation of transcription pathways involving cAMP-responsive element-binding protein (41), p38 mitogen-activated protein kinase (42), ATF2 (43), PGC-1α (44), PPARα (45), and type 2 deiodinase (45). These factors synergistically interact to achieve highly variable expression of Ucp1 and brown fat induction in the different white fat depots, but none is absolutely essential (45).
In summary, the prevailing paradigm for the relationship between thermogenesis and obesity assumes that genetic and pharmacological interventions that reduce the thermogenic capacity of the animal will increase susceptibility to obesity, whereas those that increase thermogenic capacity will reduce obesity (4, 46). The DIO phenotypes in Ucp1-/- mouse have significantly changed this paradigm. An artifactual increase in thermogenesis, as found following drug treatment or the expression of transgenes (Fig. 5), stimulates proton leaks in the mitochondrial membrane to reduce obesity in an obesogenic environment. However, as we show here, the progressive loss of essential thermogenic mechanisms to maintain body temperature does not increase susceptibility to obesity. On the contrary, it reduces the development of obesity because alternative thermogenic mechanisms must be induced if the animal is to survive. For many reasons, some just as simple as the mechanics of distributing heat from tissues, brown fat has an extraordinary level of vascularization (47), these alternative mechanisms are calorically more expensive and reduce obesity. These phenotypes of thermogenesis and the regulation of body weight in the Gdm-/-·Ucp1-/- mice have tested further the model for obesity and thermogenesis originally proposed by our laboratory (15). The enhancement of metabolic inefficiency, by pathways that are unknown, remains a key concept to reduce obesity; however, the metabolic inefficiency may come from inactivation of major thermogenic processes rather than their activation. Accordingly, inhibiting a thermogenic pathway and letting the organism adapt to its absence may be very effective in generating metabolic inefficiency.
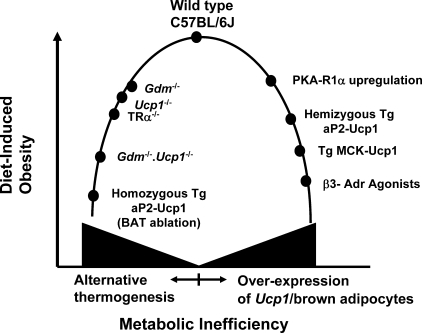
FIGURE 5.
Model describing the relationship between metabolic inefficiency and sensitivity to DIO in male C57BL/6J. On the one hand, metabolic inefficiency can be increased by inactivating major thermogenic pathways, thereby forcing the animal to utilize alternative thermogenic pathways that cost more energetically to maintain body temperature, thus reducing the level of DIO. On the other hand, overexpression and/or ectopic expression of Ucp1 or induction of brown adipocytes in white fat depots enhances the thermogenic capacity, much of which, by being unregulated, increases metabolic inefficiency and reduces diet-induced obesity. References documenting the obesity and thermogenic phenotypes are as follows: wild type C57BL/6J (48), Gdm-/- (17), Ucp1-/- (15), TRα-/- (49), Gdm-/-·Ucp1-/-, homozygous Tg aP2-Ucp1 (50), cAMP-dependent protein kinase-R1α regulation (38, 39), hemizygous aP2-Ucp1 (51), TG MCK-Ucp1 (52), and β3-Adr agonists (53–55).
Supplementary Material
Acknowledgments
We thank Tamra Mendoza and Cody Giardina for excellent technical assistance, Dr. Barbara Gawronska-Kozak for preparing photomicrographs, Drs. Margarita Teran-Garcia and William Johnson for help with statistical analysis, and Dr. Andrew Butler for a critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-HD08431. This work was also supported by Clinical Nutrition Research Unit (CNRU) and COBRE Center Grants P-30 DK072476 and P20-RR021945, respectively. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S10 and Fig. S1.
Footnotes
The abbreviations used are: UCP, uncoupling protein; DIO, diet-induced obesity; T3, triiodothyronine; T4, thyroxine; PPAR, peroxisome proliferator-activated receptor; WT, wild type; SERCA, sacroendoplasmic reticulum calcium ATPase; RER, respiratory exchange ratio.
References
- 1.Blundell, J., and Stubbs, J. (2004) in Handbook of Obesity (Bray, G., and Bouchard, C., eds) 2nd Ed., pp. 427-460, Marcel Dekker, Inc., New York
- 2.Schutz, Y., and Jequier, E. (2004) in Handbook of Obesity (Bray, G., and Bouchard, C., eds) 2nd Ed., pp. 615-629, Marcel Dekker, Inc., New York
- 3.Hill, J., Saris, W., and Levine, J. (2004) in Handbook of Obesity (Bray, G., and Bouchard, C., eds) 2nd Ed., pp. 631-653, Marcel Dekker, Inc., New York
- 4.Lowell, B. B., and Spiegelman, B. M. (2000) Nature 404 652-660 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz, M. W., Woods, S. C., Seeley, R. J., Barsh, G. S., Baskin, D. G., and Leibel, R. L. (2003) Diabetes 52 232-238 [DOI] [PubMed] [Google Scholar]
- 6.Levin, B. E. (2007) Am. J. Physiol. 293 R988-R991 [DOI] [PubMed] [Google Scholar]
- 7.Kozak, L. P., and Harper, M. E. (2000) Annu. Rev. Nutr. 20 339-363 [DOI] [PubMed] [Google Scholar]
- 8.Krauss, S., Zhang, C. Y., and Lowell, B. B. (2005) Nat. Rev. Mol. Cell. Biol. 6 248-261 [DOI] [PubMed] [Google Scholar]
- 9.Brand, M. D., and Esteves, T. C. (2005) Cell Metab. 2 85-93 [DOI] [PubMed] [Google Scholar]
- 10.Ricquier, D., and Kader, J. C. (1976) Biochem. Biophys. Res. Commun. 73 577-583 [DOI] [PubMed] [Google Scholar]
- 11.Foster, D. O., and Frydman, M. L. (1979) Can. J. Physiol. Pharmacol. 57 257-270 [DOI] [PubMed] [Google Scholar]
- 12.Nicholls, D. G., and Locke, R. M. (1984) Physiol. Rev. 64 1-64 [DOI] [PubMed] [Google Scholar]
- 13.Enerback, S., Jacobsson, A., Simpson, E. M., Guerra, C., Yamashita, H., Harper, M. E., and Kozak, L. P. (1997) Nature 387 90-94 [DOI] [PubMed] [Google Scholar]
- 14.Gambert, S., and Ricquier, D. (2007) Curr. Opin. Clin. Nutr. Metab. Care 10 664-670 [DOI] [PubMed] [Google Scholar]
- 15.Liu, X., Rossmeisl, M., McClaine, J., Riachi, M., Harper, M. E., and Kozak, L. P. (2003) J. Clin. Investig. 111 399-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ukropec, J., Anunciado, R. P., Ravussin, Y., Hulver, M. W., and Kozak, L. P. (2006) J. Biol. Chem. 281 31894-31908 [DOI] [PubMed] [Google Scholar]
- 17.Brown, L. J., Koza, R. A., Everett, C., Reitman, M. L., Marshall, L., Fahien, L. A., Kozak, L. P., and MacDonald, M. J. (2002) J. Biol. Chem. 277 32892-32898 [DOI] [PubMed] [Google Scholar]
- 18.Lardy, H., and Shrago, E. (1990) Annu. Rev. Biochem. 59 689-710 [DOI] [PubMed] [Google Scholar]
- 19.Ratner, P. L., Fisher, M., Burkart, D., Cook, J. R., and Kozak, L. P. (1981) J. Biol. Chem. 256 3576-3579 [PubMed] [Google Scholar]
- 20.Koza, R. A., Kozak, U. C., Brown, L. J., Leiter, E. H., MacDonald, M. J., and Kozak, L. P. (1996) Arch. Biochem. Biophys. 336 97-104 [DOI] [PubMed] [Google Scholar]
- 21.Labarca, C., and Paigen, K. (1980) Anal. Biochem. 102 344-352 [DOI] [PubMed] [Google Scholar]
- 22.DosSantos, R. A., Alfadda, A., Eto, K., Kadowaki, T., and Silva, J. E. (2003) Endocrinology 144 5469-5479 [DOI] [PubMed] [Google Scholar]
- 23.Rothwell, N. J., and Stock, M. J. (1979) Nature 281 31-35 [DOI] [PubMed] [Google Scholar]
- 24.Brand, M. D., Chien, L. F., Ainscow, E. K., Rolfe, D. F., and Porter, R. K. (1994) Biochim. Biophys. Acta. 1187 132-139 [DOI] [PubMed] [Google Scholar]
- 25.Liang, H., and Ward, W. F. (2006) Adv. Physiol. Educ. 30 145-151 [DOI] [PubMed] [Google Scholar]
- 26.Golozoubova, V., Hohtola, E., Matthias, A., Jacobsson, A., Cannon, B., and Nedergaard, J. (2001) FASEB J. 15 2048-2050 [DOI] [PubMed] [Google Scholar]
- 27.Satrustegui, J., Pardo, B., and Del Arco, A. (2007) Physiol. Rev. 87 29-67 [DOI] [PubMed] [Google Scholar]
- 28.Fiermonte, G., De Leonardis, F., Todisco, S., Palmieri, L., Lasorsa, F. M., and Palmieri, F. (2004) J. Biol. Chem. 279 30722-30730 [DOI] [PubMed] [Google Scholar]
- 29.Dulloo, A. G., Gubler, M., Montani, J. P., Seydoux, J., and Solinas, G. (2004) Int. J. Obes. Relat. Metab. Disord. 28 (Suppl. 4) S29-S37 [DOI] [PubMed] [Google Scholar]
- 30.Ukropec, J., Anunciado, R. V., Ravussin, Y., and Kozak, L. P. (2006) Endocrinology 147 2468-2480 [DOI] [PubMed] [Google Scholar]
- 31.Block, B. A. (1994) Annu. Rev. Physiol. 56 535-577 [DOI] [PubMed] [Google Scholar]
- 32.MacLennan, D. H., and Phillips, M. S. (1992) Science 256 789-794 [DOI] [PubMed] [Google Scholar]
- 33.Dumonteil, E., Barre, H., and Meissner, G. (1993) Am. J. Physiol. 265 C507-C513 [DOI] [PubMed] [Google Scholar]
- 34.de Meis, L., Arruda, A. P., da Costa, R. M., and Benchimol, M. (2006) J. Biol. Chem. 281 16384-16390 [DOI] [PubMed] [Google Scholar]
- 35.Guerra, C., Koza, R. A., Yamashita, H., Walsh, K., and Kozak, L. P. (1998) J. Clin. Investig. 102 412-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue, B., Rim, J. S., Hogan, J. C., Coulter, A. A., Koza, R. A., and Kozak, L. P. (2007) J. Lipid Res. 48 41-51 [DOI] [PubMed] [Google Scholar]
- 37.Soloveva, V., Graves, R. A., Rasenick, M. M., Spiegelman, B. M., and Ross, S. R. (1997) Mol. Endocrinol. 11 27-38 [DOI] [PubMed] [Google Scholar]
- 38.Cummings, D. E., Brandon, E. P., Planas, J. V., Motamed, K., Idzerda, R. L., and McKnight, G. S. (1996) Nature 382 622-626 [DOI] [PubMed] [Google Scholar]
- 39.Cederberg, A., Gronning, L. M., Ahren, B., Tasken, K., Carlsson, P., and Enerback, S. (2001) Cell 106 563-573 [DOI] [PubMed] [Google Scholar]
- 40.Hansen, J. B., Jorgensen, C., Petersen, R. K., Hallenborg, P., De Matteis, R., Boye, H. A., Petrovic, N., Enerback, S., Nedergaard, J., Cinti, S., te Riele, H., and Kristiansen, K. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 4112-4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozak, U. C., Kopecky, J., Teisinger, J., Enerback, S., Boyer, B., and Kozak, L. P. (1994) Mol. Cell. Biol. 14 59-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robidoux, J., Cao, W., Quan, H., Daniel, K. W., Moukdar, F., Bai, X., Floering, L. M., and Collins, S. (2005) Mol. Cell. Biol. 25 5466-5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao, W., Daniel, K. W., Robidoux, J., Puigserver, P., Medvedev, A. V., Bai, X., Floering, L. M., Spiegelman, B. M., and Collins, S. (2004) Mol. Cell. Biol. 24 3057-3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coulter, A. A., Bearden, C. M., Liu, X., Koza, R. A., and Kozak, L. P. (2003) Physiol. Genomics 14 139-147 [DOI] [PubMed] [Google Scholar]
- 45.Xue, B., Coulter, A., Rim, J. S., Koza, R. A., and Kozak, L. P. (2005) Mol. Cell. Biol. 25 8311-8322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirsch, J. (1997) Nature 387 27-28 [DOI] [PubMed] [Google Scholar]
- 47.Foster, D. O., and Frydman, M. L. (1978) Can. J. Physiol. Pharmacol. 56 110-122 [DOI] [PubMed] [Google Scholar]
- 48.Surwit, R. S., Kuhn, C. M., Cochrane, C., McCubbin, J. A., and Feinglos, M. N. (1988) Diabetes 37 1163-1167 [DOI] [PubMed] [Google Scholar]
- 49.Marrif, H., Schifman, A., Stepanyan, Z., Gillis, M. A., Calderone, A., Weiss, R. E., Samarut, J., and Silva, J. E. (2005) Endocrinology 146 2872-2884 [DOI] [PubMed] [Google Scholar]
- 50.Stefl, B., Janovska, A., Hodny, Z., Rossmeisl, M., Horakova, M., Syrovy, I., Bemova, J., Bendlova, B., and Kopecky, J. (1998) Am. J. Physiol. 274 E527-E533 [DOI] [PubMed] [Google Scholar]
- 51.Kopecky, J., Clarke, G., Enerback, S., Spiegelman, B., and Kozak, L. P. (1995) J. Clin. Investig. 96 2914-2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Couplan, E., Gelly, C., Goubern, M., Fleury, C., Quesson, B., Silberberg, M., Thiaudiere, E., Mateo, P., Lonchampt, M., Levens, N., De Montrion, C., Ortmann, S., Klaus, S., Gonzalez-Barroso, M. D., Cassard-Doulcier, A. M., Ricquier, D., Bigard, A. X., Diolez, P., and Bouillaud, F. (2002) J. Biol. Chem. 277 43079-43088 [DOI] [PubMed] [Google Scholar]
- 53.Champigny, O., Ricquier, D., Blondel, O., Mayers, R. M., Briscoe, M. G., and Holloway, B. R. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 10774-10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Himms-Hagen, J., Cui, J., Danforth, E., Jr., Taatjes, D. J., Lang, S. S., Waters, B. L., and Claus, T. H. (1994) Am. J. Physiol. 266 R1371-R1382 [DOI] [PubMed] [Google Scholar]
- 55.Collins, S., Daniel, K. W., Petro, A. E., and Surwit, R. S. (1997) Endocrinology 138 405-413 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.