Abstract
Whether and how mechanisms intrinsic to stem cells modulate their proliferation and differentiation are two central questions in stem cell biology. Although exogenous basic fibroblast growth factor 2 (FGF-2/Fgf-2) is commonly used to expand adult neural stem/progenitor cells (NSPCs) in vitro, we do not yet understand the functional significance or the molecular regulation of Fgf-2 expressed endogenously by adult NSPCs. We previously demonstrated that methylated CpG binding protein 1 (MBD1/Mbd1) is a transcriptional repressor of Fgf-2 and is enriched in adult brains. Mbd1 deficiency in mice selectively affected adult neurogenesis and the differentiation of NSPCs. Here we show that an Mbd1 and DNA methylation-mediated epigenetic mechanism regulated the expression of stem cell mitogen Fgf-2 in adult NSPCs. Mbd1 bound to the Fgf-2 promoter and regulates its expression in adult NSPCs. In the absence of functional Mbd1, the Fgf-2 promoter was hypomethylated, and treatment with a DNA methylation inhibitor resulted in increased Fgf-2 expression in adult NSPCs. We further demonstrated that both acute knockdown of Mbd1 or overexpression of Fgf-2 in adult NSPCs inhibited their neuronal differentiation, which could be responsible for the neurogenic deficits observed in Mbd1-deficient mice. These data indicate that intrinsic epigenetic mechanisms play critical roles in the regulation of adult NSPC functions.
Neurogenesis is a critical component of neuroplasticity in the adult brain, and its discovery presents exciting prospects for neural repair (1). Ample experimental evidence indicates that the extrinsic environment (“stem cell niche”) regulates the fate of adult neural stem/progenitor cells (NSPCs)3 (2). On the other hand, the intrinsic genetic and epigenetic mechanisms that modulate adult NSPC functions, although of potentially equal importance (3), are not fully clear (4). FGF-2 is an essential growth factor for neural development and neurogenesis (5–7). Recombinant FGF-2 can expand and maintain the undifferentiated state of isolated adult NSPCs (7–9). However, whether adult NSPCs express FGF-2, how that expression might be regulated, and the functional significance of endogenously expressed FGF-2 in adult NSPC biology remain to be investigated.
Epigenetic mechanisms, including DNA methylation, chromatin remodeling, and the noncoding RNA-mediated process, play profound regulatory roles in mammalian gene expression (10). Differential methylation at promoters is involved in the regulation of gene expression, and altered promoter methylation levels are molecular signatures for conditions ranging from cancers to psychiatric disorders (11, 12). MBD1 contains several functional domains that allow it to bind both methylated and unmethylated CpGs and subsequently repress gene expression (13). Earlier we showed that MBD1 is a transcriptional repressor for FGF-2 in human glioma cells (14). We then found that Mbd1 is expressed in adult NSPCs, and mice lacking functional Mbd1 (Mbd1-/- or KO) exhibited deficits in adult neurogenesis and in learning (15). Recently, we further demonstrated that Mbd1-/- mice also exhibited depression (16), a deficit that has been linked to reduced adult neurogenesis. We therefore investigated whether and how Mbd1 regulated the expression of Fgf-2 and the potential function of Mbd1 and DNA methylation in adult NSPCs. Here we show that Mbd1 bound to the Fgf-2 promoter in adult NSPCs. In the absence of functional Mbd1, the Fgf-2 promoter was hypomethylated, and treatment with a DNA methylation inhibitor resulted in increased Fgf-2 expression in adult NSPCs. We further demonstrated that both acute knockdown of Mbd1 and overexpression of Fgf-2 in adult NSPCs inhibited their neuronal differentiation, suggesting that intrinsic epigenetic mechanisms play critical roles in the regulation of adult NSPCs.
EXPERIMENTAL PROCEDURES
Isolation and Culturing of Adult NSPCs—The isolation of mouse or rat brain-derived NSPCs was performed as described (15, 17). All animal procedures were preapproved by the Institutional Animal Care and Use Committee at the University of New Mexico and at the Salk Institute for Biological Studies. Briefly, forebrains without the olfactory bulb (4 mice/genotype, age- and sex-matched) were dissociated mechanically, followed by enzymatic digestion using PPD (2.5 units/ml papain, 1 unit/ml DNase I, 200 mg/100 ml Dispase II) in Dulbecco's modified Eagle's medium high glucose (Cellgro). After filtering through a 70-μm cell strainer (catalog number 252350; BD Falcon), the single cell suspension was loaded onto 50% Percoll (Sigma). The NSPCs were separated from other cells by ultracentrifugation at 12,700 rpm for 30 min at 20 °C using an SW41 Rotor (Beckman, CA). The fraction containing NSPCs (immediately above the red blood cell layer in the gradient) was collected, washed with phosphate-buffered saline, and cultured with Dulbecco's modified Eagle's medium/F-12 medium containing 20 ng/ml FGF-2 (catalog number K1606; Peprotech), 20 ng/ml epidermal growth factor (catalog number A2306; Peprotech), 1% N2 supplement (catalog number 17502-048; Gibco), 1% antibiotic-antimycotic (catalog number 15240-062; Gibco), and 2 mm l-glutamine (catalog number 25030-081; Gibco) in a 5% CO2 incubator at 37 °C. Half of the medium was replaced every 2–3 days.
Western Blotting—Cells were washed twice with phosphate-buffered saline and resuspended in 400 μl of ice-cold lysis buffer containing 10 mm HEPES, 10 mm KCl, 0.1 mm EDTA (pH 8.0), 1 mm dithiothreitol, and protease inhibitor mixture (EDTA-free). Following a 15-min incubation on ice, 25 μl of 10% Nonidet P-40 was added, and then cells were briefly vortexed and centrifuged at 5000 × g for 1 min at 4 °C. The supernatant (cytoplasmic fraction) was saved and stored at -80 °C. The pellet (nuclear fraction) was resuspended in 100 μl of ice-cold nuclear extraction buffer containing 20 mm HEPES, 420 mm NaCl, 1.5 mm MgCl2, 25% (v/v) glycerol, 1 mm dithiothreitol, 200 μm EDTA, and 200 μm phenylmethylsulfonyl fluoride. The pellets were vortexed briefly and vigorously shaken on a shaker at 4 °C for 15 min. The nuclear fractions were then centrifuged for 15 min (15,000 × g, 4 °C), and the resulting supernatants (the nuclear extracts) were aliquoted and stored at -80 °C until use. Aliquots of the cytoplasmic fractions and nuclear extracts were processed for protein content determination using Bradford reagent (Bio-Rad).
For each sample, 30 μg of nuclear or 80 μg of cytoplasmic protein was resolved through a 12% or a 4–12% BisTris (catalog number NB0341BOX; Invitrogen) gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore). Nonspecific sites of the membranes were saturated by a 1-h incubation in TBST (150 mm NaCl, 20 mm Tris, pH 7.5, 1% Tween 20) containing 5% nonfat milk. The membrane was incubated overnight at 4 °C with appropriate primary antibody: either rabbit anti-Mbd1 (1:500, catalog number sc-10751; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or rabbit anti-Fgf-2 (1:250; catalog number sc-79; Santa Cruz Biotechnology) in TBST containing 1% nonfat milk. The membrane was then rinsed and incubated for 1 h at room temperature with the corresponding horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Pierce) diluted 1:2000 in TBST. Peroxidase activity was determined by the enhanced chemiluminescence detection system (ECL and SuperSignal; Pierce) and exposed for 1–5 min on x-ray films (FUJI Photo Film Co. Ltd.). The membrane was then stripped using Restore Western blot stripping buffer (catalog number 21059; Pierce) for 60 min at 37 °C, followed by incubation with a mouse antibody against β-actin (1:2000; catalog number A5441; Sigma) as a protein loading control.
Quantitative Real Time PCR and Standard Reverse Transcription-PCR—The following procedures were based on our published method (15, 18). Total RNA was isolated from cells using TRIzol according to the manufacturer's protocol (catalog number 15596-018; Invitrogen). The cDNA was synthesized by using the Superscript II First-Strand cDNA Synthesis System (catalog number 12371-019; Invitrogen). Oligonucleotide primers were designed using Primer Express software (Applied Biosystems) and ordered from Integrated DNA Technology. The primer sets and subsequent PCR products were first evaluated by gel electrophoresis to determine that a single PCR product of the predicted size was generated. The sequences of primers were as follows: Mbd1 forward, 5′-aactgagctctcccttaaagg-3′; Mbd1 reverse, 5′-tgactgctgtccactcctctg-3′; Fgf-2 forward, 5′-agtgccttacacaatggttc-3′; Fgf-2 reverse, 5′-accacgcttcttctgacatcg-3′; Gapdh (glyceraldehyde-3-phosphate dehydrogenase) forward, 5′-caatgggaagttaacaggaatgg-3′; Gapdh reverse, 5′-tgctttcacagcctccttgat-3′. A typical real-time quantitative PCR mix contained 12.5 μl of 2× SYBR Green PCR Master Mix (catalog number 4309155; Applied Biosystems), 7.5 μl of H2O, 0.5 μl of each oligonucleotide primer, and 4 μl of cDNA in a total 25-μl volume. The reactions were carried out on an ABI PRISM 7700 sequence detection system (Applied Biosystems). Each condition was acquired in triplicate at least. The standard curve was generated with a cDNA premade from a pool of mouse central nervous system tissue (a mixture of brain and spinal cord). The amount of mRNA was calculated according to the standard curve for that particular primer set. The relative amount of the tested message was finally normalized to the level of an internal control Gapdh. The results were statistically analyzed using a two-way analysis of variance post hoc Tukey test. Standard PCRs were carried out using the same cDNA and PCR primers as those used for real time PCR. The number of PCR cycles was optimized to show the relative differences in gene expression.
Mbd1-RNAi and Electroporation—Preannealed Mbd1-RNAi duplexes were purchased from Dharmacon Research, Inc. The control RNAi sequence was 5′-UAGCGACUAAACACAUCAA-3′. The Mbd1-RNAi sequence was 5′-CAACTGAGCTCTCCCTTAA-3′. The resuspended RNAi duplexes were aliquoted and stored at -20 °C. The electroporation of RNAi into adult NSPCs was performed according to the protocol provided by Amaxa (Amaxa Inc., Gaithersburg, MD) with modifications. Briefly, cultured NSPCs were trypsinized and resuspended in mouse NSPC nucleofector solution (catalog number VPG-1004; Amaxa) to a final concentration of 5 × 106 cells/100 μl. Two pmol (5 μl) of each RNAi and 2 μg of control green fluorescence protein (GFP) plasmid DNA (pmaxGFP; Amaxa) were added to the electroporation mixture. The mixture was transferred to an Amaxa cuvette and inserted into the Nucleofector device (Amaxa). A preset program, “A-33,” was used for electroporating DNA into mouse NSPCs. After the electroporation, cells were washed once with prewarmed culture medium and plated into either culture dishes for RNA and protein isolation or onto coverslips for analysis of transfection efficiency. Cells were then incubated in a 5% CO2 incubator at 37 °C, and medium was changed at 24 h postplating. Transfection efficiency was determined by counting the percentage of GFP-positive cells among the total number of cells.
Recombinant Lentivirus—Lentivirus production was as described previously (18). Briefly, lentiviral transfer vector DNA and packaging plasmid DNA were transfected into cultured 293T cells using calcium phosphate methods. The medium containing lentivirus was collected at 40, 64, and 88 h post-transfection, pooled, filtered through a 0.2-μm filter, and concentrated using an ultracentrifuge at 19,400 rpm for 2 h at 10 °C using a SW27 Rotor (Beckman). The virus was washed once and then resuspended in 150 μl of phosphate-buffered saline. We routinely obtained 0.5–1 × 109 infectious viral particles/ml, and infection of ∼0.5–5 × 106 virus particles onto 1 × 105 adult NSPCs resulted in nearly 100% infection.
The lentiviral vectors expressing either Fgf-2 or GFP driven by a cytomegalovirus promoter were generated by cloning the coding sequences of rat Fgf-2 and GFP into the SfiI and PmeI sites of the vector. For gene knockdown, the hairpin pre-RNAi sequences were generated using PCR and cloned into HpaI and ClaI sites of the lentivector. The expression of pre-RNAi was driven by the U6 promoter. The same lentivector also expresses GFP driven by the cytomegalovirus promoter. The control RNAi sequence was 5′-tctcggaatctcattcgatgcataccttcctgtcagtatgcatcgaatgagattccct-3′ (SABiosciences, Frederick, MD). The RNAi target sequences for mouse Mbd1 were as follows: A, 5′-ggtgtttgcaacggcgctgt-3′; B, 5′-gcacagagaatcgccttcaac-3′. To test the inhibitory effect of lentivirus-RNAi, 2 μg of His-tagged mouse Mbd1 expression plasmid (in pCDNA3 vector) and 2 μg of lentivirus RNAi plasmid were cotransfected into HEK293 cells using a published protocol (14). The levels of His-tagged Mbd1 protein were determined by Western blotting using an anti-His tag antibody (Qiagen). After NSPCs were infected with the lentivirus for 2 days, the infected stem cells were subjected to differentiation analysis (see below).
Chromatin Immunoprecipitation (ChIP)—The ChIP assay was performed using a ChIP kit according to the manufacturer's protocol (catalog number 17-295; Upstate Biotechnology). Briefly, cells were fixed and collected. Cell pellets were lysed using SDS lysis buffer containing protease inhibitor. After brief sonication to shear genomic DNA and centrifugation, the supernatant of cell lysate was diluted with ChIP dilution buffer. A small fraction of the lysate was then saved as “input DNA” (positive control). Cell lysate was precleaned by salmon sperm DNA/protein A-Sepharose 50% slurry to eliminate nonspecific binding to the Sepharose. After discarding protein A-Sepharose, cell lysate was incubated either with anti-Mbd1 antibody (catalog number sc-M254; Santa Cruz Biotechnology) or normal rabbit IgG (negative control) overnight. The next day, the antibody-DNA complex was precipitated by protein A-Sepharose. After washing, DNA was eluted from the Sepharose and dissolved in 30 μl of water, and 1 μl of DNA was used in PCR amplification for 40 cycles using the AmpliGold PCR system (Invitrogen) with hot start. The primers used for ChIP were as follows: mouse forward (OXZ-107), 5′-tcaggacagaggtgcagacaatc-3′; reverse (OXZ-108), 5′-aatctccagtcccgtagagcaca-3′; rat forward, 5′-tcagaacagaagcacagagaatcg-3′; reverse, 5′-aatctccagtcccctagagcaca-3′.
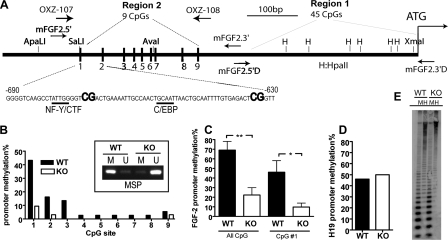
Bisulfate Treatment and Fgf-2 Promoter Methylation Analyses—Bisulfate sequencing was performed as described (19). Briefly, genomic DNA was isolated from early passage NSPCs and subjected to bisulfate treatment, which changes unmethylated “C” into “U.” The treated Fgf-2 promoter was amplified using PCR primers specific to the converted sequences (Fig. 4) and cloned into a PCRII vector. The primers used for Region 1 were as follows: Fgf 2.5′D, gttatttagttttaggatagaggtgtag; Fgf 2.3′D, caaatttctaactttctccactcctacc. The primers for Region 2 were as follows: Fgf 2.5′, caaatttctaactttctccactcctacc; Fgf 2.3′, ttcttacaataaaaccacttaaaatcctt. Each experimental condition was repeated at least three times with independent NSPC or brain samples and independent bisulfate treatment. About 10 clones from each experimental replicate were sequenced to determine the methylation status of CpGs. The methylation percentage was calculated as the number of clones with CpG methylation divided by the total number of clones analyzed.
FIGURE 4.
The Fgf-2 promoter was hypomethylated in Mbd1-/- NSPCs, and such differential methylation was specific to the Fgf-2 promoter. A, schematic view of mouse Fgf-2 promoter, focusing on two regions that were analyzed in this study. We found no difference in DNA methylation in the CpG-rich Region 1 containing 45 CpGs. Region 2 contains nine CpGs, and CpG#1 is adjacent to the putative binding sites of several transcription factors. PCR primers used for bisulfate sequencing analysis are indicated (Region 1, Fgf 2.5′D + Fgf 2.3′D; Region 2, Fgf 2.5′+ Fgf 2.3′). B, the percentage of methylation at each CpG in Region 1 was compared between WT and KO NSPCs. The analyses were done using four independent samples, and the result from one experiment is shown as an example; the reduced methylation of CpG#1 was further confirmed using methylation-sensitive PCR (inset; M, methylated product; U, unmethylated product). C, Fgf-2 promoter methylation analyzed from four independent experiments showing significant reductions in the percentage of promoter methylation at all CpG sites (All CpGs; **, p < 0.01) and at CpG#1 (*, p < 0.05) in KO NSPCs. D, the promoter of imprinted H19 genes was not differentially methylated in KO NSPCs. E, global DNA methylation was not altered in KO NSPCs, as demonstrated by Southern blotting using methylation-sensitive HpaII (H) and methylation-insensitive MspI (M) restriction enzyme-digested genomic DNA and a pericentric minor satellite probe.
Methylation-specific PCR (MSP) was performed using primers that detected the C to U conversion at CpG#1 in bisulfate-treated DNAs, based on a published method (20). The primers used were as follows: forward for the mouse Fgf-2 promoter methylated at CpG#1 (Tm = 52 °C), 5′-gTTAAgTTTATTggggTCg; forward, unmethylated at CpG#1 (Tm = 50 °C), 5′-gttaagtttattggggttg-3′. Reverse primer was 5′-cactcctaccttttaacaaaa-3′. The PCR was carried out at an annealing temperature of 50 °C. Under this condition, these primers can distinguish the methylation status of CpG#1 specifically.
Southern Blotting—Genomic Southern blot was performed with 10 μg of genomic DNA digested with either HapII (methylation-insensitive) or MspI (methylation-sensitive) (New England Biolabs). A minor satellite oligonucleotide probe was designed according to a previous study (21) and end-labeled with 32P radioactivity by T4 polynucleotide kinase (New England Biolabs).
5-Azacytidine Treatment—5-Azacytidine treatment was performed as described (19). Briefly, cultured NSPCs were treated with 5 μm 5-azacytidine (Sigma) for 2 days and collected by TRIzol, followed by RNA isolation, cDNA synthesis, and quantitative PCR analyses.
In Vitro Differentiation Analysis of Cultured NSPCs—In vitro differentiation was performed based on an established protocol (15). We performed preliminary experiments that compared 7-day differentiation using MAP2ab as a neuronal marker and 3-day differentiation using Tuj1 as a neuronal marker. We found that a shorter differentiation protocol using Tuj1 as a marker and a longer differentiation protocol using MAP2ab as a marker could detect similar neuronal differentiation phenotypes in several experimental conditions. We also found that the longer differentiation protocol yielded more cell death in the absence of mitogen FGF-2 and epidermal growth factor. Based on these preliminary data, we therefore selected the 3-day differentiation protocol and TuJI labeling method to study early neuronal differentiation. Briefly, cultured NSPCs were plated onto polyornithine- and laminin-coated coverslips in a 24-well plate at a density of 5 × 104 cells/coverslip in Dulbecco's modified Eagle's medium/F-12 medium containing 5 μm forskolin (catalog number F-6886; Sigma) and 1 μm retinoic acid (catalog number R-2625; Sigma). After 3 days, cells were fixed with 4% paraformaldehyde for 30 min, followed by washing with Tris-buffered saline, pH 7.4. Cells were then pre-blocked using Tris-buffered saline with 3% normal goat serum (catalog number S-1000; VECTOR), 0.1% Triton X-100 for 1 h, followed by overnight incubation with primary antibody against mouse neuron-specific type III β-tubulin (Tuj1, 1:4000; catalog number G712A; Promega) at 4 °C. After washing with TBS, cells were detected using goat anti-mouse Alexa568 (1:1000; catalog number A11031; Invitrogen) and counter-stained with the fluorescent nuclear dye 4′,6-diamidino-2-phenylindole (1:3000; catalog number B2261; Sigma). After the cells were mounted with VECTASHIELD (catalog number H-1000; VECTOR), the numbers of Tuj1-positive and 4′,6-diamidino-2-phenylindole-positive cells were quantified using an unbiased stereology method. The data were analyzed using two-tailed paired t tests.
RESULTS
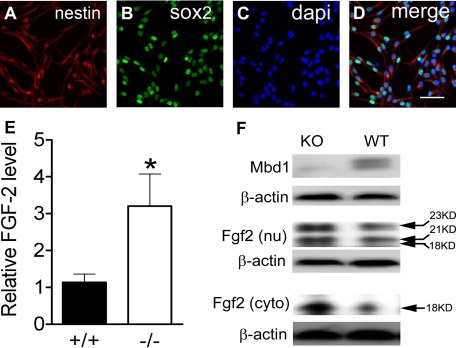
NSPCs Isolated from Adult Mbd1 Knock-out Mice Exhibit Increased Fgf-2 Expression—To determine whether Mbd1 deficiency affected the level of Fgf-2 expression in adult NSPCs, we isolated NSPCs from the brains of adult Mbd1-/- knock-out (KO) mice and their wild-type (WT) littermates. Nearly all cultured NSPCs were positive for progenitor markers Nestin and Sox2 (Fig. 1, A–D) but negative for differentiated cell markers, Tuj1 and GFAP (supplemental Fig. 1), suggesting a relative homogeneity of these primary adult NSPCs. We then examined Fgf-2 mRNA levels in both WT and KO NSPCs using real time quantitative PCR analyses and found that KO NSPCs had increased Fgf-2 mRNA levels when compared with WT cells (p < 0.05; Fig. 1E). We determined the Fgf-2 protein levels in these cells using Western blotting. As expected, Mbd1 protein was detected in the nuclear fraction of the WT but not in KO NSPCs, confirming that Mbd1 protein was not expressed in mutant cells (Fig. 1F, top). It has been shown that both high molecular mass (21–23 kDa) and low molecular mass (18 kDa) Fgf-2 protein isoforms are expressed from the same mRNA transcript by using alternative start codons (22). Since these isoforms exhibit different subcellular localizations (22, 23), we analyzed both the nuclear and the cytosolic fractions of adult NSPCs (Fig. 1B). We found that high molecular mass Fgf-2 predominantly localized in the nuclear fraction (Fig. 1F, middle), and low molecular mass Fgf-2 localized in both the nuclear and cytoplasmic fractions (Fig. 1F, bottom), consistent with published literature (22, 23). The levels of all Fgf-2 protein isoforms were higher in KO NSPCs compared with WT cells. To determine whether this increased Fgf-2 expression is specific to adult NSPCs, we analyzed the cortical tissue isolated from adult KO mice and the fibroblasts derived from embryonic KO mice and observed no significant increase in Fgf-2 expression in these tissues or cells (data not shown). Hence, Mbd1 deficiency led to increased Fgf-2 expression in adult NSPCs at both the mRNA and protein levels, and this increase in Fgf-2 expression was specific to adult NSPCs.
FIGURE 1.
Mbd1 mutant adult NSPCs exhibited increased Fgf-2 expression. A–D, NSPCs cultured under proliferating conditions expressed the neural progenitor marker Nestin (cytoplasmic; red), and Sox2 (nuclear; green). 4′,6-diamidino-2-phenylindole (dapi) (blue) stained all nuclei. E, quantitative real time PCR analyses showing that the Fgf-2 mRNA level was significantly higher in Mbd1-/- (KO) NSPCs compared with WT NSPCs (n = 3; *, p < 0.05). F, Western blotting analysis showing that Mbd1 protein was absent in the KO NSPCs (top) and that both nuclear (nu; middle) and cytoplasmic (cyto; bottom) Fgf-2 were significantly higher in KO NSPCs compared with WT NSPCs. β-Actin was used as a protein loading control.
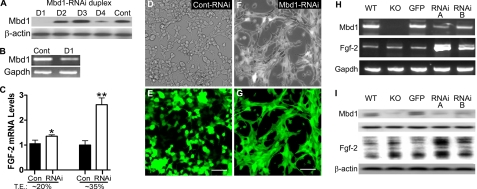
Acute Knockdown of Mbd1 in Adult NSPCs Leads to Increased Fgf-2 Expression—To determine whether the increased expression of Fgf-2 in KO NSPCs was due directly to a lack of functional Mbd1 in adult NSPCs rather than a secondary effect resulting from Mbd1 deficiency in the stem cell niche, we used small inhibitory RNAs to specifically target Mbd1 (Mbd1-RNAi) in adult NSPCs. Among four different RNAi duplexes that we tested, RNAi-D1 and, to a lesser extent, RNAi-D4 could effectively reduce the expression of Mbd1 at both the protein and mRNA levels compared with a nonsilencing control RNAi (Fig. 2, A and B). When we transfected RNAi-D1 into cultured WT adult NSPCs, we observed an increase in endogenous Fgf-2 mRNA expression, and a higher transfection efficiency of RNAi into the cells corresponded to a greater increase in Fgf-2 mRNA levels (Fig. 2C). To further confirm these data, we cloned two different Mbd1-RNAi precursors, RNAi-A and RNAi-B, into lentiviral vectors that also express enhanced GFP (18). High titer recombinant lentiviruses carrying either Mbd1-RNAi or control RNAi were used to infect cultured WT adult NSPCs. At 2 days postinfection, nearly 100% of the cells were positive for GFP fluorescence, suggesting that nearly all of the cells were infected (Fig. 2, D–G). Compared with control RNAi, both Mbd1-RNAi duplexes (Fig. 2, A and B) resulted in significant decreases in Mbd1 mRNA and protein levels and significant increases in Fgf-2 mRNA and protein levels (Fig. 2, H and I). In addition, Mbd1-RNAi-infected cells (Fig. 2, F and G) adopted a distinctive clustered morphology compared with control RNAi-infected cells (Fig. 2, D and E). Therefore, acute knockdown of Mbd1 led to an increase in the expression of endogenous Fgf-2 in adult NSPCs.
FIGURE 2.
Acute knockdown of Mbd1 led to increased Fgf-2 expression in adult NSPCs. A, transient cotransfection of a His-tagged Mbd1 expression plasmid with four different Mbd1-RNAi duplexes (D1, D2, D3, and D4) into HEK293 cells. The result indicated that the Mbd1-RNAi duplex D1 and to a lesser extent D4 significantly inhibited the expression of Mbd1 protein. Mbd1 expression was detected by an anti-His tag antibody. β-Actin was used as a control. B, semiquantitative reverse transcription-PCR showing that transfected Mbd1-RNAi D1 resulted in reduced endogenous Mbd1 mRNA levels in adult WT NSPCs. Gapdh was used as an internal control. C, quantitative real time PCR assays showing that transfected Mbd1-RNAi D1 resulted in an increased endogenous Fgf-2 mRNA level in adult NSPCs, and the higher transfection efficiency corresponded to further increases in Fgf-2 mRNA levels. T.E., transfection efficiency; *, p < 0.05; **, p < 0.01. D–G, phase-contrast (D and F) and fluorescence (E and G) images showing adult NSPCs infected with recombinant lentivirus expressing either a control RNAi (D and E) or Mbd1-RNAi (F and G). GFP was coexpressed from the lentiviral vectors. Note that recombinant lentiviruses infected adult WT NSPCs with high efficiency, and Mbd1-RNAi lentivirus-infected WT NSPCs adopted a distinctive morphology compared with control RNAi-infected cells. H and I, infection of adult WT NSPCs with two different Mbd1-RNAi lentiviruses (RNAi-A and RNAi-B) led to decreased Mbd1 expression and increased Fgf-2 expression at both mRNA levels (H; PCR) and protein levels (I; Western blotting).
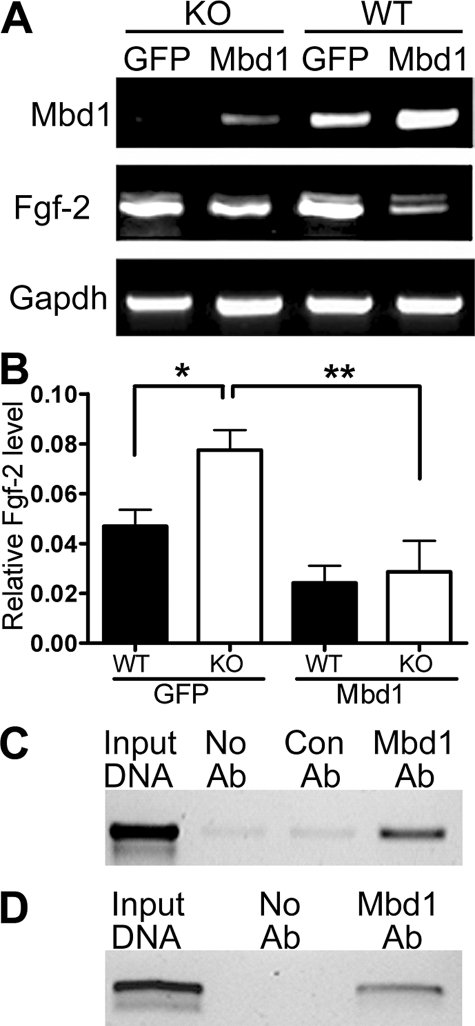
Direct Repression of Fgf-2 Expression by Mbd1—Based on the above results, we reasoned that overexpression of Mbd1 should lead to reduced Fgf-2 expression. We therefore infected cultured KO and WT NSPCs with lentiviruses expressing either Mbd1 or GFP. GFP lentivirus-infected KO cells exhibited higher Fgf-2 expression than GFP-infected WT cells (Fig. 3, A and B), consistent with the results obtained from uninfected cells (Fig. 1). Overexpression of Mbd1 resulted in decreased Fgf-2 expression in both the KO and WT NSPCs (Fig. 3, A and B). To further determine whether Mbd1 directly regulated Fgf-2 gene expression, we performed chromatin immunoprecipitation. We found that Mbd1 specifically bound to the Fgf-2 promoter in both mouse (Fig. 3C) and rat (Fig. 3D) adult NSPCs. These results support our hypothesis that Mbd1 directly regulates the expression of Fgf-2 in adult NSPCs.
FIGURE 3.
Overexpression of Mbd1 led to decreased Fgf-2 expression. A, reverse transcription-PCR analyses showing that overexpression of Mbd1 carried by lentivirus led to decreased Fgf-2 mRNA expression in both KO and WT NSPCs. B, real time quantitative PCR results confirmed that Fgf-2 mRNA levels were significantly higher in KO NSPCs compared with WT NSPCs when both of them were infected with control lentivirus (GFP), consistent with results shown in Fig. 1. Mbd1 overexpression led to decreased Fgf-2 expression in both KO and WT NSPCs (*, p < 0.05; **, p < 0.01). C and D, chromatin immunoprecipitation assays demonstrated that endogenous Mbd1 bound to Fgf-2 promoter in both mouse (C) and rat (D) adult NSPCs. Input DNA, positive control. Con Ab, an antibody against GFP was used as a negative control for immunoprecipitation. No Ab, rabbit IgG was used as negative control for immunoprecipitation. The PCR primers used to amplify precipitated Fgf-2 promoter, corresponding to “Region 2” of the Fgf-2 promoter, are indicated in Fig. 4A.
Fgf-2 Promoters in NSPCs Isolated from Adult Mbd1 Mutant Mice Are Hypomethylated—Altered promoter methylation status can significantly affect gene expression in both a cell type-specific manner and in disease conditions (11, 12). To investigate the molecular mechanism underlying the increased expression of Fgf-2 in KO NSPCs, we analyzed Fgf-2 promoter methylation status using bisulfate sequencing. After bisulfate treatment, which changed all unmethylated cytosines to uracils, the converted Fgf-2 promoter sequence was cloned using specific PCR primers (Fig. 4A). We randomly sequenced 20–30 colonies from each experimental condition. The methylation status of each CpG dinucleotide was obtained via sequencing analysis. We calculated the percentage of methylation in each CpG as the number of clones with methylation in that CpG over the total number of clones analyzed. The mouse Fgf-2 promoter had a largely hypomethylated CpG island (Fig. 4A), which was characteristic of many RNA polymerase II-transcribed genes (10). We did not find any differential methylation in the highly CpG-rich “Region 1” of the promoter, which contains 45 CpGs (Fig. 4A, Region 1). However, we found differential CpG methylation in “Region 2” of the promoter, which contains 9 CpGs (Fig. 4A, Region 2). The number of Fgf-2 promoter clones that had methylation at any of the nine CpG sites in Region 2 was 69.0 ± 9.1% in WT NSPCs but only 22.3 ± 7.6% in KO NSPCs (Fig. 4C, All CpGs, p < 0.01). Specifically, CpG#1 in Region 2 was methylated in 46.0 ± 12% clones from WT NSPCs but only 9.8 ± 4.1% clones from KO NSPCs (Fig. 4C, CpG#1, p < 0.05). CpG#2 also exhibited reduced methylation in KO NSPCs compared with WT cells, but to a lesser extent (Fig. 4B). The differential methylation at CpG#1 was further confirmed using MSP (Fig. 4B, inset). To determine whether such hypomethylation was specific to Fgf-2 promoters, we analyzed the promoter methylation status of the imprinted H19 gene and found that 50% of the H19 promoter was methylated in both WT and KO NSPCs, which was typical for imprinted genes (Fig. 4D). We also studied global DNA methylation levels in NSPCs using methylation-sensitive HpaII and methylation-insensitive MspI restriction digest and probed the genomic DNA with a pericentric minor satellite probe in Southern blotting analysis. We found that KO NSPCs had normal global DNA methylation levels compared with WT cells (Fig. 4E). To determine whether this Fgf-2 promoter hypomethylation was specific to adult NSPCs, we analyzed total cortical tissue isolated from adult mice and fibroblasts derived from embryonic mice that did not exhibit increased Fgf-2 levels (see above). We found no significant difference in Fgf-2 promoter methylation status in these tissues and cells derived from KO mice (data not shown). These data suggest that Fgf-2 promoter hypomethylation was specific to adult KO NSPCs, which might be responsible for the increased Fgf-2 expression in these cells. To further determine whether DNA methylation regulated the expression of Fgf-2, we treated NSPCs with 5 μm 5-azacytidine, an inhibitor of DNA methyltransferase. We found that the Fgf-2 mRNA level was significantly increased after 5-azacytidine treatment (treated, 0.161 ± 0.007 versus control, 0.069 ± 0.002, n = 3; p < 0.01), suggesting that DNA methylation is part of an intrinsic mechanism that regulates Fgf-2 expression in adult NSPCs.
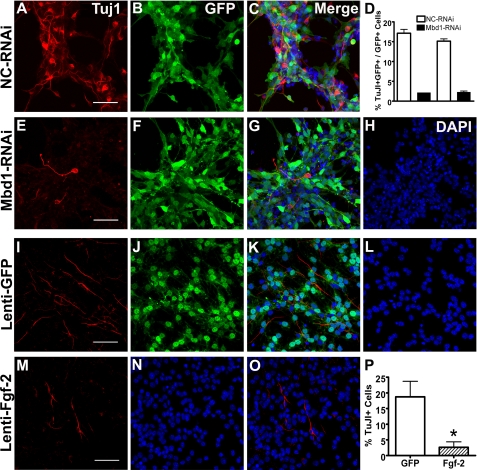
Both Down-regulation of Mbd1 and Overexpression of Fgf-2 in Adult NSPCs Result in Reduced Neuronal Differentiation—Since Mbd1 deficiency in mice leads to reduced neuronal differentiation of adult NSPCs (15), we decided to investigate whether acute knockdown of Mbd1 using RNAi could also affect neuronal differentiation of NSPCs. We performed preliminary experiments that compared a 7-day differentiation protocol using MAP2ab as neuronal marker and a 3-day differentiation protocol using Tuj1 as neuronal marker and found no significant difference between these two protocols in detecting neuronal differentiation phenotype. Since cell death was more apparent in the longer differentiation condition compared with shorter differentiation condition (data not shown), we subjected both control RNAi-infected NSPCs and Mbd1-RNAi lentivirus-infected NSPCs to differentiation for 3 days. We found that when compared with control RNAi-infected NSPCs (Fig. 5, A–C; negative control for immunostaining is included in supplemental Fig. 1), Mbd1-RNAi-infected NSPCs (Fig. 5, E–H) had significantly reduced neuronal differentiation, as assessed by the percentage of TuJ1-positive neurons (Fig. 5D, two independent experiments with triplicates each), consistent with our previous findings in NSPCs derived from Mbd1-/- (15). We know that Fgf-2 added to NSPC cultures maintains the undifferentiated state of NSPCs, possibly by inhibiting NSPC differentiation (8); however, it is unclear whether Fgf-2 expressed by adult NSPCs themselves is also important for NSPC function. We next investigated the impact of increased Fgf-2 expression on the neuronal differentiation of NSPCs. We infected NSPCs with lentivirus expressing either recombinant Fgf-2 or GFP and analyzed the neuronal differentiation capacity of NSPCs expressing high levels of Fgf-2. At 3 days postinfection, both GFP lentivirus-infected Fig. 5, I–L) and Fgf-2 lentivirus-infected (Fig. 5, M–O) NSPCs could differentiate into TuJ1-positive neurons; however, the Fgf-2 lentivirus-infected NSPCs differentiated into significantly fewer TuJ1-positive neurons compared with GFP lentivirus-infected NSPCs (Fig. 5P, n = 3; p < 0.05). These data indicate that both Mbd1 and Fgf-2 expressed intrinsically by adult NSPCs could play significant roles in the regulation of NSPC differentiation.
FIGURE 5.
Down-regulation of Mbd1 and overexpression of Fgf-2 in WT adult NSPCs inhibited neuronal differentiation. A–H, compared with lentivirus-nonsilencing (NC) control RNAi-infected NSPCs (A–C), lentivirus-Mbd1-RNAi-infected NSPCs (E–H) differentiated into fewer TuJ1-positive (red) neurons (A–D). Green, GFP coexpressed with RNAi. D, quantitative data comparing the percentages of TuJ1-positive neurons differentiated from lentivirus-infected (GFP+) NSPCs indicated that down-regulation of Mbd1 in NSPCs leads to decreased neuronal differentiation. The data were from two independent experiments with triplicates performed. I–L, nearly all NSPCs infected by lentivirus-GFP were GFP-positive. M–P, Fgf-2 lentivirus-infected WT NSPCs. Note that although both GFP lentivirus- and Fgf-2 lentivirus-infected NSPCs could differentiate into Tuj1-positive neurons (red), the percentage of Tuj1-positive cells was significantly decreased in Fgf-2-overexpressing NSPCs (E–G). H, quantitative data comparing the percentages of TuJ1-positive neurons differentiated from either GFP- or Fgf-2-overexpressing NSPCs indicated that overexpression of Fgf-2 inhibited the neuronal differentiation of NSPCs. *, p < 0.05 (scale bar, 100 μm). DAPI, 4′,6-diamidino-2-phenylindole.
DISCUSSION
In this study, we demonstrate that Mbd1 is part of the intrinsic epigenetic mechanism of adult NSPCs and modulates stem cell function via regulation of Fgf-2 expression. We provide the following evidence to support our conclusion: 1) adult NSPCs expressed the stem cell mitogen Fgf-2; 2) adult NSPCs derived from KO mice had increased endogenous Fgf-2 expression, and the Fgf-2 promoter was hypomethylated; 3) acute alterations to Mbd1 levels in adult NSPCs, either by overexpression or by RNAi knockdown, led to altered endogenous Fgf-2 expression; 4) Mbd1 bound to the promoter of Fgf-2 in adult NSPCs; and 5) expression levels of both Mbd1 and Fgf-2 by adult NSPCs had a significant impact on NSPC differentiation. Together, these data suggest that Mbd1 is part of an intrinsic mechanism in adult NSPCs that regulates the expression of Fgf-2 and modulates the functions of adult NSPCs.
FGF-2 is a critical growth factor for the development and plasticity of many cell types. In the brain, FGF-2 plays an important role in both embryonic and adult neurogenesis (5–7) and has been used to amplify endogenous NSPCs in injured adult brains for neural repairs (24, 25). Recombinant FGF-2 is commonly used to expand isolated NSPCs in vitro and is known to maintain the undifferentiated state of adult NSPCs (8, 9). Nevertheless, the molecular mechanism underlying its mitogenic effect is still unclear. In fact, abnormal proliferation and neoplastic transformation are frequently found in FGF-2-overproducing cells, such as glioma cells (26), indicating that FGF-2 expressed by target cells themselves is critical for cellular functions. Although FGF-2 is widely used to promote proliferation and inhibit differentiation of cultured NSPCs (9, 27), whether adult NSPCs express FGF-2 and what the functions of the FGF-2 expressed by adult NSPCs are remain unclear. Here we show that altering the expression levels of Fgf-2 in adult NSPCs had a significant effect on the proliferation and differentiation of adult NSPCs. Since we used relative homogeneous culture of NSPCs and GFAP-positive cells were extremely rare, the Fgf-2 was probably expressed by NSPCs themselves. Our results suggest that the regulation of NSPC proliferation and differentiation is at least in part intrinsic to adult NSPCs and that Fgf-2 is a focal point of this regulation.
The potent mitogenic effects of FGF-2 in angiogenesis and tumor progression have led to extensive studies into its transcriptional activation. Classic transcriptional activators, such as SP1 and EGR-1, are known to directly activate the FGF-2 promoter (28, 29). However, the negative regulation of FGF-2 transcription, although of critical importance, is not well understood. Recent findings indicate that SP1-activated genes, such as FGF-2, can be repressed by MBD1 in conjunction with two other epigenetic cofactors, MCAF1 (MBD1-containing chromatin-associated factor 1), also known as the human homolog of murine ATFa-associated modulator (AM), and a histone methyltransferase (SETDB1/Eset) (30). In this study, we provide strong evidence that Mbd1 is a bona fide negative regulator of Fgf-2 in adult NSPCs. Therefore, Mbd1 and DNA methylation are probably key negative transcriptional regulators of this potent mitogen.
DNA methylation, together with histone modification and noncoding RNA-mediated gene regulation, is a central component of epigenetic regulation and plays an important role in embryonic development (13), yet the role of epigenetic regulation in adult brain function has become an area of study only recently. Here we provide new evidence supporting the critical function of DNA methylation in adult neurogenesis by demonstrating neurogenic and learning deficits in Mbd1-deficient mice (15). In our earlier studies, we found that, at the cellular level, NSPCs derived from adult KO mice had reduced neuronal differentiation capacity and learning deficits (15, 16); however, the molecular mechanism underlying these deficits was unclear. In the present study, we demonstrate that, in the absence of functional Mbd1, there is an increased Fgf-2 level in adult NSPCs that could inhibit neuronal differentiation. Furthermore, using lentivirus-based gene delivery, we demonstrated that high levels of Fgf-2 expression in adult NSPCs indeed inhibit neuronal differentiation. Hence these data provide a molecular link between Mbd1 deficiency and a reduction in the neuronal differentiation of adult NSPCs, demonstrating the critical role of Mbd1 in adult neurogenesis.
CpG islands contain large numbers of CpGs and are generally hypomethylated (10). Differential methylation at CpG islands plays a major role in the regulation of gene expression levels (13), and aberrant hyper- or hypomethylation of CpG islands has been linked to diseases such as cancer and schizophrenia (11, 12). Hypermethylation and reduced MeCP2 expression are also found in patients with autism, Angelman syndrome, and several other mental retardation disorders (11, 31). Our present results show that, in the absence of functional Mbd1, the Fgf-2 promoter was hypomethylated, which could explain the increased expression of Fgf-2 in KO adult NSPCs. How Mbd1 mutation leads to Fgf-2 promoter hypomethylation and whether such promoter hypomethylation also occurs in other gene promoters are currently unknown, but these are areas of active study. Mbd1 has the ability to bind both methylated and unmethylated CpGs through its MBD domain and CXXC3 domain, respectively. The possible biological significance of such a dual DNA binding ability could be increases in binding specificity and affinity. This supposition is supported by our finding that exogenously expressed Mbd1 could repress Fgf-2 expression in KO NSPCs, although the Fgf-2 promoter is hypomethylated in these cells. Furthermore, a DNA methylation inhibitor, 5-azacytidine, induced increased expression of Fgf-2 in adult NSPCs, suggesting that both promoter methylation and Mbd1 could repress Fgf-2 expression. Whether these two repressive mechanisms belong to the same molecular process is currently under investigation. Since Fgf-2 is a potent mitogen for NSPCs and its overexpression has been associated with glioma, such negative regulation is probably of critical importance for the function of adult NSPCs and adult brains.
In summary, previous studies have revealed how the extrinsic stem cell niche can influence intrinsic gene expression through classic transcriptional regulation (2). Recently, Mbd1 expression was found to change dynamically during differentiation of mouse embryonic stem cells (32). Mbd1 interacts with Polycomb proteins that have intimate roles in stem cell differentiation (33, 34). Our data demonstrate that Mbd1 and DNA methylation are essential intrinsic modulators for adult NSPCs, supporting the emerging hypothesis that epigenetic regulation plays critical roles in adult brain function and plasticity (4). An epigenetic mechanism can translate dynamic environmental stimuli onto a fixed genome and therefore may provide molecular evidence for environmental effects on adult neurogenesis.
Supplementary Material
Acknowledgments
We thank J. Bao for contributions during the early stage of this project. We thank K. Nakashima for teaching us bisulfate sequencing and ChIP, E. Finn-Flesher for technical assistance, R. G. Summers and D. V. Schaffer for critical reading of the manuscript, and M. L. Gage and C. T. Strauss for editorial assistance. Images in this paper were generated in the University of New Mexico Cancer Center Fluorescence Microscopy Facility.
This work was supported, in whole or in part, by National Institutes of Health Grants MH080434 and MH078972. This work was also supported in part by the Oxnard Foundation, International Rett Syndrome Foundation, and University of New Mexico School of Medicine. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
Footnotes
The abbreviations used are: NSPC, neural stem/progenitor cells; BisTris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; RNAi, RNA interference; GFP, green fluorescent protein; MSP, methylation-specific PCR; KO, knock-out; WT, wild-type; ChIP, chromatin immunoprecipitation.
References
- 1.Gage, F. H. (2002) J. Neurosci. 22 612-613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma, D. K., Ming, G. L., and Song, H. (2005) Curr. Opin. Neurobiol. 15 514-520 [DOI] [PubMed] [Google Scholar]
- 3.Shi, Y., Chichung Lie, D., Taupin, P., Nakashima, K., Ray, J., Yu, R. T., Gage, F. H., and Evans, R. M. (2004) Nature 427 78-83 [DOI] [PubMed] [Google Scholar]
- 4.Cheng, L. C., Tavazoie, M., and Doetsch, F. (2005) Neuron 46 363-367 [DOI] [PubMed] [Google Scholar]
- 5.Raballo, R., Rhee, J., Lyn-Cook, R., Leckman, J. F., Schwartz, M. L., and Vaccarino, F. M. (2000) J. Neurosci. 20 5012-5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaccarino, F. M., Schwartz, M. L., Raballo, R., Nilsen, J., Rhee, J., Zhou, M., Doetschman, T., Coffin, J. D., Wyland, J. J., and Hung, Y. T. (1999) Nat. Neurosci. 2 246-253 [DOI] [PubMed] [Google Scholar]
- 7.Zheng, W., Nowakowski, R. S., and Vaccarino, F. M. (2004) Dev. Neurosci. 26 181-196 [DOI] [PubMed] [Google Scholar]
- 8.Lillien, L., and Raphael, H. (2000) Development 127 4993-5005 [DOI] [PubMed] [Google Scholar]
- 9.Palmer, T. D., Ray, J., and Gage, F. H. (1995) Mol. Cell Neurosci. 6 474-486 [DOI] [PubMed] [Google Scholar]
- 10.Bird, A. (2002) Genes Dev. 16 6-21 [DOI] [PubMed] [Google Scholar]
- 11.Grayson, D. R., Chen, Y., Costa, E., Dong, E., Guidotti, A., Kundakovic, M., and Sharma, R. P. (2006) Pharmacol. Ther. 111 272-286 [DOI] [PubMed] [Google Scholar]
- 12.Shames, D. S., Minna, J. D., and Gazdar, A. F. (2007) Curr. Mol. Med. 7 85-102 [DOI] [PubMed] [Google Scholar]
- 13.Klose, R. J., and Bird, A. P. (2006) Trends Biochem. Sci. 31 89-97 [DOI] [PubMed] [Google Scholar]
- 14.Ueba, T., Kaspar, B., Zhao, X., and Gage, F. H. (1999) J. Biol. Chem. 274 10382-10387 [DOI] [PubMed] [Google Scholar]
- 15.Zhao, X., Ueba, T., Christie, B. R., Barkho, B., McConnell, M. J., Nakashima, K., Lein, E. S., Eadie, B. D., Willhoite, A. R., Muotri, A. R., Summers, R. G., Chun, J., Lee, K. F., and Gage, F. H. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 6777-6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allan, A. M., Liang, X., Luo, Y., Pak, C., Li, X., Szulwach, K. E., Chen, D., Jin, P., and Zhao, X. (2008) Hum. Mol. Genet. 17 2047-2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer, T. D., Markakis, E. A., Willhoite, A. R., Safar, F., and Gage, F. H. (1999) J. Neurosci. 19 8487-8497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkho, B. Z., Song, H., Aimone, J. B., Smrt, R. D., Kuwabara, T., Nakashima, K., Gage, F. H., and Zhao, X. (2006) Stem Cells Dev. 15 407-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takizawa, T., Nakashima, K., Namihira, M., Ochiai, W., Uemura, A., Yanagisawa, M., Fujita, N., Nakao, M., and Taga, T. (2001) Dev. Cell 1 749-758 [DOI] [PubMed] [Google Scholar]
- 20.Herman, J. G., Graff, J. R., Myohanen, S., Nelkin, B. D., and Baylin, S. B. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 9821-9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis, K., Fan, T., Geiman, T., Yan, Q., and Muegge, K. (2001) Genes Dev. 15 2940-2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorensen, V., Nilsen, T., and Wiedlocha, A. (2006) BioEssays 28 504-514 [DOI] [PubMed] [Google Scholar]
- 23.Stachowiak, M. K., Fang, X., Myers, J. M., Dunham, S. M., Berezney, R., Maher, P. A., and Stachowiak, E. K. (2003) J. Cell. Biochem. 90 662-691 [DOI] [PubMed] [Google Scholar]
- 24.Decker, L., Picard-Riera, N., Lachapelle, F., and Baron-Van Evercooren, A. (2002) J. Neurosci. Res. 69 763-771 [DOI] [PubMed] [Google Scholar]
- 25.Tureyen, K., Vemuganti, R., Bowen, K. K., Sailor, K. A., and Dempsey, R. J. (2005) Neurosurgery 57 1254-1263 [DOI] [PubMed] [Google Scholar]
- 26.Stachowiak, E. K., Maher, P. A., Tucholski, J., Mordechai, E., Joy, A., Moffett, J., Coons, S., and Stachowiak, M. K. (1997) Oncogene 14 2201-2211 [DOI] [PubMed] [Google Scholar]
- 27.Israsena, N., Hu, M., Fu, W., Kan, L., and Kessler, J. A. (2004) Dev. Biol. 268 220-231 [DOI] [PubMed] [Google Scholar]
- 28.Biesiada, E., Razandi, M., and Levin, E. R. (1996) J. Biol. Chem. 271 18576-18581 [DOI] [PubMed] [Google Scholar]
- 29.Care, A., Silvani, A., Meccia, E., Mattia, G., Stoppacciaro, A., Parmiani, G., Peschle, C., and Colombo, M. P. (1996) Mol. Cell. Biol. 16 4842-4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichimura, T., Watanabe, S., Sakamoto, Y., Aoto, T., Fujita, N., and Nakao, M. (2005) J. Biol. Chem. 280 13928-13935 [DOI] [PubMed] [Google Scholar]
- 31.Nagarajan, R. P., Hogart, A. R., Gwye, Y., Martin, M. R., and Lasalle, J. M. (2006) Epigenetics 1 172-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayakawa, S., Miike, K., Nakao, M., and Abe, K. (2007) Genes Cells 12 447-460 [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto, Y., Watanabe, S., Ichimura, T., Kawasuji, M., Koseki, H., Baba, H., and Nakao, M. (2007) J. Biol. Chem. 282 16391-16400 [DOI] [PubMed] [Google Scholar]
- 34.Spivakov, M., and Fisher, A. G. (2007) Nat. Rev. Genet. 8 263-271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.