Abstract
We have previously shown that genistein could inhibit Akt activation and down-regulate AR (androgen receptor) and PSA (prostate-specific antigen) expression in prostate cancer (PCa) cells. However, pure genistein showed increased lymph node metastasis in an animal model, but such an adverse effect was not seen with isoflavone, suggesting that further mechanistic studies are needed for elucidating the role of isoflavone in PCa. It is known that FOXO3a and GSK-3β, targets of Akt, regulate cell proliferation and apoptosis. Moreover, FOXO3a, GSK-3β, and Src are AR regulators and regulate transactivation of AR, mediating the development and progression of PCa. Therefore, we investigated the molecular effects of isoflavone on the Akt/FOXO3a/GSK-3β/AR signaling network in hormone-sensitive LNCaP and hormone-insensitive C4-2B PCa cells. We found that isoflavone inhibited the phosphorylation of Akt and FOXO3a, regulated the phosphorylation of Src, and increased the expression of GSK-3β, leading to the down-regulation of AR and its target gene PSA. We also found that isoflavone inhibited AR nuclear translocation and promoted FOXO3a translocation to the nucleus. By electrophoretic mobility shift assay and chromatin immunoprecipitation assay, we found that isoflavone inhibited FOXO3a binding to the promoter of AR and increased FOXO3a binding to the p27KIP1 promoter, resulting in the alteration of AR and p27KIP1 expression, the inhibition of cell proliferation, and the induction of apoptosis in both androgen-sensitive and -insensitive PCa cells. These results suggest that isoflavone-induced inhibition of cell proliferation and induction of apoptosis are partly mediated through the regulation of the Akt/FOXO3a/GSK-3β/AR signaling network. In conclusion, our data suggest that isoflavone could be useful for the prevention and/or treatment of PCa.
Androgen and AR (androgen receptor) are required for both normal prostate development and prostate carcinogenesis. Androgen ablation therapy for prostate cancer, although initially effective, nearly always leads to treatment failure and progression to hormone-refractory prostate cancer (HRPC),2 for which there is no curative therapy. It is still unclear how the prostate cancer cells progress to androgen independence. However, more evidence indicates that the mechanism involved in the escape of prostate cancer (PCa) from androgen control include mutation and overexpression of the AR gene or ligand-independent AR activation by other signaling pathways, including Akt and Src (1–4).
Most HRPC cells express AR and the androgen-inducible PSA (prostate-specific antigen), suggesting that AR signaling plays an important role in the development and progression of HRPC (5). In prostate epithelial cells, ligand-free AR is sequestered in the cytoplasm. Binding of androgen to the AR induces AR conformational change. The AR then forms a homodimer and is phosphorylated. This phosphorylation stabilizes the ligand-AR complex, allowing its translocation to the nucleus for its activity (6). The activated AR then initiates gene transcription by binding to specific androgen-response elements in the promoter regions of target genes, including PSA. More evidences have shown that instead of androgen, other molecules in cell signaling pathways can also transactivate AR (7). It has been found that Akt and Src can phosphorylate the AR and transactivate the activity of AR (3, 4). In addition, Akt has also been found to phosphorylate AR and suppress AR transactivation (8). Further study has shown that Akt pathway can suppress AR activity in androgen-dependent LNCaP cells with low passage numbers; however, Akt can also enhance AR activity in LNCaP cells with high passage numbers, suggesting that Akt pathway may have distinct mechanisms to modulate AR functions in various stages of prostate cancer cells (9). Therefore, Akt could be an important regulator of AR required for the progression of PCa cells to HRPC.
The activated Akt can inhibit apoptosis and promote cell survival by direct phosphorylation of its downstream targets (10). FOXO3a (Forkhead transcription factor class O3a) is one of the main targets of activated Akt. By binding to the nuclear importer, active FOXO3a translocates to the nucleus, binds to DNA, and promotes the transcription of its target genes, including p27KIP1, inducing either cell cycle arrest or apoptosis (11). However, activated Akt regulates transcription of FOXO3a target genes through phosphorylation of FOXO3a, leading to the release of FOXO3a from DNA and translocation to the cytoplasm (11, 12). It has been found that FOXO3a also promotes the transcription of AR (13). Therefore, Akt/ FOXO3a/AR signaling appears to play important roles in the development and progression of HRPC.
GSK-3β (glycogen synthase kinase 3β) is another main Akt target gene critically involved in the induction of apoptosis and cell cycle arrest (10). GSK-3β also controls cell survival through regulation of β-catenin, one of the key molecules in Wnt signaling (14). Moreover, GSK-3β is an AR regulator that inhibits transcriptional activity of AR, forming a signal network with Akt/FOXO3a/AR (15, 16). In addition, Src, one of the important intracellular oncogenic signals, can activate Akt through the regulation of phosphatidylinositol 3-kinase (17) and transactivate AR through the phosphorylation of AR (2), suggesting that Src is also a member of the Akt/FOXO3a/GSK-3β/AR signaling network. These findings suggest that Akt/FOXO3a/GSK-3β/AR signaling network may play important roles in the development and progression of HRPC. Therefore, specific targeting of the molecules in this signaling network by a novel approach could be important for killing HRPC cells, and thus, such a strategy could be useful for the prevention and/or treatment of not only primary PCa but most importantly HRPC and metastatic disease.
We and others have previously found that genistein, one of the most active isoflavones found in soybeans, exerts a potent antiproliferative effect on various cancers, including PCa (18, 19). We have also reported that genistein induces growth inhibition and apoptosis in PCa cells through the down-regulation of Akt (20), AR, and PSA (21). Moreover, Hillman et al. (22) from our group have found that pure genistein alone could increase lymph node metastases in an orthotopic model of PCa induced by PC-3 cells; however, such a phenomenon was not found when soy isoflavone formulation was used (23). These findings suggest that isoflavone formulation is more suitable for cancer prevention and treatment studies; however, no such studies have been reported to date to elucidate the effect and molecular mechanisms of isoflavone's action on the Akt/FOXO3a/GSK-3β/AR signaling network in PCa cells, especially in HRPC cells. Recently, we found that 3,3′-diindolylmethane, one of the chemopreventive agents, could inhibit the growth of prostate cancer cells through the inhibition of Akt/FOXO3a/β-catenin signaling (24). Here we report, for the first time, that isoflavone mixture is a second class of potent agent for inducing growth inhibition and apoptotic cell death in both androgen-sensitive LNCaP and androgen-insensitive C4-2B PCa cells, which is partly mediated through the regulation of Akt/FOXO3a/GSK-3β/AR signaling. From these results, we conclude that isoflavone rather than pure genistein should be exploited for the prevention and/or treatment of PCa but most importantly in HRPC, for which there is no curative therapy.
EXPERIMENTAL PROCEDURES
Cell Lines, Reagents, and Antibodies—Human PCa cell lines, including LNCaP and C4-2B cells, were maintained in RPMI 1640 (Invitrogen) with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2 atmosphere at 37 °C. Isoflavone mixture (83.3% genistein, 14.6% daidzein, and 0.26% glycitein; manufactured by Organic Technologies and obtained from the National Institutes of Health) was dissolved in DMSO to make a stock solution containing 50 mm genistein. The concentrations of isoflavone we described in this article all refer to the concentration of genistein in the isoflavone mixture. It has been reported that the concentration of genistein in plasma can reach up to 27.46 ± 15.38 μm without clinical toxicity after a single-dose administration of soy isoflavones to healthy men (25) and that prostate tissue can concentrate genistein after oral isoflavone supplementation in PCa patients (26). Therefore, we chose 25 and 50 μm isoflavone (equivalent to 25 and 50 μm genistein) for treatments in this study. IGF-1 was purchased from R&D Systems (Minneapolis, MN). Testosterone and LY294002 were from Sigma. Anti-AR (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-Akt (Santa Cruz Biotechnology), anti-phospho-Akt (Ser473) (Cell Signaling, Danvers, MA), anti-phospho-Akt (Thr308) (Cell Signaling), anti-FOXO3a (Cell Signaling), anti-phospho-FOXO3a Ser253 (Cell Signaling), anti-phospho-FOXO3a Thr32 (Cell Signaling), anti-GSK-3β (Cell Signaling), anti-pGSK-3β Ser9 (Cell Signaling), anti-phospho-β-catenin (Cell Signaling), anti-karyopherin α (BD Biosciences), anti-karyopherin β (BD Biosciences), anti-PSA (Lab Vision, Fremont, CA), anti-Rb (Santa Cruz Biotechnology), anti-Src (Cell Signaling), anti-Src (Tyr416) (Cell Signaling), anti-Src (Tyr527) (Cell Signaling), and anti-β-actin (Sigma) primary antibodies were used for immunoprecipitation, Western blot analysis, or immunofluorescent staining.
Cell Proliferation Studies by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) and 5-Bromo-2-deoxyuridine Assays—Human LNCaP and C4-2B PCa cells were seeded in 96-well plates. After 24 h, the cells were incubated in serum-free medium supplemented with 100 ng/ml IGF-1, 50 μm isoflavone, or 50 μm isoflavone plus 100 ng/ml IGF-1 for 48 h. Control cells were treated with 0.1% DMSO (vehicle control). After treatment, the cells were subject to an MTT assay, as described previously (27), and a 5-bromo-2-deoxyuridine assay using the BrdU Labeling and Detection Kit III (Roche Applied Science), according to the manufacturer's protocol. The growth inhibition of LNCaP and C4-2B cells by isoflavone treatment was statistically evaluated with Student's t test using GraphPad StatMate software (GraphPad Software Inc., San Diego, CA).
Hoechst Staining, Histone/DNA Enzyme-linked Immunosorbent Assay, and Caspase-3 Assay for Detection of Apoptosis—LNCaP and C4-2B PCa cells were treated with 100 ng/ml IGF-1, 50 μm isoflavone, or 50 μm isoflavone plus 100 ng/ml IGF-1 for 48 h, as described above. After treatment, cells were washed with cold PBS and fixed in ethanol for 1 h. The cells were then stained with 5 μg/ml Hoechst for 30 min and visualized under a fluorescence microscope. Bright condensed, punctuate, or granular nuclei were considered apoptotic. We also quantitatively compared the apoptotic cells in control or IGF-1- or isoflavone-treated cells using the cell death detection enzyme-linked immunosorbent assay kit (Roche Applied Science) according to the manufacturer's protocol, as described previously (27). The activity of caspase-3 in each sample was measured by using the EnzChek caspase-3 assay kit (Molecular Probes, Inc., Eugene, OR), following the manufacturer's protocol. The cleavage and activation of caspase-3 were also accessed by Western blot analysis using anti-caspase-3 antibody (Cell Signaling). The induction of apoptosis by isoflavone treatment of LNCaP and C4-2B PCa cells was statistically evaluated with Student's t test using GraphPad StatMate software (GraphPad Software Inc.).
Preparation of Cytoplasmic and Nuclear Lysates—LNCaP and C4-2B PCa cells were treated with 50 μm isoflavone for 48 and 72 h. After treatment and harvesting, the cells were resuspended in lysis buffer (0.08 m KCl, 35 mm HEPES, pH 7.4, 5 mm potassium phosphate, pH 7.4, 5 mm MgCl2, 25 mm CaCl2, 0.15 m sucrose, 2 mm phenylmethylsulfonyl fluoride, 8 mm dithiothreitol) and frozen at -80 °C overnight. The cell suspension was thawed and passed through a 28-gauge needle three times. A small aliquot of the cells were checked for cell membrane breakage using trypan blue. Then the cell suspension was centrifuged, and the supernatant was saved as cytoplasmic lysate. The pellet was suspended in lysis buffer, and the nuclei were lysed by sonication. After centrifugation, supernatant was saved as nuclear lysate. The protein concentration in the lysates was measured by a Coomassie Plus protein assay (Pierce).
Immunoprecipitation—Nuclear lysate (500 μg) and cytoplasmic lysate (800 μg) were subjected to immunoprecipitation by adding 2.5–5 μg of anti-AR, anti-FOXO3a, or anti-β-catenin antibody and incubation overnight at 4 °C. After adding 50 μl of Protein G-agarose (Santa Cruz Biotechnology) and incubation for 1 h, the samples were centrifuged. The agarose pellet was then washed three times, resuspended in Laemmli buffer, and boiled for 5 min. Boiled samples were centrifuged, and supernatant was used for Western blot analysis.
Western Blot Analysis—LNCaP and C4-2B cells were treated with 25 or 50 μm isoflavone for 1–8 or 24–72 h. Whole cell lysate of the cells was prepared by sonicating the cells lysed in 62 mm Tris-HCl and 2% SDS. In another set of experiments, cytoplasmic and nuclear proteins were also extracted. The protein concentration was measured by the BCA protein assay (Pierce). Immunoprecipitates, whole cell lysates, and cytoplasmic or nuclear proteins were subjected to standard Western blot analysis, as described previously (27). The signal of Western blot was scanned and quantified by using AlphaEaseFC software (Alpha Innotech, San Leandro, CA). The signal ratio of each protein over loading control was calculated by standardizing the ratios of each control to the unit value.
Transient Transfection with Akt cDNA Constructs—pLNCX-Akt (wild type Akt), pLNCX-Myr-Akt (constitutively activated Akt), pLNCX-Akt-K179M (dominant negative), and pLNCX (control empty vector) were generously provided by Dr. Sellers (Dana-Farber Cancer Institute, Boston, MA). The pLNCX-Akt, pLNCX-Myr-Akt, pLNCX-Akt-K179M, or pLNCX was transiently transfected into LNCaP and C4-2B PCa cells using ExGen 500 (Fermentas, Hanover, MD). After 5 h, the transfected cells were washed and incubated with complete RPMI 1640 medium overnight, followed by treatment with 50 μm isoflavone for 48 h. Subsequently, the cytoplasmic and nuclear proteins from transfected and untransfected cells were extracted and subjected to Western blot analysis using the indicated antibodies.
siRNA Transfection—C4-2B and LNCaP PCa cells were transfected with Akt siRNA (Santa Cruz Biotechnology) or control RNA duplex (Santa Cruz Biotechnology) by Lipofectamine RNAiMAX (Invitrogen). After 48 h of incubation, the total cellular proteins from each sample were extracted. The level of phospho-FOXO3a, phospho-Akt, Akt, GSK-3β, phospho-AR, and PSA expression was detected by Western blot analysis. C4-2B cells were also transfected with AR, p27, and control siRNA and incubated for 48 h. The effects of siRNAs on cell proliferation were measured by an MTT assay.
Immunofluorescence Staining—LNCaP and C4-2B PCa cells were cultured on chamber slides and treated with 50 μm isoflavone for 24–72 h. Cells were then fixed with acetone for 15 min, rinsed with PBS, and incubated with 1% bovine serum albumin in PBS for 30 min. The cells were then incubated with anti-AR or anti-FOXO3a antibody at 4 °C overnight. After washing with PBS, the cells were incubated with fluorescein isothiocyanate-conjugated or Texas Red-conjugated secondary antibody (Santa Cruz Biotechnology) at 37 °C for 1 h and washed with PBS. Cell images were observed under a fluorescent microscope and photographed under the same exposure and magnification (×200) settings.
Electrophoretic Mobility Shift Assay (EMSA)—EMSA was conducted to measure the activity of FOXO3a binding to AR or p27KIP1 promoter in isoflavone-treated and -untreated cells. LNCaP and C4-2B PCa cells were treated with 50 μm isoflavone for 24–72 h. Following treatment, nuclear protein from cells was extracted and subject to DNA binding and gel analysis as we described previously (27). The sequences of IRDye-labeled oligonucleotide (LI-COR, Lincoln, NE), which contains AR or p27KIP1 promoter sequences with a consensus FOXO3a DNA binding site, are as follows: 5′-ATTATGTCCTTGTTTCAGCCTGTT-3′ for AR and 5′-ATGTGTAGCTTGTTTTCTTAGCCAC-3′ for p27KIP1 (13, 28, 29). Supershift using FOXO3a antibody was also conducted to confirm the specificity of FOXO3a DNA binding activity.
Chromatin Immunoprecipitation (ChIP) Assay—A ChIP assay was performed to test the effect of isoflavone on FOXO3a binding to AR or p27KIP1 promoter under an in vivo environment. Briefly, LNCaP and C4-2B PCa cells were treated with 50 μm isoflavone for 48 h. After formaldehyde cross-linking and quenching, the cells were incubated in cell lysis buffer (5 mm HEPES, pH 8.0, 85 mm KCl, 0.5% Nonidet P-40, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mm phenylmethylsulfonyl fluoride) on ice for 10 min. The nuclei were pelleted and resuspended in nuclear lysis buffer (50 mm Tris-HCl, pH 8.1, 10 mm EDTA, 1% SDS, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mm phenylmethylsulfonyl fluoride). After incubation on ice for 10 min, the nuclear lysates were sonicated to generate an average DNA size of ∼600 bp, and protein concentration was measured using the BCA protein assay (Pierce). 800 μg of each sample were incubated with FOXO3a antibody (Santa Cruz Biotechnology) at 4 °C overnight. The FOXO3a antibody-bound DNA complexes were pelleted with Protein G-agarose at 4 °C for 2 h. After washing and elution, the immunoprecipitated complexes and input were reversed from cross-linking. The input DNA and immunoprecipitated DNA were then precipitated and purified. Real time and conventional PCRs were performed using the purified DNA and the following primers: AR promoter (forward, 5′-AGCCTTTCCCAAATGACAATG-3′; reverse, 5′-GCACAGCCAAACATCATAGG-3′), p27KIP1 promoter (forward, 5′-GTCCCTTCCAGCTGTCACAT-3′; reverse, 5′-GGAAACCAACCTTCCGTTCT-3′), β-actin (forward, 5′-CCACACTGTGCCCATCTACG-3′; reverse, 5′-AGGATCTTCATGAGGTAGTCAGTCAG-3′). For real time PCR, data were analyzed according to the comparative Ct method, and the amount of FOXO3a-bound AR or p27KIP1 promoter DNA was normalized by input β-actin DNA. For conventional PCR, PCR product was visualized by agarose gel electrophoresis and ethidium bromide staining.
RESULTS
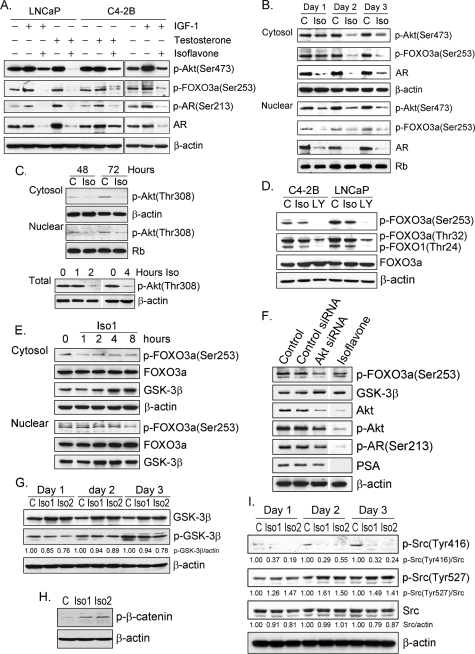
Inhibition of FOXO3a Phosphorylation, Up-regulation of GSK-3β Expression, and Down-regulation of AR Expression and Phosphorylation by Isoflavone—We have previously reported that genistein inhibits the phosphorylation of Akt in PC-3 PCa cells and the expression of PSA in LNCaP PCa cells (20, 21). To further investigate whether isoflavone could suppress the expression of PSA through the inhibition of Akt phosphorylation and the inactivation of AR, we tested the effects of isoflavone on Akt, FOXO3a, and AR in IGF-1-stimulated and -unstimulated conditions. We observed that phosphorylation of Akt, FOXO3a, and AR was up-regulated by IGF-1 in PCa cells, as expected (Fig. 1A). However, isoflavone inhibited the phosphorylation of Akt, FOXO3a, and AR in the cytoplasm and nucleus of C4-2B and LNCaP PCa cells (Fig. 1, B and C). Moreover, isoflavone abrogated the up-regulation of Akt, FOXO3a, and AR phosphorylation induced by IGF-1 (Fig. 1A). Phosphorylation of Akt at both Ser473 and Thr308 sites was inhibited by isoflavone (Fig. 1, B and C). Furthermore, we found that isoflavone inhibited the phosphorylation of FOXO3a at both Thr32 and Ser253 sites and FOXO1 at Thr24 in LNCaP and C4-2B PCa cells (Fig. 1D). We have also observed that the level of FOXO3a was increased in nuclear lysate after isoflavone treatment (Fig. 1E), suggesting that the increased ratio of FOXO3a over phospho-FOXO3a in both the cytoplasm and nucleus of PCa cells could retain a greater amount of active FOXO3a in the nuclear compartment to inhibit cancer cell growth. To further confirm the effects of isoflavone on FOXO3a and AR, we conducted studies using Akt siRNA. We found that Akt siRNA transfection decreased the levels of Akt, phospho-Akt, phospho-FOXO3a, phospho-AR, and PSA (Fig. 1F), similar to the effects of isoflavone, suggesting that the effect of isoflavone on FOXO3a and AR is mediated through the Akt signaling. We also exposed the PCa cells to a lower concentration of isoflavone for a short time (1–8 h) and observed similar results (Fig. 1E), suggesting that the down-regulation of phospho-FOXO3a by isoflavone observed in our studies was not the consequence of cell death. In addition, we also found that testosterone stimulated AR expression and phosphorylation, whereas isoflavone decreased AR expression and phosphorylation under both testosterone-stimulated and -unstimulated conditions (Fig. 1, A and B).
FIGURE 1.
Western blot analysis and siRNA assay showed that isoflavone down-regulated Akt, AR, and Src signaling and up-regulated FOXO3a and GSK-3β signaling. A, LNCaP and C4-2B PCa cells were treated with 100 ng/ml IGF-1, 100 nm testosterone, 50 μm isoflavone, 50 μm isoflavone plus 100 ng/ml IGF-1, or 50 μm isoflavone plus 100 nm testosterone for 48 h. Total lysate from each sample was subjected to Western blot analysis. B, C4-2B PCa cells were treated with 50 μm isoflavone for 24–72 h. Cytoplasmic and nuclear lysate from each sample was subjected to Western blot analysis. C, LNCaP cells were treated with 50 μm isoflavone for 1–4, 48, or 72 h. Cytosol and nuclear and total proteins were subjected to Western blot analysis (C, control; Iso, isoflavone). D, C4-2B and LNCaP PCa cells were treated with 25 μm isoflavone or 20 μm LY294002 for 48 h. Total cell lysates from each sample were subjected to Western blot analysis (C, control; Iso, isoflavone; LY, LY294002). E, C4-2B PCa cells were treated with 25 μm isoflavone (Iso1) for 1–8 h. Cytoplasmic and nuclear proteins were subjected to Western blot analysis. F, C4-2B PCa cells were transfected with Akt siRNA or control RNA duplex or treated with 25 μm isoflavone. Total cell lysates from each sample were subjected to Western blot analysis. G–I, C4-2B PCa cells were treated with 25 (Iso1) and 50 (Iso2) μm isoflavone for 24–72 h (G and I) or 24 h (H). Total cell lysates from each sample were subjected to Western blot analysis. The concentration of isoflavone is equivalent to the concentration of genistein in the isoflavone mixture.
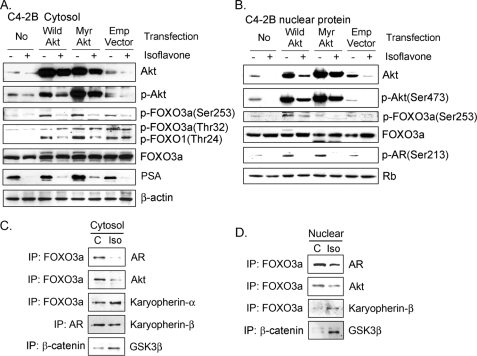
Next, we tested the effects of isoflavone on FOXO3a and AR after Akt transfection. After transfection with wild-type Akt and constitutively activated Akt cDNA, we observed up-regulation of phospho-Akt and phospho-FOXO3a proteins in the cytoplasm and nucleus of C4-2B PCa cells (Fig. 2, A and B). We also observed up-regulated AR phosphorylation in nucleus and increased level of PSA in the cytosol after Akt transfection (Fig. 2, A and B). Importantly, we found that isoflavone could abrogate the up-regulation of phospho-Akt, phospho-FOXO3a, phospho-AR, and PSA induced by wild-type Akt or constitutively activated Akt cDNA transfection and increased the levels of FOXO3a in the nuclear compartment (Fig. 2, A and B).
FIGURE 2.
Gene transfection and immunoprecipitation experiments showed that isoflavone down-regulated Akt and AR signaling and up-regulated FOXO3a and GSK-3β signaling. A and B, C4-2B PCa cells were transiently transfected with pLNCX-Akt (wild type Akt), pLNCX-Myr-Akt (constitutively activated Akt), or pLNCX (empty vector) and treated with 50 μm isoflavone for 48 h. Cytoplasmic (A) and nuclear (B) lysates from each sample were subjected to Western blot analysis. C and D, C4-2B PCa cells were treated with 50 μm isoflavone for 48 h. Cytoplasmic (C) and nuclear (D) lysates from each sample were subjected to immunoprecipitation (IP), followed by Western blot analysis, using the indicated antibodies (C, control; Iso, isoflavone). The concentration of isoflavone is equivalent to the concentration of genistein in the isoflavone mixture.
By immunoprecipitation, we found that isoflavone inhibited the formation of Akt and FOXO3a complex in the cytoplasm and nucleus of PCa cells (Fig. 2, C and D), consistent with Akt inactivation and subsequent decrease in FOXO3a phosphorylation by isoflavone treatment. We also found that isoflavone decreased the formation of FOXO3a and AR complex, which could inhibit FOXO3a activity, in both the cytoplasmic and nuclear compartments (Fig. 2, C and D), suggesting that higher levels of active FOXO3a proteins in the nuclear compartment in isoflavone-treated cells could be mechanistically linked with the antiproliferative and proapoptotic effects of isoflavone.
Moreover, we found that isoflavone enhanced the expression of GSK-3β beginning from 4–8 h and up to 72 h (Fig. 1, E and G), consistent with the down-regulation of AR by isoflavone. By immunoprecipitation, we discovered that GSK-3β binding to β-catenin was increased upon isoflavone treatment (Fig. 2, C and D). Isoflavone also inhibited the phosphorylation of GSK-3β and increased the phosphorylation of β-catenin (Fig. 1, G and H), suggesting that isoflavone could inactivate Wnt signaling to inhibit PCa cell growth. Because AR could also be transactivated by activated Src, we next investigated the effects of isoflavone on Src signaling to gain further insight as to the molecular mechanism of action of isoflavone.
Down-regulation of Src Signaling by Isoflavone—By Western blot analysis, we found that isoflavone down-regulated the expression of Src (Fig. 1I). Moreover, the level of phospho-Src (Tyr416), which is activated form of Src, was decreased by isoflavone (Fig. 1I). However, the level of phospho-Src (Tyr527), which triggers Src inactivation, was increased (Fig. 1I). These findings are consistent with the down-regulation of Akt and AR signaling by isoflavone (Fig. 1A). Because both AR and FOXO3a are transcription factors that regulate the transcription of their target genes in the nuclear compartment, we further investigated the nuclear localization of AR and FOXO3a altered by isoflavone treatment.
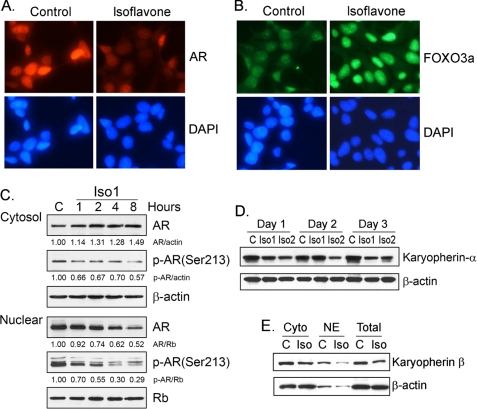
Inhibition of AR Nuclear Translocation and Enhancement of FOXO3a Nuclear Localization by Isoflavone—By immunofluorescent staining, we found that isoflavone decreased the levels of nuclear AR (Fig. 3A); however, the nuclear FOXO3a was increased upon isoflavone treatment (Fig. 3B). To confirm these results, we exposed PCa cells to 25 μm isoflavone for 1–8 h. We observed that cytoplasmic AR was increased, whereas nuclear AR was gradually decreased (Fig. 3C), suggesting the inhibition of AR nuclear translocation by isoflavone. Moreover, isoflavone treatment for 24–72 h significantly reduced the levels of both cytoplasmic and nuclear AR proteins (Fig. 1B), consistent with the results from immunofluorescent staining. We also tested the expression of karyopherin, one of the nuclear importins, and the interaction of karyopherin with AR or FOXO3a in isoflavone-treated and -untreated PCa cells. We found that isoflavone decreased the level of karyopherin protein in LNCaP (Fig. 3D) and C4-2B PCa cells (Fig. 3E). More importantly, we found that isoflavone inhibited karyopherin binding to AR (Fig. 2C), consistent with the data from immunofluorescent staining (Fig. 3A) and Western blot analysis (Fig. 3, D and E), demonstrating the inhibitory effects of isoflavone on the nuclear localization of AR. However, we observed increased binding of karyopherin to FOXO3a (Fig. 2, C and D) and increased level of FOXO3a in nuclear lysate (Fig. 1E) after isoflavone treatment, consistent with the data from immunofluorescent staining (Fig. 3B). Since FOXO3a functions as a transcription factor, which is known to regulate the transcription of its target genes, including AR and p27KIP1, we tested the effects of isoflavone on the transcriptional activity of AR and p27KIP1 regulated by FOXO3a.
FIGURE 3.
Isoflavone inhibited the nuclear translocation of AR and enhanced FOXO3a nuclear translocation. A and B, C4-2B PCa cells were treated with 50 μm isoflavone for 48 h. The samples were subjected to immunofluorescent staining using anti-AR (A) and anti-FOXO3a (B) antibodies (magnification, ×200). C, C4-2B PCa cells were treated with 25 μm isoflavone for 1–8 h. Cytoplasmic and nuclear lysates from each sample were subjected to Western blot analysis. D, LNCaPPCa cells were treated with 25 (Iso1) and 50 μm (Iso2) isoflavone for 24–72 h. Total cell lysates from each sample were subjected to Western blot analysis. E, C4-2B PCa cells were treated with 50 μm isoflavone for 48 h. Total, cytoplasmic, and nuclear lysates from each sample were subjected to Western blot analysis (C, control; Iso, isoflavone; Cyto, cytosol; NE, nuclear extract). The concentration of isoflavone is equivalent to the concentration of genistein in the isoflavone mixture. DAPI, 4′,6-diamidino-2-phenylindole.
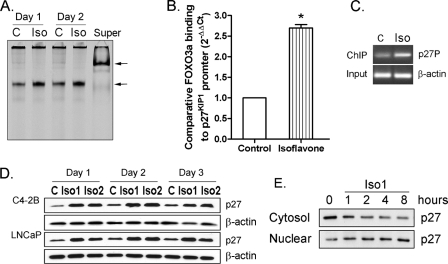
Stimulation of FOXO3a-mediated p27KIP1 Expression by Isoflavone—By EMSA, we found that isoflavone increased the activity of FOXO3a protein binding to p27KIP1 promoter DNA in C4-2B PCa cells (Fig. 4A). A ChIP assay confirmed the EMSA data, showing that isoflavone significantly enhanced FOXO3a protein binding to the p27KIP1 promoter in vivo in PCa cells (Fig. 4, B and C). Moreover, we found an increased level of p27KIP1 protein after 24 h of isoflavone treatment in LNCaP and C4-2B PCa cells (Fig. 4D), consistent with the data from EMSA and ChIP assays. Furthermore, we found that exposure of PCa cells to isoflavone for a short (1–8 h) period enhanced the nuclear translocation of p27KIP1 (Fig. 4E), suggesting that isoflavone inhibits cell proliferation partly through the nuclear accumulation of p27KIP1.
FIGURE 4.
Isoflavone up-regulated FOXO3a binding to the promoter of p27KIP1. A, C4-2B PCa cells were treated with 50 μm isoflavone for 24 and 48 h. Nuclear proteins from each sample were subjected to EMSA using p27KIP1 oligonucleotide (Super, supershift; arrow, specific DNA binding). B and C, C4-2B PCa cells were treated with 50 μm isoflavone for 48 h. The samples were then subjected to a ChIP assay, followed by real time PCR (B, bars, mean ± S.E.; *, p < 0.05 compared with control; n = 3) or conventional PCR (C, p27P, using p27KIP1 promoter primers; C, control; Iso, isoflavone treatment). D, C4-2B and LNCaP PCa cells were treated with 25 (Iso1) or 50 μm (Iso2) isoflavone for 24–72 h. Totally sates from each sample were subjected to Western blot analysis. E, C4-2BPC a cells were treated with 25 μm isoflavone for 1–8 h. Cytoplasmic and nuclear lysates from each sample were subjected to Western blot analysis. The concentration of isoflavone is equivalent to the concentration of genistein in the isoflavone mixture.
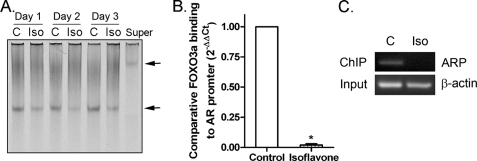
Inhibition of FOXO3a-mediated AR Transcription by Isoflavone—In contrast to the data on p27KIP1, using EMSA, we found that isoflavone inhibited the activity of FOXO3a protein binding to AR promoter DNA in C4-2B PCa cells (Fig. 5A), suggesting the inhibitory effect of isoflavone on the FOXO3a-induced transcription of AR. The data from the ChIP assay clearly showed that isoflavone treatment decreased FOXO3a protein binding to the AR promoter in vivo in C4-2B PCa cells (Fig. 5, B and C). By Western blot analysis, we found the down-regulation of AR expression by isoflavone treatment (Fig. 1, A and B), consistent with the results from EMSA and ChIP assays. Since FOXO3a, GSK-3β, p27KIP1, Src, and AR all are important factors in Akt and AR signaling that regulate cell survival, we tested the effects of isoflavone on cell proliferation and apoptosis.
FIGURE 5.
Isoflavone inhibited FOXO3a binding to the promoter of AR. A, C4-2B PCa cells were treated with 50 μm isoflavone for 24–72 h. Nuclear proteins from each sample were subjected to EMSA using AR oligonucleotide (Super, supershift; arrow, specific DNA binding). B and C, C4-2B PCa cells were treated with 50 μm isoflavone for 48 h. The samples were then subjected to a ChIP assay, followed by real time PCR (B, bars, mean ± S.E.; *, p < 0.05 compared with control; n = 3) or conventional PCR (C, ARP, using AR promoter primers; C, control; Iso, isoflavone treatment). The concentration of isoflavone is equivalent to the concentration of genistein in the isoflavone mixture.
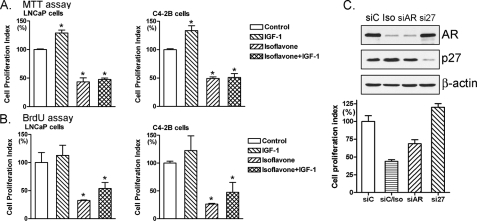
Inhibition of Cell Proliferation and Induction of Apoptosis by Isoflavone through Regulation of Akt/FOXO3a/GSK-3β/AR Signaling—Using two different methods of proliferation assays, we found that IGF-1, which activates Akt, promoted cell proliferation in LNCaP and C4-2B PCa cells (Fig. 6, A and B). Importantly, isoflavone inhibited cell proliferation and abrogated IGF-1-induced cell proliferation (Fig. 6, A and B). By Hoechst staining for testing apoptotic cells, we observed more bright condensed and granular stained nuclei in isoflavone-treated C4-2B and LNCaP PCa cells compared with control (Fig. 7A), suggesting that isoflavone could induce apoptosis. To quantitatively measure apoptotic cell death after different treatment, we conducted a histone/DNA enzyme-linked immunosorbent apoptosis assay. We found that IGF-1 protected cancer cells from apoptosis (Fig. 7B), whereas isoflavone induced apoptosis and abrogated IGF-1-mediated protection of LNCaP and C4-2B PCa cells from apoptosis (Fig. 7B). To further confirm the induction of apoptosis by isoflavone, we conducted caspase-3 activity and cleavage assays. We found increased activity of caspase-3 (Fig. 7C) and the cleaved and active caspase-3 fragment (Fig. 7D) in isoflavone-treated cells. Moreover, AR siRNA also inhibited the proliferation of prostate cancer cells, whereas p27KIP1 siRNA promoted cell growth (Fig. 6C). These results are consistent with our observation on the alterations of Akt, FOXO3a, and AR caused by IGF-1 or isoflavone treatment (Fig. 1A), suggesting that the inhibition of cell proliferation and induction of apoptosis by isoflavone is partly mediated by the regulation of Akt/FOXO3a/GSK-3β/AR signaling pathways.
FIGURE 6.
Isoflavone-inhibited cell growth. C4-2B and LNCaP PCa cells were treated with 100 ng/ml IGF-1, 50 μm isoflavone, or 50 μm isoflavone plus 100 ng/ml IGF-1 for 48 h. The concentration of isoflavone is equivalent to the concentration of genistein in isoflavone mixture. Cell proliferation was assessed by an MTT assay (A) and 5-bromo-2-deoxyuridine assay (B) (*, p < 0.05, compared with control; n = 3). C, C4-2B cells were transfected with AR, p27KIP1, or control siRNA. After 48 h, cell proliferation was assessed by an MTT assay, and protein expression was measured by Western blot analysis (siC, control siRNA; siC/Iso, control siRNA plus 50 μm isoflavone treatment for 48 h; siAR, AR siRNA; si27, p27KIP1 siRNA; p < 0.05 compared with control; n = 3).
FIGURE 7.
Isoflavone-induced apoptosis. C4-2B and LNCaP PCa cells were treated with 100 ng/ml IGF-1, 50 μm isoflavone, or 50 μm isoflavone plus 100 ng/ml IGF-1 for 48 h. Apoptotic cell death was assessed by Hoechst staining (A; arrow, condensed and granular stained nuclei in apoptotic cells), cell death detection enzyme-linked immunosorbent assay (B;*, p < 0.05, compared with control; n = 3), caspase-3 activity assay (C;*, p < 0.05, compared with control; n = 3), and Western blot analysis for cleavage of caspase-3 (D).
DISCUSSION
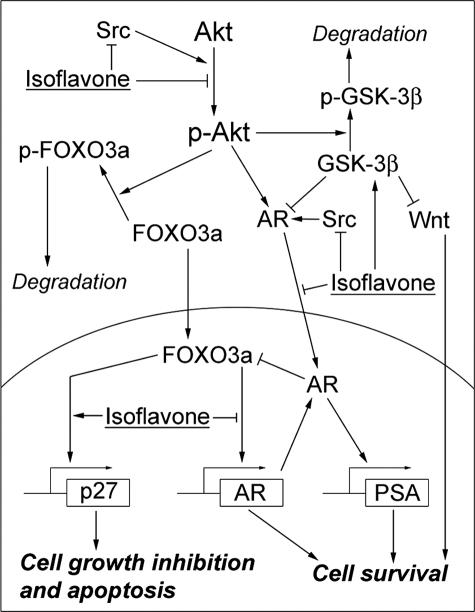
Akt/FOXO3a signal transduction is an important regulatory event in the Akt signaling pathway (30). The key step to control FOXO3a is phosphorylation of FOXO3a by Akt. The ratio of FOXO3a over phospho-FOXO3a is one of the factors that determine whether cancer cells will survive or undergo apoptotic cell death. From our series of studies, we found that isoflavone inhibited the phosphorylation and activation of Akt, leading to the lower levels of phospho-FOXO3a (Fig. 8). Moreover, isoflavone also increased nuclear translocation of FOXO3a, leading to the higher ratio of FOXO3a over phospho-FOXO3a in the nuclear compartment of isoflavone-treated cells. These results suggest that isoflavone regulates the activity of FOXO3a through the regulation of FOXO3a phosphorylation and nuclear importing. It has been shown that FOXO3a regulates the transcription of p27KIP1 and AR by binding to their promoters (13, 31). Interestingly, using EMSA and ChIP assay, we found that isoflavone was able to induce FOXO3a binding to p27KIP1 promoter and suppress FOXO3a binding to the AR promoter, resulting in the increased p27KIP1 protein expression and decreased AR protein level. Up-regulation of p27KIP1 promoter activity and protein expression by isoflavone-mediated FOXO3a regulation could lead to the growth inhibition and apoptotic cell death that we have observed in PCa cells, as reported in this paper. It has been reported that FOXO3a activates AR expression (13), whereas AR inactivates FOXO (32), forming a regulatory loop to maintain a balance between AR and FOXO. The decreased levels of AR protein and AR-FOXO3a complex after isoflavone treatment clearly suggest that isoflavone could interrupt the above mentioned regulatory loop, resulting in the inhibition of AR transactivation (Fig. 8). It is important to note that the initiation of transcription for a specific gene requires chromatin remodeling, promoter recognition, and DNA binding under the regulation of co-regulators, including activators and suppressors (33). Isoflavone-altered FOXO3a DNA binding could also be caused by the alterations in chromatin remodeling and co-regulators, such as GSK-3β, leading to increased FOXO3a binding to the p27KIP1 promoter and decreased binding to the AR promoter. In addition, the alteration of p27KIP1 protein level by isoflavone treatment could also be due to the altered activity of proteasome, as reported previously (34). Therefore, further investigations are required for establishing the cause and effect relationship between these molecules during isoflavone-induced cell death.
FIGURE 8.
Schematic representation of the molecular effects of isoflavone on the Akt/FOXO3a/GSK-3β/AR signaling network.
GSK-3β is another molecule that controls cell cycle and apoptosis in the Akt signaling pathway. GSK-3β also interacts with β-catenin to regulate cell proliferation through Wnt signaling. It has been shown that activated Akt phosphorylates and inactivates GSK-3β (35). If Akt is inactivated, GSK-3β can phosphorylate and inactivate β-catenin, leading to the inhibition of cell survival stimulated by Wnt signaling (15, 36). In this study, we found that isoflavone treatment decreased GSK-3β phosphorylation and increased GSK-3β expression and β-catenin phosphorylation, suggesting that isoflavone could down-regulate Wnt signaling. However, the regulation of Wnt signaling is a complex process and thus requires in-depth mechanistic studies that are currently being conducted in our laboratory. Importantly, it has been reported that GSK-3β can suppress the activity of AR (16), whereas Src can activate AR (2). β-Catenin also can form a complex with AR binding to the PSA promoter to initiate PSA transcription (37). In this study, we did observe increased expression of GSK-3β and decreased active Src, AR, and PSA in isoflavone-treated PCa cells. More importantly, we found that isoflavone inhibited Akt-mediated AR phosphorylation and activation. These results all suggest that isoflavone could inhibit cell survival through the regulation of Akt/GSK-3β/AR signaling (Fig. 8). Recent study by other investigators also showed that genistein in diet could inhibit Akt activation and increase GSK-3β activity in TRAMP PCa model, providing relevant evidence of isoflavone effect in vivo (38), and thus these results are consistent with our findings.
Akt, FOXO3a, p27KIP1, GSK-3β, and Src all are critical molecules in signal transduction, and they interact with each other to regulate cell survival and apoptosis (39–41). Moreover, all of them act as AR co-regulators that influence AR transactivation (13, 16, 42). It is obvious that there is significant cross-talk between Akt, FOXO3a, p27KIP1, GSK-3β, Src, and AR. Therefore, the Akt/FOXO3a/GSK-3β/AR signaling network appears to play critical roles in the control of PCa cell growth and apoptosis. In the present study, we found that isoflavone exerts its effects on each of these molecules at every step of this signaling pathway. The effect of isoflavone could begin from the inhibition of Akt activation and cause subsequent series of alterations of signal transduction in this signaling network, finally leading to the inhibition of AR transactivation and PSA expression in both androgen-sensitive and -insensitive PCa cells (Fig. 8). More importantly, it has been found that Akt, FOXO3a, and GSK-3β function as critical regulators in the development of androgen-independent PCa by regulation of the oncogenic function of AR and other genes (15, 43, 44). We found that the isoflavone mixture in the present formula that we have used could regulate these molecules and subsequently cause down-regulation of AR transactivation and decreased level of PSA, suggesting that this isoflavone mixture could be used for the prevention and/or treatment of PCa, especially HRPC. The comparisons of pure genistein, diadzin, or glycitein alone with the isoflavone mixture for the regulation of Akt/FOXO3a/GSK-3β/AR signaling pathways are needed to investigate the role of a component in the isoflavone that is responsible for negating the adverse effect of pure genistein in PCa, and thus, studies are ongoing in our laboratory, which could be important for gaining further insight as to the molecular mechanism of action of isoflavone for the prevention and/or treatment of PCa in the future.
It is known that growth factor IGF-1 activates Akt and testosterone activates AR (45, 46), suggesting that IGF-1 and testosterone could induce cell growth and inhibit apoptosis. Interestingly, we found that isoflavone abrogated the IGF-1- and testosterone-induced Akt and AR activation and cell proliferation and caused more apoptotic cell death. Isoflavone also affected Akt downstream genes, including FOXO3a, GSK-3β, and p27KIP1, due to the inactivation of Akt, resulting in the down-regulation of AR. These results suggest that isoflavone-induced inhibition of cell proliferation and induction of apoptotic cell death are in part due to the regulation of the Akt/FOXO3a/GSK-3β/AR signaling network. Our molecular mechanistic data also suggest that isoflavone could be a potent nontoxic agent for the prevention and/or treatment of hormone-dependent PCa but most importantly for HRPC.
This work was supported, in whole or in part, by National Institutes of Health, NCI, Grants 5R01CA083695 and 5R01CA101870 (to F. H. S.). This work was also supported by the Puschelberg Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HRPC, hormone-refractory prostate cancer; PCa, prostate cancer; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; IGF, insulin-like growth factor; PBS, phosphate-buffered saline; siRNA, small interfering RNA; EMSA, electrophoretic mobility shift assay; ChIP, chromatin immunoprecipitation.
References
- 1.Chen, C. D., Welsbie, D. S., Tran, C., Baek, S. H., Chen, R., Vessella, R., Rosenfeld, M. G., and Sawyers, C. L. (2004) Nat. Med 10 33-39 [DOI] [PubMed] [Google Scholar]
- 2.Guo, Z., Dai, B., Jiang, T., Xu, K., Xie, Y., Kim, O., Nesheiwat, I., Kong, X., Melamed, J., Handratta, V. D., Njar, V. C., Brodie, A. M., Yu, L. R., Veenstra, T. D., Chen, H., and Qiu, Y. (2006) Cancer Cell 10 309-319 [DOI] [PubMed] [Google Scholar]
- 3.Asim, M., Siddiqui, I. A., Hafeez, B. B., Baniahmad, A., and Mukhtar, H. (2008) Oncogene 27 3596-3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen, Y., Hu, M. C., Makino, K., Spohn, B., Bartholomeusz, G., Yan, D. H., and Hung, M. C. (2000) Cancer Res. 60 6841-6845 [PubMed] [Google Scholar]
- 5.Heinlein, C. A., and Chang, C. (2004) Endocr. Rev. 25 276-308 [DOI] [PubMed] [Google Scholar]
- 6.Edwards, J., and Bartlett, J. M. (2005) BJU Int. 95 1320-1326 [DOI] [PubMed] [Google Scholar]
- 7.Balk, S. P. (2002) Urology 60 132-138 [DOI] [PubMed] [Google Scholar]
- 8.Lin, H. K., Yeh, S., Kang, H. Y., and Chang, C. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 7200-7205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin, H. K., Hu, Y. C., Yang, L., Altuwaijri, S., Chen, Y. T., Kang, H. Y., and Chang, C. (2003) J. Biol. Chem. 278 50902-50907 [DOI] [PubMed] [Google Scholar]
- 10.Franke, T. F., Hornik, C. P., Segev, L., Shostak, G. A., and Sugimoto, C. (2003) Oncogene 22 8983-8998 [DOI] [PubMed] [Google Scholar]
- 11.Burgering, B. M., and Kops, G. J. (2002) Trends Biochem. Sci. 27 352-360 [DOI] [PubMed] [Google Scholar]
- 12.Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., Anderson, M. J., Arden, K. C., Blenis, J., and Greenberg, M. E. (1999) Cell 96 857-868 [DOI] [PubMed] [Google Scholar]
- 13.Yang, L., Xie, S., Jamaluddin, M. S., Altuwaijri, S., Ni, J., Kim, E., Chen, Y. T., Hu, Y. C., Wang, L., Chuang, K. H., Wu, C. T., and Chang, C. (2005) J. Biol. Chem. 280 33558-33565 [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi, A. (1999) Cell. Signal. 11 777-788 [DOI] [PubMed] [Google Scholar]
- 15.Mulholland, D. J., Dedhar, S., Wu, H., and Nelson, C. C. (2006) Oncogene 25 329-337 [DOI] [PubMed] [Google Scholar]
- 16.Salas, T. R., Kim, J., Vakar-Lopez, F., Sabichi, A. L., Troncoso, P., Jenster, G., Kikuchi, A., Chen, S. Y., Shemshedini, L., Suraokar, M., Logothetis, C. J., DiGiovanni, J., Lippman, S. M., and Menter, D. G. (2004) J. Biol. Chem. 279 19191-19200 [DOI] [PubMed] [Google Scholar]
- 17.Cheng, J. Q., Lindsley, C. W., Cheng, G. Z., Yang, H., and Nicosia, S. V. (2005) Oncogene 24 7482-7492 [DOI] [PubMed] [Google Scholar]
- 18.Barnes, S. (1995) J. Nutr. 125 777S-783S [DOI] [PubMed] [Google Scholar]
- 19.Li, Y., Upadhyay, S., Bhuiyan, M., and Sarkar, F. H. (1999) Oncogene 18 3166-3172 [DOI] [PubMed] [Google Scholar]
- 20.Li, Y., and Sarkar, F. H. (2002) Clin. Cancer Res. 8 2369-2377 [PubMed] [Google Scholar]
- 21.Davis, J. N., Kucuk, O., and Sarkar, F. H. (2002) Mol. Carcinog. 34 91-101 [DOI] [PubMed] [Google Scholar]
- 22.Hillman, G. G., Wang, Y., Kucuk, O., Che, M., Doerge, D. R., Yudelev, M., Joiner, M. C., Marples, B., Forman, J. D., and Sarkar, F. H. (2004) Mol. Cancer Ther. 3 1271-1279 [PubMed] [Google Scholar]
- 23.Raffoul, J. J., Banerjee, S., Che, M., Knoll, Z. E., Doerge, D. R., Abrams, J., Kucuk, O., Sarkar, F. H., and Hillman, G. G. (2007) Int. J. Cancer 120 2491-2498 [DOI] [PubMed] [Google Scholar]
- 24.Li, Y., Wang, Z., Kong, D., Murthy, S., Dou, Q. P., Sheng, S., Reddy, G. P., and Sarkar, F. H. (2007) J. Biol. Chem. 282 21542-21550 [DOI] [PubMed] [Google Scholar]
- 25.Busby, M. G., Jeffcoat, A. R., Bloedon, L. T., Koch, M. A., Black, T., Dix, K. J., Heizer, W. D., Thomas, B. F., Hill, J. M., Crowell, J. A., and Zeisel, S. H. (2002) Am. J. Clin. Nutr. 75 126-136 [DOI] [PubMed] [Google Scholar]
- 26.Rannikko, A., Petas, A., Rannikko, S., and Adlercreutz, H. (2006) Prostate 66 82-87 [DOI] [PubMed] [Google Scholar]
- 27.Li, Y., Ahmed, F., Ali, S., Philip, P. A., Kucuk, O., and Sarkar, F. H. (2005) Cancer Res. 65 6934-6942 [DOI] [PubMed] [Google Scholar]
- 28.Furuyama, T., Nakazawa, T., Nakano, I., and Mori, N. (2000) Biochem. J. 349 629-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran, H., Brunet, A., Griffith, E. C., and Greenberg, M. E. (2003) Sci. STKE 2003, RE5. [DOI] [PubMed]
- 30.Huang, H., and Tindall, D. J. (2006) Future Oncol. 2 83-89 [DOI] [PubMed] [Google Scholar]
- 31.Hu, Y., Wang, X., Zeng, L., Cai, D. Y., Sabapathy, K., Goff, S. P., Firpo, E. J., and Li, B. (2005) Mol. Biol. Cell 16 3705-3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, P., Lee, H., Guo, S., Unterman, T. G., Jenster, G., and Bai, W. (2003) Mol. Cell. Biol. 23 104-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemon, B., and Tjian, R. (2000) Genes Dev. 14 2551-2569 [DOI] [PubMed] [Google Scholar]
- 34.Kazi, A., Daniel, K. G., Smith, D. M., Kumar, N. B., and Dou, Q. P. (2003) Biochem. Pharmacol. 66 965-976 [DOI] [PubMed] [Google Scholar]
- 35.Salas, T. R., Reddy, S. A., Clifford, J. L., Davis, R. J., Kikuchi, A., Lippman, S. M., and Menter, D. G. (2003) J. Biol. Chem. 278 41338-41346 [DOI] [PubMed] [Google Scholar]
- 36.Clevers, H. (2006) Cell 127 469-480 [DOI] [PubMed] [Google Scholar]
- 37.Liu, S., Vinall, R. L., Tepper, C., Shi, X. B., Xue, L. R., Ma, A. H., Wang, L. Y., Fitzgerald, L. D., Wu, Z., Gandour-Edwards, R., vere White, R. W., and Kung, H. J. (2007) Oncogene 27 499-505 [DOI] [PubMed] [Google Scholar]
- 38.El Touny, L. H., and Banerjee, P. P. (2007) Carcinogenesis 28 1710-1717 [DOI] [PubMed] [Google Scholar]
- 39.Chong, Z. Z., Li, F., and Maiese, K. (2006) Curr. Neurovasc. Res. 3 107-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosas, M., Dijkers, P. F., Lindemans, C. L., Lammers, J. W., Koenderman, L., and Coffer, P. J. (2006) J. Leukocyte Biol. 80 186-195 [DOI] [PubMed] [Google Scholar]
- 41.Segrelles, C., Moral, M., Lara, M. F., Ruiz, S., Santos, M., Leis, H., Garcia-Escudero, R., Martinez-Cruz, A. B., Martinez-Palacio, J., Hernandez, P., Ballestin, C., and Paramio, J. M. (2006) Oncogene 25 1174-1185 [DOI] [PubMed] [Google Scholar]
- 42.Truica, C. I., Byers, S., and Gelmann, E. P. (2000) Cancer Res. 60 4709-4713 [PubMed] [Google Scholar]
- 43.Li, B., Sun, A., Youn, H., Hong, Y., Terranova, P. F., Thrasher, J. B., Xu, P., and Spencer, D. (2007) Carcinogenesis 28 572-583 [DOI] [PubMed] [Google Scholar]
- 44.Lynch, R. L., Konicek, B. W., McNulty, A. M., Hanna, K. R., Lewis, J. E., Neubauer, B. L., and Graff, J. R. (2005) Mol. Cancer Res. 3 163-169 [DOI] [PubMed] [Google Scholar]
- 45.Prueitt, R. L., Boersma, B. J., Howe, T. M., Goodman, J. E., Thomas, D. D., Ying, L., Pfiester, C. M., Yfantis, H. G., Cottrell, J. R., Lee, D. H., Remaley, A. T., Hofseth, L. J., Wink, D. A., and Ambs, S. (2007) Int. J. Cancer 120 796-805 [DOI] [PubMed] [Google Scholar]
- 46.Lawlor, M. A., and Rotwein, P. (2000) Mol. Cell. Biol. 20 8983-8995 [DOI] [PMC free article] [PubMed] [Google Scholar]