Abstract
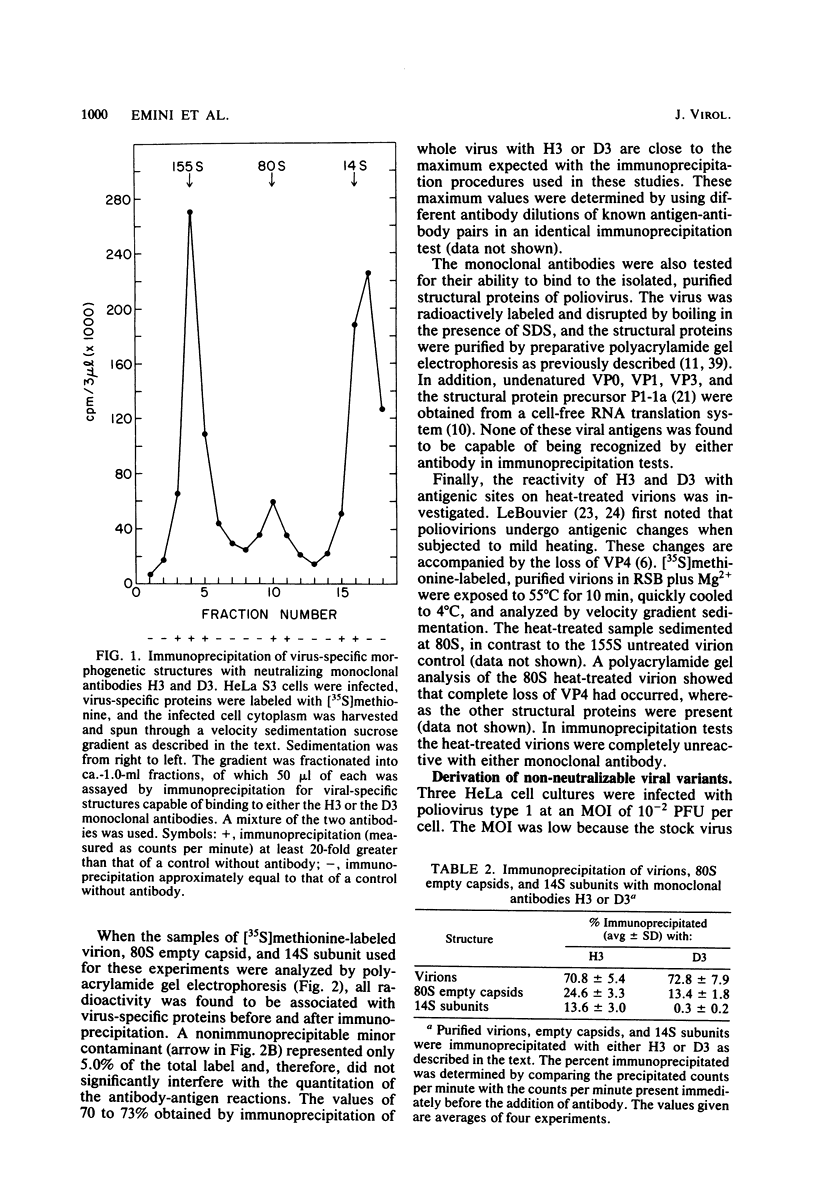
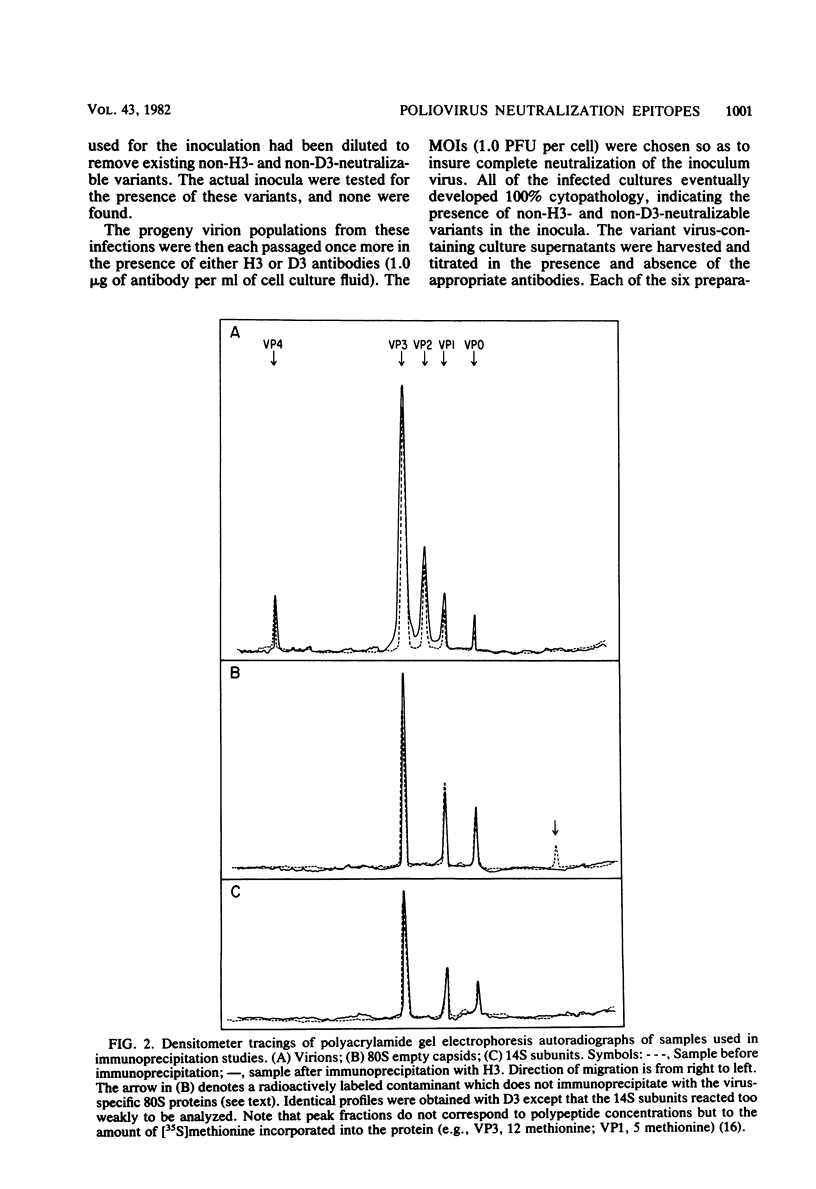
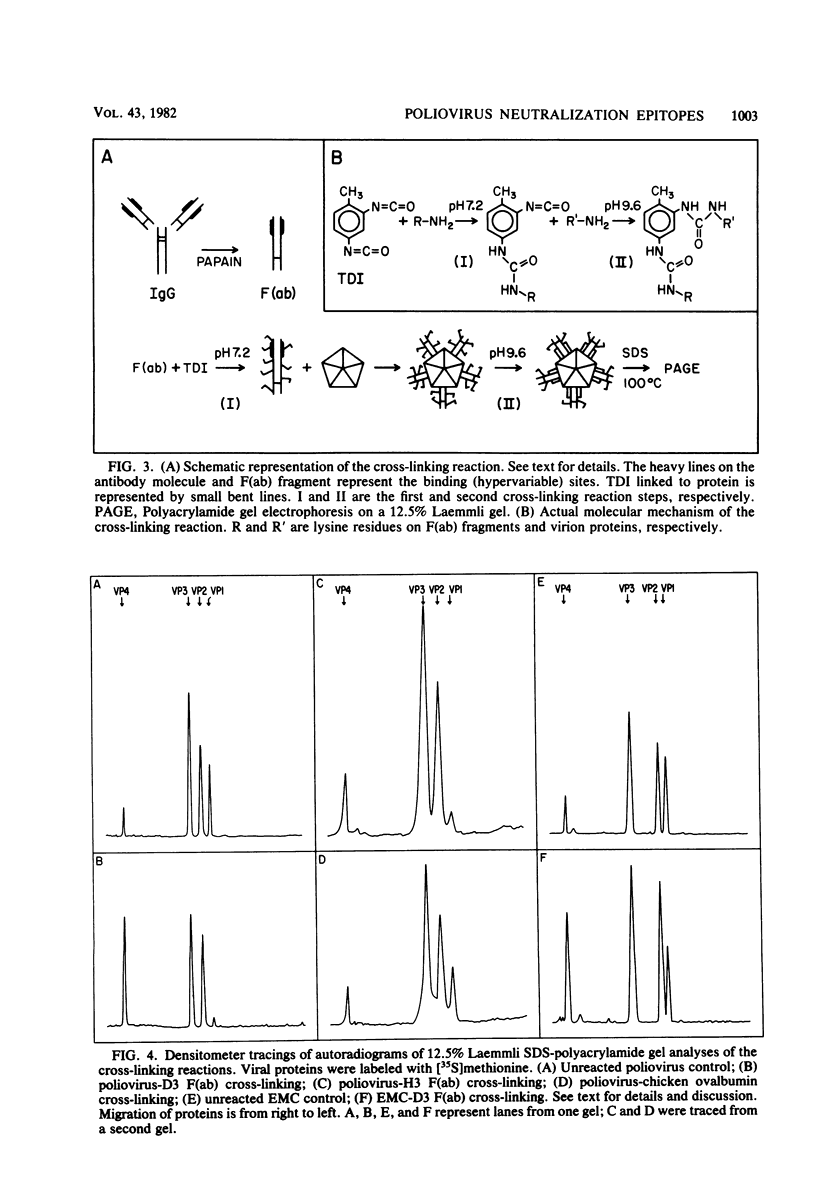
Two hybridomas (H3 and D3) secreting monoclonal neutralizing antibody to intact poliovirus type 1 (Mahoney strain) were established. Each antibody bound to a site qualitatively different from that to which the other antibody bound. The H3 site was located on intact virions and, to a lesser extent, on 80S naturally occurring empty capsids and 14S precursor subunits. The D3 site was found only on virions and empty capsids. Neither site was expressed on 80S heat-treated virions. The antibodies did not react with free denatured or undenatured viral structural proteins. Viral variants which were no longer capable of being neutralized by either one or the other antibody were obtained. Such variants arose during normal cell culture passage of wild-type virus and were present in the progeny viral population on the order of 10(-4) variant per wild-type virus PFU. Toluene-2,4-diisocyanate, a heterobifunctional covalent cross-linking reagent, was used to irreversibly bind the F(ab) fragments of the two antibodies to their respective binding sites. In this way, VP1 was identified as the structural protein containing both sites.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach H. L., Moore D. M., McKercher P. D., Polatnick J. Immune and antibody responses to an isolated capsid protein of foot-and-mouth disease virus. J Immunol. 1975 Dec;115(6):1636–1641. [PubMed] [Google Scholar]
- Beatrice S. T., Katze M. G., Zajac B. A., Crowell R. L. Induction of neutralizing antibodies by the coxsackievirus B3 virion polypeptide, VP2. Virology. 1980 Jul 30;104(2):426–438. doi: 10.1016/0042-6822(80)90345-1. [DOI] [PubMed] [Google Scholar]
- Beneke T. W., Habermehl K. O., Diefenthal W., Buchholz M. Iodination of poliovirus capsid proteins. J Gen Virol. 1977 Feb;34(2):387–390. doi: 10.1099/0022-1317-34-2-387. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyd N. D., Ottosen M. The coupling of chymotrypsinogen to bovine albumin. Immunochemistry. 1973 Oct;10(10):669–671. doi: 10.1016/0019-2791(73)90209-7. [DOI] [PubMed] [Google Scholar]
- Breindl M. The structure of heated poliovirus particles. J Gen Virol. 1971 Jun;11(3):147–156. doi: 10.1099/0022-1317-11-3-147. [DOI] [PubMed] [Google Scholar]
- Chan E. K., Boyd N. D. Preparation of covalently linked immune complexes of specific composition. J Immunol Methods. 1980;33(1):55–61. doi: 10.1016/0022-1759(80)90082-4. [DOI] [PubMed] [Google Scholar]
- Dernick R. Antigenic structure of poliovirus. Dev Biol Stand. 1981;47:319–333. [PubMed] [Google Scholar]
- Dorner A. J., Dorner L. F., Larsen G. R., Wimmer E., Anderson C. W. Identification of the initiation site of poliovirus polyprotein synthesis. J Virol. 1982 Jun;42(3):1017–1028. doi: 10.1128/jvi.42.3.1017-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Rothberg P. G., Wimmer E. The fate of VPg during in vitro translation of poliovirus RNA. FEBS Lett. 1981 Sep 28;132(2):219–223. doi: 10.1016/0014-5793(81)81164-7. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Elzinga M., Wimmer E. Carboxy-terminal analysis of poliovirus proteins: termination of poliovirus RNA translation and location of unique poliovirus polyprotein cleavage sites. J Virol. 1982 Apr;42(1):194–199. doi: 10.1128/jvi.42.1.194-199.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Icenogle J., Gilbert S. F., Grieves J., Anderegg J., Rueckert R. A neutralizing monoclonal antibody against poliovirus and its reaction with related antigens. Virology. 1981 Nov;115(1):211–215. doi: 10.1016/0042-6822(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Nottay B. K., Hatch M. H., Nakano J. H., Obijeski J. F. Multiple genetic changes can occur in the oral poliovaccines upon replication in humans. J Gen Virol. 1981 Oct;56(Pt 2):337–347. doi: 10.1099/0022-1317-56-2-337. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Adler C., Wimmer E. Structure and expression of the picornavirus genome. Ann N Y Acad Sci. 1980;354:183–201. doi: 10.1111/j.1749-6632.1980.tb27967.x. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- LE BOUVIER G. L. The D to C change in poliovirus particles. Br J Exp Pathol. 1959 Dec;40:605–620. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laporte J., Grosclaude J., Wantyghem J., Bernard S., Rouzé P. Neutralisation en culture cellulaire du pouvoir infectieuz du virus de la fièvre aphteuse par des sérums provenant de porcs immunisés à l'aide d'une protéine virale purifiée. C R Acad Sci Hebd Seances Acad Sci D. 1973 Jun 18;276(25):3399–3401. [PubMed] [Google Scholar]
- Larsen G. R., Anderson C. W., Dorner A. J., Semler B. L., Wimmer E. Cleavage sites within the poliovirus capsid protein precursors. J Virol. 1982 Jan;41(1):340–344. doi: 10.1128/jvi.41.1.340-344.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Gerhard W., Ward C. W., Dopheide T. A. Antigenic drift in type A influenza virus: sequence differences in the hemagglutinin of Hong Kong (H3N2) variants selected with monoclonal hybridoma antibodies. Virology. 1979 Oct 15;98(1):226–237. doi: 10.1016/0042-6822(79)90540-3. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Butterworth B. E. Investigation of the structure of polio- and human rhinovirions through the use of selective chemical reactivity. Virology. 1976 May;71(1):207–216. doi: 10.1016/0042-6822(76)90106-9. [DOI] [PubMed] [Google Scholar]
- Lund G. A., Ziola B. R., Salmi A., Scraba D. G. Structure of the Mengo virion. V. Distribution of the capsid polypeptides with respect to the surface of the virus particle. Virology. 1977 May 1;78(1):35–44. doi: 10.1016/0042-6822(77)90076-9. [DOI] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Meloen R. H., Rowlands D. J., Brown F. Comparison of the antibodies elicited by the individual structural polypeptides of foot-and mouth disease and polio viruses. J Gen Virol. 1979 Dec;45(3):761–763. doi: 10.1099/0022-1317-45-3-761. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Summers D. F., Maizel J. V., Jr In vitro assembly of poliovirus-related particles. Virology. 1968 Jun;35(2):216–226. doi: 10.1016/0042-6822(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Portner A., Webster R. G., Bean W. J. Similar frequencies of antigenic variants in Sendai, vesicular stomatitis, and influenza A viruses. Virology. 1980 Jul 15;104(1):235–238. doi: 10.1016/0042-6822(80)90382-7. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALK J. E. Principles of immunization as applied to poliomyelitis and influenza. Am J Public Health Nations Health. 1953 Nov;43(11):1384–1398. doi: 10.2105/ajph.43.11.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Kitamura N., Rothberg P. G., Wishart W. L., Wimmer E. Poliovirus replication proteins: RNA sequence encoding P3-1b and the sites of proteolytic processing. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3464–3468. doi: 10.1073/pnas.78.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetz K., Habermehl K. O. Topographical studies on poliovirus capsid proteins by chemical modification and cross-linking with bifunctional reagents. J Gen Virol. 1979 Aug;44(2):525–534. doi: 10.1099/0022-1317-44-2-525. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Webster R. G., Gerhard W. U. Antigenic variation in three distinct determinants of an influenza type A haemagglutinin molecule. Nature. 1979 May 17;279(5710):246–248. doi: 10.1038/279246a0. [DOI] [PubMed] [Google Scholar]


