Abstract
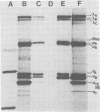
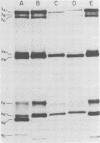
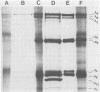
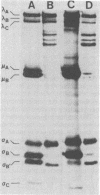
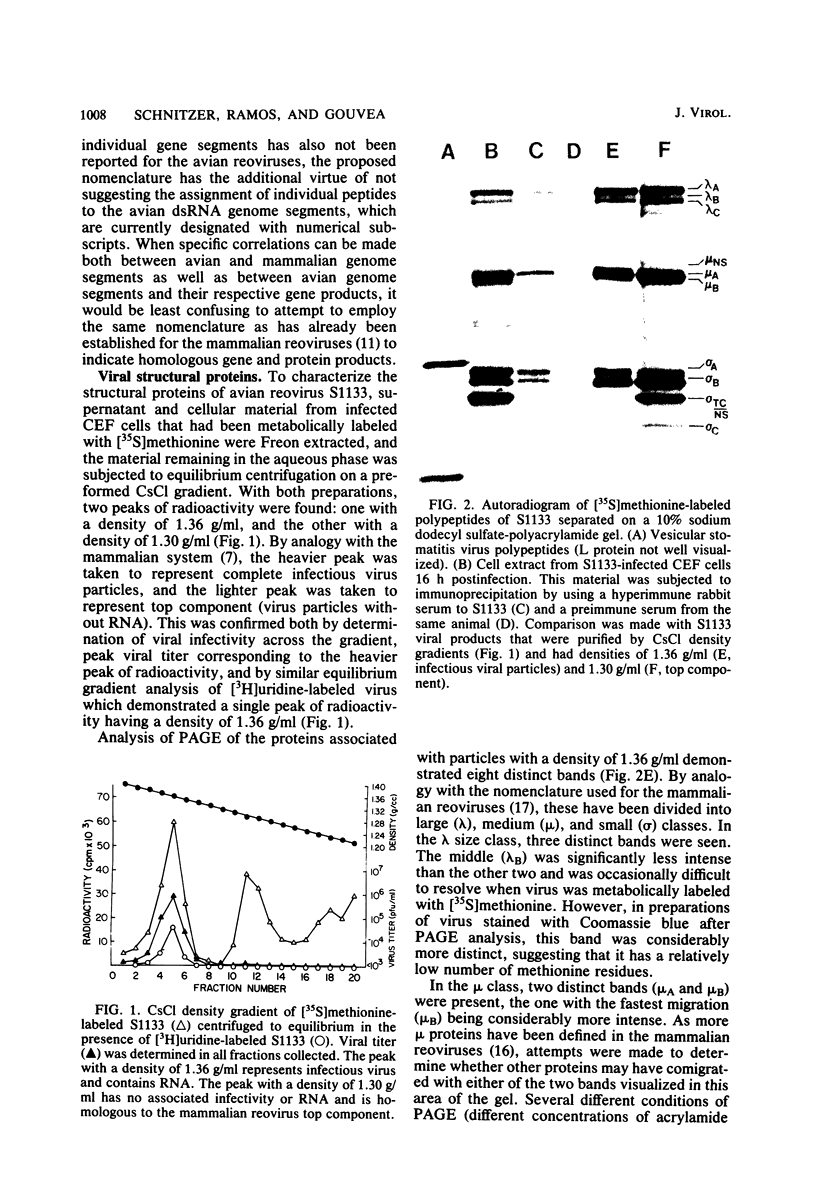
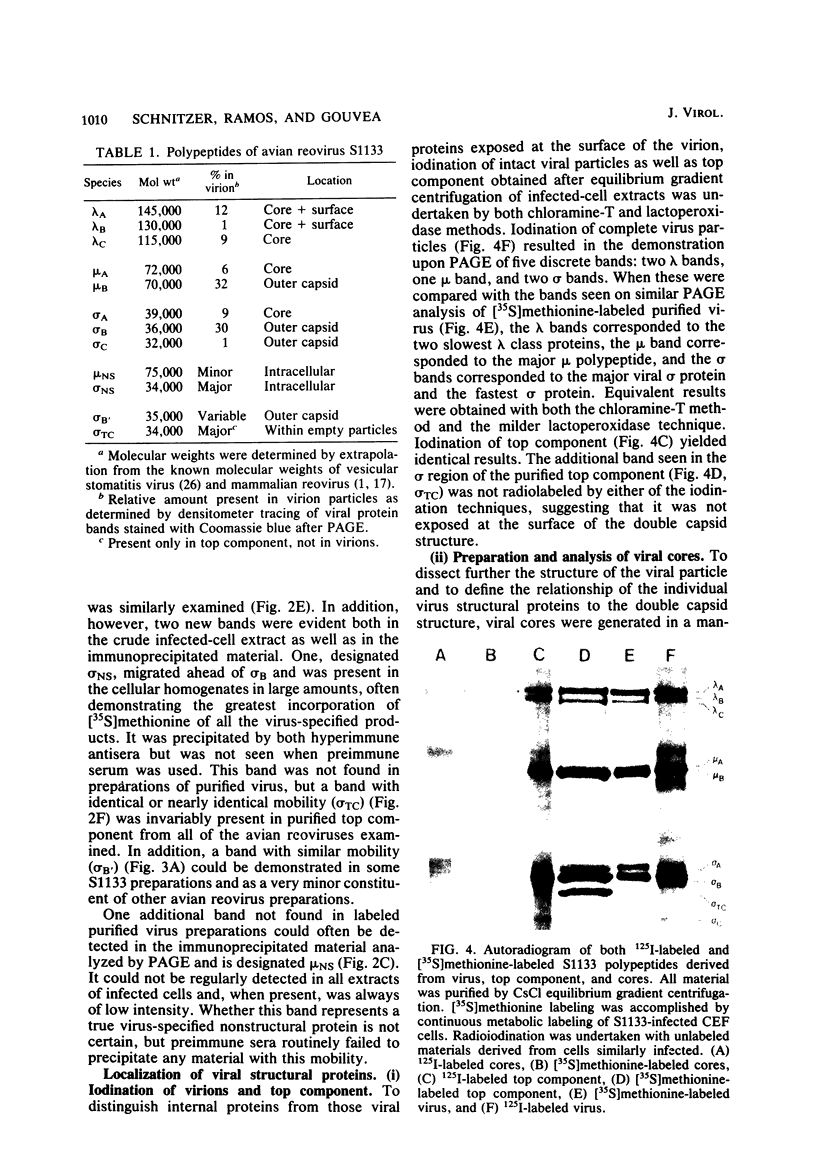
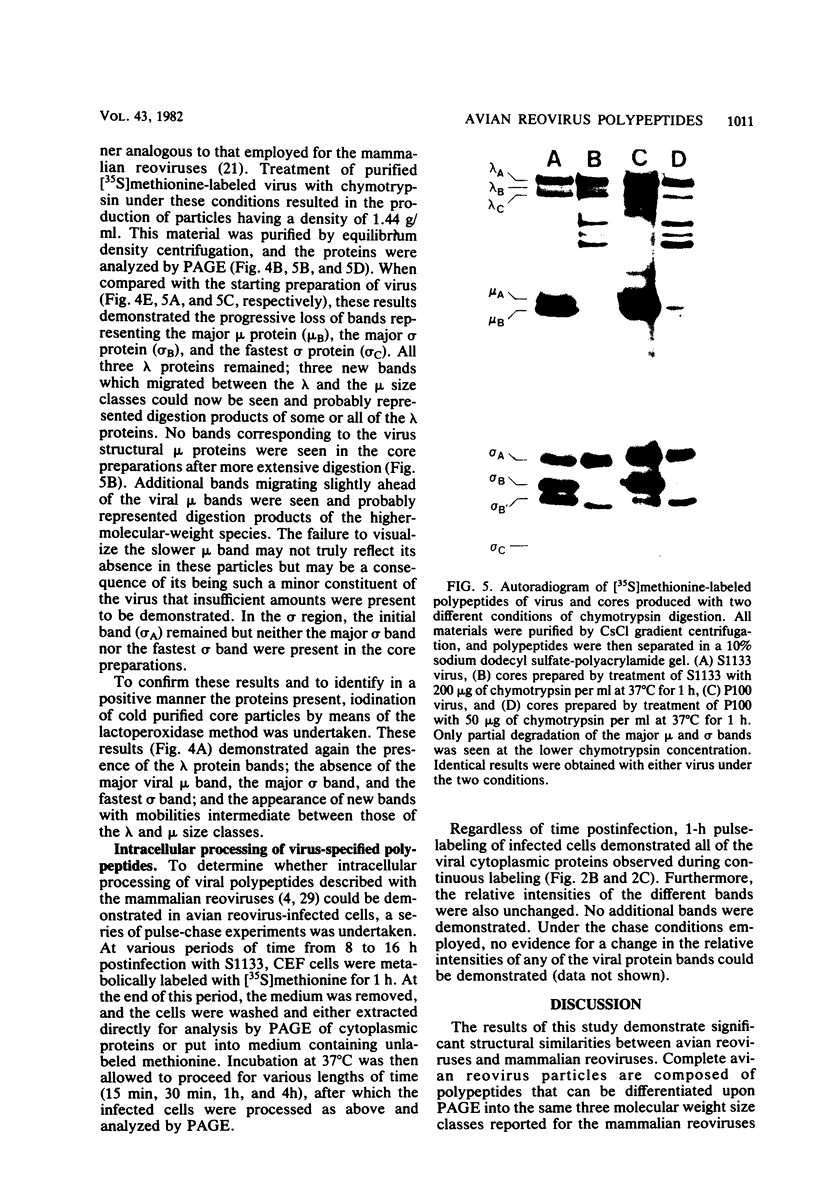
Avian reovirus-specified polypeptides can be separated into three size classes: large (λ), medium (μ), and small (σ), similar to those of the mammalian reoviruses. A nomenclature has been proposed to indicate the individual polypeptides within each size class by progressive alphabetical subscripts. Three λ polypeptides (λA, λB, and λC) are found in infectious viral particles and have molecular weights of 145,000, 130,000, and 115,000, respectively. All are present in core preparations, and two (λA and λB) appear to be exposed at the surface of the virion. Two μ polypeptides can be distinguished in purified virus (μA, 72,000 daltons; μB, 70,000 daltons), and another is occasionally evident by immunoprecipitation from infected-cell extracts (μNS). μB represents the major outer capsid protein and is structurally homologous to μ1C of the mammalian reoviruses. No additional μ proteins can be detected, and there is no evidence for a product-precursor relationship among these proteins. Three major σ proteins are evident in viral particles. σC has the lowest molecular weight, is part of the outer capsid of the virion, and appears homologous to the mammalian σ1 protein. Interestingly, it demonstrates the greatest polymorphism of all the polypeptides among the different avian reoviruses examined. σB (36,000 daltons) is a major constituent of the outer capsid and, like σC, is exposed to the surface of the virion. σA (39,000 daltons) appears to be an internal protein. An additional polypeptide band in the σ class having an apparent molecular weight of 34,000 to 35,000 can be seen under three different conditions: (i) in some S1133 reovirus preparations, particularly after prolonged storage, a new band (σB′) appears with a reduction in intensity of σB, suggesting that σB′ is a degradation product of σB; (ii) in polypeptides immunoprecipitated from infected-cell extracts, a major band (σNS) is apparent migrating just ahead of σB; (iii) in top component preparations from all avian reoviruses examined, a band (σTC) with mobility identical to that of σNS represents a major constitutent and appears to be incorporated within the particle itself. The relationship among these three bands is not currently known. Avian reovirus polypeptides are thus in general similar to those found in mammalian reoviruses, but differences do exist which may be important for understanding viral structure and assembly.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Deshmukh D. R., Pomeroy B. S. Avian reoviruses. I. Isolation and serological characterization. Avian Dis. 1969 May;13(2):239–243. [PubMed] [Google Scholar]
- Fields B. N., Laskov R., Scharff M. D. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral peptides. Virology. 1972 Oct;50(1):209–215. doi: 10.1016/0042-6822(72)90361-3. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Hayes E. C., Lee P. W., Miller S. E., Joklik W. K. The interaction of a series of hybridoma IgGs with reovirus particles. Demonstration that the core protein lambda 2 is exposed on the particle surface. Virology. 1981 Jan 15;108(1):147–155. doi: 10.1016/0042-6822(81)90534-1. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H., Tobin JO'H Congenital cytomegalovirus infection: a collaborative study on epidemiological, clinical and laboratory findings. Dev Med Child Neurol. 1978 Aug;20(4):471–482. doi: 10.1111/j.1469-8749.1978.tb15248.x. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. Genetics of reovirus: identification of the ds RNA segments encoding the polypeptides of the mu and sigma size classes. Virology. 1978 Sep;89(2):594–604. doi: 10.1016/0042-6822(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Nick H., Cursiefen D., Becht H. Structural and growth characteristics of two avian reoviruses. Arch Virol. 1975;48(3):261–269. doi: 10.1007/BF01317969. [DOI] [PubMed] [Google Scholar]
- Petek M., Felluga B., Borghi G., Baroni A. The Crawley agent: an avian reovirus. Arch Gesamte Virusforsch. 1967;21(3):413–424. doi: 10.1007/BF01241740. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Cross R. K., Fields B. N. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977 Jun;22(3):726–733. doi: 10.1128/jvi.22.3.726-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Dickson C., Weiss R. A. Morphological and biochemical characterization of viral particles produced by the tsO45 mutant of vesicular stomatitis virus at restrictive temperature. J Virol. 1979 Jan;29(1):185–195. doi: 10.1128/jvi.29.1.185-195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Christman J. K., Acs G. The reovirus replicative cycle. Annu Rev Biochem. 1976;45:375–408. doi: 10.1146/annurev.bi.45.070176.002111. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Physical and chemical characterization of an avian reovirus. J Virol. 1976 Sep;19(3):968–976. doi: 10.1128/jvi.19.3.968-976.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower B. B., Clark B. R., Rubin R. T. Preparation of 125I polypeptide hormones for radioimmunoassay using glucose oxidase with latoperoxidase. Life Sci. 1977 Oct 1;21(7):959–966. doi: 10.1016/0024-3205(77)90262-4. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Fields B. N. Neutralization of reovirus: the gene responsible for the neutralization antigen. J Exp Med. 1977 Nov 1;146(5):1305–1310. doi: 10.1084/jem.146.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., McDowell M. J., Joklik W. K. Essential and nonessential noncapsid reovirus proteins. Virology. 1971 Sep;45(3):716–723. doi: 10.1016/0042-6822(71)90185-1. [DOI] [PubMed] [Google Scholar]
- van der Heide L., Kalbac M., Hall W. C. Infectious tenosynovitis (viral arthritis): influence of maternal antibodies on the development of tenosynovitis lesions after experimental infection by day-old chickens with tenosynovitis virus. Avian Dis. 1976 Oct-Dec;20(4):641–648. [PubMed] [Google Scholar]