Abstract
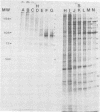
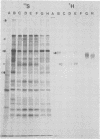
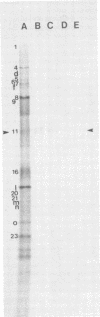
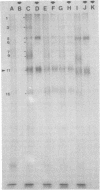
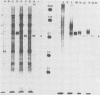
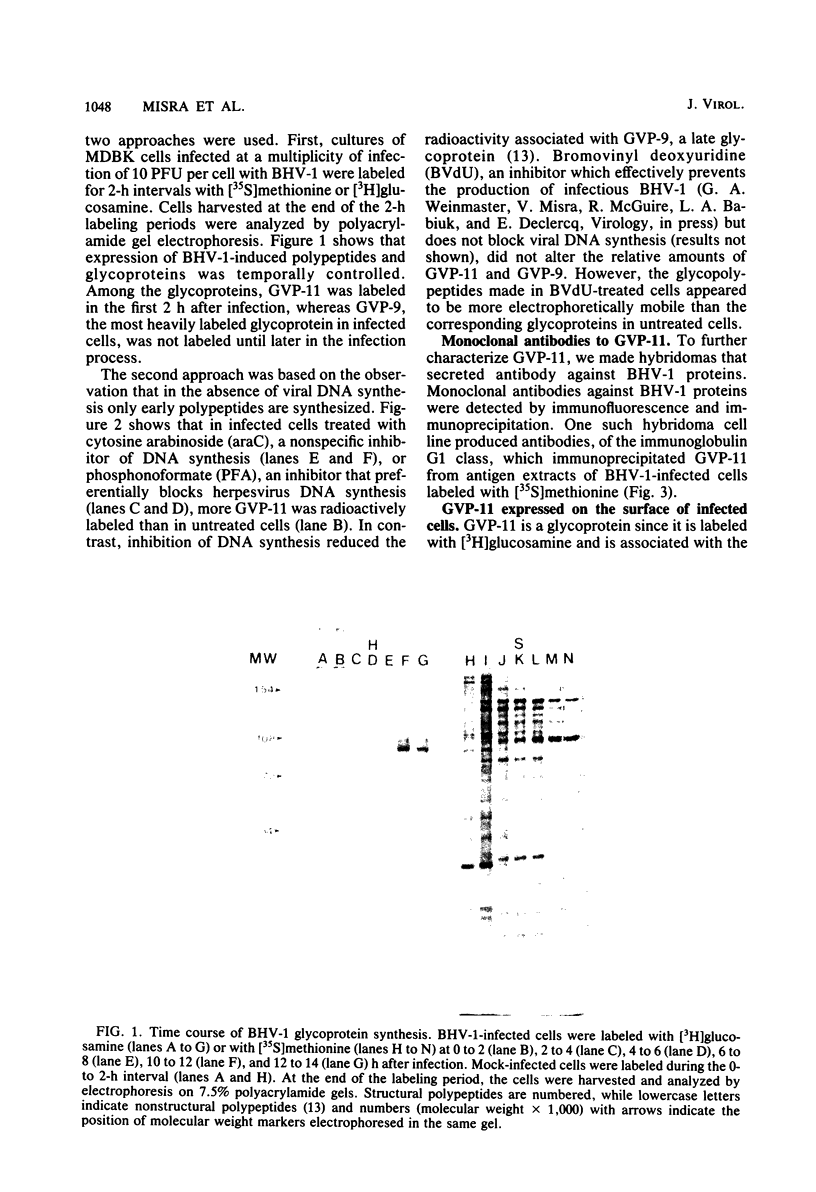
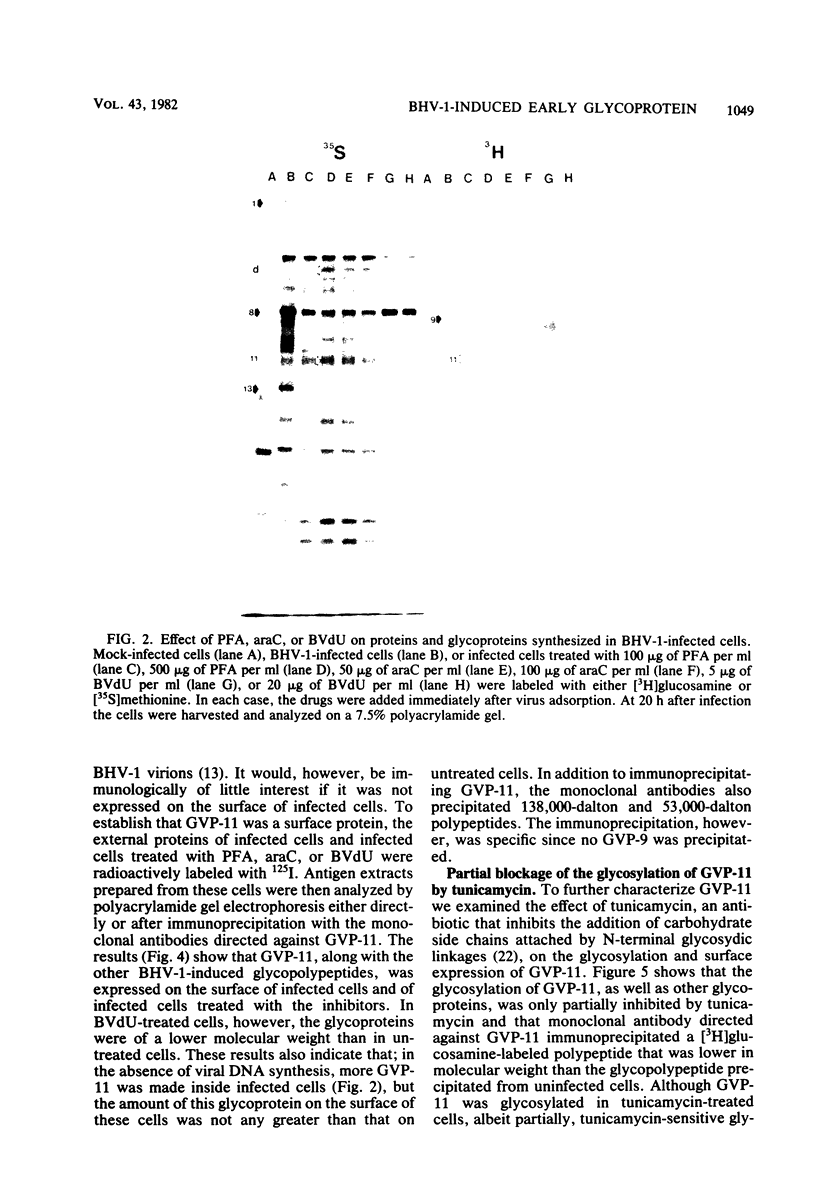
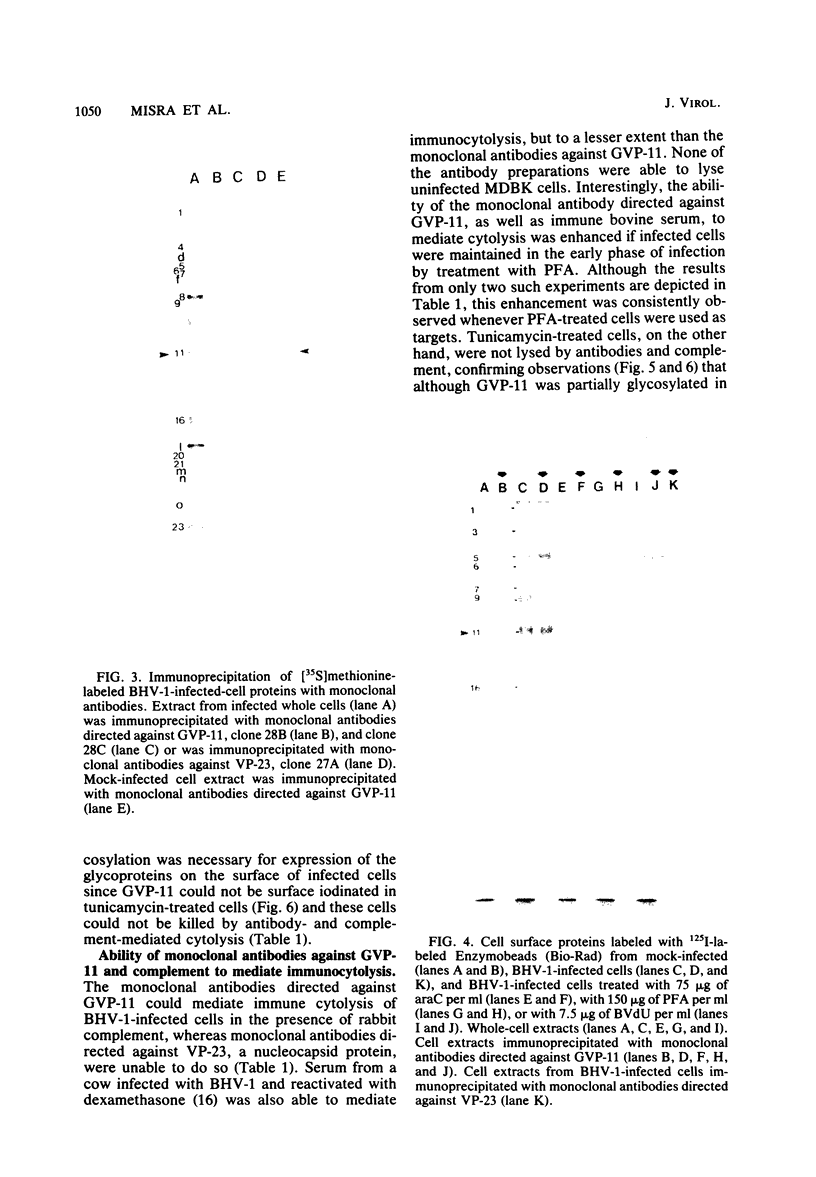
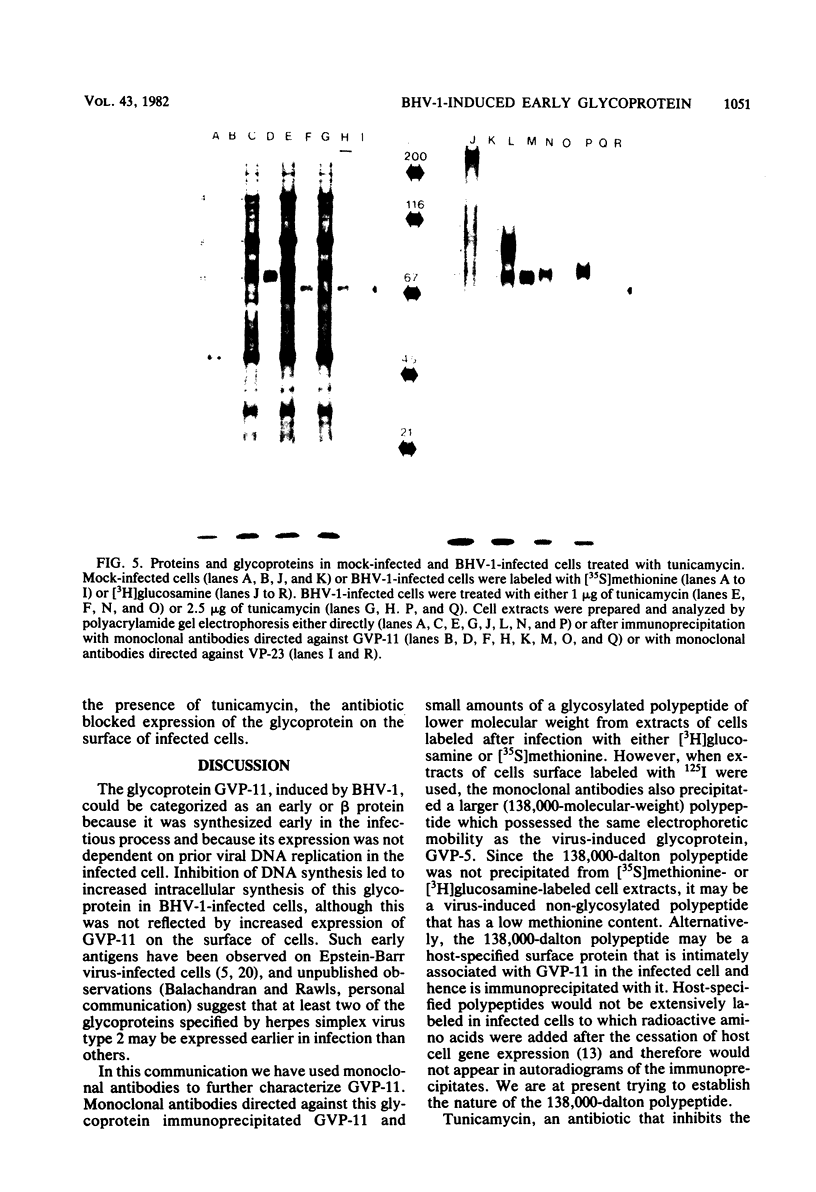
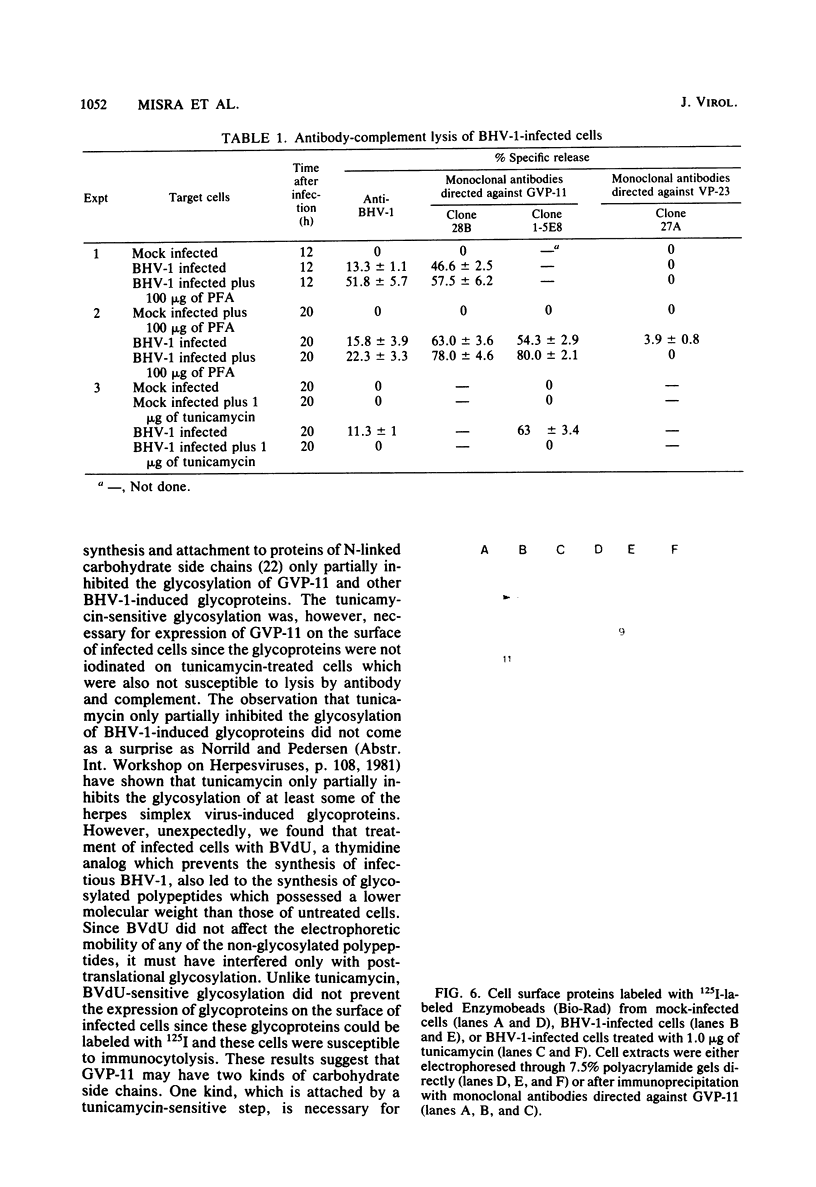
Glycoprotein GVP-11 (molecular weight, 71,500), induced by bovine herpesvirus type 1, was detected on the external surface of infected cells. It could be categorized as an "early" or "beta" class protein since it was synthesized early in the infectious process and its expression was not dependent upon prior viral DNA replication in the infected cells. Monoclonal antibodies directed against GVP-11 immunoprecipitated that glycoprotein and some low-molecular-weight polypeptides from infected cells labeled with either [35S]methionine or [3H]glucosamine. Immunoprecipitation of extracts from cells surface labeled with 125I yielded an additional 138,000-molecular-weight polypeptide. Tunicamycin- or bromovinyl deoxyuridine-treated infected cells yielded polypeptides that were smaller in size than corresponding glycoproteins in untreated cells. Tunicamycin-sensitive glycosylation appeared to be necessary for the expression of the glycoproteins on the surface of the infected cells. The monoclonal antibodies directed against GVP-11 and serum from an immune cow could participate in antibody- and complement-mediated immunocytolysis of infected cells, and this immunocytolysis could be enhanced by arresting cells in the early phase of viral gene expression by treatment with inhibitors of viral DNA synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Wardley R. C., Rouse B. T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975 Nov;12(5):958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Harnish D., Killington R. A., Bacchetti S., Rawls W. E. Monoclonal antibodies to two glycoproteins of herpes simplex virus type 2. J Virol. 1981 Aug;39(2):438–446. doi: 10.1128/jvi.39.2.438-446.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernberg I., Klein G., Kourilsky F. M., Silvestre D. Differentiation between early and late membrane antigen on human lymphoblastoid cell lines infected with Epstein-Barr virus. I. Immunofluorescence. J Natl Cancer Inst. 1974 Jul;53(1):61–65. doi: 10.1093/jnci/53.1.61. [DOI] [PubMed] [Google Scholar]
- Glorioso J. C., Smith J. W. Immune interactions with cells infected with herpes simplex virus: antibodies to radioiodinated surface antigens. J Immunol. 1977 Jan;118(1):114–121. [PubMed] [Google Scholar]
- Glorioso J. C., Wilson L. A., Fenger T. W., Smith J. W. Complement-mediated cytolysis of HSV-1 and HSV-2 infected cells: plasma membrane antigens reactive with type-specific and cross-reactive antibody. J Gen Virol. 1978 Aug;40(2):443–454. doi: 10.1099/0022-1317-40-2-443. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Knowles R. W., Person S. Effects of 2-deoxyglucose, glucosamine, and mannose on cell fusion and the glycoproteins of herpes simplex virus. J Virol. 1976 May;18(2):644–651. doi: 10.1128/jvi.18.2.644-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Misra V., Blumenthal R. M., Babiuk L. A. Proteins Specified by bovine herpesvirus 1 (infectious bovine rhinotracheitis virus). J Virol. 1981 Nov;40(2):367–378. doi: 10.1128/jvi.40.2.367-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B. Immunochemistry of herpes simplex virus glycoproteins. Curr Top Microbiol Immunol. 1980;90:67–106. doi: 10.1007/978-3-642-67717-5_4. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Cromeans T. L., Nahmias A. J. Participation of three major glycoprotein antigens of herpes simplex virus type 1 early in the infectious cycle as determined by antibody-dependent cell-mediated cytotoxicity. Infect Immun. 1980 Apr;28(1):38–44. doi: 10.1128/iai.28.1.38-44.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoret P. P., Babiuk L. A., Misra V., Griebel P. Reactivation of temperature-sensitive and non-temperature-sensitive infectious bovine rhinotracheitis vaccine virus with dexamethasone. Infect Immun. 1980 Aug;29(2):483–488. doi: 10.1128/iai.29.2.483-488.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Kozak M., Honess R. W., Hayward G. Regulation of herpesvirus macromolecular synthesis: evidence for multilevel regulation of herpes simplex 1 RNA and protein synthesis. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):687–701. doi: 10.1101/sqb.1974.039.01.083. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairenji T., Hinuma Y., Sekizawa T., Yoshida M. Appearance of early and late components of Epstein-Barr virus-associated membrane antigen in Daudi cells superinfected with P3HR-1 virus. J Gen Virol. 1978 Jan;38(1):111–120. doi: 10.1099/0022-1317-38-1-111. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Cromeans T. L., Norrild B. Early damage of herpes-infected cells by antibody-dependent cellular cytotoxicity: relative roles of virus-specified cell-surface antigens and input virus. J Immunol. 1979 Nov;123(5):2239–2244. [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J Virol. 1978 Jun;26(3):686–701. doi: 10.1128/jvi.26.3.686-701.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino K., Isono N., Tada A., Urayama M. Studies on the neutralization of herpes simplex virus. X. Demonstration of complement-requiring neutralizing (CRN) and slow-reacting CRN (s-CRN) antibodies in late IgG. Microbiol Immunol. 1979;23(10):975–985. doi: 10.1111/j.1348-0421.1979.tb00528.x. [DOI] [PubMed] [Google Scholar]