Abstract
Learning deficits may be part of the early symptoms of Huntington's disease (HD). Here we characterized implicit and explicit aspects of sequence learning in eleven pre-symptomatic HD gene carriers (pHD) and eleven normal controls. Subjects moved a cursor on a digitizing tablet and performed the following tasks: SEQ: learning to anticipate the appearance of a target sequence in two blocks; VSEQ: learning a sequence by attending to the display without moving for one block, and by moving to the sequence in a successive block (VSEQtest). Explicit learning was measured with declarative scores and number of anticipatory movements. Implicit learning was measured as a strategy change reflected in movement time. By the end of SEQ, pHD had a significantly lower number of correct anticipatory movements and lower declarative scores than controls, while in VSEQ and VSEQtest these indices improved. During all three tasks, movement time changed in controls, but not in pHD. These results suggest that both explicit and implicit aspects of sequence learning may be impaired before the onset of motor symptoms. However, when attentional demands decrease, explicit, but not implicit, learning may improve.
Keywords: Reaching movements, Motor sequence, Basal ganglia
1. Introduction
HD is an autosomal dominant degenerative disease of variable onset characterized by motor abnormalities, cognitive decline, personality changes and psychiatric disturbances. Most frequently, the disease becomes manifest in the third to fifth decade of subjects with 40 or more CAG repeats on chromosome 4 and leads to death 15 or 20 years after onset. HD is usually diagnosed when motor symptoms first appear [1]. Cognitive dysfunction becomes more evident with disease progression [2-4]. However, the severity, the order of appearance and the time course of the different symptoms are very variable [5] and not entirely predictable by the number of CAG repeats [6-8]. Several neuropsychological studies have investigated whether cognitive deficits might be already present in the pre-clinical stages, when motor symptoms are not yet evident, with mixed results [9-11]. Recent studies have shown that pre-clinical cognitive deficits might include impaired executive functions, attention, working memory, organization, sequencing, regulation, perception, and episodic memory [12, 13]. Because of this pattern of cognitive impairment, it is reasonable to expect that explicit learning of sequences might be also altered in absence of overt motor symptoms. Indeed, we have recently found that this was the case [14, 15].
In this paper, we expand the results of our previous studies by, first, ascertaining whether in subjects with pre-symptomatic HD (pHD), implicit and explicit aspects of sequence learning are differentially impaired. Secondly, by using a sequence learning task that requires less attentional and working memory resources, we determine whether learning improves. We use tasks where subjects are explicitly asked to learn and anticipate the order of appearance of eight targets. We have previously shown that, with these tasks, normal subjects typically learn the order of simple repeating sequences in ninety seconds or less, either when learning occurs while reaching for targets or when, in a less demanding situation, the sequence order is first learned visually, without moving [16-18]. Learning of the sequence order is reflected by decreases of onset time, as subjects move out of reaction-time mode and anticipate target appearance, and measured with discrete variables, such as the number of correct anticipatory movements. In addition, while learning the sequence, subjects prolong their movement time and improve spatial accuracy [17]. The change in movement time, which is accompanied by decreases in the amplitude of peak accelerations, is a measure of skill or implicit learning, as it happens without explicit requests and subject's awareness. This implicit learning occurs in tasks where target anticipation is possible and is seen as a shift from a time-saving (as in reaction time tasks) to an energy-saving (as in timed-response tasks) strategy. In summary, in our sequence learning tasks, the order of the elements is mostly learned explicitly and can be quantified with the number of correct anticipatory movements, while the ability to modulate movement time and thus, to change motor strategy is learned without awareness, implicitly, and can be measured with movement time changes [16-18].
2. Methods
2.1 Subjects
We studied eleven right-handed pHD subjects (5 men and 6 women; mean age: 45.8 ± 11.0 years, range from 33 – 62; CAG repeat length: 41.6 ± 1.8; range: 39-45). They underwent a standardized neurological exam and were administered the functional assessment scale of the Unified Huntington's Disease Rating Scale (UHDRS) [19]. Their scores were: motor: 7.6±9.7; behavioral: 9.5±10.1; independence scale: 100±0; total functional capacity: 12.9±0.3. Magnetic resonance imaging was performed to exclude potential structural brain lesions.
Controls were 11 neurologically normal subjects (6 men and 5 women; mean age: 46.1 ± 12.9 years, range from 28–66 years, Mini Mental State Examination [20] scores >27). Controls and pHD subjects were matched for age and education. Written informed consent was obtained from all participants under a protocol approved by the institutional review boards.
2.2 Motor tasks
General features of the motor tasks have been detailed previously [16, 17]. Briefly, subjects moved a cursor on a digitizing tablet with their right hand out and back from a central starting point to one of eight radially arrayed targets at 1.6 cm distance. Subjects were instructed to make uncorrected movements with sharp reversal inside each target. Targets appeared on a screen in synchrony with a tone at a constant interval of 1 s. Testing was done in separate trial blocks of 90 seconds each, for a total of 88 movements (eleven complete movement cycles). All subjects learned to perform the tasks in one or two training sessions the day before testing. Training was complete when performance became stable.
The following tasks were administered:
CCW: targets appeared in a predictable counterclockwise order. Subjects had to reach the target in synchrony with the tone. Thus, they had to initiate each movement before target and tone presentation.
RAN: targets were presented in a non-repeating and unpredictable order. Instructions were to reach for each target “as soon as possible”, minimizing reaction time but avoiding target anticipation. For each subject, the floor value of the reaction time distribution, i.e., the lowest onset time, was used to define anticipatory movements in SEQ [17].
SEQ: The eight targets appeared in a repeating order. Subjects were informed that a sequence was to be presented, instructed to learn the order of the sequence while reaching for targets and to anticipate target appearance in two successive blocks (SEQ1 and SEQ2). At the end of each block, they were reported the sequence order and declarative scores (from 0 to 8) were computed [17].
VSEQ: A repeating sequence of eight targets was presented for 11 cycles. Subjects were asked to learn the sequence order without moving. Learning was assessed in a subsequent block, VSEQtest, where subjects were asked to reach for that target sequence as in SEQ.
Data Analysis
For each movement we computed: (1) Spatial error, the distance from the center of the target to the movement reversal point; (2) Movement time, the time from movement onset to the reversal point; (3) Onset time, the time from target and tone presentation to movement onset.
For SEQ1, SEQ2 and VSEQtest we quantified the number of anticipatory movements, i.e., those with onset times lower than RAN floor value, directed to the correct targets. This number reflects explicit learning and is a good predictor of declarative scores [17].
Statistical Analysis
To assess learning across cycles and the differences between groups and tasks we used mixed model analysis of variance (ANOVA) followed, when appropriate, by post hoc comparisons. Results were considered significant for p<0.05 with Bonferroni correction for multiple comparisons.
2.3 Neuropsychological Tests
pHD subjects underwent a battery of neuropsychological tests (Table 1). With the exception of Dementia Rating Scale (DRS), results of all tests were converted in T scores. Scores were considered abnormal when outside 1 SD of the normal range.
Table 1.
| Neuropsychological Tests | # of abnormal pHD |
|---|---|
| A. General Cognition: | 0 |
| Dementia rating scale (DRS), National Adult Reading Test (NART) | |
| B. Processing efficiency and working memory: | 3 |
| Symbol Digit Modality Test (SDMT) written and oral, Stroop word, Stroop color, Trial A, Brief test of attention | |
| C. Shifting and inhibition: | 1 |
| Stroop “color & word”, Trial B | |
| D. Memory tests: | 4 |
| California Verbal Learning Test delayed recall score (CVLT LD FR), Rey Figure Copy Delay | |
| E. Visuo-spatial tests: | 0 |
| Hooper visual organization, Rey Copy | |
| F. Verbal Tests: | 5 |
| Controlled Oral Word Association (COWA), Boston Naming, Token test |
3. Results
3.1 Predictable and random sequences
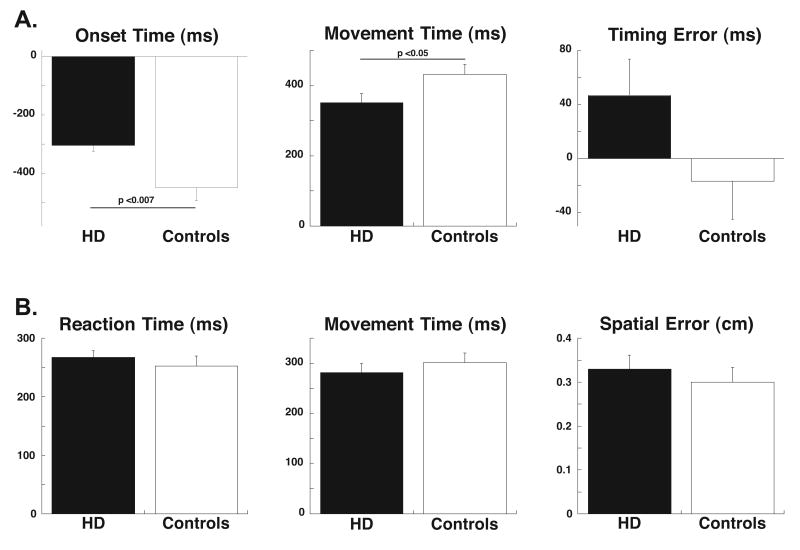
During CCW, pHD and control subjects made straight out and back movements and anticipated target appearance, with no significant difference in spatial accuracy (pHD: 0.25±0.03 cm; controls: 0.21±0.02 cm). In both groups, onset times, temporal and spatial accuracy significantly improved during the block, as shown previously [21]. However, movements in pHD started later than in controls, had shorter duration but reached the target at the same time as controls (Figure 1A).
Figure 1.
Average values (±S.E.) for pHD subjects and normal controls are shown as black and white columns, respectively. A. Predictable timed response sequence (CCW). Onset times occurred later (F(1,10)=9.4, p<0.007) and movement times were shorter (F(1,10)=4.9, p<0.05) in pHD than in controls. No differences between groups were found for timing error (F(1,10) = 2.9, p=0.11). B. Movements to random targets (RAN). No differences were found between the two groups for reaction time, movement time, and spatial error.
Movements in RAN were also straight in all subjects. In both groups, reaction and movement times were stable across each block, while spatial accuracy increased (F (1,200)=6.98, p<0.0001). Reaction time, movement times and spatial accuracy of pHD were not significantly different from those of controls (Figure 1B).
3.2 Sequence Learning
The results of ANOVA performed for all variables are summarized in Tables 2 and 3. The results of post-hoc comparisons are reported in the main text.
Table 2.
| Variable | F, p | F, p | F, p | F, p | F, p | F, p | F, p |
|---|---|---|---|---|---|---|---|
| Group (df=1, 60) | Task (df=2, 60) | Group X Task (df=1, 2) | Cycle (df=10,600) | Cycle X Task (df=10, 20) | Cycle X Group (df=10, 600) | Cycle X Task X Group (df=20, 600) | |
| Onset time | 36.6, <0.0001 | 12.4, <0.0001 | 0.4, >0.9 | 30.0, <0.0001 | 2.0, <0.008 | 1.5, >0.1 | 3.8, <0.0001 |
| Anticipatory Mvt. | 21.5, <0.0001 | 12.1, <0.0001 | 0.8, >0.4 | 30.1, <0.0001 | 2.9, <0.0001 | 1.2, >0.2 | 3.8, <0.0001 |
| Movement Time | 27.8, <0.0001 | 0.8, >0.4 | 0.5, >0.5 | 1.9 <0.04 | 0.5, >0.9 | 2.1, <0.03 | 2.1, <0.004 |
| Spatial error | 5.4, <0.03 | 1.6, >0.8 | 1.7, >0.2 | 27.9, <0.0001 | 1.1, >0.3 | 0.8 >0.6 | 1.4, <0.08 |
Table 3.
| Variable | Group (df=1, 200) F, p | Cycle (df=10, 200) F, p | Group X Cycle (df=1, 20) F, p |
|---|---|---|---|
| Task: SEQ1 | |||
| Onset time | 15.7, 0.0008 | 21.7, <0.0001 | 5.7, <0.0001 |
| Anticipatory Mvt. | 15.0, 0.0008 | 23.6, <0.0001 | 4.5, <0.0001 |
| Movement Time | 6.9, 0.016 | 0.68, 0.7 | 4.1, <0.0001 |
| Spatial error | 0.3, 0.56 | 9.8, <0.0001 | 1.7, 0.09 |
| Task: SEQ2 | |||
| Onset time | 7.9, 0.01 | 7.3, <0.0001 | 2.1, 0.02 |
| Anticipatory Mvt. | 4.3, 0.05 | 6.3, <0.0001 | 2.2, 0.02 |
| Movement Time | 7.6, 0.01 | 1.1, 0.40 | 0.9, 0.54 |
| Spatial error | 0.3, 0.85 | 9.8, <0.0001 | 0.6, 0.76 |
| Task: VSEQtest | |||
| Onset time | 16.4, 0.0006 | 5.5, <0.0001 | 1.5, 0.12 |
| Anticipatory Mvt. | 4.8, 0.04 | 5.6, <0.0001 | 1.8, 0.05 |
| Movement Time | 8.6, 0.008 | 2.2, 0.017 | 1.3, 0.20 |
| Spatial error | 5.3, 0.03 | 10.4, <0.0001 | 1.3, 0.2 |
3.2.1 Concurrent visual and motor sequence learning
As subjects attempted to anticipate the upcoming target, some movements were in the wrong direction. The number of correct movements per cycle was similar in the control (7.2 ± 0.55) and pHD (7.1± 0.43).
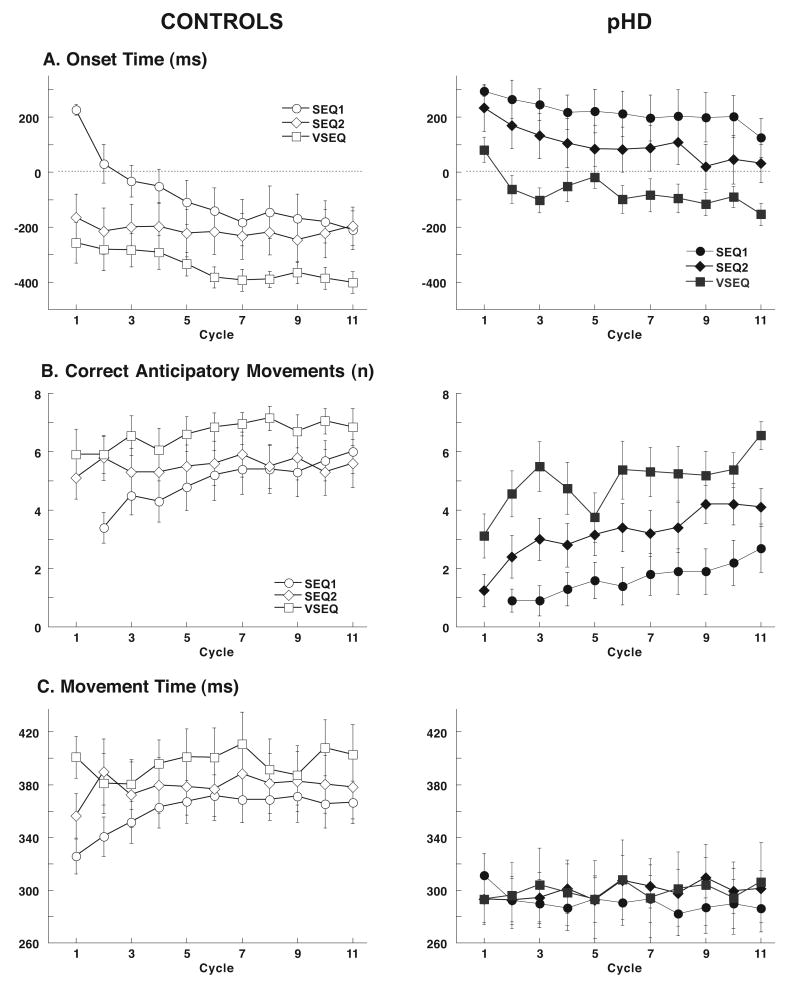
Sequence learning was evident as a progressive decrease in SEQ1 onset time (Figure 2A). In controls, this reduction was more conspicuous and occurred more rapidly than in pHD (Figure 2A). The group differences persisted in SEQ2.
Figure 2.
Sequence learning in controls (empty symbols) and pHD (filled symbols). A. Onset time, plotted as a function of movement cycles, significantly decreases in SEQ1 (circles) but at more rapid pace in controls than in pHD. The group difference persisted in SEQ2 (diamonds). After visual exposure, pHD onset times for the sequence performed in VSEQtest (squares) were lower than SEQ2 (F(1,40)=4.9, p<0.05), but were still different than controls. B. Number of correct anticipatory movements per cycle increases during SEQ1 block with a significant difference between controls and pHD. Group differences were less evident in SEQ2 and VSEQ. In VSEQtest, the number of anticipatory movements was significantly higher than in SEQ2 only for the pHD group (F(1,40)=4.2, p<0.05). C. In normal subjects movement time increased during SEQ1 and further raise, although not significantly so, inSEQ2. In VSEQtest it increased similarly to SEQ1. In pHD, movement times of SEQ1 first cycle were not different from controls; however, unlike in controls, there was no increase in the course of either SEQ1, SEQ2 or VSEQtest blocks.
Onset time change in our tasks is the sum of both explicit and implicit learning aspects of a sequence [17]. The number of correct anticipatory movements and declarative scores represent the explicit learning of sequence order; the progressive increase in movement times captures an implicit aspect of skill learning [17]. In the following paragraphs, we describe these two types of learning for the two groups.
Explicit sequence learning
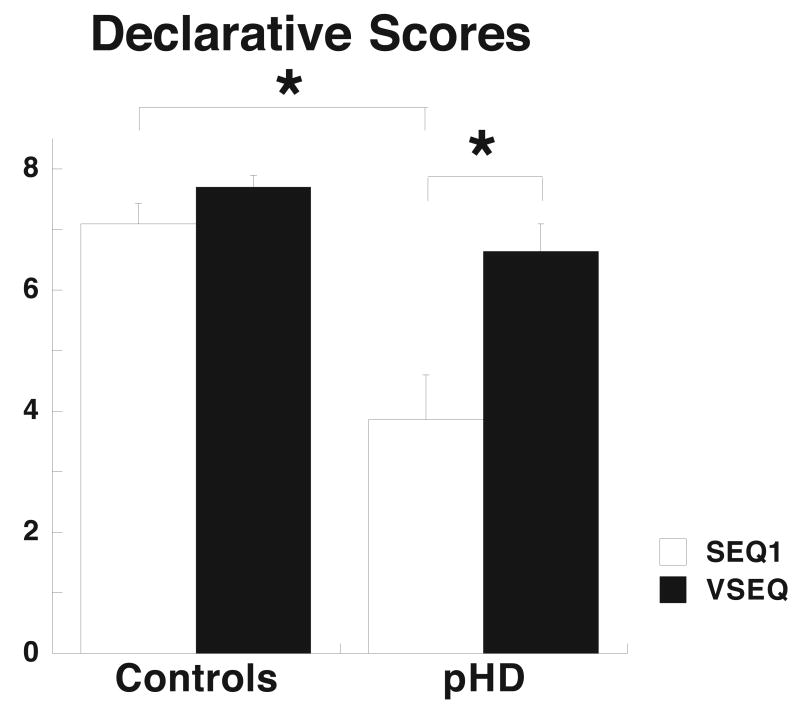
Declarative scores at the end of SEQ1 were significantly lower in pHD compared to controls (Figure 3). The number of correct anticipatory movements increased across cycles in SEQ1 for both groups, but at a more rapid rate in controls than in pHD. This group difference persisted in SEQ2 (Figure 2B).
Figure 3.
Mean declarative scores of controls and pHD after SEQ1 (empty columns) and VSEQ (black columns). ANOVA disclosed a significant effect of group (F(1,40)=20.0, p<0.0001) and task (F(1,40)=4.9, p=0.03) with a significant interaction group x task (F(1,40)=4.1, p<0.05). Post hoc test showed that there was a significant difference between SEQ1 and VSEQ in pHD (p=0.002), but not in controls. Moreover, there was a significant difference between controls and pHD for SEQ1 (p=0.0007), but not VSEQ.
Implicit sequence learning
During SEQ1, movement times were different in the two groups: in controls, they increased significantly (p<0.005, Figure 2C), while in pHD, they were shorter than in controls and showed a small, although not significant, decrement across SEQ1 (p=0.09). In SEQ2, the difference between the two groups persisted. Spatial errors decreased across SEQ1 and SEQ2 cycles in both groups, and were higher, although not significantly so, in pHD than in controls (controls, SEQ1: 0.26±0.02 cm, SEQ2: 0.24±0.02 cm; pHD, SEQ1: 0.29±0.02 cm, SEQ2: 0.27±0.01 cm).
These results indicate that pHD subjects attained some levels of explicit sequence learning, but fail to modulate movement time and, thus, to switch from a time-saving to an energy-saving strategy.
3.2.3 Separate visual and motor sequence learning
Explicit learning of sequence order after visual exposure
Learning was first measured after 88 target presentations with declarative scores. These scores were compared with those obtained after a block of SEQ1, where visual learning occurred together with motor performance. VSEQ declarative scores were similar in the two groups, as were higher than for SEQ1 only in pHD (p<0.003), and did not change in controls (Figure 3).
VSEQtest
Subjects then performed the visually learned sequence. On average, controls anticipated target appearance from the first movement cycle. In pHD, mean onset time of the first cycle was below reaction time threshold and became negative from the second cycle. However, it never reached the range of the controls (Figure 2A). In both controls and pHD, onset times were generally lower in VSEQtest than in SEQ2 (Figure 2A; p<0.05).
Explicit sequence learning in VSEQtest
The number of correct anticipatory movements increased in the course of VSEQtest in both controls and pHD, with a significant difference between the two groups. However, by the last cycle, this number was the same in the two groups (p=0.4, Figure 2B). In controls, there was no difference between VSEQtest and SEQ2 (p=0.2). In pHD, the number of these movements was higher than in SEQ2 (p<0.05).
These findings suggest that the decrease in task demands, as in VSEQtest, had a beneficial effect on pHD explicit learning.
Implicit motor sequence learning in VSEQtest
During VSEQtest, movement times were different in the two groups (Figure 2C): in controls, they increased significantly (p<0.02) similarly to SEQ1 and SEQ2 (p=0.8). In pHD, they still were shorter than in controls and, like SEQ1 and SEQ2, did not significantly change across cycles (p=0.8). Spatial errors decreased in VSEQtest in both groups with a significant difference between controls and pHD (controls: 0.23±0.02 cm; pHD: 0.31± 0.03 cm).
Thus, reduced attentional requirements of VSEQtest produced a beneficial effect on explicit learning of sequence order in pHD, but did not improve implicit aspects of sequence learning.
3.2.4 Neuropsychological tests
All pHD had normal DRS scores. However, six pHD had abnormal results in at least one of the test sets, with language and memory sets being more frequently altered (Table 1). Learning indices of these six subjects did not differ from those of the five with normal neuropsychological scores. However, we found significant correlations (r2=0.70, p<0.02) between SEQ1 anticipatory movements and the scores of Stroop test, oral SDMT and attention matrix indexes and between VSEQ declarative scores and the same neuropsychological tests.
4. Discussion
The current study is the first that simultaneously characterizes implicit and explicit learning attributes in pHD and found that both are abnormal prior to the clinical onset of the clinical symptoms. This is observed either when a sequence is learned while reaching for targets or, in a less demanding task, when a similar sequence is first learned visually. Interestingly, the development of implicit motor learning, defined as a switch from a time-saving to an energy-saving strategy is equally impaired in the two tasks with a relative sparing of spatial accuracy, whereas in the latter task, explicit learning of a sequence order is better than in the former.
4.1 Explicit and implicit aspects of motor sequence learning are impaired in pHD
Previous studies in both HD and pHD have reported abnormalities in a variety of explicit and implicit learning tasks and in neuropsychological tests [12, 13, 22-25], suggesting that HD affects circuits involving dorsolateral prefrontal cortex (DLPFC) and the striatum even before the clinical manifestation of motor signs. The few studies on implicit motor learning in HD and pHD have shown skill learning impairment with rotary pursuit [26-28], mirror-reversed reading [29], prism [30] and force field adaptation [31]. More variable results were obtained with implicit sequence learning tasks using serial reaction time paradigms [32, 33]. Our results show, for the first time, impairment of both the implicit and explicit components of sequence learning. In our motor learning task, subjects are required to learn the sequence order and to anticipate target appearance with fast movements. Thus, the learning of the sequence order, the explicit component, is evident as a progressive increase in the number of correct anticipatory movements, which is a sensitive measure of explicit and declarative learning because of its high correlation with declarative scores [17]. A second type of learning occurs implicitly together with sequence order: in the course of the block, without awareness, normal subjects prolong movement time, decrease peak acceleration and increase endpoint accuracy. The temporal and spatial changes are measures of skill acquisition as they reflect implicit processes that optimize movement spatial and temporal accuracy [17]. The explicit and implicit learning indexes show different patterns of memory stabilization or consolidation (Ghilardi, unpublished data) and, as also suggested by other works [34], probably encompass separate neural substrates. We have previously found that abnormal explicit sequence learning in pHD is accompanied by increased activation in the caudate and orbito-frontal cortex, as possible compensatory mechanisms for a deficient activation of DLPFC [15]. The correlation between explicit learning indexes and scores of neuropsychological tests of working memory and attention further substantiated the involvement of DLPFC in the explicit acquisition of the sequence order. The results of the present study additionally suggest that implicit learning of temporal aspects, akin to skill formation, are impaired in pHD. The neural substrate for this type of implicit skill learning includes areas such as SMA, pre-SMA and prefrontal cortex [34-36]. All these cortical areas and DLPFC are part of parallel loops that include the putamen and the caudate [37], two regions that show robust decreases of gray matter volumes in the early HD [38]. The alterations of both implicit and explicit aspects of learning could be attributed to early changes in the caudate and putamen of pHD, as suggested by Hikosaka and coworkers [34]. They postulate that sequence learning is possibly based on the activity and interaction of two systems, the visual and the kinetic, a categorization that parallels our division in “explicit” and “implicit” learning attributes. The neural counterparts of these systems are basal ganglia loops: the visual system is based on the DLPFC loop that encompasses the anterior striatum, while the “kinetic” or motor system includes SMA and the putamen loop and involves the primary motor cortex, the pre-frontal cortex and pre-SMA. Their model predicts that early atrophy in those basal ganglia regions might manifest with impairment in both the explicit, visually-based, and the implicit, kinetically-based, learning, as we have found in pHD.
4.2 Decreased attentional demands benefit explicit but not implicit learning
Another important result of this study is that the indexes of explicit learning improved when pHD subjects were required first, to learn the sequence order and then, to perform the motor sequence. Their declarative score at the end of the visual exposure was higher compared to SEQ1 and the number of correct anticipatory movements in VSEQtest, increased compared to SEQ2, almost reaching the controls' range. Thus, explicit learning in pHD might benefit from decreased demands. These results further suggest that attentional and working memory capacities are decreased and point to early alterations of the DLPFC loop.
In contrast to the partial recovery of explicit learning, implicit learning in VSEQtest did not improve. This dissociation suggests that, even before the appearance of clinical motor symptoms, the kinematic loop responsible for skill formation is altered so that, despite the learning of a substantial portion of the sequence order, pHD subjects cannot properly modulate movement time and spatial accuracy. This result points to a dysfunction of the feedback control mechanisms and confirms previous findings of alterations of on-line error correction during adaptation to force fields in pHD and HD [31, 39].
We also found that reaction and movement times as well as spatial accuracy of pHD are relatively preserved in movements to randomly appearing targets (Figure 1B). However, CCW movement times are significantly lower in pHD than in controls (Figure 1A) and spatial errors are slightly increased. Follow-up studies are needed to verify whether these changes represent an initial disruption of feedback control mechanisms. The neural bases of the implicit learning deficits and the alteration in trajectory formation probably reside in the motor loop that encompasses putamen, SMA, primary motor and the pre-frontal cortices.
4.3 Conclusions
Implicit and explicit aspects of sequence learning are affected in pHD, even when motor symptoms are not clinically present. Explicit but not implicit indexes improve by decreasing attentional and working memory demands. Altogether, these results point to an early involvement of multiple basal ganglia loops in pHD.
Acknowledgments
We thank Drs. Mark Guttman and Jane S. Paulsen for patient referrals. We thank Drs. C. Ghez, P. Mazzoni and J. Krakauer for countless discussions. Supported by NIH NS01961 and McDonnell Foundation (MFG), and NIH NS 37564 (DE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirkwood SC, Siemers E, Stout JC, Hodes ME, Conneally PM, Christian JC, et al. Longitudinal cognitive and motor changes among presymptomatic Huntington disease gene carriers. Arch Neurol. 1999;56:563–68. doi: 10.1001/archneur.56.5.563. [DOI] [PubMed] [Google Scholar]
- 2.Josiassen RC, Curry L, Roemer RA, DeBease C, Mancall EL. Patterns of intellectual deficit in Huntington's disease. J Clin Neuropsychol. 1982;4:173–83. doi: 10.1080/01688638208401127. [DOI] [PubMed] [Google Scholar]
- 3.Taylor HG, Hansotia P. Neuropsychological testing of Huntington's patients. Clues to progression. J Nerv Mental Dis. 1983;171:492–96. doi: 10.1097/00005053-198308000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Brandt J, Butters N. The neuropsychology of Huntington's disease. Trends Neurol Sci. 1986;9:118–20. [Google Scholar]
- 5.Folstein SE. Huntington's disease a disorder of families. Baltimore: Johns Hopkins University Press; 1989. [Google Scholar]
- 6.Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nature Genetics. 1993;4:398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- 7.Ashizawa T, Wong LJ, Richards CS, Caskey CT, Jankovic J. CAG repeat size and clinical presentation in Huntington's disease. Neurology. 1994;44:1137–43. doi: 10.1212/wnl.44.6.1137. [DOI] [PubMed] [Google Scholar]
- 8.Simpson SA, Davidson MJ, Barron LH. Huntington's disease in Grampian region: correlation of the CAG repeat number and the age of onset of the disease. J Medic Genetics. 1993;30:1014–17. doi: 10.1136/jmg.30.12.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butters N, Sax D, Montgomery K, Tarlow S. Comparison of the neuropsychological deficits associated with early and advanced Huntington's disease. Arch Neurol. 1978;35:585–89. doi: 10.1001/archneur.1978.00500330033006. [DOI] [PubMed] [Google Scholar]
- 10.Blackmore L, Simpson SA, Crawford JR. Cognitive performance in UK sample of presymptomatic people carrying the gene for Huntington's disease. J Medic Genetics. 1995;32:358–62. doi: 10.1136/jmg.32.5.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, Garron DC. Psychological features of Huntington's disease and the problem of early detection. Social Biol. 1980;27:11–19. doi: 10.1080/19485565.1980.9988399. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence AD, Hodges JR, Rosser AE, Kershaw A, ffrench-Constant C, Rubinsztein DC, et al. Evidence for specific cognitive deficits in preclinical Huntington's disease. Brain. 1998;121:1329–41. doi: 10.1093/brain/121.7.1329. [DOI] [PubMed] [Google Scholar]
- 13.Robins Wahlin TB, Lundin A, Dear K. Early cognitive deficits in Swedish gene carriers of Huntington's disease. Neuropsychology. 2007;21:31–44. doi: 10.1037/0894-4105.21.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Ghilardi MF, Silvestri G, Feigin A, Eidelberg D. Trajectory control and motor learning in pre-symptomatic Huntington's disease (pHD): a follow up study. Neurology. 2003;60 [Google Scholar]
- 15.Feigin A, Ghilardi MF, Huang C, Ma Y, Carbon M, Guttman M, et al. Preclinical Huntington's disease: compensatory brain responses during learning. Ann Neurol. 2006;59:53–59. doi: 10.1002/ana.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54:102–09. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- 17.Ghilardi MF, Eidelberg D, Silvestri G, Ghez C. The differential effect of PD and normal aging on early explicit sequence learning. Neurology. 2003;60:1313–19. doi: 10.1212/01.wnl.0000059545.69089.ee. [DOI] [PubMed] [Google Scholar]
- 18.Ghilardi MF, Feigin AS, Battaglia F, Silvestri G, Mattis P, Eidelberg D, et al. L-Dopa infusion does not improve explicit sequence learning in Parkinson's disease. Parkinsonism Relat Disord. 2007;13:146–51. doi: 10.1016/j.parkreldis.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Unified Huntington's Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 1996;11:136–42. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Ghilardi MF, Alberoni M, Rossi M, Franceschi M, Mariani C, Fazio F. Visual feedback has differential effects on reaching movements in Parkinson's and Alzheimer's disease. Brain Res. 2000;876:112–23. doi: 10.1016/s0006-8993(00)02635-4. [DOI] [PubMed] [Google Scholar]
- 22.Bylsma FW, Rebok GW, Brandt J. Long-term retention of implicit learning in Huntington's disease. Neuropsychologia. 1991;29:1213–21. doi: 10.1016/0028-3932(91)90035-7. [DOI] [PubMed] [Google Scholar]
- 23.Maki PM, Bylsma FW, Brandt J. Conceptual and perceptual implicit memory in Huntington's disease. Neuropsychology. 2000;14:331–40. doi: 10.1037//0894-4105.14.3.331. [DOI] [PubMed] [Google Scholar]
- 24.Randolph C. Implicit, explicit, and semantic memory functions in Alzheimer's disease and Huntington's disease. J Clin Exp Neuropsychol. 1991;13:479–94. doi: 10.1080/01688639108401065. [DOI] [PubMed] [Google Scholar]
- 25.Sprengelmeyer R, Canavan AG, Lange HW, Homberg V. Associative learning in degenerative neostriatal disorders: contrasts in explicit and implicit remembering between Parkinson's and Huntington's diseases. Mov Disord. 1995;10:51–65. doi: 10.1002/mds.870100110. [DOI] [PubMed] [Google Scholar]
- 26.Gabrieli JD, Stebbins GT, Singh J, Willingham DB, Goetz CG. Intact mirror-tracing and impaired rotary-pursuit skill learning in patients with Huntington's disease: evidence for dissociable memory systems in skill learning. Neuropsychology. 1997;11:272–81. doi: 10.1037//0894-4105.11.2.272. [DOI] [PubMed] [Google Scholar]
- 27.Heindel WC, Butters N, Salmon DP. Impaired learning of a motor skill in patients with Huntington's disease. Behav Neurosci. 1988;102:141–47. doi: 10.1037//0735-7044.102.1.141. [DOI] [PubMed] [Google Scholar]
- 28.Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer's, Huntington's, and Parkinson's disease patients. J Neurosci. 1989;9:582–87. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martone M, Butters N, Payne M, Becker JT, Sax DS. Dissociations between skill learning and verbal recognition in amnesia and dementia. Arch Neurol. 1984;41:965–70. doi: 10.1001/archneur.1984.04050200071020. [DOI] [PubMed] [Google Scholar]
- 30.Paulsen J, Butters N, Salmon D, Heindel W, Swenson M. Prism adaptation in Alzheimer and Huntington disease. Neuropsychology. 1993;7:73–81. [Google Scholar]
- 31.Smith MA, Brandt J, Shadmehr R. Motor disorder in Huntington's disease begins as a dysfunction in error feedback control. Nature. 2000;403:544–49. doi: 10.1038/35000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knopman D, Nissen MJ. Procedural learning is impaired in Huntington's disease: evidence from the serial reaction time task. Neuropsychologia. 1991;29:245–54. doi: 10.1016/0028-3932(91)90085-m. [DOI] [PubMed] [Google Scholar]
- 33.Willingham D, Koroshetz W. Evidence for dissociable motor skills in Huntington's disease patients. Psychobiology. 1993;21:173–82. [Google Scholar]
- 34.Nakahara H, Doya K, Hikosaka O. Parallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences - a computational approach. J Cogn Neurosci. 2001;13:626–47. doi: 10.1162/089892901750363208. [DOI] [PubMed] [Google Scholar]
- 35.Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Putz B. Presupplementary motor area activation during sequence learning reflects visuo-motor association. J Neurosci. 1999;19:RC1. doi: 10.1523/JNEUROSCI.19-10-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Putz B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci. 1998;18:1827–40. doi: 10.1523/JNEUROSCI.18-05-01827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 38.Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J. Executive dysfunction in early stages of Huntington's disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study. J Neurol Sci. 2005;239:11–19. doi: 10.1016/j.jns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–21. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]