Abstract
Context
The percentage of cancer patients ≥80 years old is expected to increase in the next few years. However data on the use of chemotherapy in these patients are limited.
Objective
We conducted a retrospective review to define the profile of patients ≥ 80 years old who received chemotherapy at our center and assess their survival.
Design, Setting and Participants
Patients ≥ 80 years treated with chemotherapy between 1/1/2000 to 12/31/2004 were included in this analysis.
Results
Of the 4689 patients treated with chemotherapy over the 5 year period, 133 patients (3%) were ≥ 80 years old. The median age was 83 years. 61% were females and 39% were males. 16% had hematologic tumors and 84% had solid tumors. Gynecological (32%) and aerodigestive cancers (27%) were the most common sites, and lung cancer (22%) was the most common cancer. During the first regimen, 512 cycles of chemotherapy were delivered with a median of 3 cycles (range 1–24 cycles). 49% received single and 51% multidrug regimens. Carboplatin was the most common single agent and carboplatin and paclitaxel was the most common combination among solid tumor patients. 19% of solid tumor patients received radiation with chemotherapy. The 1 year survival among hematologic cancer and solid tumor patients was 65% and 48%, respectively. Stage of disease was the only statistically significant factor predicting survival.
Conclusions
In cancer patients ≥ 80 years old selected for chemotherapy, both single and multi-agent therapy appeared to be feasible.
Keywords: cancer, chemotherapy, elderly, 80 years old
II INTRODUCTION
It is well recognized that cancer occurs predominantly in older individuals. More than 60% of new cancers and more than 70% of cancer deaths occur in patients over the age of 65 years. Moreover this age group is growing rapidly and therefore the number of cancer patients in this age group will continue to rise1. It is estimated that during the next 20 years cancer patients ≥ 75 years will triple, and cancer patients ≥ 80 years will double.1 Hence treatment of the elderly cancer patient is an increasingly important issue for the treating physician, and for the whole health care system.
The management of elderly cancer patients is a challenging and a complex problem that is influenced by different issues. Elderly patients often have decreased organ function from the aging process and commonly have comorbid illnesses that make them more vulnerable to toxicity from conventional cytotoxic agents.2 In addition, the presence of co-morbid illnesses increases the probability of drug interactions that could lead to adverse effects and reduced efficacy.2,3 Elderly patients also have an unique set of social and financial issues that make receiving cancer care more challenging.4 The use of cytotoxic chemotherapy in the elderly is also limited by lack of adequate clinical data due to under-representation of elderly patients in therapeutic trials. This is particularly true of cancer patients ≥ 75 years. 5
Advances in oncologic supportive care, including the availability of improved antiemetic agents and hematopoietic growth factors, has decreased toxicities associated with chemotherapy treatment. Hence there is an increasing trend towards offering systemic chemotherapy to elderly patients but the percentages are still smaller when compared to younger patients.6 It has been suggested that “selected fit elderly patients” should be treated with standard dose and schedule of chemotherapeutic medications as younger patients.7 However, there are data showing that elderly patients have increased hematologic toxicity associated with chemotherapy regimens.8
We decided to conduct a retrospective review of patients ≥ 80 years old who received systemic chemotherapy at Karmanos Cancer Institute. Our objectives were to define the demographic profile and tumor types of these patients. We also wanted to assess the common chemotherapy drugs and regimens administered and the duration of chemotherapy in these patients. Finally we also assessed their survival and factors that may affect their survival.
III. MATERIALS AND METHODS
Retrospective analysis of patients who received systemic chemotherapy at the Karmanos Cancer Institute in Detroit, MI between 1/1/2000 to 12/31/2004 was performed using computer generated pharmacy records. Patients with diagnosis of cancer and age 80 or above who received their first systemic chemotherapy in this center were included in the analysis; patients receiving only supportive care and hormonal therapy were excluded from the study. Patients were excluded from the study if they received: (1) any clinical study drug; (2) only supportive medications like erythropoietin, G-CSF, GM-CSF, bisphosphonates; (3) only oral chemotherapy; or (4) only hormonal therapy. Since there was heterogeneity of tumors among patients in this retrospective review, stage of solid tumors were categorized into three categories: local, regional, or distant in order to achieve uniformity regarding stage of the tumors. Tumors were considered local if there was no evidence of regional lymph node involvement or distant metastasis.
Statistical Methods
The patient demographics, the distribution of tumor types, the chemotherapy regimen including single or polychemotherapy, drug types, and survival data were summarized with descriptive statistics. Rates of second cycle chemotherapy delay were compared using Fisher’s exact test (2-sided). Exact, minimum-width 90% confidence intervals (CI) for response and toxicity rates were calculated using the Casella method 9 as implemented in StatXact software.10 Overall survival (OS) was measured from treatment start date to the date of death from any cause; patients still alive were censored as of their most recent date of vital status determination. Standard Kaplan-Meier estimation of the censored OS distribution was computed. Survival statistics (e.g., median) were estimated more conservatively using linear interpolation among successive event times on the Kaplan-Meier curve. 11 Length of censored OS was compared by subgroups using the log-rank test.
IV. RESULTS
A total of 4,689 patients received intravenous chemotherapy at the Karmanos Cancer Institute over a 5 year period between 2000 and 2004. Among these, 159 patients were 80 years or above when they received their first treatment. 26 patients were excluded from the study because 2 patients received study drugs, 3 patients received only growth factor support, 21 patients received either single agent bisphosphonates (zoledronic acid) or hormonal therapy alone (fulvestrant, leuprolide, or goserelin). Hence, data were analyzed for 133 patients (3% of all patients) treated in this period.
Patient demographics are detailed in Table 1. The median age of this group was 83 years (range 80 to 92); 31% of the patients were 85 years or older. There were more female patients (61%) in this group than males. Racial distribution of this group was similar to the overall population of patients treated at the center. Of the 133 patients, 112 (84%) had solid tumors and 21 patients (16%) had hematologic malignancy (see Table 1). The majority of patients (94%) treated had regional disease or distant metastasis while only 6% received adjuvant treatment. The tumor types are detailed in Table 2. Gynecological malignancies were the most common (32%) solid tumor type in this patient population. Among single organ tumors, lung cancer (22 %) and ovarian cancer (19%) were the most common tumors.
Table 1.
Patient demographics (N = 133)
| Characteristics | N (% of patients) |
| Gender | |
| Male | 52 (39) |
| Female | 81 (61) |
| Ethnicity | |
| White | 65 (49) |
| Black | 41 (31) |
| Other * | 18 (13) |
| Unknown | 9 (7) |
| Age (years) | Median 83 years (range: 80–92) |
| Tumor type | N (% of patients) |
| Solid | 112 (84) |
| Non-solid | 21 (16) |
| Stage | |
| Local | 8 ( 6) |
| Regional | 54 (41) |
| Distant | 50 (38) |
| Non-solid | 21 (16) |
Other includes Hispanic and Asian
Table 2.
Location of tumors
| Location | N (% of patients) | (% of solid tumor patients) |
| Solid tumors | 112 | |
| Gynecological | 36 (27) | (32) |
| Ovarian | 21 (16) | (19) |
| Thoracic | 25 (19) | (22) |
| Gastrointestinal | 19 (14) | (17) |
| Head/Neck | 12 ( 9) | (11) |
| Genitourinary | 11 ( 8) | (10) |
| Other | 9 ( 7) | ( 8) |
| Hematological | 21 (16) | N/A |
| Total | 133 (100) | (100) |
N/A = Not applicable.
About half of the patients (68 of 133) were treated with multi-agent chemotherapy (Table 3). Twenty one patients with solid tumors (19%) received either concurrent or sequential radiation therapy with systemic chemotherapy. The most common sites for radiation treatment were the head/neck and cervix. The median number of cycles of chemotherapy delivered in this elderly group was 3 (range: 1–24 cycles) with 42% receiving at least 4 cycles of chemotherapy. The median number of cycles delivered was same among patients who received single agent or multi-agent chemotherapy. Of the 133 patients, 48 patients (36%) had a delay in the start of the second cycle. Interestingly 45% (30/67) of patients who received single agent chemotherapy had a delay in the start of the second cycle while only 27% (18/66) of patients who had multi-agent chemotherapy had the same delay (p=0.047) Among the patients who had solid tumors and received single agent chemotherapy, carboplatin was the most common drug used, followed by gemcitabine and cisplatin. For combination treatments, carboplatin and paclitaxel (38%) was the most common regimen among solid tumor patients. There were 20 (15%) patients who went on to receive a second chemotherapy regimen. The median number of cycles administered in these 20 patients was 3 (range: 1–15 cycles).
Table 3.
Characteristics of Chemotherapy [Median- 3 cycles (range: 1–24 cycles)]
| Single agent | N=65 | (% of patients) |
| Carboplatin | 13 | (20) |
| Cisplatin | 8 | (12) |
| Gemcitabine | 8 | (12) |
| Docetaxel | 6 | ( 9) |
| Other | 30 | (46) |
| Multiple agents | N=68 | |
| Carboplatin/paclitaxel | 26 | (38) |
| CHOP/R-CHOP* | 10 | (14) |
| Carboplatin/etoposide | 7 | (10) |
| Other | 25 | (37) |
Cyclophosphamide, vincristine, adriamycin, predinsone, Rituxmab
By December 2005, 95 patients (71.5%) had died and 12 patients (9%) were lost to follow-up. The survival rates of the 133 patients at 6 months, 1 year and 3 years were 73%, 52% and 24%, respectively. The median OS was 14.3 months (95% CI: 10.3 – 19.1). The 1 year OS rates of the 92 patients age 80–84 and the 41 patients age 85–92 were 53% (95% CI: 0.43 – 0.64) and 50% (95% CI: 0.34 – 0.65), respectively. The median survivals of male and female patients were 10.9 months (95% CI: 7.6 – 21.2) and 15.1 months (95% CI: 10.6 – 21.7), respectively. The 1 year survival rates among hematologic cancer and solid tumor patients were 65% and 48%, respectively. Stage of disease (local, regional, distant) was the only statistically significant factor associated with OS (p = 0.0002 for the 3-group comparison) (Figure 1).
Figure 1.
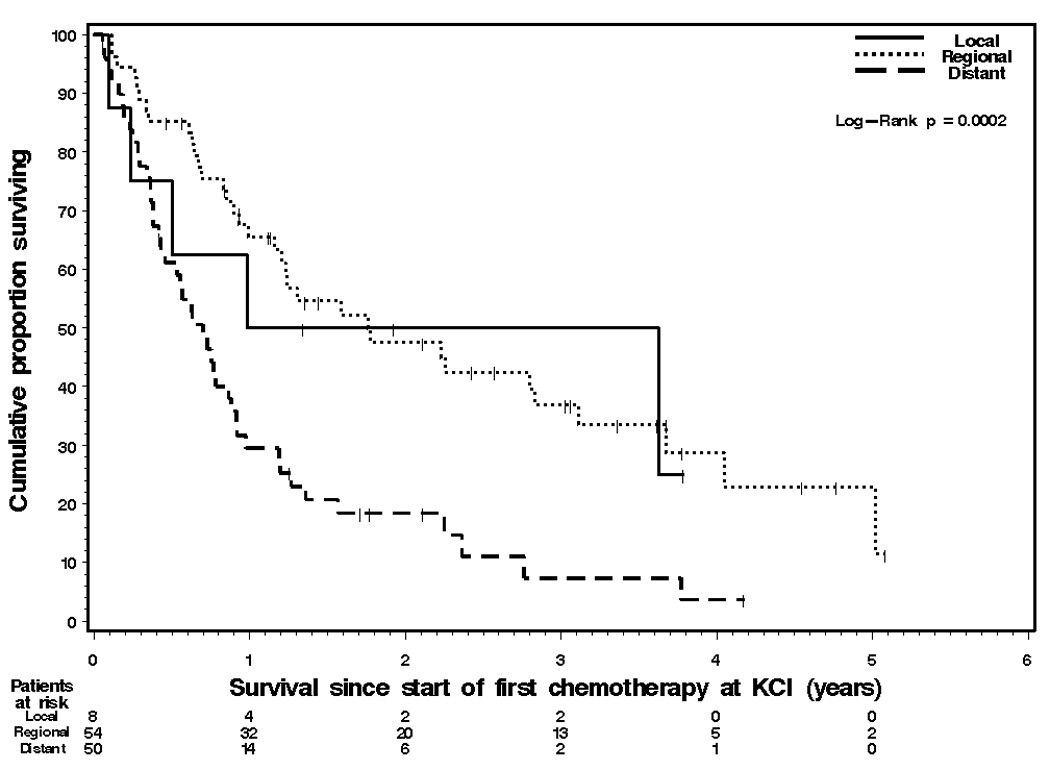
Kaplan-Meier graph of overall survival of the 112 solid tumor patients by stage of disease. Median survivals were: 11.8 months (95% CI: 2.8 – not reached) for local disease patients; 21.0 months (95% CI: 14.3 – 37.2) for regional disease patients; and 7.8 months (95% CI: 4.6 – 10.0) for distant disease patients. One-year survival rates were 50% (95% CI: 11%89), 65% (95% CI: 52–79), and 29% (95% CI: 17–42), respectively.
V. DISCUSSION
Elderly cancer patients need special attention when considering active systemic therapy because of the pathophysiological changes of aging, and also because of the changes in pharmacokinetics and pharmacodynamics of chemotherapy agents. Although there is no age limit for delivering systemic chemotherapy, the barriers to delivery of systemic chemotherapy are greater with increasing age. We therefore decided to conduct a retrospective review of the patients 80 years and older who received systemic chemotherapy at our cancer center, in an attempt to define the profile of such patients.
About 3 % of all the patients who received systemic chemotherapy at our cancer center over a 5 year period were ≥ 80 years. We do not know the number of patients in this age group evaluated during the same time period and therefore are unable to determine the percentage of patients ≥ 80 years not considered for cytotoxic chemotherapy. In an analysis from the Surveillance, Epidemiology and End Results (SEER) registries of lung cancer patients adjusted for factors such as co-morbidities and socio-economic status, the percentage of patients ≥ 75 years who received therapy was 32%. In the same analysis the percentage of patients ≤ 50 years who received therapy was 75%.12 It is likely that this lower use of cytotoxic therapy in the elderly lung cancer patients extends to patients with other tumor types. Multiple factors explain the limited use of cytotoxic therapy in the elderly including physician concerns regarding tolerability of cytotoxic therapy, patient or family preference not to undergo chemotherapy particularly with advanced disease, and various social and financial issues specific to the elderly.
There were more females than males in this cohort (61% vs. 39%), but the gender distribution was more even (47% v. 53%) after excluding gender specific tumors. According to a recent report by the Southwest Oncology Group, the proportion of females in the US population of cancer patients is 43%.13 The higher percentage of female patients in this elderly cohort may be explained by longer life expectancy in women. In addition the response rates to cytotoxic chemotherapy in female specific tumors such as breast and ovarian cancers are higher than many other tumors, possibly leading to greater use of such therapy in elderly female patients as compared to male patients.
The median number of cycles of chemotherapy delivered in this cohort was 3. The number of cycles administered does vary between different tumors. In lung cancer (non-small cell and small cell) the number of cycles of chemotherapy delivered is usually 4, where as in ovarian cancer and in non-Hodgkin’s lymphoma 6 cycles or more are administered during front line therapy. The relatively low median number of cycles in this cohort may reflect the limitation of delivering chemotherapy in patients ≥ 80 years. However, 42% of elderly patient were able to receive more than 4 cycles of chemotherapy and 22% of patient received more than 6 cycles of treatment. Hence there is subset of elderly patients who can tolerate more treatment than the general population.
We assessed time to 2nd cycle of chemotherapy as a surrogate marker for feasibility of continuing chemotherapy in this elderly group. In our cohort 64% of the patients had no delay in the start of the second cycle. However we did not assess whether the dosages during the first cycle in these patients were different than the recommended dosages for the particular regimen used. We observed that the rate of delay in the start of the second cycle was significantly higher among patients who received single agent therapy. It is possible that patients who received single agent chemotherapy had more co-morbidities or poor performance status and therefore were selected for single agent treatment. It is also possible that dosages of multi-agent regimens were lower than the standard dosages, where as single agent therapy was delivered at full dose.
The most common drug used in this cohort was carboplatin either as single agent or as part of a combination. Carboplatin dosing is based on the Calvert formula that incorporates age and renal function. However there are concerns about the accuracy of the Cockcroft-Gault equation, utilized to estimate the glomerular filtration rate (GFR) when calculating carboplatin dose by the Calvert formula, in patients ≥ 65 years.14,15 Therefore, close monitoring of elderly patients on carboplatin is essential, particularly with regard to blood counts. Paclitaxel, the other drug commonly used in this cohort, has modest but significant decreased clearance of total paclitaxel with increasing age but without adverse clinical sequelae.16, 17 The decrease in clearance seems to be partly related to the decreased clearance of Cremphor, the formulation vehicle, in the elderly. Unbound paclitaxel maybe a better predictor of clinically relevant exposure than total paclitaxel and may explain the lack of clinical sequelae despite the lower clearance. The pharmacokinetics of docetaxel, the other taxane commonly used in cancer patients, is not influenced by age, though the elderly seem to be more susceptible to the adverse effects of the drug.18,19
Heterogeneity of tumors and the retrospective nature of the analysis limit the interpretation of the survival data. We also did not collect data on all the 4689 patients to conduct comparative analysis of the younger and older patients. However the 6 month survival rate of 73% suggests that the treatment related death rate was low and the treatment was feasible in these elderly patients. In addition, their 1 year survival rate of 52% suggests that systemic chemotherapy may have been beneficial since these survival rates are consistent with the survival rates observed in many of the major cancers. Even the oldest patients (age 85–92) in our study had survival duration similar to that of the older (age 80–84) patients. As expected stage of disease was the only predictive factor of survival.
The results of this analysis have several limitations. Since this is a retrospective analysis we were unable to determine the number of patients ≥ 80 years who were assessed at our cancer center during the time period of this analysis and were not offered any systemic therapy for their cancer. We were also unable to assess the chemotherapy associated toxicities for these patients and the reasons for discontinuation of therapy. Since the data for this analysis was derived from the cancer center’s pharmacy records, no data is available on patients who were treated with oral agents since these agents are dispensed through outpatient pharmacy and not through the cancer center’s pharmacy.
In conclusion, a small minority (3%) of all patients treated with systemic intravenous chemotherapy at our center were ≥ 80 years old. The majority of these patients were females and gynecological tumors were the most common tumor types observed. Single agent and multi-agent treatments were equally utilized and appeared to be feasible in elderly patients.
Acknowledgement
Everyone who contributed to this work has been listed as an author.
Grant Support:
Supported in part by NIH Cancer Center Support Grant, CA-22453
Biographies
Dr. Minsig Choi completed his medical school in 1994 at the Far Eastern University, Manila, Philippines. He then completed an Internal Medicine residency at the Chicago Medical School in Chicago followed by a Hematology-Oncology fellowship at the Wayne State University/Karmanos Cancer Institute in Detroit, USA. He currently works at Jackson VAMC and also holds a faculty position at the University of Mississippi, Jackson, USA. His main focus is research in the area of geriatric cancer and gastrointestinal malignancies.
Dr. Shirish Gadgeel completed medical school in 1989 at the University of Bombay, Bombay (Mumbai) India. He then completed Internal Medicine residency in 1992 at the same university. He then did internship in Internal Medicine at LaGuardia Hospital in New York from 1993–1994 and subsequently Internal Medicine residency at Wayne State University, Detroit, Michigan from 1996–2000. Since October of 2000 he is on faculty at Karmanos Cancer Institute/Wayne State University and member of the Thoracic Oncology program at the institute. His current designation is Associate Professor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 42nd Annual meeting of the American Society of Clinical Oncology
Conflict of Interest:
None of the authors have any conflict of interest to disclose
REFERENCES
- 1.Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Repetto L, Balducci L. A case for geriatric oncology. Lancet Oncol. 2002;3:289–297. doi: 10.1016/s1470-2045(02)00730-1. [DOI] [PubMed] [Google Scholar]
- 3.Di Maio M, Perrone F, Gallo C, et al. Supportive care in patients with advanced non-small-cell lung cancer. Br J Cancer. 2003;89:1013–1021. doi: 10.1038/sj.bjc.6601236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtman SM, Balducci L, Aapro M. Geriatric Oncology: A field coming of age. J Clin Oncol. 2007;25:1821–1823. doi: 10.1200/JCO.2007.10.6567. [DOI] [PubMed] [Google Scholar]
- 5.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 6.Dale D. Poor prognosis in elderly patients with cancer: the role of bias and undertreatment. J Support Oncol. 2003 (4 Suppl 2):11–17. [PubMed] [Google Scholar]
- 7.Wasil T, Lichtman S. Treatment of elderly cancer patients with chemotherapy. Cancer Invest. 2005;23:537–547. doi: 10.1080/07357900500202770. [DOI] [PubMed] [Google Scholar]
- 8.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 9.Casella G. Refining binomial confidence intervals. Canadian J. Statistics. 14;1987:113–129. [Google Scholar]
- 10.Mehta C, Patel N. StatXact 6: Statistical software for exact nonparametric inference, user manual. Cambridge, MA: Cytel Software Corporation; 2004. pp. 1–29. [Google Scholar]
- 11.Lee E. Statistical Methods for Survival Data Analysis. ed 3rd. New York: Wiley & Sons, Inc; 2003. pp. 76–91. [Google Scholar]
- 12.Potosky AL, Saxman S, Wallace RB, et al. Population variations in the initial treatment of non-small cell lung cancer. J Clin Oncol. 2004;22:3261–3268. doi: 10.1200/JCO.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 13.Unger JM, Coltman CA, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24:141–144. doi: 10.1200/JCO.2005.02.8928. [DOI] [PubMed] [Google Scholar]
- 14.Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 15.Verhave JC, Fesler P, Ribstein J, et al. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis. 2005;46:233–241. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Lichtman SM, Hollis D, Miller AA, et al. Prospective evaluation of the relationship of patient age and paclitaxel clinical pharmacology: Cancer and Leukemia Group B (CALGB 9762) J Clin Oncol. 2006;24:1846–1851. doi: 10.1200/JCO.2005.03.9289. [DOI] [PubMed] [Google Scholar]
- 17.Smorenburg CH, ten Tije AJ, Verweij J, et al. Altered clearance of unbound paclitaxel in elderly patients with metastatic breast cancer. Eur J Cancer. 2003;39:196–202. doi: 10.1016/s0959-8049(02)00611-1. [DOI] [PubMed] [Google Scholar]
- 18.Bruno R, Hille D, Riva A, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 19.ten Tije AJ, Verweij J, Carducci MA, et al. Prospective Evaluation of the Pharmacokinetics and Toxicity Profile of Docetaxel in the Elderly. J Clin Oncol. 2005;23:1070–1077. doi: 10.1200/JCO.2005.03.082. [DOI] [PubMed] [Google Scholar]


