Abstract
Background
Nutritional concerns in HIV-infected children have evolved, from wasting to obesity and insulin resistance. However, little is known about the diet of these children during this evolution.
Objective
We analyzed dietary macronutrient intake in HIV-infected children over nearly 10 y.
Design
HIV-infected children underwent periodic longitudinal nutritional assessments between 1995 and 2004. Sex-specific initial and final means or proportions and time trends in macronutrient intakes were estimated with regression analyses.
Results
Three hundred thirty nutritional records from 49 males and 411 from 67 females were analyzed. Caloric intake exceeded the estimated energy requirement (EER) for ideal body weight in 1995 by 62% for males and 39% for females and decreased by 3% of the EER per year in males (P = 0.02) and by 2% in females (P = 0.004). In 2004, caloric intake still remained >19% above the EER in both groups. Protein intake was nearly 400% of the recommended dietary allowance (RDA) for ideal body weight in 1995 among both males and females and decreased by 13% of the RDA per year for males (P = 0.001) and by 21% per year for females (P < 0.001). However, daily protein intake still exceeded the RDA by >60% in both groups in 2004. Females consumed more energy from carbohydrates (P = 0.05) and sugar (P = 0.10) and less from monounsaturated (P = 0.04), polyunsaturated (P = 0.05), saturated (P = 0.03), and total (P = 0.10) fat in 2004 than in 1995.
Conclusion
Excessive caloric intake and a shift in dietary composition toward carbohydrates in females suggest that continued monitoring of diet in HIV-infected children is important to avoid increased nutritional risk.
INTRODUCTION
One critical component of HIV-associated morbidity and mortality is nutritional status. The relation between proteinenergy malnutrition and adverse effects on the immune system has been recognized for many years (1). Before highly active antiretroviral therapy (HAART), the primary nutritional concern for HIV-infected individuals was wasting syndrome (2–4). In addition, many children were unable to consume the calories required for growth because of pancreatitis, malabsorption, or general anorexia associated with the disease (5–8), and they often required nutritional supplementation, such as high-calorie liquid complete nutrition, to maintain appropriate growth (9).
Although weight loss and wasting may still be important concerns for some HIV-infected patients in the HAART era (10), studies show that, overall, HAART increases body cell mass and decreases wasting in both adults and children (2, 11, 12). Because virological and immunologic variables improve in many children with HIV, nutritional supplements are less often required for growth. Instead, new nutritional and metabolic challenges are now associated with HAART, such as lipodystrophy and insulin resistance (13–15).
The prevalence of overweight among all children and adolescents is increasing in the United States. The National Health and Nutrition Examination Surveys (NHANES) have documented increases in the number of 12- to 19-y-old adolescents with a body mass index (BMI; in kg/m2) above the 95th percentile, from 10.5% between 1988 and 1994 to 16.1% between 1999 and 2002 (16, 17). This increase in the prevalence of obesity has been attributed, in part, to dietary and lifestyle factors. The concern is that the nutritional characteristics of HIV-infected children may become similar to their uninfected counterparts as they become healthier with HAART. As a result, HIV-infected children may not only be less wasted and malnourished, but they may move to the opposite extreme of overweight and obesity and experience the associated sequelae.
To date, there has been little systematic evaluation of the evolution of dietary intake in HIV-infected children in the HAART era. Given the new potential for an elevated risk of lipodystrophy and cardiovascular disease in HIV-infected children, understanding their diets has become more important. Accordingly, we evaluated changes in the intake of macronutrients among HIV-infected children between 1995 and 2004.
SUBJECTS AND METHODS
Study cohort
HIV-infected children were enrolled in a prospective, longitudinal study on growth and nutrition at the Children’s Hospital AIDS Program (CHAP), Boston MA, and the University of Rochester Pediatric HIV Program, Rochester NY. The Institutional Review Boards at both institutions approved all research protocols, and procedures for informed consent and assent were followed.
Inclusion criteria
To be included in the study, children must have been between 3 and 20 y of age and eating exclusively by mouth. They must not have been given megestrol acetate in the 90 d before enrollment. Data from visits at which measurements of kilocalories, carbohydrate, fat, protein, weight, height, triceps skinfold thickness (TSF), and midarm circumference (MAC) were not made were excluded. In 7 cases, many children meeting the inclusion criteria were biologically related or shared a household. We selected a single subject from each of these clusters using a pseudorandom number generator to maintain independence between subjects without inducing bias.
Data collection
Data were collected at scheduled clinical care visits to each center’s program, typically every 3 to 6 mo. Data were collected by registered dietitians during dietary monitoring as part of routine clinical care. Clinical data included disease stage [per the Centers for Disease Control and Prevention (CDC);18] and antiretroviral use at the time of the clinical visit. CD4+ T lymphocyte frequency (CD4%) and HIV RNA (viral load) were measured as clinically indicated. Current clinical practice guidelines for HIV management recommend measuring CD4+ T lymphocytes and viral load every 3 mo (19). However, at the beginning of the study, these laboratory studies were not widely available and were done only intermittently. For this reason, HIV RNA was not included in the analysis because of limited availability of data early in the study. The closest CD4% value obtained within 365 d of a nutritional assessment before HAART exposure or within 90 d of an assessment after HAART exposure was used if CD4% was not obtained at a particular assessment.
Registered dietitians conducted dietary assessment interviews with each child and their primary caretaker using open-ended questions about meal timing, food selection, and portion size. At each visit, the previous day’s dietary intake, or, if the previous day was atypical by report of the patient and caretaker, a typical day’s dietary intake was verbally requested and recorded (20). Each 24-h recall was immediately read back to the patient and caretaker and revised as needed to increase accuracy and completeness of the record. Food models were used to assist patients and caretakers in identifying correct portion sizes and food selections. Food records were then reviewed a final time with the patient and caretaker, with dietary counseling directed to advance intake toward dietary goals. Nutrition education individualized to each patient’s nutritional status was initiated or continued, as appropriate, after the dietary recall.
Total caloric and macronutrient (fat, carbohydrate, fiber, protein, and sugar) intakes were calculated by using nutrient analysis software (NUTRITIONIST IV, version 4.0 and NUTRITIONIST V, version 2.3; First Data Bank, San Bruno, CA). We determined total daily calorie and protein intakes as a percentage of the age- and sex-specific estimated energy requirement (EER)/recommended dietary allowance (RDA), respectively, at ideal weight-for-height (21), fiber intake as a percentage of the ageand sex-specific adequate intake (21), cholesterol intake as a percentage of the US Department of Agriculture (USDA) guideline (22), and percentage of total calorie intake from carbohydrate (total and simple sugar), fat (total, monounsaturated, polyunsaturated, and saturated), and protein by using Atwater general factors (23). The ideal body weight for actual height was determined by the age- and sex-specific median BMI from the CDC growth curves (24). In calculating the EER, we assumed a physical activity level consistent with completing the regular activities of daily living without additional exercise (21), because we lacked detailed information from which to estimate the actual physical activity level of the patients.
Height, weight, MAC, and TSF were measured by registered dietitians trained in applying standardized anthropometric methods (25). Weight (recorded to the nearest 0.1 kg) and standing height (recorded to the nearest 0.1 cm) were measured by using recommended techniques. BMI was calculated as weight (kg)/ height2 (m) (24). Age- and sex-specific weight, height, and BMI z scores were calculated from the CDC growth curves (24). TSF (a measure of fat stores) was measured with skin calipers (Lange, Cambridge, MD), and MAC was measured using standard techniques. The TSF and MAC measurements were used to derive arm-muscle circumference (AMC). Age- and sex-adjusted percentiles for TSF and AMC were calculated on the basis of the work by Frisancho (26). These percentiles were dichotomized at the 85th percentile for analysis to avoid problems caused by censoring at the lowest (5th) and highest (95th) reported empirical percentiles (26).
Statistical methods
Outcomes were either age-independent or standardized to be age-independent so that outcomes for healthy children would not change during the study because of somatic growth or changes in the age distribution of the cohort. To describe sex-specific trends in the continuous and dichotomous outcomes of interest, we fit generalized linear models including center, sex, years since the beginning of the study (14 February 1995), and the interaction between years and sex. Estimates were obtained by using generalized estimating equations with an exchangeable working correlation structure to account for potential correlations between repeated measurements on the same individuals (27). A working independence correlation structure was used to model the proportion of HAART-exposed children because of convergence problems with an exchangeable working correlation structure.
These models yielded estimates of the sex-specific means or proportions at the beginning and end of the study period with 50% weight on each center. They also yielded estimates of sex-specific per annum changes in the means or odds of an outcome over the study period. Two-sided Wald tests with 1 df were used to test the following null hypotheses for each outcome: 1) no sex difference in the estimated mean or proportion at the beginning of the study period, 2) no change over time for males, 3) no change over time for females, 4) no sex difference in the change over time, and 5) no sex difference in the estimated mean or proportion at the end of the study period. An intersection-union test (28) was used to test the null hypothesis that the mean percentage of energy from a given macronutrient was not within the range recommended for children aged 4–18 y in the dietary reference intakes (21) against the alternative that it was by rejecting the null at the 0.025 significance level if the 95% CI was entirely within the recommended range.
The regression model allowed each treatment center to have an additive (main) effect on the estimated mean or proportion to account for systematically higher or lower outcomes at one center over the entire study period as a result of differences in the populations served. However, the model assumed that there was no center interaction with changes over time, sex differences at the beginning of the study period, or sex differences in the changes over time to combine the data from Rochester with the data from the larger Children’s Hospital Boston cohort. Because 6 y of concurrent data from both centers were available, this assumption could be tested empirically. The Simes test (29) of the joint null hypothesis of no center interactions in our regression model for all outcomes was performed by using the 21 P values from the 3-df generalized score tests for each outcome. All analyses were performed by using SAS/STAT software, version 9.1.3 of the SAS System for WINDOWS (SAS Institute Inc, Cary, NC).
RESULTS
Demographic and clinical characteristics at enrollment
Between 1995 and 2004, 86 children were enrolled from Boston and 30 from Rochester (Table 1). Approximately 50% of both cohorts were black, non-Hispanic, and most had acquired HIV vertically. A greater proportion of patients from Boston were classified in CDC category B for clinical HIV disease status. More patients in Boston were enrolled earlier in the study period (when HAART and prenatal HIV screening were not as widely available), as indicated by the lower median enrollment time and the younger median age at entry in Boston (6.6 compared with 8.5 y). Other clinical and demographic characteristics were similar in the 2 cohorts and appear to be representative of the pediatric HIV-infected population during the period.
TABLE 1.
Demographic and clinical characteristics of 116 HIV-positive children at enrollment in an observational study of macronutrient intake1
| Characteristic | Boston cohort (n = 86) |
Rochester cohort (n = 30) |
|---|---|---|
| Female sex [n (%)] | 52 (60) | 15 (50) |
| Race [n (%)] | ||
| Black, non-Hispanic | 41 (48) | 15 (50) |
| Hispanic | 15 (17) | 5 (17) |
| White, non-Hispanic | 27 (31) | 8 (27) |
| Other | 3 (3) | 2 (7) |
| CDC stage [n (%)]2 | ||
| N (1–3) | 2 (2) | 2 (7) |
| A (1–3) | 18 (21) | 16 (53) |
| B (1–3) | 44 (52) | 6 (20) |
| C (1–3) | 21 (25) | 6 (20) |
| Route of infection [n (%)] | ||
| Vertical | 79 (92) | 29 (97) |
| Blood products | 6 (7) | 0 (0) |
| Hemophilia | 1 (1) | 0 (0) |
| Sexual transmission | 0 (0) | 1 (3) |
| HAART-exposed | 21 (24) | 12 (40) |
| Age at enrollment (y) | 6.6 (3.1, 18.5)3 | 8.5 (3.1, 19.0) |
| Enrollment time (y)4 | 0.4 (0.0, 9.0) | 2.9 (2.5, 8.5) |
| Length of follow-up (y) | 4.1 (0.0, 9.2) | 2.7 (0.0, 6.0) |
| CD4 (%)5 | 21.0 (0.0, 53.0) | 21.0 (9.0, 42.0) |
Enrollment is defined as the first qualifying dietary intake. CDC, Centers for Disease Control and Prevention; HAART, highly active antiretroviral therapy.
The initial CDC stage was missing for one child from the Boston cohort.
Medians; range in parentheses (all such values).
Years after the beginning of the study (14 February 1995) that a patient’s first qualifying dietary intake occurred.
n = 79 for the Boston cohort, and n = 12 for the Rochester cohort.
Characteristics of the regression analysis
The regression analysis was based on 330 nutritional records from 49 males and from 411 records from 67 females. The median interval between qualifying nutritional records was 147 d [minimum: 15 d; quartile (Q)1: 98; Q3: 252; maximum: 2590 d], and the median number of nutritional records per child was 5 (minimum: 1; Q1: 2; Q3: 10; maximum: 24 records). There was no evidence of center interactions with per annum trends or sex effects apart from random variation for any outcomes (Simes, P = 0.44). Therefore, we proceeded to estimate combined time trends and sex effects using our model with only a center main effect.
Regression estimates of baseline sex-specific characteristics
Means and proportions at the beginning of the study (early 1995) with 50% weight on each center were estimated with regression analysis (Table 2). Mean CD4% was higher in females (P = 0.003). Mean height and weight z scores were both below normal in males. Mean height z score was below normal in females, whereas mean BMI z score was above normal. The percentage of females at or above the 85th percentile for AMC exceeded 15% at the beginning of the study.
TABLE 2.
Regression estimates of anthropometric and dietary characteristics among 116 HIV-positive children in 19951
| Characteristic | Males (n = 49 children, 330 observations) | Females (n = 67 children, 411 observations) | P2 |
|---|---|---|---|
| HAART-exposed (%)3 | 13.4 (5.4, 29.6) | 7.5 (3.0, 17.9) | 0.35 |
| CD4 (%) | 16.9 (12.2, 21.5) | 25.1 (21.3, 28.9) | 0.003 |
| Height (z score) | -1.02 (-1.50, -0.54) | -0.49 (-0.88, -0.10) | 0.07 |
| Weight (z score) | -0.60 (-1.03, -0.17) | -0.14 (-0.48, 0.20) | 0.08 |
| BMI (z score) | 0.18 (-0.19, 0.55) | 0.32 (0.01, 0.63) | 0.57 |
| BMI ≥ 85th percentile (%)3 | 9.0 (3.7, 20.3) | 20.2 (11.6, 32.8) | 0.09 |
| BMI ≥ 95th percentile (%)3 | 1.8 (0.5, 6.5) | 11.8 (4.7, 26.8) | 0.009 |
| Triceps skinfold ≥ 85th percentile (%)3 | 14.1 (5.0, 33.6) | 21.5 (11.7, 36.0) | 0.43 |
| AMC ≥ 85th percentile (%)3 | 15.2 (6.3, 32.4) | 27.3 (15.9, 42.8) | 0.15 |
| Kilocalories (% of EER) | 161.6 (148.3, 174.9) | 139.5 (128.8, 150.1) | 0.008 |
| Carbohydrate (% of energy) | 53.7 (51.7, 55.8) | 52.0 (50.0, 54.1) | 0.23 |
| Sugar (% of energy) | 21.9 (19.4, 24.4) | 20.8 (18.3, 23.3) | 0.51 |
| Fat (% of energy) | 33.3 (31.3, 35.3) | 34.0 (32.1, 36.0) | 0.58 |
| Monounsaturated (% of energy) | 10.0 (9.1, 10.8) | 10.2 (9.4, 11.1) | 0.66 |
| Polyunsaturated (% of energy) | 4.7 (4.0, 5.4) | 5.1 (4.5, 5.7) | 0.40 |
| Saturated (% of energy) | 12.6 (11.7, 13.5) | 12.7 (11.7, 13.7) | 0.87 |
| Protein (% of energy) | 14.5 (13.5, 15.5) | 15.4 (14.6, 16.2) | 0.15 |
| Protein (% of RDA) | 377.8 (330.0, 425.7) | 357.7 (322.5, 392.9) | 0.51 |
| Dietary fiber (% of AI) | 45.7 (39.3, 52.1) | 39.7 (33.3, 46.0) | 0.19 |
| Cholesterol (% of USDA guideline) | 83.4 (64.5, 102.3) | 61.2 (46.8, 75.5) | 0.06 |
| Known oral supplement use (%)3 | 22.1 (10.3, 41.3) | 32.4 (18.6, 50.1) | 0.34 |
All values are means unless designated as proportions and expressed as percentages (see footnote 3); 95% CIs in parentheses (all such values). For some variables, the number of male/female subjects (observations) was different from that indicated in the table header. CD4: 45/60 (275/353); sugar: 46/62 (216/273); monounsaturated and saturated fat: 47/66 (284/341); polyunsaturated fat: 45/65 (275/331); dietary fiber: 48/66 (287/350); and cholesterol: 48/66 (290/353). Sex-specific means or proportions at the beginning of the study period with 50% weight on each center and their 95% CIs were estimated with generalized linear models including center, sex, years since the beginning of the study (14 February 1995), and the interaction between years and sex. HAART, highly active antiretroviral therapy; AMC, arm-muscle circumference; EER, estimated energy requirement; RDA, Recommended Dietary Allowance; AI, adequate intake; USDA, US Department of Agriculture.
Estimates were obtained by using generalized estimating equations with an exchangeable working correlation structure to account for potential correlations between repeated measurements on the same individuals. The estimated proportion was calculated using the logit link and applying backtransformation to the sex-specific intercepts. A working independence correlation structure was used to model the proportion of HAART-exposed children because of convergence problems with an exchangeable working correlation structure. P value for the 2-sided Wald test of no sex difference in the estimated mean or proportion at the beginning of the study period (no sex difference in intercepts in the generalized linear model).
Estimated proportion from the generalized linear model expressed as a percentage.
Mean caloric and protein intakes substantially exceeded requirements at baseline. The mean percentages of energy derived from carbohydrate, sugar, and protein were within the ranges recommended for children aged 4–18 y in the dietary reference intakes. The adequacy and macronutrient composition of the diet were similar in males and females at the beginning of the study, but males consumed an additional 22% of the EER for the ideal weight per day (P = 0.008). On average, cholesterol consumption by the females was below the USDA guideline, but fiber consumption was inadequate.
Estimates of dietary evolution during the study
Means and proportions at the end of the study (late 2004; Table 3) with 50% weight on each center and per-annum changes in means and odds ratios during the study (Table 4) were estimated with regression analysis. At the end of the study, females derived a higher mean percentage of calories from carbohydrate (P = 0.01) and a lower mean percentage of calories from monounsaturated (P = 0.01), saturated (P = 0.09), and total (P = 0.06) fat than did males, although the mean percentages of energy derived from carbohydrate, fat, and protein for the females were within the ranges recommended for children aged 4–18 y. For males, the mean percentages of energy derived from carbohydrate, sugar, and protein remained within the recommended ranges at the end of the study.
TABLE 3.
Regression estimates of anthropometric and dietary characteristics among 116 HIV-positive children in 20041
| Characteristic | Males (n = 49 children, 330 observations) | Females (n = 67 children, 411 observations) | P2 |
|---|---|---|---|
| HAART-exposed (%)3 | 98.8 (85.2, 99.9) | 97.2 (84.3, 99.6) | 0.63 |
| CD4 (%) | 31.6 (26.3, 36.9) | 27.7 (23.9, 31.4) | 0.24 |
| Height (z score) | -0.92 (-1.32, -0.51) | -0.29 (-0.77, 0.19) | 0.05 |
| Weight (z score) | -0.38 (-0.91, 0.15) | 0.11 (-0.23, 0.44) | 0.13 |
| BMI (z score) | 0.01 (-0.55, 0.57) | 0.25 (-0.02, 0.51) | 0.46 |
| BMI ≥ 85th percentile (%)3 | 27.3 (11.3, 52.7) | 22.5 (12.2, 37.7) | 0.69 |
| BMI ≥ 95th percentile (%)3 | 4.0 (0.9, 15.9) | 4.4 (1.3, 13.8) | 0.91 |
| Triceps skinfold ≥ 85th percentile (%)3 | 16.8 (3.4, 53.7) | 14.6 (5.9, 31.7) | 0.87 |
| AMC ≥ 85th percentile (%)3 | 15.0 (4.9, 37.6) | 20.9 (10.8, 36.6) | 0.59 |
| Kilocalories (% of EER) | 134.9 (117.7, 152.1) | 119.4 (110.8, 128.0) | 0.11 |
| Carbohydrate (% of energy) | 49.9 (46.5, 53.4) | 55.2 (53.2, 57.2) | 0.01 |
| Sugar (% of energy) | 21.5 (18.8, 24.2) | 24.7 (21.6, 27.7) | 0.13 |
| Fat (% of energy) | 35.0 (31.8, 38.1) | 31.3 (29.5, 33.2) | 0.06 |
| Monounsaturated (% of energy) | 10.6 (9.4, 11.8) | 8.8 (8.0, 9.6) | 0.01 |
| Polyunsaturated (% of energy) | 4.8 (3.9, 5.6) | 4.2 (3.6, 4.7) | 0.21 |
| Saturated (% of energy) | 12.3 (11.0, 13.6) | 10.9 (9.9, 11.9) | 0.09 |
| Protein (% of energy) | 15.9 (14.4, 17.5) | 14.6 (13.6, 15.6) | 0.13 |
| Protein (% of RDA) | 252.4 (197.5, 307.4) | 160.1 (129.6, 190.7) | 0.002 |
| Dietary fiber (% of AI) | 43.5 (36.8, 50.3) | 45.8 (39.6, 52.1) | 0.62 |
| Cholesterol (% of USDA guideline) | 99.8 (74.2, 125.4) | 72.8 (61.0, 84.7) | 0.07 |
| Known oral supplement use (%)3 | 38.0 (14.6, 68.8) | 5.1 (1.3, 18.6) | 0.01 |
All values are means unless designated as proportions and expressed as percentages (see footnote 3); 95% CIs in parentheses (all such values). For some variables, the number of male/female subjects (observations) was different from that indicated in the table header. CD4: 45/60 (275/353); sugar: 46/62 (216/273); monounsaturated and saturated fat: 47/66 (284/341); polyunsaturated fat: 45/65 (275/331); dietary fiber: 48/66 (287/350); and cholesterol: 48/66 (290/353). Sex-specific means or proportions at the end of the study period with 50% weight on each center and their 95% CIs were estimated with generalized linear models including center, sex, years since the beginning of the study (14 February 1995), and the interaction between years and sex. HAART, highly active antiretroviral therapy; AMC, arm-muscle circumference; EER, estimated energy requirement; RDA, Recommended Dietary Allowance; AI, adequate intake; USDA, US Department of Agriculture.
Estimates were obtained by using generalized estimating equations with an exchangeable working correlation structure to account for potential correlations between repeated measurements in the same individuals. The estimated proportion was calculated by using the logit link and applying backtransformation to the sex-specific linear predictors at the end of the study period.A working independence correlation structure was used to model the proportion of HAART-exposed children because of convergence problems with an exchangeable working correlation structure. P value for the two-sided Wald test of no sex difference in the estimated mean or proportion at the end of the study period (no sex difference in linear predictors from the generalized linear model at the end of the study period).
Estimated proportion from the generalized linear model expressed as a percentage.
TABLE 4.
Regression estimates of per-annum changes in anthropometric and dietary characteristics among 116 HIV-positive children from 1995 to 20041
| Characteristic | Males (n = 49 children, 330 observations) |
Females (n = 67 children, 411 observations) |
|||
|---|---|---|---|---|---|
| Per annum change (95% CI)2 |
P2,3 | Per annum change (95% CI)2 |
P2,3 | P for sex difference2,4 | |
| HAART-exposed5 | 1.93 (1.36, 2.75) | <0.001 | 1.89 (1.45, 2.47) | <0.001 | 0.93 |
| CD4 (%) | 1.5 (0.8, 2.3) | <0.001 | 0.3 (-0.1, 0.6) | 0.13 | 0.002 |
| Height (z score) | 0.01 (-0.04, 0.07) | 0.69 | 0.02 (-0.04, 0.08) | 0.50 | 0.81 |
| Weight (z score) | 0.02 (-0.06, 0.10) | 0.57 | 0.03 (-0.02, 0.07) | 0.21 | 0.95 |
| BMI (z score) | -0.02 (-0.10, 0.07) | 0.68 | -0.01 (-0.04, 0.03) | 0.70 | 0.82 |
| BMI ≤ 85th percentile5 | 1.15 (0.99, 1.34) | 0.08 | 1.01 (0.93, 1.10) | 0.73 | 0.16 |
| BMI ≤ 95th percentile5 | 1.09 (0.89, 1.34) | 0.41 | 0.89 (0.79, 1.01) | 0.07 | 0.10 |
| Triceps skinfold ≤ 85th percentile5 | 1.02 (0.78, 1.34) | 0.87 | 0.95 (0.84, 1.08) | 0.43 | 0.64 |
| AMC ≤ 85th percentile5 | 1.00 (0.84, 1.19) | 0.99 | 0.96 (0.86, 1.08) | 0.52 | 0.73 |
| Kilocalories (% of EER) | -2.8 (-5.1, -0.5) | 0.02 | -2.1 (-3.5, -0.7) | 0.004 | 0.61 |
| Carbohydrate (% of energy) | -0.4 (-0.9, 0.1) | 0.13 | 0.3 (0.0, 0.7) | 0.05 | 0.02 |
| Sugar (% of energy) | 0.0 (-0.5, 0.4) | 0.85 | 0.4 (-0.1, 0.9) | 0.10 | 0.18 |
| Fat (% of energy) | 0.2 (-0.3, 0.7) | 0.47 | -0.3 (-0.6, 0.1) | 0.10 | 0.12 |
| Monounsaturated (% of energy) | 0.1 (-0.1, 0.3) | 0.46 | -0.1 (-0.3, 0.0) | 0.04 | 0.07 |
| Polyunsaturated (% of energy) | 0.0 (-0.1, 0.1) | 0.92 | -0.1 (-0.2, 0.0) | 0.05 | 0.22 |
| Saturated (% of energy) | 0.0 (-0.2, 0.2) | 0.75 | -0.2 (-0.4, 0.0) | 0.03 | 0.21 |
| Protein (% of energy) | 0.1 (-0.1, 0.4) | 0.16 | -0.1 (-0.2, 0.1) | 0.29 | 0.08 |
| Protein (% of RDA) | -13.2 (-21.2, -5.2) | 0.001 | -20.8 (-25.3, -16.2) | -0.001 | 0.11 |
| Dietary fiber (% of AI) | -0.2 (-1.2, 0.7) | 0.64 | 0.6 (-0.4, 1.7) | 0.24 | 0.23 |
| Cholesterol (% of USDA guideline) | 1.7 (-2.2, 5.7) | 0.39 | 1.2 (-1.1, 3.5) | 0.29 | 0.83 |
| Known oral supplement use5 | 1.08 (0.88, 1.33) | 0.44 | 0.80 (0.65, 0.97) | 0.02 | 0.03 |
For some variables, the number of male/female subjects (observations) was different from that indicated in the table header. CD4 (%): 45/60 (275/353); sugar: 46/62 (216/273); monounsaturated and saturated fat: 47/66 (284/341); polyunsaturated fat: 45/65 (275/331); dietary fiber: 48/66 (287/350); cholesterol: 48/66 (290/353). OR, odds ratio; HAART, highly active antiretroviral therapy; AMC, arm-muscle circumference; EER, estimated energy requirement; RDA, Recommended Dietary Allowance; AI, adequate intake; USDA, US Department of Agriculture.
Sex-specific per-annum changes or ORs and their 95% CIs were estimated with generalized linear models including center, sex, years since the beginning of the study (14 February 1995), and the interaction between years and sex. Estimates were obtained by using generalized estimating equations with an exchangeable working correlation structure to account for potential correlations between repeated measurements in the same individuals. The logit link was used for proportions. A working independence correlation structure was used to model the proportion of HAART-exposed children because of convergence problems with an exchangeable working correlation structure.
P value for the 2-sided Wald test of no per-annum trend (zero sex-specific years effect in the generalized linear model).
P value for the 2-sided Wald test of no sex difference in per-annum trends (no interaction between years and sex in the generalized linear model).
Estimated per-annum OR from the generalized linear model.
The proportion of HAART-exposed children increased during the study and was accompanied by a concomitant increase in CD4%. Mean anthropometric z scores all had estimated per-annum changes near zero, indicating negligible changes in these measurements in the average child. The odds of being at or above the 85th percentile for BMI increased 15% per year in males (P = 0.08).
Caloric intake decreased between 2% and 3% of the EER per year in both males (P = 0.02) and females (P = 0.004), but consumption exceeded requirements on average in both sexes at the end of the study. Protein intake was nearly 400% of the RDA for ideal body weight at the start of the study among both males and females and decreased by ≈13% of the RDA per year for males (P = 0.001) and by 21% of the RDA per year for females (P < 0.001). However, both groups continued to consume in excess of the requirements on average at the end of the study period (Figure 1).
FIGURE 1.
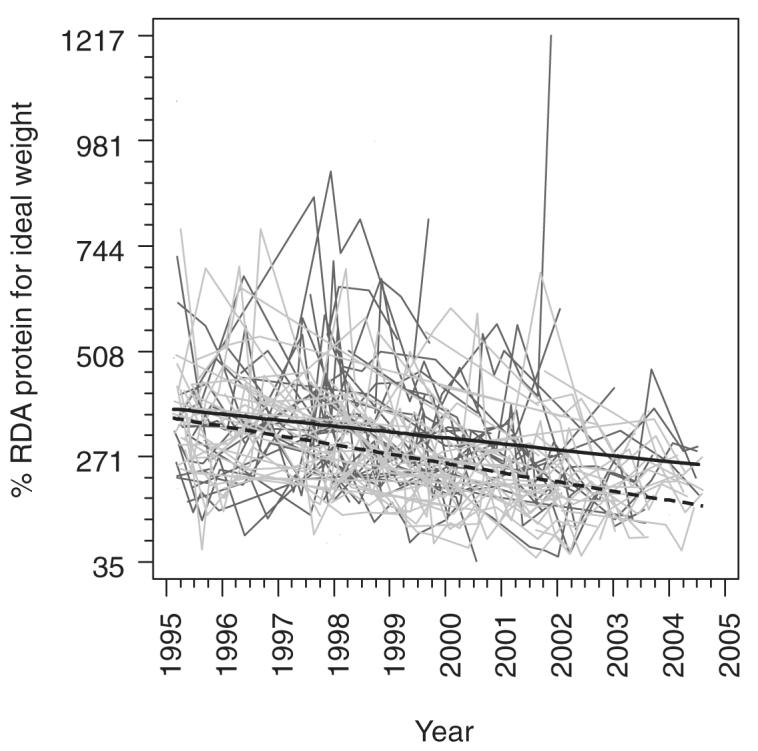
Individual trajectories for the percentage Recommended Dietary Allowance (RDA) of protein consumed in male (dark gray) and female (light gray) subjects and fitted means for males (solid black line) and females (dashed black line) with 50% weight on each center from a generalized linear model including center, sex, years since the beginning of the study (14 February 1995), and the interaction between years and sex. Estimates were obtained by using generalized estimating equations with an exchangeable working correlation structure to account for potential correlations between repeated measurements in the same individuals.
The mean percentage of energy from carbohydrate (P = 0.05) and sugar (P = 0.10) increased over the study in females (Figure 2). Females had a concomitant decrease in the mean percentages of calories from monounsaturated (P = 0.04), polyunsaturated (P = 0.05), saturated (P = 0.03), and total (P = 0.10) fat over the study period.
FIGURE 2.
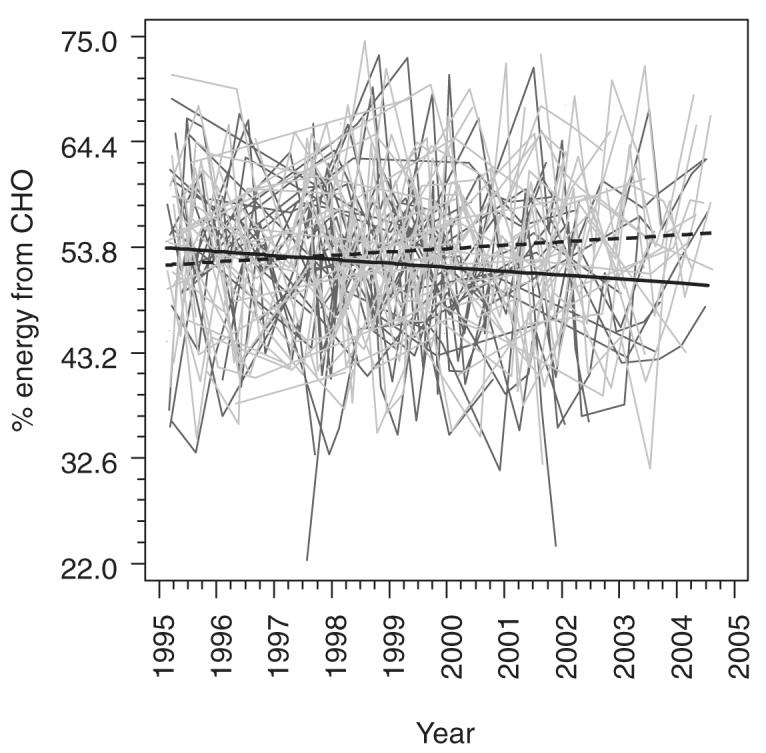
Individual trajectories for the percentage of energy from carbohydrate in male (dark gray) and female (light gray) subjects as well as fitted means for males (solid black line) and females (dashed black line) with 50% weight on each center from a generalized linear model including center, sex, years since the beginning of the study (14 February 1995), and the interaction between years and sex. Estimates were obtained by using generalized estimating equations with an exchangeable working correlation structure to account for potential correlations between repeated measurements in the same individuals.
On average, cholesterol consumption in the females remained below the USDA guideline, but fiber consumption remained inadequate. The odds of known oral supplementation decreased 20% per annum in females (P = 0.02), but there was little change over time in males. At the end of the study, a larger proportion of males than females was known to be receiving oral supplementation (P = 0.01).
DISCUSSION
In this study, we described changes in the dietary intake of macronutrients in HIV-infected children during a time in which HIV care was rapidly evolving. Total caloric and protein intakes that initially exceeded recommendations decreased significantly over time but continued to exceed recommended intakes by the end of the study. Dietary composition among females may be shifting away from fats and toward carbohydrates, although the mean percentages of energy from various macronutrients mostly remained within the accepted ranges by the end of the study. Regression estimates indicated poor growth characteristics at the beginning of the study, but we found few clinically important changes in anthropometric measures in males and in females over the study period.
Before HAART, high levels of circulating HIV virus resulted in poor weight gain as a result of a state of chronic negative energy balance and frequent opportunistic infections (2–4). In contrast, both NHANES III and the Bogalusa Heart Study data indicate an increase in the prevalence of overweight in otherwise healthy US children over time (30, 31). Our hypothesis was that as HIV-infected children improved their immunologic and disease status, their diet and growth would come to reflect the patterns seen in healthy children.
Our baseline data are consistent with research showing lower than standard weights and heights in HIV-infected children before HAART (3, 4). Per-annum changes in age-standardized height, weight, BMI, and percentage fat mass over the study period were near zero, despite a concomitant improvement in CD4%. Although HAART has been associated with increased growth in individual patients, our study suggests that it did not substantially change the growth characteristics among populations of HIV-infected children. Our cohort, on average, consumed greater than the EER for the activities of daily living without additional exercise, but losses through gastrointestinal malabsorption, higher energy utilization because of chronic immune activation, or vomiting and diarrhea may have partially accounted for the imbalance between energy intake and growth. Furthermore, because HAART was generally prescribed at the nadir of the clinical course during the period over which our study was conducted, patient-specific estimates of changes in growth characteristics after HAART from other observational studies may simply reflect rebounds to an earlier level or regression to the mean after HAART initiation in the sickest patients.
Our findings regarding dietary intake are similar to those in healthy children. Dietary intake data from NHANES III show that overall energy intake has changed little since NHANES I and II in most children; however, energy intake in adolescent females has increased markedly over time (32). These studies also show that the contribution of both total fat and saturated fat to energy intake has declined over time in all age and sex groups. However, consumption of sweetened beverages, particularly soft drinks, has increased in all groups and may account for the observed weight gains. Data from the Bogalusa Heart Study and data from the Continuing Survey of Food Intakes by Individuals described similar findings (30, 33).
Contrary to the trend in healthy US children, our HIV-infected cohort had a decrease in protein intake over time, but they started and ended the study period consuming substantially more than the RDA on average. We speculate that as HAART improved the disease status of these children, nutritional counseling changed. The children no longer required a high-calorie, high-protein diet to prevent wasting, so intakes of both energy and protein decreased substantially relative to the reference standards. Females in our study, in particular, reduced their use of high-calorie liquid complete nutrition supplements over time. Although protein intake decreased, it still remained substantially higher than the RDA, as it does in healthy children. Notably, a higher proportion of males remained on oral liquid supplementation at the end of the study, and protein intake was also higher in males than in females relative to the RDA for protein.
In our HIV-infected female children, the mean percentages of energy derived from carbohydrates and sugars increased during the study, whereas the mean percentages of energy from various fats decreased. Although total caloric intake decreased in our patients, it was still higher than that recommended in both males and females. It will be critical to continue to follow carbohydrate, simple sugar, and total caloric intakes over time in HIV-infected patients to determine whether these children ultimately follow the dietary pattern of otherwise healthy children and to determine whether these dietary patterns will translate into increases in weight and BMI with continued observation.
An important question that arises in our evaluation of nutritional intake and growth measures is whether there was a causal relation between changes in macronutrient intake and growth status in HIV-infected children. However, because of the observational design of this study, we were not able to directly assess this relation. The changes in growth measures observed in our sample during this period reflect the effects of many independent trends, the most important of which were probably the introduction of HAART therapy as well as changes in dietary composition in the general population. The contemporaneous nature of these independent trends possibly induced spurious associations and colinearity between the time-dependent covariates reflecting them. Moreover, because time-dependent covariates reflecting these trends are also potentially associated with outcomes, they confound one another as well, so we cannot reliably separate the degree to which changes in growth status were uniquely attributable to a single trend, such as changes in macronutrient intake, using standard regression techniques. To do this, we would need to have followed the sample over a period during which there were no time-dependent changes in variables that affect growth measures other than macronutrient intakes.
Limitations of the study
Our study had some potential limitations. We could not specifically assess lifestyle factors such as exercise, socioeconomic status, and eating habits (eg, excessive intake of sugary beverages, skipping breakfast, snacking instead of eating full meals), although these factors affect obesity in children and adolescents (30, 32–34). Additionally, because we combined data from 2 different centers that were collected at different times, center confounding was possible. However, our models allowed the centers to have an additive effect on the estimated mean or proportion to account for systematically higher or lower outcomes at one center over the entire study as a result of differences in the populations served. Also, the Simes test provided no evidence of center interaction for any of the outcomes, suggesting that our statistical model for combining the data from the 2 centers was reasonable.
Finally, some outcome variables had a large percentage of missing data as a result of the observational nature of the study. In general, these missing data resulted from time-dependent changes in best clinical practice, time- and center-dependent variation in the data collection protocol, and other factors unrelated to previous outcomes or the values of the unobserved outcomes. Thus, we believe that any missing data are likely missing completely at random, conditional on time and center, meaning that a generalized estimating equation analysis of all complete observations using a model including time and center should provide valid inferences (35).
Conclusions
Current evidence indicates that HAART, and possibly HIV infection itself, independently increase the risk of cardiovascular disease through metabolic complications in HIV-infected individuals (36). Although there is not yet a cure for HIV or an alternative to critical antiretroviral therapy, diet is a potentially modifiable factor that can alter metabolic risk for children with HIV infection. Given the early dietary trends observed in this study, continued monitoring of caloric and carbohydrate intakes is essential to avoid future increases in adiposity that may contribute to the cardiovascular disease risk of HIV-infected children.
Acknowledgments
We thank Carolina Vasquez and Daniela Neri for helping with the data collection and entry and E John Orav for providing statistical advice and consultation.
Footnotes
From the Divisions of Infectious Diseases (TSS) and Gastroenterology and Nutrition (CD, LB, and LF), Children’s Hospital, Harvard Medical School, Boston, MA; the Division of Pediatric Clinical Research, Department of Pediatrics, Miller School of Medicine, University of Miami, Miami, FL (DDK and TLM); the Divisions of Infectious Disease (GAW) and Gastroenterology (JN), Golisano Children’s Hospital at Strong, University of Rochester School of Medicine, Rochester, NY; and the Department of Medicine, Family Medicine and Community Health, Tufts—New England Medical Center, Tufts University School of Medicine, Boston, MA (SLG).
Supported by grants P01DK45734, MO1-RR00054, M01 RR00044, and M01 RR02172 (to TLM) from the National Institutes of Health, Bethesda, MD, and The Green Family Foundation Initiative in Pediatric Infectious Disease and International Health, University of Miami Miller School of Medicine (to TSS, TLM, and DDK).
Reprints not available. Address correspondence to TL Miller, University of Miami Miller School of Medicine, Batchelor Children’s Research Institute, Room 542, 1580 NW 10th Avenue, Miami, FL 33136. E-mail: tmiller2@med.miami.edu.
REFERENCES
- 1.Chandra RK, Kumari S. Nutrition and immunity: an overview. J Nutr. 1994;124(suppl 8):1433S–5S. doi: 10.1093/jn/124.suppl_8.1433S. [DOI] [PubMed] [Google Scholar]
- 2.Miller TL, Easley KA, Zhang W, et al. Maternal and infant factors associated with failure to thrive in children with vertically transmitted human immunodeficiency virus-1 infection: the prospective P2C2 human immunodeficiency virus multicenter study. Pediatrics. 2001;108(6):1287–96. doi: 10.1542/peds.108.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller TL, Evans SJ, Orav EJ, McIntosh K, Winter HS. Growth and body composition in children infected with the human immunodeficiency virus-1. Am J Clin Nutr. 1993;57:588–92. doi: 10.1093/ajcn/57.4.588. [DOI] [PubMed] [Google Scholar]
- 4.Arpadi SM, Horlick MN, Wang J, Cuff P, Bamji M, Kotler DP. Body composition in prepubertal children with human immunodeficiency virus type 1 infection. Arch Pediatr Adolesc Med. 1998;152:688–93. doi: 10.1001/archpedi.152.7.688. [DOI] [PubMed] [Google Scholar]
- 5.Miller TL, Orav EJ, Martin SR, Cooper ER, McIntosh K, Winter HS. Malnutrition and carbohydrate malabsorption in children with vertically transmitted human immunodeficiency virus 1 infection. Gastroenterology. 1991;100:1296–302. [PubMed] [Google Scholar]
- 6.Yolken RH, Hart W, Oung I, Shiff C, Greenson J, Perman JA. Gastrointestinal dysfunction and disaccharide intolerance in children infected with human immunodeficiency virus. J Pediatr. 1991;118:359–63. doi: 10.1016/s0022-3476(05)82147-x. [DOI] [PubMed] [Google Scholar]
- 7.Carroccio A, Fontana M, Spagnuolo MI, et al. Pancreatic dysfunction and its association with fat malabsorption in HIV infected children. Gut. 1998;43:558–63. doi: 10.1136/gut.43.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfaro MP, Siegel RM, Baker RC, Heubi JE. Resting energy expenditure and body composition in pediatric HIV infection. Pediatric AIDS HIV Infect. 1995;6:276–80. [PubMed] [Google Scholar]
- 9.Miller TL, Awnetwant EL, Evans S, Morris VM, Vazquez IM, McIntosh K. Gastrostomy tube supplementation for HIV-infected children. Pediatrics. 1995;96:696–702. [PubMed] [Google Scholar]
- 10.Mangili A, Murman DH, Zampini M, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the Nutrition for Healthy Living cohort. Clin Infect Dis. 2006;42:836–42. doi: 10.1086/500398. [DOI] [PubMed] [Google Scholar]
- 11.Ferrando SJ, Rabkin JG, Lin S, McElhiney M. Increase in body cell mass and decrease in wasting are associated with increasing potency of antiretroviral therapy for HIV infection. AIDS Patient Care. 2005;19(4):216–23. doi: 10.1089/apc.2005.19.216. [DOI] [PubMed] [Google Scholar]
- 12.Verweel G, van Rossum AMC, Hartwig NG, Wolfs TFW, Scherpbier HJ, de Groot R. Treatment with highly active antiretroviral therapy in human immunodeficiency type-1 infected children is associated with a sustained effect on growth. Pediatrics. 2002;109(2):e25. doi: 10.1542/peds.109.2.e25. [DOI] [PubMed] [Google Scholar]
- 13.Miller T. Nutritional aspects of HIV-infected children receiving highly active antiretroviral therapy. AIDS. 2003;17(suppl):S130–40. doi: 10.1097/00002030-200304001-00016. [DOI] [PubMed] [Google Scholar]
- 14.Brambilla P, Bricalli D, Sala N, et al. Highly active antiretroviral-treated HIV-infected children show fat distribution changes even in absence of lipodystrophy. AIDS. 2001;15:2415–22. doi: 10.1097/00002030-200112070-00009. [DOI] [PubMed] [Google Scholar]
- 15.Vigano A, Mora S, Testolin C, et al. Increased lipodystrophy is associated with increased exposure to highly active antiretroviral therapy in HIV-infected children. JAIDS. 2003;32:482–9. doi: 10.1097/00126334-200304150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegel KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Flegel KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention 1994 Revised classification system for human immunodeficiency syndrome in children less than 13 years of age. MMWR. 1994;43(RR12):1–10. [Google Scholar]
- 19.Bartlett J, Cheever L, Johnson M, Paauw D, editors. A guide to primary care of people with HIV/AIDS. US Department of Health and Human Services, Health Resources and Service Administration, HIV/AIDS Bureau; Washington, DC: 2004. [Google Scholar]
- 20.Thompson FE, Byers T. Dietary assessment resource manual. J Nutr. 1994;124(11S):2245S–50S. doi: 10.1093/jn/124.suppl_11.2245s. [DOI] [PubMed] [Google Scholar]
- 21.Panel on Macronutrients. Panel on the Definition of Dietary Fiber. Subcommittee on Upper Reference Levels of Nutrients. Subcommittee on Interpretation and Uses of Dietary Reference Intakes. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board . Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients) National Academies Press; Washington, DC: 2005. [Google Scholar]
- 22.US Department of Health and Human Services. US Department of Agriculture . Dietary guidelines for Americans, 2005. 6th US Government Printing Office; Washington, DC: 2005. [Google Scholar]
- 23.Atwater WO, Woods CD. Experiment Stations Bulletin 28. US Government Printing Office; Washington, DC: 1896. The chemical composition of American food materials. US Office of Experiment Stations. [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. [accessed 12 July 2006];Advance data from vital and health statistics. 2000 314:1–27. http://www.cdc.gov/nchs/about/major/nhanes/ growthcharts/datafiles.htm [PubMed]
- 25.Lohman GT, Roche AR, Martorell R. Anthropometric standardization reference manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- 26.Frisancho AR. Triceps skin fold and upper arm muscle size norms for assessment of nutrition status. Am J Clin Nutr. 1974;27(10):1052–8. doi: 10.1093/ajcn/27.8.1052. [DOI] [PubMed] [Google Scholar]
- 27.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 28.Berger RL, Hsu JC. Bioequivalence trials, intersection-union tests and equivalence confidence sets. Stat Sci. 1996;11(4):283–319. [Google Scholar]
- 29.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73(3):751–4. [Google Scholar]
- 30.Nicklas TA, Elkasabany A, Srinivasan SR, Berenson G. Trends in nutrient intake of 10-year-old children over two decades (1973 – 1994) The Bogalusa Heart Study. Am J Epidemiol. 2001;153:969–77. doi: 10.1093/aje/153.10.969. [DOI] [PubMed] [Google Scholar]
- 31.Troiano RP, Flegel KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics. 1998;101:497–504. [PubMed] [Google Scholar]
- 32.Troiano RP, Briefel RR, Carroll MD, Bialostosky K. Energy and fat intakes of children and adolescents in the United States: data from the National Health and Nutrition Examination Surveys. Am J Clin Nutr. 2000;72(suppl):1343–53S. doi: 10.1093/ajcn/72.5.1343s. [DOI] [PubMed] [Google Scholar]
- 33.Enns CW, Mickle SJ, Goldman JD. Trends in food and nutrient intakes by children in the United States. Family Econ Nutr Rev. 2002;14(2):56–68. [Google Scholar]
- 34.Miech RA, Kumanyika SK, Stettler N, Link BG, Phelan JC, Chang V. Trends in the association of poverty with overweight among US adolescents, 1971-2004. JAMA. 2006;295:2385–93. doi: 10.1001/jama.295.20.2385. [DOI] [PubMed] [Google Scholar]
- 35.Hardin JW, Hilbe JM. Generalized estimating equations. Vol. 127. Chapman & Hall/CRC; New York, NY: 2003. [Google Scholar]
- 36.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with HIV disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]


