SUMMARY
Studies in mice and humans have demonstrated a role for the immune system in preventing the growth of tumors. Deciphering the mechanisms involved in the immune response to tumors is essential to our understanding of immune recognition and cancer progression. Here we report an innate immune response to tumors in Drosophila melanogaster. We found that circulating blood cells, termed hemocytes, adhere to tumors upon detection of basement membrane disruption, and subsequently counter their growth. Basement membrane components are remarkably conserved throughout the animal kingdom, providing a unique structure for the immune system to sense tissue integrity. Further, we show that tissue damage activates JNK signaling in both tumors and aseptic wounds, causing expression of JAK/STAT-activating cytokines. Cytokine secretion from the injured tissue is amplified into a systemic response through the induction of additional cytokine expression in the hemocytes and the fat body, resulting in hemocyte proliferation. Our findings reveal common mechanisms in the response to tumors and wounds in flies. A similar innate reaction may underlie the response to tumors and tissue damage in vertebrates and humans.
INTRODUCTION
Evidence in mice and humans supports the once controversial notion that immune responses, in addition to defending the organism against external threats, have a role in preventing the growth of tumors (Dunn et al., 2002). This function of the immune system as an extrinsic tumor suppressor is known as tumor immune surveillance. The interactions between tumors and the immune system of cancer patients, involving both adaptive and innate mechanisms, are complex and not well understood. It is known that the adaptive immune system can react to altered tumor antigens (Boon et al., 1994); however, studies have shown that cells of the innate immune system are also required for effective tumor surveillance (Girardi et al., 2001; Smyth et al., 2000).
The similarities between wound healing and the formation of the tumor stroma led to the prediction that many aspects of tumor biology would be shared between the two processes, and that insights into tumor-host interactions could be gained by thinking of tumors as chronic wounds (Balkwill and Mantovani, 2001; Chang et al., 2005; Dvorak, 1986). It is believed that innate immune sensing of the distress that tumors cause in a tissue is crucial for the initiation of the response (Dunn et al., 2002). The specific mechanisms by which tumors or tissue damage stimulate innate immune cells are largely unknown. Furthermore, the immune recognition of tumors and tissue damage raises the important question, essential for both immunologists and cancer biologists, of how self tissue is targeted by the immune system.
Innate immunity is the most ancient form of immune defense in animals; the origin of many of its mechanisms dates back to a common ancestor of insects and vertebrates in the metazoan lineage (Hoffmann et al., 1999). Insect immunity, despite some evidence for primed responses (Pham et al., 2007), lacks true memory and therefore relies completely on innate mechanisms for protection against external threats. Drosophila melanogaster has proven to be a powerful model for the study of innate immunity, because mammalian immune signaling pathways, such as the Toll, NF-κB and Janus tyrosine kinase/signal transducer and activator of transcription (JAK/STAT) pathways, are also crucial for the regulation of immune responses in flies (Lemaitre and Hoffmann, 2007).
Insects have a comparatively simple and robust innate immune system that protects the animal against different kinds of pathogens and parasites by orchestrating a number of defensive responses (Brennan and Anderson, 2004; Lemaitre and Hoffmann, 2007). Circulating blood cells, known as hemocytes, are the cellular arm of the fly immune system (Evans et al., 2003; Meister and Lagueux, 2003). Insect hemocytes share many characteristics of their development and function with mammalian blood cells, and probably share a common origin in evolution (Evans et al., 2003; Hartenstein, 2006). As effectors of the fly immune response, hemocytes phagocytose and kill invading microbes in a similar way to vertebrate macrophages, by encapsulating parasites and other foreign bodies, mediating coagulation in open wounds and controlling melanization reactions that release toxic oxygen species. In addition, secretion of JAK/STAT-activating cytokines by hemocytes has been shown to serve a regulatory role in the humoral response to septic injury (Agaisse et al., 2003).
In this study, we have used a Drosophila tumor model to explore the interactions between tumors and the immune system. We report that malignant tumors in Drosophila that are derived from imaginal discs elicit an innate response from hemocytes. Furthermore, we show that common mechanisms underlie the immune response to tumors and tissue damage in flies.
RESULTS
Hemocytes adhere to RasV12/scrib−/− and scrib tumors
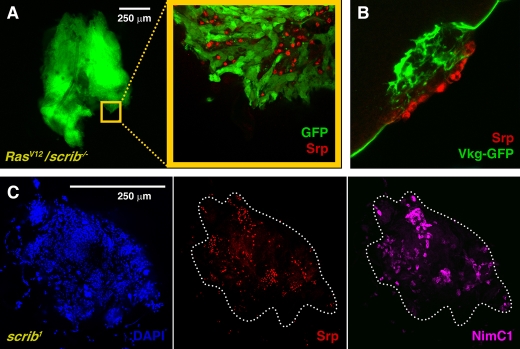
Clones of cells with a mutant polarity determinant scribble (scrib), that simultaneously express an oncogenic form of the Ras protein (RasV12/scrib−/− clones), generate tumors when induced in the eye-antennal imaginal discs of Drosophila larvae (Pagliarini and Xu, 2003). This tumor model, previously established in our laboratory, reproduces crucial steps in the progression of human cancers. The tumors dramatically overproliferate, outcompete wild-type cells, and give rise to masses of cells, which degrade the basement membrane (BM), invade contiguous tissues, metastasize to distant organs to form secondary tumors, and finally kill the host (Igaki et al., 2006; Pagliarini and Xu, 2003). Our work on the immune response to tumors in Drosophila started following the observation that RasV12/scrib−/− tumors (GFP-positive) display many cells on their surface that do not belong to the tumor (Fig. 1A). These GFP-negative cells are hemocytes, as revealed by their expression of the GATA transcription factor Serpent (Srp), a marker for hemocytes (Lebestky et al., 2000) (Fig. 1A).
Fig. 1. Adhesion of hemocytes to tumors.
(A) GFP-expressing RasV12/scrib−/−tumors (green) in the eye-antennal discs display GFP-negative cells on their surface (yellow square magnified) that express the transcription factor Serpent (Srp), a hemocyte marker (anti-Srp staining, red nuclei). (B) A confocal section of a RasV12/scrib−/− tumor, perpendicular to the surface, shows hemocytes (anti-Srp staining, red) adhered to an area of the tumor where the BM, visualized with a collagen IV GFP protein trap (Vkg-GFP, green), is disrupted. (C) scrib tumors (all cell nuclei stained with DAPI, blue), resulting from fusion of the wing, third leg and haltere imaginal discs, showing hemocytes on their surface (anti-Srp staining, red). These hemocytes are plasmatocytes, as revealed by their expression of the plasmatocyte-specific protein NimC1 (P1 antibody staining, purple).
One feature of RasV12/scrib−/− tumors that makes their surface different from that of normal imaginal discs, is the degradation of the BM (Pagliarini and Xu, 2003; Srivastava et al., 2007). Hemocytes were preferentially found in areas of the RasV12/scrib−/− tumors where the BM was disrupted; these areas were visualized with a GFP protein-trap inserted into the Drosophila collagen IV gene viking (Morin et al., 2001) (Fig. 1B). In contrast, hemocytes did not attach to RasV12 benign overgrowths (data not shown), which preserve epithelial character and show no sign of BM degradation (Pagliarini and Xu, 2003).
Srp-positive cells are also found on the surface of scrib tumors (Fig. 1C). scrib tumors, although not capable of forming secondary foci, share important characteristics with RasV12/scrib−/− tumors, including loss of cell polarity, tissue overgrowth (Bilder and Perrimon, 2000), and extensive disruption of the BM (supplementary material Fig. S1). Three types of hemocytes exist in Drosophila: plasmatocytes, lamellocytes and crystal cells (Meister and Lagueux, 2003). Plasmatocytes constitute more than 95% of the total hemocytes present in a healthy larva (Zettervall et al., 2004). We found that all hemocytes that were adhered to tumors stained positive for the P1 antibody (Fig. 1C), which detects the membrane protein Nimrod C1, a plasmatocyte-specific differentiation marker (Kurucz et al., 2007). Expression of markers for the two other hemocyte subtypes [L1 antibody for lamellocytes (Vilmos et al., 2004) and Lz-GAL4 for crystal cells (Lebestky et al., 2000)] was not detected (data not shown).
Tumors induce an increase in the number of circulating hemocytes
Hemocytes are the cellular branch of the fly immune system, and are known to mediate several responses to pathogens. The attachment of hemocytes to the surface of RasV12/scrib−/− and scrib tumors led us to speculate that an immune reaction might be taking place in these larvae. Thus, we decided to look for additional signs of an immune response to the tumors. Most hemocytes in wild-type larvae are found circulating in the hemolymph, a blood-like liquid filling the body cavity, which is set in circulation by the pumping of the heart. We found that the number of circulating hemocytes in RasV12/scrib−/− and scrib larvae were dramatically increased when compared with wild-type larvae (Fig. 2A). Hemocyte counts were also increased in RasV12/scrib−/− and scrib larvae when compared with the metamorphosis-defective ecdysoneless1 (ecd1) mutant (Garen et al., 1977) (Fig. 2A), which was used as a control for the extended larval development that animals hosting tumors undergo. The proportions of the three hemocyte subtypes remained similar to those of wild-type animals (see Methods section). In addition to scrib tumors, we also analyzed the effect in hemocytes of tumors caused by mutations in two other polarity determinants, lethal giant larvae (lgl) and discs large (dlg). Similar results were obtained in animals with dlg, lgl, RasV12/dlg−/−, and RasV12/lgl−/− tumors, with elevated numbers of circulating hemocytes but no observable changes to subtype proportions (data not shown).
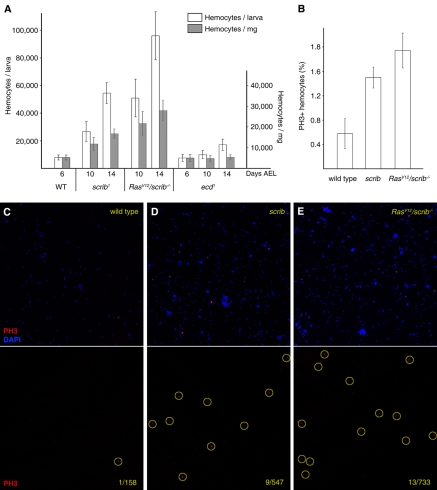
Fig. 2. Increase in the number of circulating hemocytes in response to tumors.
(A) Hemocyte density and absolute counts in larvae with tumors. In addition to wild-type animals, ecd1 mutant larvae were used as a control for extended larval development. Densities were estimated as hemocytes per mg of fresh larval weight. Only plasmatocyte counts are represented (see Methods section for details). (B–E) Circulating hemocytes of wild-type (C), scrib (D) and RasV12/scrib−/− (E) larvae stained with an anti-PH3 antibody, which detects nuclei undergoing mitotic prophase (yellow circles). The proportion of PH3-positive cells is quantified for each type of larvae in (B). Error bars in these and all other graphs throughout the report represent 95% confidence intervals (1.96 × s.e.m.).
Next, we addressed the question of how circulating hemocyte counts are elevated in RasV12/scrib−/− and scrib larvae. In addition to the circulating hemocytes, a population of undifferentiated hemocytes resides in the lymph glands that, normally, is only released to the hemolymph during metamorphosis (Holz et al., 2003). Early lymph gland emptying in larvae is part of a well-characterized immune response to eggs injected by parasitic wasps (Lemaitre and Hoffmann, 2007). In RasV12/scrib−/− or scrib larvae the BM surrounding the lymph gland lobes remains intact, and we did not observe any sign of lymph gland emptying (supplementary material Fig. S2). This rules out an early release of hemocytes as a cause for their greater number in circulation, and suggests that the increase is the result of increased proliferation induced by the tumors. Indeed, anti-phospho-histone 3 (PH3) staining revealed that a significantly higher proportion of circulating hemocytes were undergoing mitosis in larvae hosting scrib and RasV12/scrib−/− tumors (Fig. 2B–E). These data indicate that the presence of tumors stimulates proliferation of hemocytes and that, in addition to the local presence of hemocytes on the surface of the tumor, a systemic reaction is taking place in these larvae.
Hemocytes restrict tumor growth
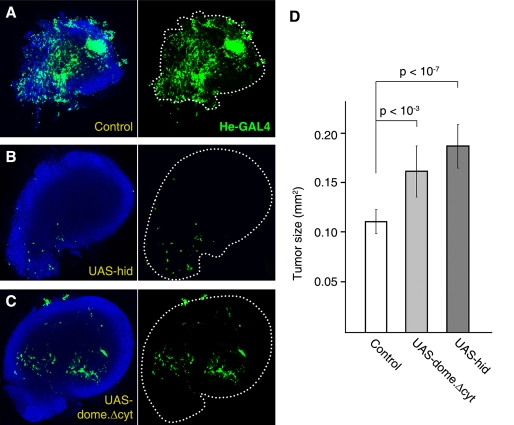
In humans, the immune system is thought to play a role in preventing the development of cancer (Dunn et al., 2002); at the same time, inflammatory responses triggered by tumors can promote their own development (de Visser et al., 2006). We therefore decided to test whether the immune response we observed had an effect on the tumors. To do this, we compared the sizes of tumors from control scrib larvae with scrib larvae in which hemocyte numbers were reduced by overexpression of the proapoptotic protein Hid (Zhou et al., 1997) (supplementary material Fig. S3). Tumors within the Hid-expressing larvae were significantly larger than in scrib controls (Fig. 3A,B,D). Therefore, the immune response of blood cells does counter the growth of tumors.
Fig. 3. Hemocytes restrict tumor growth.
(A–C) Tumors from a control scrib larva (A), a scrib larva in which the number of circulating hemocytes is reduced by expression of the proapoptotic protein Hid (He-Gal4>UAS-hid) (B) and a scrib larva with reduced JAK/STAT signaling in hemocytes (He-Gal4>UAS-domeΔcyt) (C) Hemocytes are marked by He-Gal4-driven expression of GFP (green). White dots outline the perimeter of the tumor. Confocal sections of tumors have been averaged to show both tumor size and hemocytes on the surface. (D) Quantification of tumor size differences in control scrib (n=29), scrib / He>hid (n=21) and scrib/ He>domeΔcyt (n=34) larvae. Tumor size was estimated as the area that the tumor occupied on the slide. P-values are from two-tailed t-tests.
Hemocytes react to tissue damage
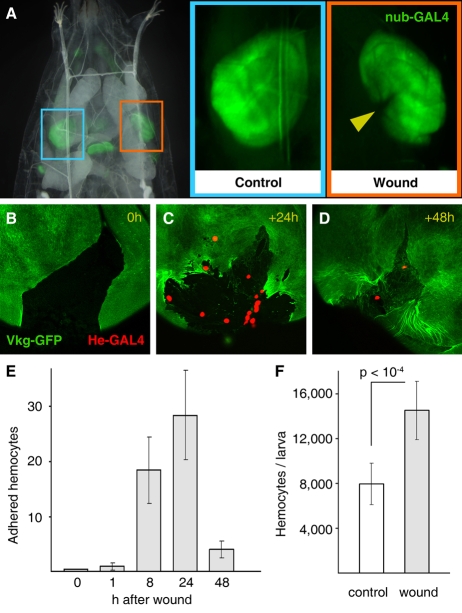
We tried to dissect the mechanisms by which the innate immune system in flies recognizes the presence of tumors. As noted above, hemocytes were preferentially found in areas of the RasV12/scrib−/− and scrib tumors where the BM of the disc was disrupted (Fig. 1B,D). This raises the possibility that hemocytes actually recognize and react to tissue damage in the tumor. We therefore asked whether tissue damage in imaginal discs was able to trigger an immune response from hemocytes, similar to the one observed in larvae hosting tumors. To answer this question, we devised a technique for aseptically wounding wing discs in situ (Fig. 4A) and examined the effect of mechanical damage. Briefly, we performed wounding operations in living larvae by closing a pair of forceps over the wing disc, avoiding any damage to the overlying larval epidermis or any other larval organs. These wounding operations did not yield any sign of melanization, contrasting with other modes of wounding that we assayed, which resulted in damage to the larval epidermis or the gut, and subsequent exposure of the hemolymph to air or food, respectively (not shown).
Fig. 4. Response of hemocytes to tissue damage.
(A) In situ wounding of wing imaginal discs. Aseptic in situ wounding is performed in living larvae without further damaging the animal. nub-Gal4-driven expression of GFP marks the wing blade region in control (not wounded, blue rectangle) and wounded (orange rectangle) discs. The yellow arrowhead points to the incision caused by the operation. (B–D) Adhesion of hemocytes to wounds. Wounding of the disc results in rupture of the BM (Vkg-GFP). Discs dissected 24 and 48 hours after wounding show hemocytes adhered to the damaged tissue (He-Gal4-driven expression of red fluorescent protein). (E) Quantification of hemocytes adhered to wounds. At least twenty discs were examined for each time point. (F) Number of circulating hemocytes in control larvae and larvae wounded in their wing imaginal discs. Wounded larvae were bled 24 hours after the operation.
In wing discs that were dissected at different times after wounding, hemocytes were shown to adhere to the damaged tissue (Fig. 4B–D). The number of adherent hemocytes peaked at 24 hours after wounding (Fig. 4C,E). At 48 hours, BM healing was evident and concurrent with fewer adherent hemocytes (Fig. 4D,E), further suggesting a link between disruption to BMs and hemocyte attachment. Moreover, we observed that, similar to larvae hosting tumors, the number of circulating hemocytes in wounded larvae was significantly increased 24 hours after the operation (Fig. 4F). These experiments show that tissue damage causes a reaction similar to the response observed in tumors. We conclude from the wounding experiments that hemocytes are capable of reacting to tissue damage.
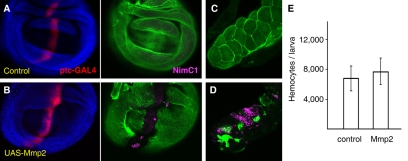
We decided to specifically test the possibility that BM disruption was sufficient to trigger the response from hemocytes. In order to do this, we examined imaginal discs where the BM had been degraded by targeted expression of matrix metalloproteases (MMPs); these enzymes are capable of degrading most extracellular matrix components (Mott and Werb, 2004). Both our group and others have shown that MMPs are involved in BM degradation in RasV12/scrib−/− tumors (Srivastava et al., 2007; Uhlirova and Bohmann, 2006). Expression of Mmp2, one of two MMP proteins present in Drosophila (Llano et al., 2002), which is driven by patched-Gal4 (ptc-Gal4) in a stripe of cells across the wing disc, degrades the underlying BM (Fig. 5A,B). We found that hemocytes adhered to the disc in the region where Mmp2 expression had degraded the BM (Fig. 5A,B). In these same animals, adhesion of hemocytes was also observed in the salivary glands (Fig. 5C,D), where ptc-Gal4 is also expressed. The BM in the salivary glands had been similarly disrupted, thus showing that hemocyte adhesion to regions of BM disruption is not a phenomenon restricted to imaginal discs. We infer from the above experiments that BM disruption alone is sufficient to cause recruitment of hemocytes.
Fig. 5. BM disruption causes hemocyte adhesion, but not an increase in hemocyte number.
(A,B) Adhesion of hemocytes (NimC1, purple) to a wing disc expressing MMP (ptc-Gal4>UAS-Mmp2). The BM (visualized with Vkg-GFP, green) is degraded as a result of MMP expression. Cells expressing ptc-Gal4 are marked by expression of UAS-myrRFP (red). (C,D) Adhesion of hemocytes to the salivary glands of ptc-Gal4>UAS-Mmp2 larvae. (E) Number of circulating hemocytes in control and ptc-Gal4>UAS-Mmp2 larvae.
To ascertain whether BM disruption was also sufficient to trigger the production of a proliferative response in hemocytes, we bled these larvae and measured their hemocyte content. No significant difference was observed when compared with control larvae (Fig. 5E), indicating that although disruption of the BM was enough to produce adhesion of hemocytes, it is not sufficient to induce their proliferation. This suggests that in addition to BM disruption, one or more signals, which are probably common to tumors and wounds, are responsible for the proliferation of hemocytes in response to tissue damage.
JAK/STAT signaling increases hemocyte numbers in response to tissue damage
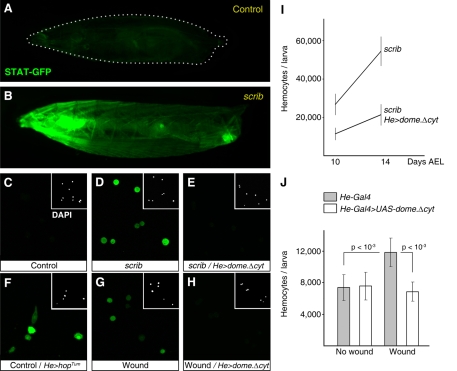
Among the signaling pathways already known to promote hemocyte proliferation (Harrison et al., 1995), we found that scrib larvae exhibit high levels of JAK/STAT activity throughout the third instar (Fig. 6A,B and supplementary material Fig. S4). This was revealed by using a STAT-GFP reporter (Ekas et al., 2006), which expresses GFP under the control of binding sites for the STAT transcription factor. The reporter was highly expressed in the tumors (supplementary material Fig. S5), but was also expressed systemically. Importantly, STAT-GFP reporter activity was detected in the circulating hemocytes of scrib larvae, but not in the hemocytes of wild-type larvae (Fig. 6C,D). The specificity of the STAT-GFP reporter to detect JAK/STAT signaling in hemocytes was confirmed by overexpression of a dominant negative mutant form of Domeless (DomeΔCYT, deletion of the cytoplasmic domain), a receptor from the JAK/STAT pathway (Brown et al., 2001), driven by He-Gal4. This was effective at inhibiting reporter expression in the hemocytes of scrib larvae (Fig. 6E). Conversely, expression of a constitutively active form of the JAK kinase Hopscotch (HopTum) (Harrison et al., 1995) induced STAT-GFP expression in the hemocytes of wild-type larvae (Fig. 6F). In addition, we examined STAT-GFP activation in the circulating hemocytes of wild-type larvae, 24 hours after performing in situ wounding of their wing discs. In situ wounding results in STAT-GFP reporter expression in circulating hemocytes (Fig. 6G) and DomeΔCYT expression, as seen in scrib larvae, was able to abolish STAT-GFP expression (Fig. 6H).
Fig. 6. JAK/STAT signaling drives hemocyte proliferation in response to tissue damage.
(A,B) Expression of STAT-GFP (green), an activity reporter for the JAK/STAT pathway, in a living third-instar larva (A) and a 14-day AEL scrib larva (B). (C–E) Expression of STAT-GFP in circulating hemocytes bled from wild-type larvae (C), scrib larvae (D) and scrib larvae expressing a dominant negative form of the JAK/STAT pathway receptor Domeless (He-Gal4>UAS-domeΔcyt) (E). (F) Expression of STAT-GFP in the circulating hemocytes of larvae that express a constitutively active form of the JAK kinase Hopscotch (He-Gal4>UAS-hopTum). (G,H) Expression of STAT-GFP in hemocytes of wild-type (G) and He-Gal4>UAS-domeΔcyt (H) larvae bled 24 hours after in situ wounding of their wing discs. (I) Circulating hemocyte counts in scrib larvae (control) and scrib larvae expressing DomeΔCYT in hemocytes. (J) Circulating hemocyte counts in wild-type unwounded larvae, unwounded larvae expressing DomeΔCYT in hemocytes, wild-type wounded larvae (24 hours after wounding) and wounded larvae expressing DomeΔCYT. P-values are from two-tailed t-tests.
Once we knew that JAK/STAT signaling was active in hemocytes in response to wounds and tumors, we wanted to investigate the effect of inhibiting the JAK/STAT signaling in these hemocytes. In scrib larvae, DomeΔCYT expression lessened hemocyte expansion caused by the tumors (Fig. 6I). Furthermore, this reduction in the number of circulating hemocytes resulted in significantly larger tumors (Fig. 3C,D). DomeΔCYT expression also prevented the increase in hemocyte numbers observed in wounded larvae (Fig. 6J); whereas, consistent with previous reports (Zettervall et al., 2004), it had no effect on hemocyte numbers in unwounded controls. Together, these data indicate that JAK/STAT signaling is activated in hemocytes in response to wounds and tumors, and that this activity is required for their increased proliferation following these insults.
An amplification loop in JAK/STAT signaling mediates the systemic response to tissue damage
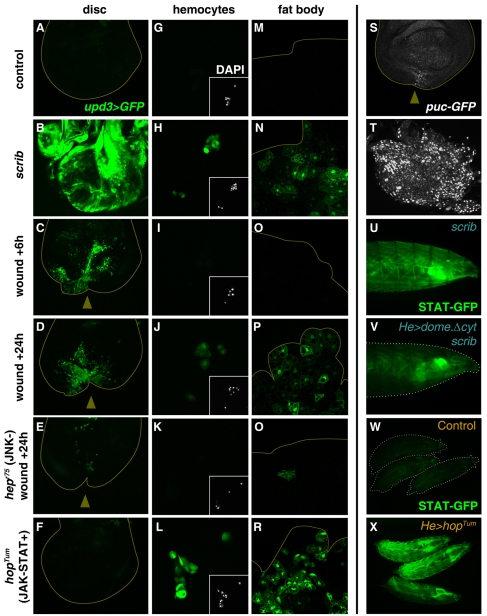
Having established the importance of JAK/STAT signaling in the response to tumors and wounds, we wanted to study how its activity is regulated to achieve a systemic reaction in response to local damage. The JAK/STAT pathway in Drosophila is activated by three closely related cytokines, with homology to human interleukins, encoded by the genes unpaired, unpaired 2 and unpaired 3 (upd, upd2 and upd3) (Agaisse et al., 2003). Therefore, we decided to investigate the expression pattern of these genes in tumors and wounds. We found that expression of upd3, monitored with an upd3-Gal4 reporter (Agaisse et al., 2003), was clearly upregulated in both scrib tumors (Fig. 7A,B) and mechanically wounded discs (Fig. 7C,D). Similarly, upd and upd2 were upregulated (supplementary material Fig. S6), indicating that tissue damage, caused by either tumors or wounds, induces local expression of all three Drosophila JAK/STAT-activating cytokines.
Fig. 7. An amplification loop in JAK/STAT signaling mediates the systemic response to tissue damage.
(A–R) Expression of upd3 (upd3-Gal4>UAS-GFP, green) in the discs (A–F), hemocytes (G–L) and fat body (M–R) of control wild-type larvae (A,G,M); scrib larvae (B,H,N); wounded larvae dissected 6 hours (C,I,O) and 24 hours (D,J,P) after wounding; wounded hepr75/Y larvae (E,K,Q); and unwounded hopTum/Y larvae (F,L,R). (S,T) Expression of the JNK target gene puckered (pucG462-GFP, white) in a wing disc 24 hours after wounding (S) and in a scrib tumor (T). (U,V) Expression of STAT-GFP (green), an activity reporter for the JAK/STAT pathway, in a living third-instar scrib larva (U) and a scrib larva with reduced JAK/STAT signaling in hemocytes (He-Gal4>UAS-domeΔcyt) (V). (W,X) Expression of STAT-GFP (green) in third-instar wild-type larvae (W) and larvae where JAK/STAT signaling in hemocytes is increased (He-Gal4>UAS-hopTum) (X).
As a possible pathway involved in expression of the Unpaired cytokines in response to tissue damage, we tested the JNK mitogen-activated protein kinase (MAPK) signaling cascade. JNK signaling has been shown to increase locally, in external wounds in the larval epidermis (Ramet et al., 2002) and in cultured imaginal discs wounded ex vivo (Bosch et al., 2005). In our in vivo system of aseptic wounding, we confirmed that JNK activity is upregulated (Fig. 7S and supplementary material Fig. S7), as shown by expression of reporters for the puckered (puc) gene, a JNK-phosphatase that lies downstream of JNK and is a negative regulator of the pathway (Martin-Blanco et al., 1998; Morin et al., 2001; Pastor-Pareja et al., 2004). Further, JNK activity was dramatically increased in scrib tumors (Fig. 7T). We checked expression of upd3 after wounding by using hepr75 larvae, which contain a strong hypomorphic mutant in the gene encoding the Drosophila JNK-kinase hemipterous (hep) (Glise et al., 1995). Twenty-four hours after wounding, expression of upd3 is either absent or reduced at the wound site in hepr75 mutant discs (Fig. 7E). Also, upd upregulation, monitored with an upd-lacZ reporter (Chao et al., 2004), was prevented when the level of JNK activity was reduced by expression of Puckered (supplementary material Fig. S8). Conversely, expression of a constitutively active form of the JNK-kinase (Hep.CA) induced ectopic expression of the upd-lacZ reporter (supplementary material Fig. S9). These results show that local activation of JNK signaling is required, and sufficient, for expression of Unpaired cytokines in wounds and tumors.
When examining upd3 expression in wounds and scrib tumors, we noticed that upd3 was expressed in the hemocytes and fat body of these larvae (Fig. 7H,J,N,P). Strong upd3 upregulation is detected in wounded wing discs within 6 hours of wounding (Fig. 7C); however, upd3 upregulation was not detected this early in the hemocytes and fat body (Fig. 7I,O), showing that upd3 expression in the damaged tissue precedes its upregulation in the hemocytes and fat body. Since JAK/STAT activity is systemically elevated as a result of wounds or tumors, we decided to examine the possibility that JAK/STAT signaling itself could be activating upd3 expression in the hemocytes and fat body in a positive feedback loop. To test this, we examined upd3 expression in hopTum larvae; this gain-of-function mutant causes abnormally high levels of JAK/STAT signaling (Harrison et al., 1995). We found that the fat body and hemocytes of hopTum mutant larvae constitutively expressed upd3 (Fig. 7L,R), indicating that activation of JAK/STAT signaling in these two cell types can induce upd3 expression. upd3 ectopic expression in hopTum larvae was not observed in imaginal discs (Fig. 7F) or any other tissues (not shown), suggesting that hemocytes and fat body cells are specifically involved in a positive feedback loop to amplify JAK/STAT activity.
Altogether, the above data support a model in which JNK signaling in damaged tissues activates the expression of JAK/STAT-activating Unpaired cytokines; this local response is subsequently amplified by additional expression of the Unpaired cytokines from the hemocytes and the fat body. Consistent with this model, reduced expression of JAK/STAT-activating cytokines at the local site of damage in hepr75 mutants resulted in reduced, or nonexistent, upd3 expression in the fat body and circulating hemocytes (Fig. 7K,O). In addition, expression of DomeΔCYT in hemocytes reduces systemic expression of the STAT reporter in larvae hosting scrib tumors (Fig. 7U,V), providing further support for the existence of this positive feedback loop in JAK/STAT signaling. Conversely, expression of HopTum in hemocytes, driven by He-GAL4, causes high systemic activation of the STAT-GFP reporter (Fig. 7W,X).
DISCUSSION
An innate immune response to tumors in Drosophila
We investigated interactions between tumors and the immune system in Drosophila, and found that malignant tumors derived from imaginal discs elicit an innate immune response in their hosts. The fly hemocytes have a central role in this response. In the presence of tumors, circulating hemocytes attach to the tumor surface (Fig. 1), increase in number (Fig. 2) and restrict tumor growth (Fig. 3). Our experiments, which were designed to further dissect the phenomenon, revealed that hemocytes react in a similar way to aseptic wounds in imaginal discs (Fig. 4) and that they attach to tumors and wounds as a consequence of BM disruption (Fig. 5).
Previous studies have suggested that Drosophila hemocytes are able to discriminate intact, self BM from disrupted or non-self BM, and showed that lamellocytes in tu(1)Sz1 mutant larvae encapsulate transplanted damaged tissues, as well as undamaged tissues from sufficiently distant drosophilid species (Rizki and Rizki, 1980). However, tu(1)Sz1 is a mutation that causes a constitutive autoimmune encapsulation response; similar reactions were not observed when transplants were made into wild-type hosts (Rizki and Rizki, 1980), leaving the relevance of these findings to the normal function of the immune system unclear. Migration of hemocytes towards septic wounds in the body wall of Drosophila embryos has previously been reported (Wood et al., 2006). Our experiments show, unambiguously, that the lack of an intact BM causes recruitment of hemocytes to a tissue. Given the vigorous circulation of the hemolymph in larvae, this recruitment of hemocytes probably results from the capture of randomly impacting hemocytes by the damaged tissue, rather than from directed hemocyte migration. In addition, hemocytes are capable of adhesion to undamaged imaginal discs; a population of hemocytes is consistently found under the posterior region of the eye imaginal disc throughout the third larval instar. In very late third-instar larvae/white prepupae, we also noticed occasional adhesion of hemocytes to wing, leg and haltere imaginal discs. The reason for normal hemocyte adhesion is not known.
The structure and components of the BM are remarkably conserved in species ranging from flies to vertebrates (Fessler and Fessler, 1989). Our work suggests that the BM, which surrounds every organ, has a general role as an indicator of tissue integrity, thus allowing the immune system to sense damage by assessing BM status. However, the proliferation of circulating hemocytes is not stimulated by BM disruption, but is actually a consequence of JAK/STAT activation within the hemocyte (Fig. 6). Consistent with this, MMP-mediated BM degradation failed to induce upd expression in the wing disc (supplementary material Fig. S9).
Our data indicate that the initial expression of Unpaired cytokines in the damaged tissue is amplified through a positive feedback loop (Fig. 7) in which JAK/STAT activation in hemocytes and the fat body causes expression of Upd 3, which then increases JAK/STAT signaling. This amplification loop causes systemic activation of JAK/STAT signaling following local damage. Since hemocytes are capable of expressing Upd 3, the resulting increase in the number of hemocytes caused by JAK/STAT activity may act as another positive input in the amplification loop. Although not addressed in this study, there are probably additional effects of systemic JAK/STAT signaling in response to tissue damage. The fat body, given its role as the main source of antimicrobial peptides in the humoral response to infection, could secrete the same products in response to both tissue damage and infection or, it could secrete tissue damage-specific products. In larvae with tumors, we observed an increase in the size of the lymph glands’ secondary lobes (not shown), suggesting that although the lymph glands were not releasing hemocytes, the hemocytes there were reacting to tissue damage by increasing their proliferation rate.
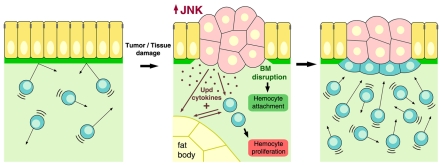
Our results show that hemocyte adhesion and hemocyte proliferation are dependent on different stimuli, namely BM disruption and secretion of JAK/STAT-activating cytokines, respectively. BM degradation by MMPs caused local recruitment of hemocytes, but did not cause an increase in the number of circulating hemocytes (Fig. 5E). Conversely, hemocytes expressing DomeΔCYT were less abundant in circulation, but still able to adhere to tumors (Fig. 3C) and wounds (not shown), suggesting that JAK/STAT activation does not affect their adhesive properties. Overall, the results support a model in which convergence of the two stimuli determines a fully effective tissue damage response (TDR), increasing the number of available hemocytes and localizing them to the damaged tissue (Fig. 8).
Fig. 8. The tissue damage response (TDR).
Schematic model depicting the TDR. Tissues damaged by tumors or wounding trigger an immune response in blood cells. The TDR involves at least two aspects. First, the damaged tissue elevates JNK activity and secretes JAK/STAT-activating cytokines (Upd, Upd2, and Upd3). JAK/STAT cytokines released from the damaged tissue activate JAK/STAT signaling in hemocytes and the fat body, which in turn activates more cytokine expression, thus amplifying the response and inducing hemocyte proliferation. Second, hemocytes adhere to the damaged region as a consequence of BM disruption. The TDR restricts tumor growth and could aid defense against infection following wounding.
In the event of a wound, the activation of an immune response to tissue damage could clearly benefit the animal by priming the immune response in preparation for the entrance of pathogens that would normally follow. Our experiments indicate that the TDR counters tumor growth (Fig. 3), although further experiments are needed to address this. Our previous work, and studies by other research groups, found that BM degradation in tumors, mediated by MMP expression (Srivastava et al., 2007; Uhlirova and Bohmann, 2006), enhances tumor growth. Insect hemocytes, unlike imaginal disc cells, are able to secrete BM components (Fessler and Fessler, 1989), providing a possible mechanism through which the immune reaction to tumors could restrict tumor growth by healing the BM. Hemocytes may also influence the growth of the tumor through phagocytic activity; hemocytes are known to phagocytose apoptotic cells during normal embryonic development (Tepass et al., 1994). We found that the incidence of apoptosis in the imaginal discs of scrib and RasV12/scrib−/− flies was not significantly different to that found in wild-type flies (not shown). However, it is known that apoptotic stimuli in immortalized Drosophila imaginal disc cells dramatically promote autonomous and non-autonomous proliferation causing aberrant overgrowths (Ryoo et al., 2004). Therefore, the removal of only a few of these half-dying or ‘undead’ cells could have a big effect in preventing further tumor growth. Additional or alternative mechanisms, involving for instance the production of reactive oxygen species or other more specific signals sent from the hemocytes to the tumor to prevent its growth, remain an interesting possibility for further studies.
Common mechanisms in the response to tumors and tissue damage
The data presented here uncovers the existence of an innate mechanism for the detection of, and response to, tissue damage in Drosophila, at work in both tumors and wounds. It has been proposed that the immune system, beyond recognition of the self versus non-self paradigm (Janeway, 1989), may not only respond to foreign or abnormal antigens, but also to so-called ‘danger signals’ (Matzinger, 1994; Matzinger, 2002). According to this model, known as the ‘danger hypothesis’, the immune system would be alerted by endogenous stress signals released from injured tissues. Recent findings seem to confirm the activation of the immune system by this proposed kind of signals (Ogura et al., 2006; Shi et al., 2003).
In species ranging from yeast to vertebrates, JNK signaling has been implicated in the response to many forms of environmental stress, including radiation, osmotic stress, redox stress and nutrient imbalance (Weston and Davis, 2007). In particular, in Drosophila imaginal discs, it has been shown that JNK activation can be induced by loss of cell-cell adhesion (Igaki et al., 2006), and by abrupt discontinuities in positional values (Adachi-Yamada et al., 1999) or proliferation rates (Moreno et al., 2002) across the tissue, resulting in apoptosis. High levels of JNK signaling are observed in the damaged tissue of both aseptic wounds and tumors. JNK signaling subsequently induces the expression of the Unpaired cytokines, thus promoting hemocyte proliferation. Physical damage from a wound naturally indicates a breakage of the BM; however, in tumors, BM degradation requires MMP expression, which is induced by JNK signaling (Srivastava et al., 2007; Uhlirova and Bohmann, 2006), suggesting that JNK activation is sufficient to induce the whole TDR program. Defining the precise molecular mechanisms by which JNK is activated in all these different situations could lead to the identification of common, or different, danger signals and should yield insights into how these seemingly diverse phenomena take place. It could also help to ascertain whether the homology between wounds and tumors extends upstream of JNK activation.
Insects and vertebrates share many innate immune mechanisms (Hoffmann et al., 1999); the response to damaged tissues, or ragged cells such as tumors, is crucial to all multicellular organisms. Spontaneous tumors in wild specimens of all the major invertebrate phyla, including insects, have been widely documented by field naturalists (Harshbarger and Taylor, 1968; Scharrer and Lochhead, 1950), a fact often overlooked in contemporary discussions of the powers and limitations of lower organisms as cancer models. Given that the main factors involved in the Drosophila TDR, which we have characterized, such as MMPs (Page-McCaw et al., 2003), BM molecules (Fessler and Fessler, 1989), stress sensing through JNK (Stronach and Perrimon, 1999) and cytokine-activated JAK/STAT signaling (Hombria and Brown, 2002), are all conserved in humans, similar TDR mechanisms may exist in humans and other vertebrates. Based on our findings, we believe that Drosophila will provide a powerful system to further the understanding of innate immune reactions to cancers and wounds in humans.
METHODS
Fly strains and culture
The following strains were used: y w,ey-FLP1;AyGAL4,UAS-GFP.S65T;FRT82B,Tub-Gal80; w;UAS-RasV12;FRT82B,scrib1/TM6B; y w,ey-FLP1;AyGAL4,UAS-myrRFP,vkgG454/CyO;FRT82B,Tub-Gal80; w;FRT82B,scrib1/TM6B; w1118; ecd1 st1 ca1; w;nub-GAL4.K; w;UAS-GFP.S65T; w;vkgG454/CyO; w;He-GAL4.85; w;UAS-myr-RFP/TM6B; w;UAS-myr-RFP (II); w;ptc-GAL4; w;10XSTAT92E-GFP.1; w;UAS-dome. Δcyt.2.1; y w;UAS-hopTum/CyO; w,upd-lacZ; w,UAS-hid; y w hepr75/FM7i,act-GFP; w;UAS-puc (III); w;UAS-Mmp2 (III); w;upd3-GAL4,UAS-GFP.S65T/CyO; y w hepr75/FM7i,act-GFP; y v hopTum-l/ Basc; w;pucG462/TM6B; w;UAS-GFP.S65T;pucE69-I-Gal4/TM6B; w;UAS-hep.CA (III).
He-Gal4 was chosen as a driver for hemocyte marking and overexpression. Using He-Gal4 to drive GFP expression under the set conditions of our experiments (heterozygous, 25°C), we did not observe GFP signals in other tissues that could be involved in the immune response, such as the imaginal discs, fat body or larval epidermis. We confirmed this by crossing w;He-Gal4,UAS-GFP flies to a strain bearing a flip-out cassette (actin-FRT-y+-FRT-Gal4) and a UAS-Flipase construct, which amplifies and permanently marks all of the lineage of cells that express Gal4 during development. Additionally, these tissues, unlike hemocytes, were unaffected when expression of Hid was driven with He-Gal4, further supporting the lack of a significant amount of Gal4 expression.
The genotypes of the animals employed in each experiment are detailed in the supplementary material. Whenever staging of the larvae was required, parental flies were transferred to a fresh vial and left there to lay eggs for 1 day; we considered the time of removing the flies from the vial to be 12 hours (±12) AEL (after egg laying). Careful attention was given to avoiding overcrowding in these vials, since this can cause delayed and asynchronous development of the larvae. Cultures were maintained at 25°C, except for ecd1 mutant cultures, which were placed at 29°C (restrictive temperature) for 5 days AEL.
Hemocyte counts
Larvae were washed in water, dried and bled by tearing the larval epidermis with two pairs of forceps into an 11 μl drop of PBS placed over a Sylgard (Dow Corning) plate. To maximize the recovery of hemolymph and circulating cells, the larvae were torn inside out, into the drop. The liquid was mixed and loaded into a hemocytometer for immediate counting. For each sample, the hemocytes in five squares (0.1 mm3 each) were counted and added up. Lamellocyte counts in RasV12/scrib−/− and scrib larvae represented 1-2% of the total number of circulating hemocytes (not shown), similar to wild-type and ecd1 animals. Crystal cells in circulation in scrib and RasV12/scrib−/− larvae were very rare, as for wild-type flies, which was consistent with the fact that signs of melanization were not observed in these animals. At least 14 larvae were bled and analyzed for each genotype, time point or wounding experiment. In all cases, the distributions of the measurements were found to fit a normal distribution according to K-S tests. We found it very hard to reliably estimate the amount of hemolymph in a larva, owing to its small volume and the interstitial liquid retained by the larval tissues after bleeding. To measure hemocyte density, we used the number of hemocytes per mg of fresh larval weight. To measure larval weight, we weighed 12 larvae together in a Mettler AE100 microbalance and averaged the figure to obtain the following typical larval weights (mg): wild-type 6 days AEL, 1.51; ecd1 6 days AEL, 1.53, 10 days AEL, 2.13, 14 days AEL, 3.19; scrib 10 days AEL, 2.37, 14 days AEL, 3.46; RasV12/scrib−/− 10 days AEL, 2.28, 14 days AEL, 3.28. We used these weights to normalized the absolute hemocyte counts shown in Fig. 2A.
In situ wounding
Mid and late third-instar larvae were operated on whilst immersed in water in a petri dish that also contained ice to decrease larval movement. In situ wounding was performed by gently holding the larva in position with one pair of forceps while closing a second pair of forceps (Dumont #5 straight tips) over the larval epidermis and the underlying disc. Operated larvae were transferred to a fresh vial and left to develop at 25°C. Most larvae reach pupation around 24 hours after performing the operation. Wounded larvae were able to develop into normal adults that present defects only in the operated wings.
Stainings and imaging
Staining tissues with antibody was performed according to the standard procedures used for imaginal discs. The following primary antibodies and dyes were used: rabbit polyclonal anti-Srp (1:200 dilution, Deborah Keiko-Hoshizaki), mouse monoclonal P1 antibody (1:1000, Istvan Ando), mouse monoclonal L1 antibody (1:1000, Istvan Ando), mouse polyclonal anti-βgal (1:500, Sigma), goat Alexa 635-conjugated anti-mouse IgG (1:200, Molecular Probes), goat Alexa 545-conjugated anti-mouse IgG, goat Alexa 488-conjugated anti-mouse IgG, phalloidin-TxR (1:50, stock solution dissolved in DMSO instead of methanol, Molecular Probes). The samples were mounted in Vectashield or DAPI-Vectashield (Vector Labs). Confocal images were taken in a Zeiss LSM510 Meta confocal microscope.
For anti-PH3 staining of circulating hemocytes, four larvae of every genotype were each bled into 5 μl of PBS on a separate glass slide (12 slides). Hemocytes were allowed to settle and attach to the glass for 10 minutes and then fixed with 4% formaldehyde for an additional 10 minutes. Hemocytes were then stained with rabbit polyclonal anti-PH3 (Ser10) antibody (1:1000, Upstate). Four different 10× micrographs per animal were taken and hemocytes counted (>5000 per genotype).
STAT-GFP expression in living hemocytes was imaged by bleeding larvae into 10 μl of PBS on a slide and placing a coverslip on top of the liquid, after adding and mixing 1 μl of DAPI-Vectashield for immediate imaging. The same results were obtained using fixed hemocytes.
Living larvae were immobilized by the tension created by water between a slide and a coverslip, and imaged in a Leica MZ FLIII fluorescence stereomicroscope with a Leica DFC300FX camera.
Tumor size measurement
Tumors were mounted in DAPI-Vectashield and confocal micrographs were taken of the DAPI signal at sample mid-depth. The area occupied by the tumor was measured with the program ImageJ (NIH). Measurement distributions passed K-S tests for normality.
Supplementary Material
ACKNOWLEDGEMENTS
We thank E. Bach, A. Page-McCaw, D. Keiko-Hoshizaki, I. Ando, H. Agaisse, L. Cooley and the Bloomington Stock Center for providing antibodies and fly strains, and R. A. Flavell, H. Agaisse, T. Igaki and T. Xu lab members for reading and discussing the manuscript. J.C.P.-P. was funded by a Spanish Ministry of Education postdoctoral fellowship. M.W. is a Yale predoctoral fellow. This work was supported by a grant from NIH/NCI to T.X. T.X. is a Howard Hughes Medical Institute Investigator.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
J.C.P.-P., M.W. and T.X. designed research. J.C.P.-P. and M.W. performed research. J.C.P.-P. and T.X. wrote the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/content/1/2-3/144/suppl/DC1
REFERENCES
- Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K. (1999). Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature 400, 166–169 [DOI] [PubMed] [Google Scholar]
- Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. (2003). Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell 5, 441–450 [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. (2001). Inflammation and cancer: back to Virchow? Lancet 357, 539–545 [DOI] [PubMed] [Google Scholar]
- Bilder D, Perrimon N. (2000). Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676–680 [DOI] [PubMed] [Google Scholar]
- Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. (1994). Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 12, 337–365 [DOI] [PubMed] [Google Scholar]
- Bosch M, Serras F, Martin-Blanco E, Baguna J. (2005). JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 280, 73–86 [DOI] [PubMed] [Google Scholar]
- Brennan CA, Anderson KV. (2004). Drosophila: the genetics of innate immune recognition and response. Annu. Rev. Immunol. 22, 457–483 [DOI] [PubMed] [Google Scholar]
- Brown S, Hu N, Hombria JC. (2001). Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr. Biol. 11, 1700–1705 [DOI] [PubMed] [Google Scholar]
- Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, van’t Veer LJ, Bartelink H, et al. (2005). Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc. Natl. Acad. Sci. USA 102, 3738–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JL, Tsai YC, Chiu SJ, Sun YH. (2004). Localized Notch signal acts through eyg and upd to promote global growth in Drosophila eye. Development 131, 3839–3847 [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. (2006). Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 6, 24–37 [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. (2002). Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 [DOI] [PubMed] [Google Scholar]
- Dvorak HF. (1986). Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 [DOI] [PubMed] [Google Scholar]
- Ekas LA, Baeg GH, Flaherty MS, Ayala-Camargo A, Bach EA. (2006). JAK/STAT signaling promotes regional specification by negatively regulating wingless expression in Drosophila. Development 133, 4721–4729 [DOI] [PubMed] [Google Scholar]
- Evans CJ, Hartenstein V, Banerjee U. (2003). Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell 5, 673–690 [DOI] [PubMed] [Google Scholar]
- Fessler JH, Fessler LI. (1989). Drosophila extracellular matrix. Annu. Rev. Cell Biol. 5, 309–339 [DOI] [PubMed] [Google Scholar]
- Garen A, Kauvar L, Lepesant JA. (1977). Roles of ecdysone in Drosophila development. Proc. Natl. Acad. Sci. USA 74, 5099–5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. (2001). Regulation of cutaneous malignancy by gamma delta T cells. Science 294, 605–609 [DOI] [PubMed] [Google Scholar]
- Glise B, Bourbon H, Noselli S. (1995). hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell 83, 451–461 [DOI] [PubMed] [Google Scholar]
- Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. (1995). Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14, 2857–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshbarger JC, Taylor RL. (1968). Neoplasms of insects. Annu. Rev. Entomol. 13, 159–190 [Google Scholar]
- Hartenstein V. (2006). Blood cells and blood cell development in the animal kingdom. Annu. Rev. Cell Dev. Biol. 22, 677–712 [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. (1999). Phylogenetic perspectives in innate immunity. Science 284, 1313–1318 [DOI] [PubMed] [Google Scholar]
- Holz A, Bossinger B, Strasser T, Janning W, Klapper R. (2003). The two origins of hemocytes in Drosophila. Development 130, 4955–4962 [DOI] [PubMed] [Google Scholar]
- Hombria JC, Brown S. (2002). The fertile field of Drosophila Jak/STAT signalling. Curr. Biol. 12, R569–R575 [DOI] [PubMed] [Google Scholar]
- Igaki T, Pagliarini RA, Xu T. (2006). Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16, 1139–1146 [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr (1989). Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54 Pt 1, 1–13 [DOI] [PubMed] [Google Scholar]
- Kurucz E, Markus R, Zsamboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, Udvardy A, Krausz I, Lukacsovich T, Gateff E, et al. (2007). Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr. Biol. 17, 649–654 [DOI] [PubMed] [Google Scholar]
- Lebestky T, Chang T, Hartenstein V, Banerjee U. (2000). Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 288, 146–149 [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. (2007). The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743 [DOI] [PubMed] [Google Scholar]
- Llano E, Adam G, Pendas AM, Quesada V, Sanchez LM, Santamaria I, Noselli S, Lopez-Otin C. (2002). Structural and enzymatic characterization of Drosophila Dm2-MMP, a membrane-bound matrix metalloproteinase with tissue-specific expression. J. Biol. Chem. 277, 23321–23329 [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. (1998). puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12, 557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. (1994). Tolerance, Danger, and the Extended Family. Annu. Rev. Immunol. 12, 991–1045 [DOI] [PubMed] [Google Scholar]
- Matzinger P. (2002). The danger model: a renewed sense of self. Science 296, 301–305 [DOI] [PubMed] [Google Scholar]
- Meister M, Lagueux M. (2003). Drosophila blood cells. Cell Microbiol. 5, 573–580 [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, Morata G. (2002). Cells compete for Decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755–759 [DOI] [PubMed] [Google Scholar]
- Morin X, Daneman R, Zavortink M, Chia W. (2001). A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA 98, 15050–15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JD, Werb Z. (2004). Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 16, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Sutterwala FS, Flavell RA. (2006). The inflammasome: first line of the immune response to cell stress. Cell 126, 659–662 [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Serano J, Sante JM, Rubin GM. (2003). Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev. Cell 4, 95–106 [DOI] [PubMed] [Google Scholar]
- Pagliarini RA, Xu T. (2003). A genetic screen in Drosophila for metastatic behavior. Science 302, 1227–1231 [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Grawe F, Martín-Blanco E, García-Bellido A. (2004). Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev. Cell 7, 387–399 [DOI] [PubMed] [Google Scholar]
- Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. (2007). A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 3, e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramet M, Lanot R, Zachary D, Manfruelli P. (2002). JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol. 241, 145–156 [DOI] [PubMed] [Google Scholar]
- Rizki RM, Rizki TM. (1980). Hemocyte responses to implanted tissues in Drosophila melanogaster larvae. Dev. Genes Evol. 189, 207–213 [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. (2004). Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev. Cell 7, 491–501 [DOI] [PubMed] [Google Scholar]
- Scharrer B, Lochhead MS. (1950). Tumors in the invertebrates: a review. Cancer Res. 10, 403–419 [PubMed] [Google Scholar]
- Shi Y, Evans JE, Rock KL. (2003). Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521 [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. (2000). Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191, 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. (2007). Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc. Natl. Acad. Sci. USA 104, 2721–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach BE, Perrimon N. (1999). Stress signaling in Drosophila. Oncogene 18, 6172–6182 [DOI] [PubMed] [Google Scholar]
- Tepass U, Fessler LI, Aziz A, Hartenstein V. (1994). Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development 120, 1829–1837 [DOI] [PubMed] [Google Scholar]
- Uhlirova M, Bohmann D. (2006). JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25, 5294–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilmos P, Nagy I, Kurucz E, Hultmark D, Gateff E, Ando I. (2004). A rapid rosetting method for separation of hemocyte sub-populations of Drosophila melanogaster. Dev. Comp. Immunol. 28, 555–563 [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. (2007). The JNK signal transduction pathway. Curr. Opin. Cell Biol. 19, 142–149 [DOI] [PubMed] [Google Scholar]
- Wood W, Faria C, Jacinto A. (2006). Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J. Cell Biol. 173, 405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. (2004). A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101, 14192–14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR. (1997). Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc. Natl. Acad. Sci. USA 94, 5131–5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.